3.2. Bioink Materials
Two major types of bioink materials have been used in bioprinting of 3D tissue and organ constructs (Ozbolat, 2015a). The first and most common one is scaffold-based bioink where cells are loaded in hydrogels or similar exogenous materials and bioprinted into 3D constructs. Cell-laden hydrogels allow cell proliferation and growth and facilitate formation of tissue. In the second type of bioink, cells are bioprinted without the use of an exogenous biomaterial, in a scaffold-free process mimicking embryonic development. Cells are first formed into neotissues that are engineered for bioprinting processes; resulting neotissues are then deposited in specific patterns where they fuse and mature for fabrication of larger scale functional tissues.
3.2.1. Scaffold-Based Bioink Materials
3.2.1.1. Hydrogels
A class of crosslinked polymeric substances capable of absorbing and retaining large quantities of water are generally referred to as hydrogels. Hydrogels in tissue engineering are classified into two groups: naturally derived hydrogels such as gelatin, fibrin, collagen, chitosan, and alginate and synthetically derived hydrogels such as Pluronic® or polyethylene glycol (PEG). They are used in biofabrication and tissue engineering for a wide array of applications such as drug delivery (Yang et al., 2011), contact lenses (White et al., 2011), and wound dressings (Kamoun et al., 2015). Some are able to mimic the native tissue environment as they possess several essential features of the native ECM components (Tibbitt and Anseth, 2009). These ECM-like properties allow cell encapsulation in a highly hydrated, mechanically strong 3D environment; however, both natural and synthetic hydrogels have some limitations. Natural hydrogels generally have weak mechanical properties, while synthetic counterparts lack major components such as bioactive molecules for cell adhesion or migration (Zhu and Marchant, 2011). Biocompatibility of hydrogels is defined by their hydrophilicity. Hydrogels can absorb up to 1000 times their original weight in aqueous medium without dissolving (Ahmed, 2013), making them ideal for cell encapsulation. Because they are highly permeable to oxygen, nutrients, and other water-soluble compounds, hydrogels are attractive materials for fabrication of tissue constructs (Thomas et al., 2009; Zhu and Marchant, 2011). Finally, hydrogels provide 3D niches for embedded cells, which mimic their native tissue environment (Rajan et al., 2006; Drury and Mooney, 2003; Yamamoto et al., 2010; Benedikt et al., 2000).Distinct features of natural and synthetic hydrogels cannot be clearly stated; however, most of natural hydrogels are cell friendly.
3.2.1.1.1. Bioprintability of Hydrogels
Over the past few decades, numerous hydrogels have been prepared by altering the chemical backbone of polymers for tissue engineering applications. However, not all hydrogels can be considered “bioprintable.” Bioprintability of hydrogels is governed by their rheological properties and the target bioprinting modality. According to their bioprinting mechanisms, bioprinting processes can be classified under three major modalities: extrusion-based bioprinting (EBB), droplet-based bioprinting (DBB), and laser-based bioprinting (LBB) (Gudapati et al., 2016; Ozbolat and Hospodiuk, 2016). Each bioprinting modality has different bioink requirements according to the bioprinting mechanism employed (see Fig. 3.1).
EBB employs pneumatic-, mechanical-, or solenoid-driven microextrusion along with a computer-controlled writing process. Hydrogels used in EBB broadly fall under the category of non-Newtonian fluids, where viscosity is strongly dependent upon shear rate (Jungst et al., 2015). Hydrogels with shear thinning and thixotropic behavior are well suited for EBB processes. In shear thinning hydrogels, shear forces align the random polymer chains in a favorable direction making them extrudable. Thixotropy, a time-dependent shear thinning behavior, enables the bioink to assume a stable form at rest in the barrel, exhibit low viscosity inside the nozzle tip during extrusion, and regain its stability postbioprinting. In addition, the bioink should possess low adhesion and surface tension properties to eliminate its attachment on the surface of the nozzle tip and easily overcome the surface tension–driven droplet formation enabling successful filament extrusion. In addition, the bioink should have rapid gelation characteristics so that it can retain its shape without spreading. Moreover, appropriate substrate (with high surface roughness and low wettability) should be in place so that the bioink can stick to the substrate and retain its shape.
DBB utilizes various energy sources such as electric, sound, and heat to generate droplets of bioink in a high-throughput manner. According to their droplet generation mechanisms, DBB processes can be classified under four groups: inkjet (thermal, piezoelectric, or electrostatic) bioprinting, electrohydrodynamic jetting, acoustic droplet election, and microvalve bioprinting. In general, the bioink used in DBB should have low viscosity and a nonfibrous nature so that it can easily flow through the tubing system and nozzle without clogging problems. In addition, the bioink needs to possess a rheopectic behavior, which is a time-dependent dilatant behavior resulting in increased viscosity as shear is applied triggering droplet formation due to an increase in viscosity following ejection. The bioink should also have appropriate surface tension. It should have sufficient wettability to travel through the cartridge correctly but not leak out, flooding of the print head and wetting the exterior of the nozzle tip. In addition, the droplets should solidify immediately after landing. Droplet–substrate interactions are also important as appropriate substrate surface properties are needed to prevent spreading, splashing, or rebounding of droplets as extensively discussed in Chapter 5.
LBB utilizes laser energy for fabrication of tissue constructs or high-precision patterning of biologics and can be classified under two groups: processes based on cell transfer [i.e., laser-guided direct writing (Odde and Renn, 1999), matrix-assisted pulsed laser evaporation-direct write (MAPLE-DW) (Riggs et al., 2011), or laser-induced forward transfer (LIFT) (Michael et al., 2013)] and processes involving photopolymerization [i.e., stereolithography (SLA) (Arcaute et al., 2010), dynamic optical projection stereolithography (DOPsL) (Zhang et al., 2012), or two-photon polymerization (2PP) (Ovsianikov et al., 2014)]. In the former approach, the bioink is transferred from a cartridge to a substrate by laser-induced jet formation; however, in the latter approach, the laser beam selectively solidifies a photocurable bioink material (in a vat) through polymerization. The bioink used for cell transfer processes should possess sufficient adhesion and low surface tension characteristics so that it can uniformly spread on the intermediate layer and adhere to it without dripping. The bioink should easily transfer thermal energy into kinetic energy and exhibit high viscoelasticity so that well-defined jets can be formed with rest of the bioink maintained in the cartridge. The bioink needs to have a rapid gelation capability so jets can solidify without spreading. Moreover, jet–substrate interactions are also important (similar to DBB); therefore, an appropriate substrate should be selected to prevent spreading and splashing of the jets. For bioink in processes involving photopolymerization, photopolymerizable hydrogels should be used. The bioink should be further reinforced with nontoxic water-soluble photoinitiators and light absorbers to initiate photopolymerization and enable fabrication of tissue constructs with uniform layer thickness. Stability and high-mechanical strength as well as the ability to retain cells uniformly distributed in the precursor solution are other important requirements of such bioink selection.
In addition, the bioink should possess an appropriate gelation mechanism driven by chemical, physical, or enzymatic crosslinking as outlined in the following section.
3.2.1.1.2. Crosslinking Mechanisms of Hydrogels
3.2.1.1.2.1. Physical Crosslinking
In the field of tissue engineering, there has been a growing interest in polymers which can be effectively crosslinked without the use of any exogenous agents, thus minimizing the risk of chemical contamination or chemically induced toxicity (Hennink and van Nostrum, 2012). Apart from obvious advantages, such polymeric hydrogels foster a more congenial environment for embedded cells, proteins, and other biologics. Ionic, hydrophobic, and hydrogen bonding interactions, stereocomplexation, self-assembly of amphiphilic peptides or polymers into micellar structures are some of the well-established mechanisms which are known to drive physical crosslinking of hydrogels (Jungst et al., 2015).
Ionic crosslinking involves the association of polymer chains by noncovalent interactions. A crosslinked hydrogel network is formed when molecules containing opposite charges are blended, e.g., polyelectrolyte solution and multivalent ions (Gulrez et al., 2011).The ions of opposite charges electrostatically attract each other giving rise to a crosslinked polymeric network. The network can also be disrupted by using specific chelators to remove the multivalent ions from the polymeric network to reverse the gelation process.
In some hydrogels, hydrophobic interactions or hydrogen bonding interactions play a significant role in crosslinking. These interactions often tend to be temperature dependent and alter the rheology of the hydrogel network with changing temperature. Some hydrogels exhibit a random coil conformation at high temperatures; upon lowering the temperature, the polymer chains adapt a more ordered conformation forming junction points and consequently aggregating as physical gels (Jeong et al., 2002). Others undergo the reverse process. In reverse gelation, polymeric strands mostly contain both hydrophobic and hydrophilic regions (Pradines et al., 2015). At low temperatures, water (solvent) molecules dissolve the hydrophilic parts of the molecule leading to complete dissolution of monomer chains. There is a strong binding affinity between water molecules and monomeric chains, leading to highly ordered water molecules along the monomeric chains. The forces of interaction here are primarily hydrogen bonding forces, where the binding is an enthalpy-driven process; however, when the temperature increases, entropy overcomes enthalpy as water molecules become randomly oriented and hydrophobic interactions between the monomeric chains become predominant. Thus the chains dehydrate as the water molecules break free. With the increase in temperature, the polymeric interactions become more dominant as compared to the hydrogen bonding forces.
