Bioprinter Technologies∗
Abstract
Since the first cytoscribing of cells demonstrated by Klebe in 1986, three dimensional (3D) bioprinting has made a substantial leap forward, particularly in the last decade. As bioprinting has gained great interest in the medical and pharmaceutical communities, the demand for bioprinters has risen dramatically. Several companies emerged to commercialize advanced bioprinter technologies in addition to a myriad of bioprinters developed at various research institutions worldwide. This chapter prefaces the evolution of the bioprinter technologies and presents the components and working mechanisms of bioprinters under three major modalities including extrusion-, droplet-, and laser-based bioprinting. A comparative evaluation is performed for commercially available bioprinters; limitations of the current bioprinter technologies are discussed thoroughly and future prospects for bioprinters are provided to the reader.
Keywords
Logic will get you from A to B. Imagination will take you everywhere
Albert Einstein
7.1. Introduction
7.2. Bioprinters
7.2.1. Components of Bioprinters
7.2.2. Bioprinter Types
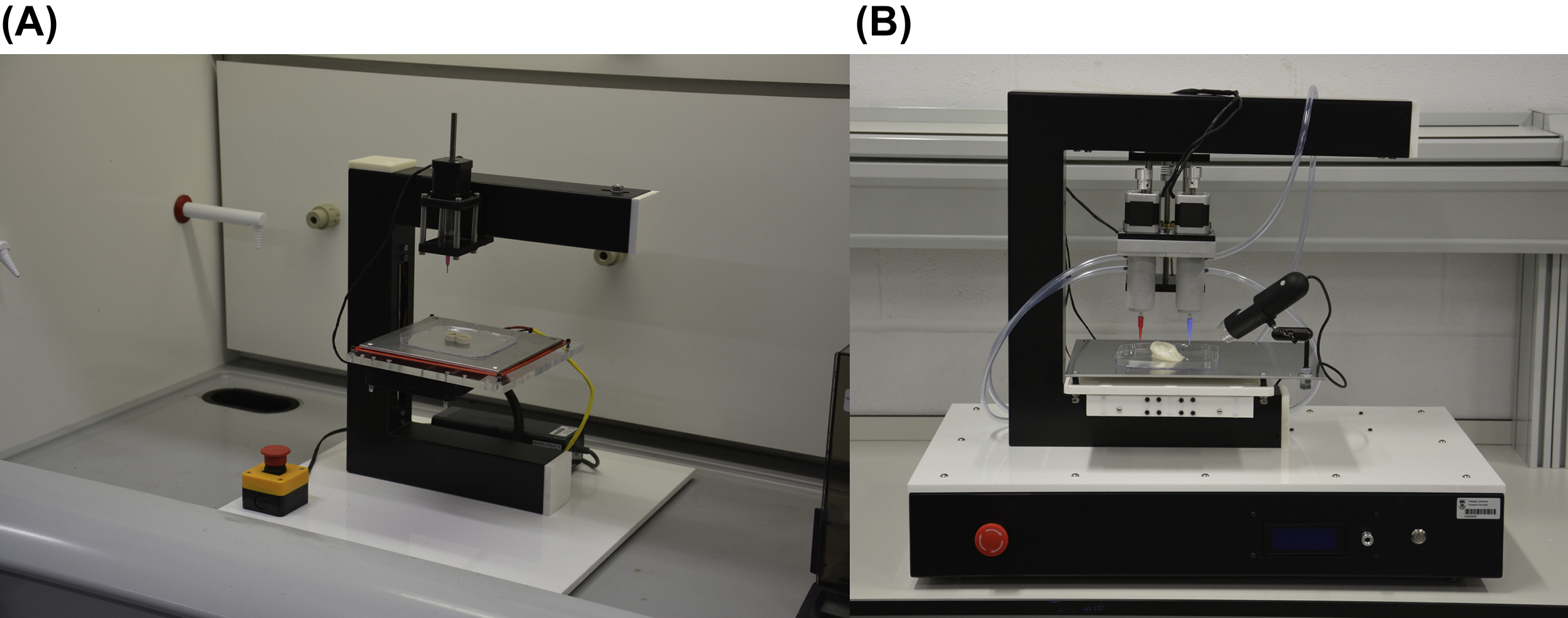
7.2.2.1. Extrusion-Based Bioprinters
Table 7.1
Noncommercial Extrusion-Based Bioprinters
| Bioprinter Name | Country | Bioprinting Mechanism | University/Research Institution | Use | Dual Bioink Printability | |
| Extrusion-Based Bioprinters | Multi-Arm BioPrinter | USA | Mechanical or pneumatic | The University of Iowa, Penn State University | In situ bone printing (Ozbolat, 2015a), vascular tissue (Yu et al., 2014) | + |
| Modular tissue printing platform | USA and South Korea | Pneumatic microextrusion | Harvard Medical School and KAIST | Skin (Lee et al., 2009a), fluidic channels (Lee et al., 2010a), vascular network (Lee et al., 2014) | + | |
| Multihead tissue/organ building system | South Korea | Pneumatic microextrusion | The Catholic University of Korea | Liver (Pati et al., 2015), heart and adipose tissue (Pati et al., 2014), bone and cartilage (Shim et al., 2012) | + | |
| Three-dimensional (3D) axis bioprinting system | South Korea | Mechanical microextrusion | Korea University | Cell-free scaffold (Song et al., 2010), skin (Hong et al., 2013) | + | |
| Multinozzle system | USA | Pneumatic, piezoelectric, and solenoid microextrusion | Drexel University | Fibroblasts (Khalil et al., 2005) | + | |
| Palmetto Printer | USA | Pneumatic microextrusion | Medical University of South Carolina | Adipose-derived stem cells (Jia et al., 2014) | + | |
| Multimaterial 3D bioprinting | USA | Pneumatic microextrusion | The Wyss Institute, Harvard | Vasculature (Kolesky et al., 2014) | + | |