The association of disordered molecules into an ordered state by virtue of its nature or by electrostatic or covalent interactions is referred to as “self-assembly,” which is a primary mechanism of gelation observed in amphiphilic peptide molecules (Hauser et al., 2015). Synthetic peptides with a cationic polar head group and anionic groups along its backbone undergo rapid self-assembly from a random coil configuration to form ordered α helices and β sheets. With increased concentration, β sheets undergo further self-assembly to form nanofibrous networks that bear close resemblance to the native ECM. Some of the most commonly employed physically crosslinked hydrogels in bioprinting are agarose, alginate, chitosan, collagen, gelatin, Matrigel™, and Pluronic®, which are further discussed in Section 3.2.1.1.3 in detail.
3.2.1.1.2.2. Chemical Crosslinking
Chemically crosslinked hydrogels, characterized by covalent bonding between polymer chains, often provide better mechanical stability compared to physically crosslinked ones. Chemical crosslinking can involve exogenous crosslinking agents or formation of reactive species by photoirradiation; however, the use of a crosslinker can induce undesirable reactions with the hydrogel surface or result in cytotoxicity (Hennink and van Nostrum, 2012). Some of the widely studied mechanisms for chemical crosslinking of polymers include condensation reactions, crosslinking by Schiff base formation (a compound formed by the nucleophilic addition of an amine and a carbonyl group) (Dragan, 2014), or photo-crosslinking (Wang et al., 2015b).
Schiff base is a compound formed by the nucleophilic addition of an amine to a carbonyl functional group. Amine containing amino acids, natural polysaccharides, or other synthetic polymers can be easily crosslinked under mild reaction conditions in the presence of crosslinkers containing aldehyde functionalities such as glutaraldehyde or other polyaldehydes (Hennink and van Nostrum, 2012). Other complementary functional groups that can also react with aldehydes to form crosslinked hydrogels include alcohol and hydrazides (Hennink and van Nostrum, 2012). The degree of crosslinking depends on the concentration of the crosslinker used. A high degree of crosslinking results in a hydrogel with strong mechanical properties; however, this reduces the degradation time of the hydrogel. Release of drugs, growth factors, or other biologics immobilized in a bioprinted hydrogel matrix might take longer to diffuse into the surrounding tissue region due to stronger encapsulation between the chemically crosslinked polymer chains. Some frequently used crosslinking agents (i.e., glutaraldehyde) are used to induce chemical crosslinking reactions between complementary functional groups. While glutaraldehyde might induce cytotoxicity (Takigawa and Endo, 2006), genipin is a benign naturally derived crosslinker, which has been used to crosslink gelatin (Bigi et al., 2002), collagen (Yan et al., 2010), chitosan (Chen et al., 2004; Moura et al., 2011), and fibrin (Gamboa-Martinez et al., 2015). The amine groups of the amino acids present in fibrin undergo a nucleophilic, ring closure type of reaction to form crosslinked hydrogels with genipin (Gamboa-Martinez et al., 2015).
Photopolymerization is one of the widely used crosslinking mechanisms, where low molecular weight monomers or oligomers undergo a process called curing to form a crosslinked polymeric network, when exposed to radiation29. The crosslinking process is initiated in the presence of a photoinitiator that forms excited molecular species on irradiation with light and onsets the polymerization process. Often pure hydrogels do not crosslink by themselves unless an external initiator is added or the molecule is chemically modified with functional groups capable of undergoing free radical polymerization. The physical properties of the hydrogel can be efficiently controlled by proper modulation of the rate and degree of crosslinking by photopolymerization. Some commonly used photoinitiators in bioprinting include Irgacure (2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl]-2-methyl-1-propanone) (Aubin et al., 2010), (Wang et al., 2015b), VA-086 (2, 2′-azobis [2-methyl-N-(2-hydroxyethyl) propionamide]) (Billiet et al., 2014), and Biokey (lithium phenyl-2,4,6-trimethylbenzoylphosphinate) (Fairbanks et al., 2009). While some photoinitiators produce free radicals via unimolecular bond cleavage, others undergo bimolecular reactions by producing excited species which undergo collision with another initiator molecule to generate free radicals. Irgacure is the most widely used photoinitiator in the bioprinting community, but it is only nontoxic below a certain threshold quantity. Even at concentration of 0.5% (w/v), this initiator has proven to be extremely detrimental to cells unless the excess photoinitiator is leached out of fabricated constructs (Arcaute et al., 2006). A few important requirements for photoinitiators are their water solubility, type of radiation, and exposure time required to generate free radicals. Although Irgacure is water soluble, it requires the use of ultraviolet (UV) light which is detrimental to cells with prolonged exposure. Long-term exposure to UV can damage the DNA, can induce undesired crosslinking, or affect cell functionality. In contrast, the use of photoinitiators such as Eosin Y and Biokey is advantageous as crosslinking can occur in visible light within a short span of time (Fairbanks et al., 2009). Using a low concentration (<0.1%) photoinitiator, as recommended by some studies (Fedorovich et al., 2009), can minimize toxicity but diminishes mechanical properties. Low mechanical properties often give rise to increased swelling and enhanced depth of curing, which deteriorates the bioprinted construct quality. Methacrylated gelatin (GelMA) and PEG are two of commonly used polymers capable of undergoing photopolymerization.
3.2.1.1.2.3. Enzymatic Crosslinking
The most popular enzymatically -crosslinked hydrogel in tissue engineering is fibrin (Scheraga, 2004; Benedikt et al., 2000). It is composed of fibrinogen and thrombin, the major precursors involved in blood clotting. Thrombin is a serine protease that converts fibrinogen, a complex glycoprotein, into fibrin. Cytocompatibility and cell adhesive properties make fibrin a widely used bioink in bioprinting (Ehsan et al., 2014; Yu et al., 2015; Lee et al., 2010b). It is possible to manipulate the integrity of other hydrogels, such as gelatin, by using transglutaminase, a bacterial enzyme (Gómez-Guillén et al., 2011). Transglutaminase catalyzes the formation of isopeptide bonds by a transamidation-mediated reaction between the gamma-carbonyl group of a glutamine residue and the epsilon-amino group of a lysine residue (Sakai et al., 2009). Thrombin and transglutaminase are Ca2+-dependent enzymes.
3.2.1.1.3. Hydrogels Used in Bioprinting
Bioprintability of hydrogels is a key attribute to the success of 3D bioprinting; each hydrogel brings different properties and requirements to the bioprinting process. Hydrogels used in bioprinting technology are herein described in detail with respect to their chemical content, crosslinking behavior, biocompatibility, and bioprintability under EBB, DBB, and LBB.
3.2.1.1.3.1. Natural Hydrogels
Agarose is a naturally derived polysaccharide molecule which undergoes gradual gelation at low temperatures and liquefies at the temperatures ranging from 20 to 70°C, depending on the hydroxyethylation (Serwer et al., 1983; Tako and Nakamura, 1988). It has been suggested that the gelation mechanism goes through three stages, namely initiation, nucleation, and pseudoequilibrium (Tako and Nakamura, 1988). In solid state, agarose is brittle, but maintains its shape for a long period of time at a broad range of temperatures. Agarose is not as cell friendly as alginate and Pluronic®, as cell proliferation rate and the biosynthesis of cell components is limited (Fedorovich et al., 2008). Low cell adhesion and spreading suggest that agarose is a poor material for cell culture; however, it serves well as a mold material for 3D culture of cell aggregates (Livoti and Morgan, 2010). Its biological properties can, however, be improved by blending with other hydrogels such as collagen (Duarte Campos et al., 2014). Agarose has been used in EBB providing superior stability and construct thickness when compared to collagen/agarose blends. Cell viability was maintained; however, cells retained a round as opposed to a spread morphology (Duarte Campos et al., 2014). In addition, agarose has been printed for construction of support structures to facilitate aggregation of bioprinted cell aggregates (see Fig. 3.2A). Agarose has a thermally reversible feature and can be used as a sacrificial material in a bulky scaffold of a thermally stable hydrogel. It can be liquefied and drained, leaving a hollow, perfusable structure (Lee et al., 2010a). Agarose has been used in DBB, particularly in microvalve bioprinting of mammalian cells yielding high cell viability (Xu et al., 2005). For microvalve bioprinting, a temperature controller is required to keep agarose in liquid state. In general, its viscous nature does not allow inkjet bioprinting as it can easily clog the nozzle. Agarose is a promising candidate for LBB due to its viscoelastic nature and rapid gelation mechanism. It has been deposited using LIFT technology, successfully maintaining high cell viability (Koch et al., 2010).
Alginate is a popular hydrogel used in bioprinting processes due its biocompatibility, various choices of crosslinking and bioprinting methods, low price, and ease of use in the creation of 3D structures (Ozbolat and Hospodiuk, 2016). It is a polysaccharide made of alternating β-D-mannuronate (M) and its C-5 epimer α-L-guluronate (G) units (Jungst et al., 2015). The G subunits are responsible for forming the gel phase of alginate. As the number of G subunits increase, the degree of gelation increases. Alginate undergoes ionic crosslinking in calcium chloride (CaCl2) or calcium sulfate (CaSO₄) solutions. The divalent calcium ions form a bridge, due to the attraction of negatively charged carboxylic acid groups between two neighboring alginate chains (Lai et al., 2016). Alginate has been widely used for cell encapsulation, including neural stem cells (Purcell et al., 2009), skeletal myoblasts (Hill et al., 2006), and articular chondrocytes (Alsberg et al., 2002; Ab-Rahim et al., 2013). Moreover, alginate provides favorable conditions for adipogenic differentiation and does not alter cell morphology (Galateanu et al., 2012). In addition to cell encapsulation, alginate has been successfully used as a 3D matrix for in vitro culture of organoids and embryos, i.e., pancreatic islets (Tomei et al., 2014). However, despite the intrinsic properties of alginate that make it a favorable material for tissue engineering applications, chemical modifications are often required to promote desirable cellular functions, provide a greater range of mechanical properties, and facilitate controlled release of encapsulated factors. It was shown that, due to the highly hydrophilic nature of alginate, proteins are minimally adsorbed thus hampering cell attachment (Rowley et al., 1999). This can be overcome by modifying the alginate surface with peptides, such as RGD, to provide molecule binding sites for cell adhesion. EBB of alginate has been one of the most popular techniques in the bioprinting community (Ahn et al., 2012; Ozbolat and Koc, 2010; Yu et al., 2013). Alginate has several advantages for use in EBB, including the ease of crosslinking and a wide range of concentrations with superior mechanical properties. Alginate can be extruded in one of two forms, precursor and pre–crosslinked, depending on the application. Alginate in 2–4% (w/v) is extrudable, structurally stable and biologically acceptable and, after contacting the crosslinker, solidifies rapidly and maintains its 3D shape (see Fig. 3.2B) (Zhang et al., 2013a,b; Zhang et al. 2015; Ozbolat et al., 2014; Dolati et al., 2014). The concentration of alginate determines the viscosity of the solution, porosity and crosslinking time. It is possible to form pre-crosslinked alginate by mixing with low concentrations of the crosslinker, which increases its printability (Cohen et al., 2010b). Depending on the purpose, the bioprinted constructs can be strengthen by further addition of the crosslinker. DBB of alginate is feasible as long as its concentration allows for droplet formation [in general <2% in inkjet bioprinting (Gudapati et al., 2016)]. Exploiting the crosslinking mechanism of alginate with CaCl2, heterogeneous tissue constructs comprised of amniotic fluid–derived stem cells (AFSCs), detrusor smooth muscle cell, and biliary epithelial cells were fabricated (Xu et al., 2013). Mechanical properties of bioprinted constructs can also be improved by combining alginate with other functional materials. For example, bifurcated vascular tissue constructs were fabricated within fluorocarbon employing a blend of 3% (w/v) low melting agarose and 3% (w/v) low viscosity alginate (Blaeser et al., 2013). The buoyant forces of the fluorocarbon solution supported soft tissue constructs. Similarly, zigzag cellular constructs were fabricated by printing 3T3 fibroblast-loaded alginate solution into a CaCl2 crosslinker pool, where the crosslinker provided buoyancy support (Xu et al., 2012). Using an opposite configuration, another work also demonstrated bioprinting of alginate; heartlike tissue constructs were fabricated by bioprinting the crosslinker (CaCl2) solution into an alginate pool using a modified HP inkjet printer (Xu et al., 2009). LBB with alginate of different concentrations has been widely described using MAPLE-DW, where an alginate suspension containing cells was placed on the ribbon then pulsed with a laser (Guillotin et al., 2010; Yan et al., 2013; Gudapati et al., 2014). Successful bioprinting depended upon alginate concentration and viscosity, surface tension, the bioink layer thickness coated onthe intermediate layer, gelation times, wettability, and laser fluence (Gudapati et al., 2014).

Figure 3.2 Bioprinted Hydrogels.
(A) Agarose filaments bioprinted to enclose tissue spheroids (Reproduced/adapted with permission from Norotte et al. (2009)); (B) three-dimensional (3D) printed alginate in brain shape with recapitulated anatomical features (Reproduced/adapted from Hinton et al. (2015)); (C) chitosan scaffold after 4 weeks of culture (Reproduced/adapted with permission from Ye et al. (2014)); (D) collagen type I construct for skin tissue regeneration implanted on a wound (Reproduced/adapted from Michael et al. (2013)); (E) bioprinted fibrin into 3D tubular scaffolds (Reproduced/adapted from Hinton et al. (2015)); (F) 3D bioprinted “half-heart” scaffold using a blend of alginate/gelatin (Reproduced with permission from Xu et al. (2009)); (G) gelatin bioprinted by a laser direct-write technique resulted in precise deposition of cells (Reproduced/adapted with permission from Raof et al. (2011)); (H) bioprinted hyaluronic acid into a mesh structure (Reproduced/adapted with permission from Pescosolido et al. (2011))/Copyright (2011) American Chemical Society); (I) human mammary epithelial cells bioprinted in Matrigel™ in zigzag shape (Reproduced/adapted with permission from Snyder et al. (2011)); (J) a 3D confocal image of cells in a methacrylated gelatin (GelMA) scaffold fabricated by laser-based bioprinting (Reproduced/adapted with permission from Gauvin et al. (2012)); (K) a bioprinted Pluronic® F-127 fluorescent tube (Reproduced/adapted with permission from Müller et al. (2015)); (L) Polyethylene glycol hydrogel bioprinted into an aortic valve construct (Reproduced/adapted with permission from Hockaday et al. (2012)).
Chitosan, a linear polysaccharide molecule obtained from deacetylation of chitin, has a wide range of applications in tissue engineering such as cartilage regeneration, devices for hemostatic and antibacterial activity, formation of sponge scaffolds, and fabrication of wound dressings (Croisier and Jérôme, 2013). The best concentration for optimal biological response is 1.5% (w/w) with a degree of acetylation between 30% and 40% (Montembault et al., 2006). Due to its unstable mechanical properties and limited bioprintability (see Fig. 3.2C), chitosan is an appropriate material for cell encapsulation, but not for the formation of large-scale scaffolds (Geng et al., 2005). Chitosan has been used in EBB for bioprinting of perfusable vessel-like microfluidic channels, where chitosan was crosslinked by an ionic crosslinker, sodium hydroxide (NaOH) (Zhang et al., 2013a,b). The polymer and the crosslinker solution were printed simultaneously using a coaxial nozzle unit with the crosslinker solutions in the inner core diffusing out toward the polymer resulting in hollow tubular constructs. Bioprinting of chitosan using DBB and EBB has not yet been attempted.
Collagen type I is a triple helical biocompatible protein obtained from natural sources which has been extensively used in bioprinting (Ferreira et al., 2012). It is one of the major components of connective tissues and occupies about 25% of the entire protein mass in most mammals. Collagen is a highly conserved protein cross-species causing minimal immunological reactions. Collagen matrix facilitates not only cell adhesion but also enhances cell attachment and growth due to abundant integrin-binding domains. Although collagen type I has been used in bioprinting, it has limitations as it remains in a liquid state at low temperatures and forms a fibrous structure with increased temperature or neutral pH. Complete gelation can take up to half an hour at 37°C. This slow gelation rate makes bioprinting of 3D constructs difficult, since deposited collagen remains liquid for more than 10 min. Also, cells deposited in collagen are not homogeneously distributed, as gravity pulls down the cells before gelation takes place. Low mechanical properties and instability along with the abovementioned issues necessitate the use of supportive hydrogels for collagen. EBB has utilized collagen alone as a bioink (Smith et al., 2004); however, mixing it with Pluronic® has generated promising results (Homenick et al., 2011). The cell number increased significantly after 24 h postbioprinting demonstrating that collagen is suitable for cell growth. Fully crosslinked, bioprinted constructs can even be perfused (Lee et al., 2014b). DBB also takes advantage of collagen as a bioink material; however, collagen needs to be deposited before the onset of crosslinking (Deitch et al., 2008). In one study, Boland’s group used collagen as a bioink constituent to investigate cell adhesion and proliferation on collagen-coated cell repellant substrates (Roth et al., 2004). Additionally, the same group fabricated a bilayer skin graft that generated neoskins on mice that are near-identical to native skin with microvessels (Yanez et al., 2014). As collagen possesses a fibrous microarchitecture, its use in inkjet bioprinting is highly limited, therefore microvalve bioprinting has been preferred. For example, fibrin-collagen bioink incorporating one of two cell types, AFSCs or mesenchymal stem cells (MSCs), was bioprinted using a valve-based bioprinter into wound sites as a treatment for skin burns (Skardal et al., 2012). Similarly, LBB has been successfully used to coat thin layers of collagen on a laser-absorbing material; due to collagen’s sticky nature, it can be easily transferred using a laser source for fabrication of skin tissue constructs (see Fig. 3.2D) (Michael et al., 2013).