| 3D Scaffold printer | Germany | Mechanical microextrusion | Fraunhofer Institute for Materials Research and Beam Technology | Acellular Scaffolds (Wu et al., 2011) | N/A | |
| 3D integrated organ printer | USA | Pneumatic microextrusion | Wake Forest Institute for Regenerative Medicine | Keratinocytes (Murphy et al., 2013) | + |
7.2.2.1.1. 3D Bioplotter®
7.2.2.1.2. The Alpha and Omega Bioprinters
7.2.2.1.3. BioAssemblyBot
7.2.2.1.4. Biobot
7.2.2.1.5. Biofactory and 3D Discovery®
7.2.2.1.6. Bio3D SYNˆ and Bio3D Explorer
7.2.2.1.7. Fab@Home
7.2.2.1.8. Inkredible
7.2.2.1.9. NovoGen MMX™
7.2.2.1.10. REGEMAT 3D
7.2.2.1.11. Regenovo
7.2.2.1.12. nScrypt
7.2.2.2. Droplet-Based Bioprinters
Table 7.2
Commercially Available Extrusion-Based Bioprinters
| Bioprinter Name | Country | Bioprinting Mechanism | Company | Use | Dual Bioink Printability | Features | |
| Extrusion-based bioprinters | Novogen MMX™ | USA | Mechanical microextrusion | Organovo | Bone (Edwin et al. n.d.), liver (Robbins et al., 2013), breast cancer (King et al., 2013), vascularization (Bertassoni et al., 2014) | + | Precision: 20 μm (Root Analysis, 2014) |
| BioScaffolder | Germany | Pneumatic microextrusion | SYS + ENG | Encapsulated proteins (Poldervaart et al., 2013), cartilage (Visser et al., 2013), vascularization (Fedorovich et al., 2011), bone (Seyednejad et al., 2011) | + | Stepper motor with 5 μm/step (SYS + ENG n.d.) | |
| 3D BioPlotter | Germany | Pneumatic microextrusion | EnvisionTec | Bone (Fedorovich et al., 2008), acellular scaffolds (Chien et al., 2012) | + | Axis resolution: 1 μm (x–y–z axis) (EnvisionTEC) Speed: 0.1–150 mm/s (EnvisionTEC), price > $145,000§ | |
| Regenovo | China | Extrusion | Regonovo Biotechnology Co., Ltd | β-TCP bone scaffold (Zou et al., 2016), 3D gelatin/alginate scaffolds (Wang et al., 2016) | + | Resolution 20 μm (Bodrum et al., 2015) | |
| BioAssembly tool (BAT) | USA | Pneumatic microextrusion | Sciperio/nScrypt | Vascularization and skin (Smith et al., 2004) | + | Price range: $200,000–$400,000 (Root Analysis, 2014) | |
| 3D Discovery | Switzerland | Pneumatic microextrusion | RegenHU | Cartilage (Markstedt et al., 2015) | + | Printing materials up to viscosity 10,000 mPa (Root Analysis, 2014) | |
| BioFactory | Switzerland | Pneumatic microextrusion | RegenHU | Air-blood tissue barrier (Horváth et al., 2015) | + | Printing resolution: < 5 μm (Horváth et al., 2015) | |
| BioBot 1 | USA | Pneumatic micro-extrusion | BioBots | GelMA hydrogel extrusion optimization (Ersumo and Spiller, 2015) | + | Printing resolution: 100 μm (BioBots, n.d.) Price: $10,000 (BioBots, n.d.) | |
Bioassembly Bot | USA | Pneumatic microextrusion | Advanced solutions | Human heart (Root Analysis, 2014) | + | Repeatedly up to 20 μm (Advanced Solutions n.d.) | |