Fibrin is a hydrogel formed by the enzymatic reaction between thrombin and fibrinogen, the key proteins involved in blood clotting. It supports extensive cell growth and proliferation (Cui and Boland, 2009), plays a significant role in wound healing, and has been used in fabrication of skin grafts (Skardal et al., 2012; Yanez et al., 2014). Rheology of gelled fibrin has been extensively studied because of its nonlinear elasticity (Janmey et al., 2009). The fibrin network is comprised of filaments forming as soft complex that allows a high degree of deformation without breakage (Janmey et al., 2009). Human umbilical vein endothelial cells (HUVECs) exhibit angiogenic behavior when cocultured with fibroblasts in fibrin. This is an essential factor in the vascularization of large tissue constructs and provides an effective in vitro model for analysis of the fundamentals of the angiogenic process (Nakatsu et al., 2003). The disadvantage of using fibrin for in vivo applications is the possibility of a severe immune reaction or transmission of infectious diseases when heterologous proteins are used. To increase the efficacy and safety of fibrin hydrogels, bacteria and viruses must be inactivated or fibrinogen and thrombin produced as recombinant proteins by mammalian cell lines. In addition, the degradation of fibrin is rapid, which is not conductive to long-term culture. Due to the non–shear-thinning nature of fibrinogen and thrombin, fibrin is rarely extruded (see Fig. 3.2E). However, despite these drawbacks, a few studies have utilized fibrin (Gruene et al., 2011). Weak mechanical properties do not allow manipulation on fibrin after gelation rendering EBB of precrosslinked fibrin a challenge. However, bioprinting of the two components of fibrin is an ideal option for DBB. Bioprinting of thrombin is feasible using inkjet (piezo- or thermal inkjet) bioprinting, but fibrinogen leads to clogging issues with unstable droplet formation due to its fibrous nature. In general, fibrinogen at low concentrations (<2 mg/mL) is usable, but such a concentration range limits the mechanical properties of bioprinted constructs. Therefore, microvalve bioprinting is more convenient for DBB of fibrinogen (Gudapati et al., 2016). For example, thermal-inkjet bioprinting was employed for bioprinting human microvascular endothelial cells (HMVECs), but cell were loaded in culture media and precisely bioprinted on crosslinked fibrin (Cui and Boland, 2009). Cells aligned in fibrin and formed into an extensive capillary network after 21 days of culture. Fibrinogen and thrombin can be bioprinted from separate cartridges and combined on a platform to form a hydrogel; alternating layers of neural cells and fibrin gel were bioprinted generating viable neural constructs with a potential in neural engineering applications (Xu et al., 2006). Because of its prolonged crosslinking time and dependence on thrombin concentration, fibrin is generally difficult to print into a designed shape. Fibrin is not a suitable bioink material for LBB due to its delicate structure; however, it can be mixed with other LBB compatible hydrogels such as hyaluronic acid (HA) (Gruene et al., 2011).
Gelatin, a denatured form of collagen protein, is used in food and pharmaceutical industries (Liang et al., 2004; Gómez-Guillén et al., 2011). It is extracted from bones, skin, and connective tissues of animals (Gómez-Guillén et al., 2002). At low temperatures, gelatin strands self-associate to form helical structures leading to a gel-like form, which reverts back to a random coil conformation as temperature increases (Chiou et al., 2008). Gelatin retains the Arg–Gly–Asp (RGD) sequence from its precursor, is less immunogenic, and promotes cell adhesion, differentiation, migration, and proliferation (Sakai et al., 2009).Gelatin in gel form can be obtained by thermally induced crosslinking, and it can easily liquefy at 37°C. The weight of unmodified gelatin gel decreases by 50% after around 10 h of incubation, and the gel completely dissolves within 24 h (Sakai et al., 2009). To avoid dissolution, a variety of chemical crosslinking methods have been examined; however, such methods are problematic in in situ and in vivo applications as crosslinking agents are toxic to cell-laden constructs (Liang et al., 2004). Popular enzymatic crosslinkers, such as transglutaminase, have been successfully used (Jin and Dijkstra, 2010). Other crosslinkers include horseradish peroxidase (HRP) and hydrogen peroxide (H2O2) (Sakai et al., 2009). Gelatin can be successfully gelled in vivo and remained intact for 1 week without inducing necrosis in surrounding tissues (Sakai et al., 2009). Gelatin-encapsulated cells demonstrate long-term viability, but limited cell elongation (Benton et al., 2009; Nichol et al., 2010). Gelatin is rarely bioprinted in its native form due to its poor mechanical properties. To employ gelatin for bioprinting, it has been chemically crosslinked by the addition of agents such as glutaraldehyde (Hellio and Djabourov, 2006). The free amine groups along the gelatin polymer backbone undergo a nucleophilic addition reaction followed by dehydration with the two aldehyde groups of glutaraldehyde that form an imine functional group commonly referred to as a Schiff base (Hennink and van Nostrum, 2012). Often the degree of gelation depends on the pH of the medium. Molecules which are capable of accepting or losing protons with an increase or decrease in pH are sensitive to a change in the local pH environment. Using EBB, hepatocyte-laden gelatin was bioprinted into spatially defined 3D structures. Hepatocytes remained viable and conducted their biological functions for more than 2 months (Wang et al., 2006). Gelatin is, however, not a popular hydrogel for DBB. Some studies have been performed by blending fibrin with gelatin for liver tissue biofabrication (Xu et al., 2007). Boland et al. (2007) presented DBB of gelatin/alginate blend; however, CaCl2 solution was selectively printed onto the blended solution for fabrication of cardiac constructs as can be seen in Fig. 3.2F. Gelatin has been successfully used in LBB due to its viscoelastic properties, stability, and ability to hold cells in precise positions without damaging cells (see Fig. 3.2G) (Raof et al., 2011). Additionally, its thermosensitive properties allow for cell transfer and ease of removal postprinting to reveal an application-specific growth surface.
Hyaluronic acid, a linear nonsulfated glycosaminoglycan, is ubiquitous in almost all connective tissues and a major ECM component of cartilage (Zhang et al., 2009). It behaves similar to collagen type I and has properties that can be beneficial in the treatment of osteoarthritis (Migliore and Granata, 2008). It is comprised of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine moieties linked by alternating β-1,4 and β-1,3 glycosidic linkages (Zhong et al., 1994). The three major functional groups, which govern the chemical activity of HA, are the glucuronic carboxylic acid group, the secondary hydroxyl group, and the N-acetyl group. Hyaluronic acid is widely used in tissue engineering due to its excellent biocompatibility and ability to form flexible hydrogels (Luo et al., 2000). Chemical modification of HA is an illustrative example of a bioink molecule polymerized by photo-crosslinking. To use HA as a bioink, chemical modifications of these groups are often carried out to enhance its rheological properties (Burdick and Prestwich, 2011); however, HA has slow gelation rate and poor mechanical properties. Hyaluronic acid is favored due to its role in early embryonic development, cell-friendly nature, and controllable mechanics, architecture, and degradation. Human embryonic stem cells encapsulated in HA, as opposed to 2D culture cells, maintained their phenotype, normal karyotype, and full differentiation capability (Gerecht et al., 2007). Additionally, the high molecular weight of HA adds an essential structural and organizational element into the ECM of native tissues (Kreger and Voytik-Harbin, 2009; Toole, 2004). It is instrumental in cell migration (Baier et al., 2007; Turley et al., 2002), nerve regeneration (Seidlits et al., 2010; Faroni et al., 2015; Ikeda et al., 2003), neuronal (Mészár et al., 2008; Banerjee and Toole, 1991; Margolis et al., 1975; Rauch et al., 2005; Baier et al., 2007) and glial development (Back et al., 2005; Liu et al., 2004), and wound healing (Aya and Stern, 2014). Hyaluronic acid has only minor cross-species variation and excellent biocompatibility (Dana et al., 2004; Leach et al., 2003). Pescosolido et al. (2011) developed a semiinterpenetrating network (semi-IPN) bioink by combining HA with a photopolymerizable dextran derivative (see Fig. 3.2H). In that study, a hydroxyethyl-methacrylate (HEMA) derivative of dextran was prepared by activating the HEMA group with N,N′-carbonyldiimidazole and coupled it to dextran, forming dex-HEMA. Hyaluronic acid/dextran-HEMA solutions were exposed to UV radiations in the presence of a photoinitiator inducing polymerization of the dextran chains. Hyaluronic acid moieties became entangled in the chemically crosslinked dextran network. Hyaluronic acid has been used in EBB but is generally blended with other hydrogels to enhance its bioprintability and solidification ability. For example, Skardal et al., 2010c used hyaluronic hydrogels crosslinked with tetrahedral PEG derivatives for bioprinting vessel-like constructs. To form extrudable ECM hydrogels, the symmetrical tetra-PEG molecules were acrylated to form tetraacrylates (TetraPAcs) then cocrosslinked with thiolated gelatin and HA derivatives. In another study conducted by the same group (Skardal et al., 2010a,b,c), methacrylated derivatives of gelatin and hyaluronic acid were synthesized separately and photo-crosslinked by a two-step process, before and after extrusion, to form more compact firm structures. Dynamic crosslinking of thiol-modified HA by gold nanoparticles was also studied to develop a hydrogel capable of reforming itself during and after bioprinting (Skardal et al., 2010a,b,c). Use of HA in DBB has not been demonstrated so far due to its viscous nature and slow gelation rate; however, it has been employed in LBB when combined with other hydrogels such as fibrin (Gruene et al., 2011) to facilitate faster crosslinking. The bioink consisting of human adipose-derived stem cells or endothelial colony-forming cells combined with hyaluronic acid and fibrinogen was coated on the donor slide and bioprinted using LIFT. The collector slide was blade-coated with HA-fibrinogen as well and subsequently crosslinked by thrombin. Alternating layers of HA-fibrinogen combined with both cell types were bioprinted to study the interaction between the two cell types.