| Fab@Home | USA | Mechanical microextrusion | Seraph Robotics | Aortic valves (Duan et al., 2013), filling chondral and osteochondral defects (Cohen et al., 2010a), and ear (Mannoor et al., 2013) | + | Positioning resolution: 15.8 μm per full step, a nominal top speed of 25 mm/s (x–y–z axes) (Malone and Lipson, 2007) | |
| Table Continued | |||||||

| Bioprinter Name | Country | Bioprinting Mechanism | Company | Use | Dual Bioink Printability | Features | |
| The Alpha Bioprinter | UK | Pneumatic extrusion | 3Dynamic Systems (3DS) | NI | − | Accuracy: ± 75 μm (3Dynamic Systems n.d.) Price: £9,480§ | |
| The Omega Bioprinter | UK | Pneumatic extrusion | 3Dynamic Systems (3DS) | NI | + | Accuracy: ±50 μm (3Dynamic Systems n.d.) Price: £14,480§ | |
| Regemat 3D V1 | Spain | Pneumatic extrusion | Regemat 3D | NI | + | ||
| Fab@Home MD4 | USA | Pneumatic extrusion | Seraph Robotics | NI | + | 50 μm Gantry (Seraph Robotics n.d.) | |
| Scientist 3D printer | USA | Pneumatic extrusion | Seraph Robotics | NI | + | 5–10 μm Gantry (Seraph Robotics n.d.) | |
| Bio3D SYNˆ | Singapore | Microextrusion | Bio3D Technologies | NI | + | 1 μm (x–y–z resolution) (Bio3D technologies n.d.) Speed: 10–300,000 μm/min (Bio3D technologies n.d.) | |
| Bio3D Explorer | Singapore | Microextrusion | Bio3D technologies | NI | + | 5 μm (x–y–z resolution) (Bio3D technologies n.d.) | |
| Inkredible | USA | Microextrusion | Cellink | NI | + | x–y resolution per microstep: 10 μm z resolution per microstep: 2.5 μm Layer resolution: 50–100 μm (Cellink n.d.) |

7.2.2.2.1. Autodrop Compact and AD-P-8000
Table 7.3
| Availability | Bioprinter Name | Country | Bioprinting Mechanism | University/Company | Use | Dual Bioink Printability | |
| Droplet-based bioprinters | Commercial | Fujifilm Dimatrix Printer | USA | Piezoelectric drop-on-demand | Fujifilm Dimatix, Inc. | Study of cell-to-cell communications among bioprinted bacterial cells (Choi et al., 2011) | − |
| MicroFab JetLab II | USA | Piezoelectric drop-on-demand | MicroFAB Technologies, Inc. | Cell and biologics 3D bioprinting including silk nest arrays for hosting cells (Escherichia coli) for biosensing (Suntivich et al., 2014) | + | ||
| MicroFab JetLab 4 | USA | Piezoelectric drop-on-demand | MicroFAB Technologies, Inc. | Bioprinting | + | ||
| Autodrop Compact | Germany | Piezoelectric drop-on-demand | Microdrop Technologies | Bioprinting | + | ||
| Autodrop AD-P-8000 | Germany | Piezoelectric drop-on-demand | Microdrop Technologies | Bioprinting | + | ||
| Cluster Technology DeskViewer | Japan | Piezoelectric drop-on-demand | Cluster Technology Co., Ltd. | Human liver tissue chips comprising of hepatocytes (HepG2) and human umbilical vein endothelial cells (HUVECs) (Matsusaki et al., 2013) | + | ||
| Cell Jet Cell Printer | USA | synQUAD drop-by-drop technology (micro-valve and syringe pump) | Digilab, Inc. | Cell printing | + | ||