Matrigel™ is a gelatinous ECM protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells (Kleinman and Martin, 2005). It promotes cellular outgrowth from tissue fragments, vascularization, and differentiation of various cell types (Gaetani et al., 2012; Kleinman and Martin, 2005). One of the major advantages of culturing cells on Matrigel™ is that it promotes complex cellular behavior which is difficult to observe on other surfaces. For example, endothelial cells create capillary sprouts in Matrigel™ (Kleinman and Martin, 2005). Molecules promoting endothelial cell network formation (Paul et al., 2013) are potential candidates for use in tissue regeneration therapies while the molecules that inhibit such formation can be used for anticancer therapies. Matrigel™ has been vital in the development of tumor xenograft models in rodents for discovery of novel cancer treatments (Benton et al., 2011). Matrigel™ is an expensive material; however, it has the ability to create mechanically strong, 3D bioprinted constructs with higher cell survival rates than other popular hydrogels such as agarose and alginate (Melchels et al., 2012). Thermal gelation properties of Matrigel™ and collagen type I are similar; however, gelation of Matrigel™ is thermally reversible. Crosslinking occurs between 24 and 37°C, and gelation takes about half an hour, beginning at temperatures above 4°C. In EBB, Matrigel™ needs to be bioprinted before becoming fully crosslinked; thus, a cooling chamber is essential. To accelerate the crosslinking process after deposition and maintain the printed shape, a heated plate should be employed. In EBB, Matrigel™ has been used in a radioprotection study on human hepatic carcinoma and human mammary epithelial cells (see Fig. 3.2I) (Snyder et al., 2011), and osteo- and endothelial-progenitor cell printing (Fedorovich et al., 2011). For DBB, Matrigel™ has not been used as a bioink material but employed as a substrate to place and pattern cells (Horváth et al., 2015). Its thermal crosslinking property and optimal viscosity make it an attractive bioink for LBB. Matrigel™ has been used for developing cellular constructs with precisely placed cells using MAPLE-DW cell transfer. A study conducted by Schiele et al. (2009) involved encapsulation of many cell types, such as dermal fibroblasts, neural stem, myoblasts, breast cancer cells, and bovine pulmonary artery endothelial in Matrigel™. The donor slide was coated with Matrigel™ containing cells while the receiver was coated only with Matrigel™. In a similar study, 3D cellular constructs were fabricated using multiple layers of human osteosarcoma cells bioprinted onto Matrigel™ substrates, using LIFT technique (Barron et al., 2004).
3.2.1.1.3.2. Synthetic Hydrogels
While natural polymers offer a favorable environment similar to native ECM for tissue engineering applications, synthetic polymers can be adapted according to the requirements of bioprinting processes. Synthetic polymers can be chemically modified not only with crosslinkable functional groups, but also with domains capable of enhancing the structural and mechanical properties of bioprinted constructs. This enables one to carefully engineer the design of the polymer backbone with either cell adhesive domains containing the RGD sequence or with domains responsive to external electric or magnetic stimulus.
Methacrylated gelatin, the denatured form of collagen consisting of methacrylate (MA) groups conjugated to its amine side groups, has been used for tissue engineering due to its favorable biological and adjustable mechanical characteristics (Nichol et al., 2010; Benton et al., 2009). Despite low cell proliferation rates, GelMA has relatively high mechanical strength and low swelling ratio and is amenable to blending with other hydrogels to increase cell survival. In 5% micropatterned GelMA, cells elongated, migrated, and formed interconnected networks with surrounding cells (Nichol et al., 2010). At higher concentrations, 10% or 15%, cells do not migrate; however, individual cells showed limited elongation and some multicellular networks formed. Methacrylated gelatin is frequently used in EBB studies such as bioprinting of chondrocytes (Schuurman et al., 2013), liver cells (Visser et al., 2013; Schuurman et al., 2013; Bertassoni et al., 2014; Billiet et al., 2014), and MSCs (Du et al., 2015). It has low viscosity at room temperature and is easy to extrude, and its crosslinking rate can be manipulated by the length of exposure to UV light. Methacrylated gelatin forms a biomimetic (Nichol et al., 2010) as well as an enzymatically degradable (Hutson et al., 2011) hydrogel that is mechanically strong when photo-crosslinked in the presence of a photoinitiator. For example, Demirci’s group bioprinted bone morphogenetic protein-2 (BMP-2) and transforming growth factor (TGF-β1) using DBB on a GelMA substrate to mimic a native fibrocartilage microenvironment. This substrate differentiated bioprinted human MSCs toward osteogenic and chondrogenic lineages in a spatial manner (Gutkan, 2014). Methacrylated gelatin has also been combined with other synthetic polymers such as polyethylene glycol diacrylate (PEGDA) and photo-crosslinked by Eosin Y-based photoinitiator (Wang et al., 2015b). Crosslinking ability with visible light, long-term biocompatibility, bioprintability, and enhanced rheology are some of the advantageous features of this bioink formulation. Scaffolds with a porous architecture based on GelMA have also been bioprinted using LBB, where HUVECs spread over scaffold within 4 days (Gauvin et al., 2012) (see Fig. 3.2J). Recently, Ovsianikov et al. (2014) demonstrated the first bioprinting of cells using 2PP. MG63 cells were encapsulated in GelMA hydrogel loaded with water-soluble photoinitiators. Ying-Yang structures were then bioprinted with solid and hollow parts, where cells exposed to the laser were damaged; however, they were able to proliferate and filled all the available space in 3 weeks of in vitro culture.
Pluronic® F-127 is a trade name for synthetic polymer poloxamer-based polymeric compound. It exhibits a polymeric architecture consisting of two hydrophilic blocks between a hydrophobic block making it an effective surfactant (Mesa et al., 2005). There are 11 types of Pluronic® polymers which differ by molar mass, percentage of composites, functionality, and temperature of crosslinking which can vary from 10 to 40°C (Wang et al., 2015a). Pluronic® undergoes reverse gelation as it starts to crosslink with increasing temperature. Pluronic® copolymer structures erode quickly and cannot hold structural integrity for longer than a few hours; however, Pluronic®, in combination with other hydrogels such as PEG, is useful for drug delivery and controlled release applications (Gong et al., 2009). Increase of mechanical strength was shown also by addition of methacrylated hyaluronic acid (MeHA) and then bioprinted into a fluorescent tube (see Fig. 3.2K) (Müller et al., 2015). The study performed by Vashi et al. (2008) showed that bone marrow–derived MSCs were able to interact with each other in a 3D Pluronic® environment and adipogenic induction started after 4 days of culture. Pluronic®, without any additives, can maintain the viability of cells for up to 5 days with a dramatic decrease thereafter. However, when supplemented with 60 nM hydrocortisone, cell viability was preserved even in higher concentrations of Pluronic® (Khattak et al., 2005). Pluronic® can be cured by UV light for bioink applications; however, the radiation type and duration can impact cell viability, and the concentration of the photoinitiator can affect the metabolic activity of cells. Chemical crosslinking can make the gel more resistant to thermal degradation33. Pluronic® can be crosslinked enzymatically whereby two ends of PEO side chains can be formed into self-assembled micelles (Lee et al., 2011). Pluronic® does not lose its thermally reversible properties, but its mechanical strength increases with enzymatic crosslinking. In EBB, acceptable bioprintability of Pluronic® is obtained above 20°C as it becomes more viscous and exhibits shear-thinning behavior. Therefore, Pluronic®, depending on its concentration, requires a heating system around the needle to transition from liquid to gel state; a heated plate to maintain the temperature of the construct after deposition should also be considered. Nonetheless, it is more advantageous to maintain Pluronic® in a semiliquid state to preserve cell viability in homogeneous suspension. In the case of prolonged bioprinting times, a cooling system might be needed to maintain a lower temperature in the bioink reservoir. The reversible properties of Pluronic® can be useful in fabrication of complex constructs. Pluronic® in solid form (at room temperature or higher) can be surrounded by a second type of hydrogel and then placed at 4°C to liquefy the Pluronic®. This procedure creates perfusable channels within bulky cell-laden constructs (Wu et al., 2011). The thermosensitive nature of Pluronic® and its high viscosity is problematic for DBB and so has not been used. LBB has not been attempted as Pluronic® is not viscoelastic, maintains a solid coating on the quartz support, and cannot transfer thermal energy to kinetic energy, which is essential for jet formation.
PEG has been widely used in medical and nonpharmaceutical products (Lin and Anseth, 2009; Pasut et al., 2008; Alexander et al., 2013; Wang et al., 2015a). It is a linear polyether hydrophilic compound which can be conjugated with proteins (Alconcel et al., 2011; Zhang et al., 1998), enzymes (Pasut et al., 2008), liposomes (Ishida et al., 2005), and other biomolecules. It has been employed as a drug delivery agent (Veronese and Pasut, 2005; Lin and Anseth, 2009; Alconcel et al., 2011) and used in biosensing or signaling (Sharma et al., 2004) and pharmaceutical applications (Alconcel et al., 2011). Because PEG is a hydrophilic polymer, it resists protein adsorption and cell adhesion. Hence, some cells, particularly chondrocytes and osteoblasts, encapsulated in PEG survive well even without addition of biological constituents (Benoit et al., 2006). Other cell types may require additional cell adhesion components, such as RGD peptides or fibronectin coating. Fibronectin precoating improves cell adhesion, spreading, and the organization of the actin cytoskeleton (Vladkova et al., 1999). PEGylation inhibits protein adsorption making PEG a very useful synthetic polymer for bioprinting of tissue constructs for regenerative medicine. PEG is water soluble, and its mechanical properties can be manipulated through variation of its chemistry (Gao et al., 2015). Altering the composition of PEG-based hydrogels in tandem with photo-crosslinkage in the presence of a photoinitiator allows alterations to the structural, functional, and mechanical properties of fabricated tissues. One of the major limitations of PEG is its poor mechanical strength; therefore, addition of diacrylate (DA) of MA is highly beneficial. However, additives such as DA and MA require photo-crosslinking by exposure to UV light for a specific length of time to obtain the desired mechanical properties. Excessive exposure to UV light can dramatically reduce cell viability. For example, rat aortic smooth muscle cells have been successfully photoencapsulated in PEG with 20 s of UV exposure (Mann et al., 2001). PEG was also used in creation of microgels that were assembled in seconds using acoustic waves (Xu et al., 2011). PEGDA and polyethylene glycol methacrylate (PEGMA) hydrogels are used in all types of bioprinting modalities: EBB (Hockaday et al., 2012; Wüst et al., 2014; Bertassoni et al., 2014; Skardal et al., 2010c), DBB (Cui et al., 2012a,b), and LBB (Hribar et al., 2014). Photopolymerization of PEG-based hydrogels with tunable mechanical properties has been widely demonstrated using EBB. Hockaday et al. (2012) performed 3D printing of anatomically correct aortic valve scaffolds using PEGDA hydrogels (see Fig. 3.2L). Scaffolds were then seeded with porcine aortic valve interstitial cells, where cells demonstrated adhesion with minimum spreading or proliferation in 21 days. Earlier work, however, demonstrated that the immobilization of cell adhesion sites and growth factors during EBB promoted cell spreading and proliferation (Zhang et al., 1998; Fedorovich et al., 2007). Due to greater mechanical stiffness compared to naturally derived polymers, such as alginate, fibrin, agarose, and collagen type I, PEG has been extensively used in DBB. Using thermal inkjet bioprinting, Cui et al. (2012a,b) bioprinted human articular chondrocytes in PEGDMA into osteochondral plugs in a layer-by-layer fashion with UV exposure following each layer, producing a 3D scaffold with uniform allocation of cells and neocartilage formation. In a similar study, acrylated-PEG was bioprinted with acrylated-RGD peptide, followed by photopolymerization of the bioprinted layer (Gao et al., 2015). Bone marrow–derived human MSCs were suspended in PEGDMA in conjunction with hydroxyapatite (HA) nanoparticles and bioactive glass and bioprinted using a thermal inkjet bioprinter (Gao et al., 2014), which conferred spatial control of the distribution of cells and bioactive ceramic materials in the fabricated bone tissue constructs. PEG-based hydrogels have been widely used in LBB, including SLA and DOPsL. Bioprinting using SLA was first demonstrated with a commercial SLA 3D printer (SLA-250, 3D Systems), where Chinese hamster ovary cells were encapsulated in PEGDMA with over 90% cell viability (Dhariwala et al., 2004). In a similar approach, the same 3D printer equipped with He–Cd laser was used for encapsulation of 3T3 fibroblasts in PEGDMA to fabricate well-defined cylindrical scaffolds with multiple channels (Arcaute et al., 2006). In addition to SLA applications, DOPsL enabled fabrication of vascularized tissue construct using PEGDA hydrogels to study the migration behavior of HeLa cells (Huang et al., 2014). It was demonstrated that HeLa cells migrated significantly when the vasculature diameter was decreased.
Table 3.1 provides sample bioprinting and bioink formulation parameters along with bioprinters used in EBB, DBB, and LBB of all the hydrogels presented in this chapter.
Table 3.1
Detailed Characteristic of Bioprinting Parameters of Hydrogels Using EBB, DBB, and LBB
| Process Type | Crosslinking Properties | ConcenTration | Bioprinting Parameters | Construct Size | Cell-Related Attributes | BioprintAbility | Bioprinter Used | References | ||||
| Type | Time | Type | Density (Million/mL) | Viability (%) | ||||||||
| Agarose | EBB | Thermal | Minutes | 0.3% | n/a | 2.5 mm | MSCs | 0.1 | 98.8 | High | custom-made | (Duarte Campos et al., 2014) |
| DBB | Thermal | 1–2 h | 1.5% | n/a | 6 mm × 6.5 mm | CHO: motoneurons | 5: 2 | 90 | High | HP Desktop 550C | (Xu et al., 2005) | |
| Alginate | EBB | Ionically: CaCl2 | Seconds | 2% | 200 kPa, 17 mm/s | 430 μm diameter | BMSC | 0.25 | 95 | High | Bioplotter | (Fedorovich et al., 2008) |
| Ionically: CaSO4 | 10 min | 2% | 72.3 kPa, 10 mm/s | 19 mm × 6 mm | n/a | n/a | n/a | High | Fab@Home | (Cohen et al., 2010a) | ||
| Ionically: CaCl2 | Precrosslinked | 3% | n/a | 20 mm × 20 mm × 3 mm | gMSCs | 10 | n/a | High | Bioscaffolder | (Poldervaart et al., 2013) | ||
| n/a | 1–2% | 50–300 kPa, 1–30 mm/s | 20 mm × 20 mm × 2 mm | BMSCs | 0.25 | 95 | High | Bioplotter | (Fedorovich et al., 2008) | |||
| DBB | Ionically: CaCl2 | n/a | 1% | 60 Hz, echo and dwell time 45 μs | 10 mm × 5 mm | NIH 3T3 | 5 | 90.8 | High | Platform-assisted 3D inkjet bioprinting system | (Christensen et al., 2015) | |
| LBB | Ionically: CaCl2 | n/a | 0.1–1% | 4.5–9 μJ, 160-80 cm/s | 3 mm × 1.5 mm | HUVECs | 40–100 | n/a | High | Custom-made | (Guillotin et al., 2010) | |
| Seconds | 1–8% | 193 nm, 85% transmit., 1.5 mm/s | 100 μm | n/a | n/a | n/a | High | MAPLE-DW | (Yan et al., 2013) | |||
| Chitosan | EBB | pH-mediated: CH3COOH | n/a | 3% | 89.6 kPa, 15 cm/min | 10 mm × 10 mm × 5 mm | Adipose-derived stem cells | 0.75 | n/a | High | Custom-made | (Ye et al., 2014) |
| 2 h | 200 kPa, 6 mm/s | 2 × 2 cm | Porcine bone marrow cells | 0.5 | n/a | High | Custom-made | (Geng et al., 2005) | ||||
| Collagen Type I | EBB | pH-mediated | Minutes | 0.3% | 11.7 kPa, 20 mm/s | 200 μm | BAECs | 5–20 | 33 | Average | BioAssembly Tool | (Smith et al., 2004) |
| 10.3 kPa, 15 mm/s | 800 μm | 86 | Low | |||||||||
| pH: NaHCO3 and cooled under 20°C | 1 min | 0.223% | 0–13.8 kPa, Valve operating time, 600-150 μs | 1 mm × 10 mm × 10 mm | Fibroblasts | 1 | 95 | High | custom-made | (Lee et al., 2010a) | ||
| DBB | pH-mediated and thermal | 1–2 h | 0.1% | n/a | 6 mm × 6.5 mm | CHO, motoneurons | 5;2 | 90 | High | HP Desktop printer HP 550C | (Xu et al., 2005) | |
| pH-mediated | <2 h | 0.025–0.5% | n/a | 23 mm × 3 mm | Rat cardiomyocytes | 0.25 | n/a | Low | HP DeskJet 500 | (Deitch et al., 2008) | ||
| Table Continued | ||||||||||||