| Noncommercial | Custom printer with MicroJet™ piezoelectric actuator with MicroFab Technologies nozzle | USA | Piezoelectric drop-on-demand | Carnegie Mellon University and University of Pittsburgh Medical Center (UPMC) | FGF-2 dose impact on human MG-63 osteosarcoma cell response (Campbell et al., 2005; Miller et al., 2006), BMP-2 to evaluate spatially controlled differentiation of MDSCs (Phillippi et al., 2008) | − | |
| Custom printer with MicroFab MJ-ABP-01 piezoelectric nozzle | Canada | Piezoelectric drop-on-demand | University of British Columbia | MCF-7 movement within in the nozzle during the printing process (Cheng et al., 2014) | − | ||
| Table Continued | |||||||

| Availability | Bioprinter Name | Country | Bioprinting Mechanism | University/Company | Use | Dual Bioink Printability | |
| Custom printer with MicroFab MJ-ABL-01-120-6MX piezoelectric nozzle | USA | Piezoelectric drop-on-demand | Clemson University and University of Florida | Complex tubular tissue (NIH 3T3 cells and alginate) constructs with bifurcations (Xu et al., 2012; Christensen et al., 2015) | − | ||
| Modified Canon Bubble Jet printer (BJC-2100) and modified Hewlett–Packard Deskjet printers (HP 550C, HP 500, and HP 340) | USA | Thermal drop-on-demand | Clemson University and the University of Texas at El Paso | Collagen scaffolding patterns (Roth et al., 2004), mammalian cell (CHO cells and rat primary embryonic motoneural cells) constructs (Xu et al., 2005), neural cell (rat hippocampal and cortical cell) constructs (Xu et al., 2006), alginate 3D constructs (Boland et al., 2007), cardiac 3D constructs (feline and H1 cardiomyocytes with alginate), vascular (HMVECs and fibrin) constructs (Cui and Boland, 2009), skin transplants (NHDF and NHEK) with built-in vascular networks (HMVECs) for in vivo wound healing studies (Yanez et al., 2014) | + | ||
| Modified Hewlett–Packard Deskjet printer (HP 550C) | USA | Thermal drop-on-demand | Wake Forest University | Alginate microspheres with single-encapsulated cells (beta-TC6) (Xu et al., 2008), complex heterogeneous 3D tissue models with hAFCS, dSMCs, and bECs (Xu et al., 2013a) | + | ||
| Modified Hewlett–Packard Deskjet printer (HP 500) | USA | Thermal drop-on-demand | The Scripps Research Institute | In situ bioprinting of chondrocytes and PEGDMA hydrogel for direct cartilage repair (Cui et al., 2012a), cartilage constructs (human articular chondrocytes) to study FGF-2 and TGF-β1 growth factors impact on printed cartilage formation (Cui et al., 2012b) | + | ||
| Modified Hewlett–Packard ink-jet printer | USA | Thermal drop-on-demand | TeVido BioDevices | Breast tissue | + | ||
| Table Continued | |||||||