| Process Type | Crosslinking Properties | ConcenTration | Bioprinting Parameters | Construct Size | Cell-Related Attributes | BioprintAbility | Bioprinter Used | References | ||||
| Type | Time | Type | Density (Million/mL) | Viability (%) | ||||||||
| Fibrin | DBB | Enzymatic fibrinogen [F]–thrombin [T] | Seconds | 10 mg/mL [F]; 20 U/mL [T] | 250,000 drops/s | 25 mm × 5 mm × 1 mm | NT2 neurons | 2 | 74.27 | Low | HP Desktop 550 | (Xu et al., 2006) |
| 6 min | 60 mg/mL [F]; 50 U/mL [T] | Droplets 130 pl; 10 μs pulse | 8 mm × 1.8 mm | Human microvascular endothelial cell | 1–8 | n/a | Low | HP Deskjet 400 | (Cui and Boland, 2009) | |||
| Gelatin | EBB | Thermal | 5 min | 20% | 5 psi | 5 mm × 5 mm × 3 mm | Hepatocytes | 1 | 90 after 45 days | Average | n/a | (Wang et al., 2006) |
| n/a | 7% | 41.4–89.6 kPa, 450–750 μs valve operating | 1 mm × 10 mm × 10 mm | Fibroblasts | 1 | n/a | High | Custom-made | (Lee et al., 2010a) | |||
| LBB | Thermal | 7 min | 20% | 1.0 J/cm2 | 1 mm × 1.5 mm | Fibroblasts | 0.6 | 91 | High | MAPLE-DW | (Schiele et al., 2011) | |
| n/a | n/a | 107 × g, 122 m/s | n/a | Schwann, astroglial cells | 2–6 | 80–85 | Average | AFA-LIFT | (Hopp et al., n.d.) | |||
| Gelatin / Alginate | EBB | Ionically: CaCl2 | Precrosslinking | 0.06% | 5 mm/s | 3 × 1 mm | NIH 3T3 | 10 | 84.6 | High | Fab@Home | (Duan et al., 2013) |
| DBB | 90% in 5s | 2 wt% | 96.5–2000 Pa | 250 μm × 300 μm × 3.5 mm | n/a | Average | MicroDrop | (Pataky et al., 2012) | ||||
| Hyaluronic Acid | EBB | Photopolymerization; pH-mediated | 3 min | 1.5% | n/a | 2 mm ring | HepG2, C3A, Int-407, NIH 3T3 | 25 | n/a | Average | Fab@Home | (Skardal et al., 2010a,b,c) |
| Photopolymerization | 10 min | 2–6% | n/a | n/a | Articular cartilage | 5 | n/a | Average | Bioscaffolder | (Pescosolido et al., 2011) | ||
| MatrigelsTM | EBB | Thermal | n/a | 1:1 ratio with cells | 11.7 Pa, 1 cm/s | 0.75 cm × 1 cm | HepG2 and M10 | 1 | n/a | Low | Roland DXY-1100 plotter | (Snyder et al., 2011) |
| n/a | n/a | 174 kPa, 30 cm/min | 10 mm × 20 mm × 1 mm | gEPCs and MNCs | 5 | n/a | Low | Bioscaffolder | (Fedorovich et al., 2011) | |||
| DBB | 30 min | n/a | 24.8 kPa, 20 mm/s, 17.86 Hz | 1.12 mm | Alveolar EC, HUVECs | 4.5 | n/a | Low | BioFactory | (Horváth et al., 2015) | ||
| LBB | n/a | n/a | 193 nm, 300 Hz | 3 × 3 array | Fibroblast, myoblast, neural stem cells, breast cancer cells | n/a | n/a | Average | MAPLE DW | (Schiele et al., 2009) | ||
| Table Continued | ||||||||||||

| Process Type | Crosslinking Properties | ConcenTration | Bioprinting Parameters | Construct Size | Cell-Related Attributes | BioprintAbility | Bioprinter Used | References | ||||
| Type | Time | Type | Density (Million/mL) | Viability (%) | ||||||||
| GelMA | EBB | Photopolymerization | Minutes | 10–20% | 96.5–400 kPa, 30–70 cm/min | 150–200 μm | HepG2 | 1.5 | >97 | High | Bioplotter pneumatic | (Billiet et al., 2014) |
| 10–60 s | 5–15% | 2 mm/s | 750 μm | HepG2, NIH3T3 | 1–6 | >75 | High | NovoGen MMX Bioprinter | (Bertassoni et al., 2014) | |||
| DBB | 10 min | 10–20% | 300 dpi, 3.6 kHz | 4 mm × 2 mm | Articular cartilage | 5 | 63.2 | High | HP Deskjet 500 | (Cui et al., 2012a,b) | ||
| 30 s | 5% | 6.9 mW/cm2 | 8 mm × 8 mm | MSCs, growth factors | 1 | 90 | High | custom-made | (Gutkan, 2014) | |||
| LBB | 20 s | 10–15% | 50 mW/cm2 | 5 mm × 5 mm × 1 mm | HUVEC | 0.02 | n/a | High | Custom-made projection stereolithography system | (Gauvin et al., 2012) | ||
| Pluronic F-127 | EBB | Thermal | Minutes | 30%; 27% with cells | 250 μm/s | 2 mm × 2 mm × 1.5 mm | Human primary fibroblasts | 0.8 | 60 | High | BioAssembly Tool | (Smith et al., 2004) |
| 25% | 50–300 kPa, 1–30 mm/s | 20 mm × 20 mm × 2 mm | BMSCs | 0.25; 0.5 | 85 | High | Bioplotter | (Fedorovich et al., 2008) | ||||
| n/a | <10%; 20% | 16.5 mW/cm2, 3–6 mm/s | 22 mm × 25 mm, 17 mm × 20 mm, 12 mm × 14 mm | PAVICs | 0.5 | 91.3 | High | Fab@Home | (Hockaday et al., 2012) | |||
| PEG | EBB | Photopolymerization | Up to 19 min | 10% | 300 dpi, 3.6 kHz | 4 mm | hMSCs | 6 | 90 | High | HP 51626A | (Gao et al., 2015) |
| DBB | 10 min | 10–20% | 4–8 mW/cm2 | 5 mm | Human articular cartilage | 5 | 89 | High | HP Deskjet 500 | (Xiaofeng Cui, Breitenkamp, Finn, et al., 2012a) | ||

3.2.1.2. Decellularized Matrix Components
Cells secrete specific molecules to create ECM, a localized environment which fosters cell attachment, proliferation, signaling, and tissue development. In an attempt to recapitulate this natural tissue environment, a bioink material based on decellularized extracellular matrix (dECM) has been developed. Preparation of dECM bioink requires removal of cellular material using chemical, physical, and enzymatic processes without damage or loss of ECM (Ott et al., 2008). To determine if the matrix is fully decellularized, samples are evaluated by DNA quantification assays. Successful decellularization methods remove about 98% of the cellular content (Pati et al., 2014). The dECM is then further processed to generate a gel-like substance for use in bioprinting (see Fig. 3.3A1–A2). A recent study showed that bioprinted constructs of dECM and stem cells did not induce cytotoxicity or inflammation after in vivo implantation (Pati et al., 2015). Pati et al. printed a decellularized adipose tissue matrix loaded with human stem cells in tandem with melt extrusion of PCL (see Fig. 3.3B). As the printed dECM could not retain its shape postbioprinting, the printed PCL provided the structural frame necessary to maintain the stability of dECM (see Fig. 3.3C). dECM bioink supported high cell viability over 2 weeks in culture and promoted the expression of adipogenic genes without additional supplementation.
3.2.1.3. Microcarriers
Microcarriers are supportive structures for cell growth and expansion; they are made of synthetic (i.e., dextran, plastic, glass) or natural (cellulose, gelatin, and collagen) materials with specific engineered porosities (Malda and Frondoza, 2006). Typically, they have been used as reinforcement blocks in bioprinting (Turner and Flynn, 2012). The shape of microcarriers, interconnected pores in a spherical architecture, allows cells to attach and grow on the surface (see Fig. 3.4A). They provide an expansive monolayer environment, vastly improving the cell culturing process (Goh et al., 2013). Nutrient and gas transfer between the media and attached cells is more efficient than in 2D culture. Large numbers of cell-laden microcarriers can be suspended in cell media and cultured in environmentally controlled bioreactors. The surface of 1 g of microcarriers is equivalent to the surface of 15 75 cm2 culture flasks (Malda and Frondoza, 2006). Moreover, they improve the compressive modulus of constructs, support differentiation of stem cells to desired lineages, and preserve phenotypic stability at high cell concentrations (Levato et al., 2014). Using EBB, microcarriers can be loaded and bioprinted in hydrogels, where a representative bioprinting process is illustrated in Fig. 3.4B. The ability to use high cell concentrations without compromising viability makes this a promising approach. Bioprinting MSCs preseeded on polylactic acid (PLA) microcarriers, Levato et al. (2014) showed that cells merely suspended in hydrogels exhibited less interaction, aggregation, and osteogenic differentiation than cell-loaded microcarriers in hydrogels (see Fig. 3.4C).

Figure 3.3 Bioprinting of Decellularized Extracellular Matrix (dECM).
(A1–A2) Sol–gel transition of a dECM-based bioink prepared from porcine cartilage; (B–C) bioprinting of dECM required a PCL structural frame to support gelation of the bioink (Reproduced/adapted with permission from Pati et al. (2014)).

Figure 3.4 Bioprinting of Microcarriers.
(A) A fluorescence image of a cell-laden microcarrier, where cell surface and nucleus were stained in green and red, respectively (Reproduced/adapted with permission from Jakob et al. (2016)). (B) A representative extrusion-based bioprinting process for deposition of microcarrier-laden hydrogels. (C) Bioprinted microcarriers loaded in GelMA hydrogels facilitates better cell–cell interactions (Reproduced/adapted with permission from Levato et al. (2014)).
3.2.2. Scaffold-Free Bioink Materials
Evolution of organ development is based on cellular self-assembly mechanisms (Rivron et al., 2009). The cellular environment of a tissue construct needs to resemble its native counterpart for cells to maintain their phenotype, establish appropriate cell–cell interactions, and express tissue-specific proteins. 3D cell aggregate configurations provide a more hospitable environment for tissue self-assembly to occur than monolayer cell cultures. Tissue morphogenesis is dependent upon the formation of multicellular aggregates bound by cadherin molecules (Heilshorn et al., 2005; Albelda and Buck, 1990). Cadherin facilitates strong intercellular adhesion enabling signal transduction and an increase in integrin expression and binding to RGD motifs in the deposited ECM components (Behrens, 1999).
3.2.2.1. Tissue Spheroids
Tissue spheroids constitute a scaffold-free bioink type, where cells are organized spherically into 200- to 400-μm diameter cell aggregates that can serve as building blocks and tissue models for tissue engineering and pharmaceutics, respectively. Several techniques have been demonstrated for fabrication of tissue spheroids. One of the most popular methods is culturing cells in microwells with rounded ends on a cell adhesion inert mold made of hydrogels such as agarose (Dean et al., 2007; Mehesz et al., 2011; Livoti and Morgan, 2010), MeHA (Wheeldon et al., 2010), and alginate (Yamada et al., 2011). In this approach, thousands to millions of cells are seeded into an array of microwells and cultured for 24–48 h to facilitate cell aggregation (see Fig. 3.5A). Cells fall to the bottom of the microwells and settle in close contact with each other, driving the cells to spontaneously adhere to one another to minimize free energy and develop into a neotissue (Athanasiou et al., 2013). Over time, as tissue spheroids are formed, their size diminishes due to radial contraction; this is attributed to intracellular cytoskeletal reorganization from cadherin-mediated cell–cell binding (Akkouch et al., 2015). Other approaches are also used in tissue spheroid fabrication. One is the hanging drop method (Del Duca et al., 2004), which relies on self-assembly driven by gravity. A cell suspension in a small volume (15–30 μl) is pipetted onto the lid surface of a tissue culture plate. After inverting the lid, the droplets remain attached; however, gravity concentrates the cells at the bottom of droplet facilitating aggregation of cells. Microfluidic-assisted technology is another approach enabling cell aggregation inside cell traps (Jin et al., 2011; Torisawa et al., 2009; Zhang et al., 2014). Usually constructed by polydimethylsiloxane (PDMS) stamping, U-shape traps are created on an angled perfusion channel. During perfusion, cells are entrapped within U-shape and begin aggregation on contact. The device can be set in the vertical position to increase the effect of gravity. The major advantage of this method is the improved oxygen and media flow around the aggregates, which prevents necrosis. The major disadvantage is the difficulty of extraction of intact aggregates from PDMS. Another recent method is acoustic wave–assisted cell assembly (Guo et al., 2015) where cells are deposited onto a membrane, which vibrates in a specific frequency to form defined shapes.
Bioprinting of tissue spheroids has been performed using EBB only (Mironov et al., 2009); as shown in Fig. 3.5B, tissue spheroids within the gel medium can be loaded into a dispensing tip using slight mechanical pressure and extruded one by one to generate a precise spatial conformation (see Fig. 3.5C) for a construct in “T” shape. The speed of this process is driven by a mechanical ram which extrudes the bioink through a pipette unit. Along with spheroids, an agarose support structure (mold) is also printed to support fusion and maturation of the deposited spheroids. Over time, spheroids fuse to each other creating larger scale tissues that can be easily removed from the mold (Norotte et al., 2009). Instead of using a mold support, a recent work demonstrated the bioprinting of spheroids by picking them from a reservoir and placing and skewering them on a needle array (Itoh et al., 2015).
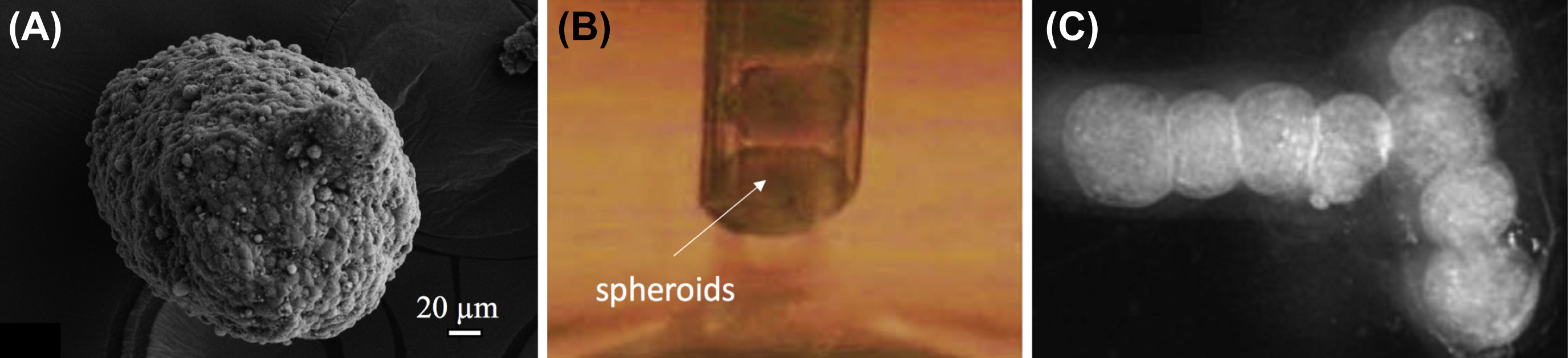
Figure 3.5 Bioprinting of Tissue Spheroids.
(A) A sample heterocellular tissue spheroid made of beta (β)-TC3 mouse insulinoma and rat heart microvascular endothelial cells. (B) Tissue spheroids can be bioprinted one by one using a piston-driven mechanism in a pipette tip (C) for fabrication of larger scale tissue constructs (Reproduced/adapted with permission from Mironov et al. (2009)).
3.2.2.2. Cell Pellet
The cell pellet technique is based on centrifugal or gravitational forces that concentrate cells at the bottom of conical tube (Khalil et al., 2001). The pellet can then be transferred to a micropipette or other mold where intercellular interactions are further established to improve cohesion (see Fig. 3.6A). The cell pellet technique is a practical method to create aggregates without the need for sophisticated systems; however, medium and oxygen circulation is limited, and cell viability decreases markedly after 24 h. One notable exception is chondrocytes, where a low oxygen level is desirable for enhanced production of ECM, including collagen type II and chondrogenic proteins (Schrobback et al., 2012). Cell pellet–based bioink has been used in EBB where the pellet was extruded into a confined space generated by 3D printing of a supporting agarose mold as demonstrated in Fig. 3.6B (Ozler et al., 2015). As shown in Fig. 3.6C, the agarose support mold facilitated aggregation of cells resulting in tissue constructs [i.e., nerve grafts (Owens et al., 2013) and aortic constructs (Kucukgul et al., 2015)].
3.2.2.3. Tissue Strands
Tissue strands can be defined as cylindrical neotissues that are engineered for bioprinting of scale-up tissues (Yu et al., 2016; Akkouch et al., 2015). The process for tissue strand formation has been recently established (Yu and Ozbolat, 2014). Cells at very high density are injected and packed into hollow alginate tubes. Semipermeable alginate tubes allow the exchange of medium with nutrition and oxygen; the cells grow in given shape as they do not attach to the alginate luminal surface. When cells aggregate into a neotissue (see Fig. 3.7A), the tube is dissolved using a decrosslinker solution. The formed tissue strand is then placed into a custom-made bioprinter head and extruded mechanically (see Fig. 3.7B). Tissue strands must be optimally matured to ensure appropriate bioprinting conditions. Immature strands that are not fully aggregated may not form the desired shape and can disintegrate during bioprinting. Tissue strands that are overmature may not be malleable enough for deposition. A low level of oxygen in the core of strands may be a drawback for some tissue constructs; however, this method is especially suitable for cartilage formation (see Fig. 3.7C1–C3), as chondrocytes preferentially proliferate in hypoxic conditions (Schrobback et al., 2012).

Figure 3.6 Bioprinting Cell Pellet.
(A) Cell pellet can be injected into in a custom syringe to increase its cohesiveness. (B) Bioprinting of the cell pellet requires three-dimensional printing of an agarose mold structure for facilitation of cell aggregation and tissue maturation, (C) where the bioprinted heterocellular pellet (consisting of mouse aortic smooth muscle cells, 3T3 mouse fibroblasts, and human umbilical vein endothelial cells) aggregated into a tissue construct (Reproduced/adapted with a permission from Ozler et al. (2015)).
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.