| Availability | Bioprinter Name | Country | Bioprinting Mechanism | University/Company | Use | Dual Bioink Printability | |
| Modified Hewlett–Packard deskjet printer | USA, Germany, Japan, and China | Thermal drop-on-demand | Stemorgan Therapeutics, Technical University Munich, The Scripps Research Institute, Tokyo University of Science, Rensselaer Polytechnic Institute, Wuhan University of Technology | Stem cell tissue constructs (hMSCs with PEG) and their directed differentiation into bone and cartilage (Gao et al., 2014; Gao et al. 2015) | + | ||
| Modified Hewlett–Packard 5360 printer | USA and China | Thermal drop-on-demand | University of Texas at El Paso, Shanghai Jiao Tong University, Sun Yat-sen University, and Texas Tech University Health Sciences | High-throughput miniature drug-screening platform employing bioprinted E. coli-laden alginate and three different antibiotics (penicillin/streptomycin, antimycotic, and kanamycin sulfate) (Rodríguez-Dévora et al., 2012) | + | ||
| Lab-on-a-printer | Canada | Microchannel-based thermal inkjet | Aspect Biosystems | 3D tissue fabrication, drug testing, toxicity testing | + | ||
| Modified Hewlett–Packard (HP 660C) printer with add-on piezoelectric pump | USA | Piezoelectric drop-on-demand | Clemson University | Protein (bovine serum albumin and streptavidin) and cell (bovine aortal endothelial cell) 2D constructs (Wilson and Boland, 2003) | N/A | ||
| Custom printer with Epson SEA-Jet printhead | Japan | Electrostatic drop-on-demand | University of Toyama and Kanagawa Academy of Science and Technology | 3D tissue (HeLa cells) constructs (hollow tubes) (Nishiyama et al., 2008) | − | ||
| Custom printer | Ireland and Germany | Piezoelectric drop-on-demand | University of Freiburg, Trinity College and Women and Infants University Hospital | Microspheres with single-encapsulated cells (HeLa cells) (Yusof et al., 2011) | − | ||
| Custom printer with Xaar-126 piezoelectric printhead | Australia | Piezoelectric drop-on-demand | University of Wollongong | 2D tissue (C2C12 and PC12 cells) constructs (Ferris et al., 2013) | + | ||
| Custom EHD printer using commercially available subsystems | UK | Electrohydrodynamic (EHD) jetting | University of London | EHD as a viable bioprinting strategy using Jurkat cells (Jayasinghe et al., 2006), CAD (Cath.-a-differentiated) mouse neural cells (Eagles et al., 2006), human astrocytoma cells (Jayasinghe and Townsend-Nicholson, 2006), white blood cells, erythrocytes (Mongkoldhumrongkul et al., 2009), and THP-1 cells with alginate and collagen (Workman et al., 2014) | − | ||
| Table Continued | |||||||

| Availability | Bioprinter Name | Country | Bioprinting Mechanism | University/Company | Use | Dual Bioink Printability | |
| Custom EHD printer using commercially available subsystems | Singapore | EHD jetting | National University of Singapore and Molecular Engineering of Biological and Chemical Systems | Microencapsulation of cells (hepatocytes G2 cells) with alginate (Xie and Wang, 2007) | − | ||
| Custom EHD printer using commercially available subsystems | South Korea | EHD jetting | Yonsei University | Collagen scaffold patterns (Kim et al., 2007) | − | ||
| Custom EHD printer using commercially available subsystems | USA | EHD jetting | University of Illinois at Urbana–Champaign, University of Michigan, and Rensselaer Polytechnic Institute | Rabbit Immunoglobulin-G and fibronectin scaffold patterns (Poellmann et al., 2011) | − | ||
| Custom EHD printer using commercially available subsystems | Italy | EHD jetting | University of Trento | Microencapsulation of cells (B50 rat neural cells) with alginate (Gasperini et al., 2013), 3T3 fibroblasts and alginate constructs (Gasperini et al., 2015) | − | ||
| Custom acoustic picoliter droplet ejection system | USA | Acoustic droplet ejection | Harvard University | Encapsulation of a single to multiple cells (mESC, RAJI, HL-1, 3T3, and AML-12) (Demirci and Montesano, 2007) | + | ||
| Custom acoustic droplet ejection system | USA | Acoustic droplet ejection | University of Michigan | 2D heterogeneous tissue (MDA MB 231 breast cancer cells and HEK 239 cells with dextran) constructs (Fang et al., 2012) | − | ||
| Custom printer with TechElan solenoid valve ejector (G100-150300nj) | USA and Finland | Microvalve (Solenoid) | Harvard University, Massachusetts Institute of Technology, Clemson University, and University of Helsinki | Cells (mESC, RAJI, HL-1, 3T3, and AML-12) encapsulation, cell encapsulation (rat bladder smooth muscle cells with collagen) (Xu et al., 2010), 3D tissue constructs (rat bladder smooth muscle cells and collagen) fabrication (Moon et al., 2010), heterogeneous tissue (NIH: OVCAR-5 human ovarian cancer cells and MRC-5 normal human fibroblasts) constructs (Xu et al., 2011), 3D fibrocartilage tissue models by bioprinting mesenchymal stem cells with GelMA precursor solution and photointiator (Gurkan et al., 2014) | + | ||
| Table Continued | |||||||

| Availability | Bioprinter Name | Country | Bioprinting Mechanism | University/Company | Use | Dual Bioink Printability | |
| Custom printer with Fritz Gyger SMLD solenoid valve ejector | USA and South Korea | Microvalve (Solenoid) | Harvard Medical School, Rensselaer Polytechnic Institute, Albany Medical College, and Korea Advanced Institute of Science and Technology (KAIST) | 3D skin tissue (human dermal fibroblasts, human epidermal keratinocytes, and collagen) constructs (Lee et al., 2009a), 2D neural tissue (rat astrocytes, neurons, and collagen) constructs (Lee et al., 2009b), VEGF-releasing fibrin gel scaffolds for neural stem cell (murine NSC) culture (Lee et al., 2010b), Angiogenic sprouting of vascular networks at cellular level through bioprinted HUVECs and NHLFs (Lee et al., 2014) | + | ||
| Custom printer with Offshore Solutions solenoid inkjet valve | USA | Microvalve (Solenoid) | Wake Forest University | Cartilage tissue constructs (chondrocytes, fibrinogen, and collagen) (Xu et al., 2013b) | − | ||
| Custom printer with Lee Products VHS Nanoliter dispense valve with Lee Products Minstac Nozzle | UK | Microvalve (Solenoid) | Heriot-Watt University and Roslin Biocentre | Tissue (HEK293 and hESC cells) spheroids (Faulkner-Jones et al., 2013), 3D hepatocyte constructs (HLCs differentiated from hESCs and hiPSCs with alginate) (Faulkner-Jones et al., 2015), DNA based hydrogel bioprinting (Li et al., 2015a,b) | + | ||
| Custom printer | Australia | Microvalve (Solenoid) | University of Wollongong | 2D tissue (C2C12 cells) constructs (Ferris et al., 2013) | − |

7.2.2.2.2. MicroFab Jetlab®
7.2.2.3. Laser-Based Bioprinters
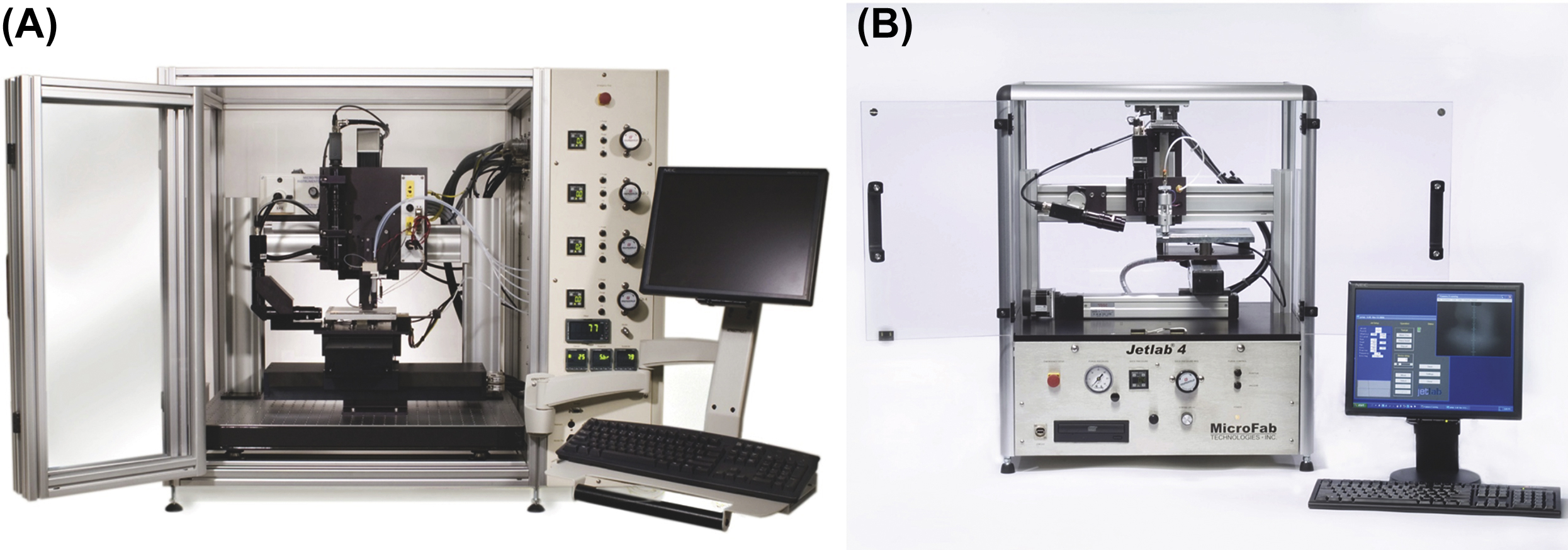
Table 7.4
Laser-Based Bioprinters∗
| Availability | Bioprinter Name | Country | Bioprinting Mechanism | University/Institution | Use | Dual Bioink Printability | |
| Laser-based bioprinters | Noncommercial | Custom-built laser-based bioprinter | USA | LGDW | University of Minnesota and Michigan Technological University | Embryonic chick spinal cord cells to demonstrate LGDW capabilities (Odde and Renn, 2000), 2D and 3D HUVEC patterns on Matrigel (Nahmias et al., 2005) | − |
| Custom-built laser-based bioprinter | USA | LGDW | Michigan Technological University and University of Missouri–Columbia | Evaluation of laser-induced damage on bioprinted avidin biomolecules (proteins) (Xu et al., 2003) | − | ||
| Custom-built laser-based bioprinter based on ArF excimer laser | USA | LIFT and MAPLE-DW | US Naval Research Laboratory | Biotinylated bovine serum albumin (BSA) protein microarray fabrication by using MAPLE-DW (Ringeisen et al., 2002), 2D patterning of human osteosarcoma and rat cardiac cells by using MAPLE-DW (Barron et al., 2004c), 3D patterning of human osteosarcoma cells by using BioLP (Barron et al., 2004c), single cell patterns of human osteosarcoma cells by using BioLP (Barron et al., 2005) | − | ||
| Custom-built laser-based bioprinter based on ArF excimer laser | MAPLE-DW | US Naval Research Laboratory, University of North Carolina at Chapel Hill, Georgia Institute of Technology, Paul Scherrer Institute, Laboratory for Functional Polymers EMPA Swiss Federal Laboratories for Materials Testing and Research Uberlandstrasse, Hungarian Academy of Sciences and University of Szeged, National Institute for Laser and Plasma and Radiation Physics (Romania) | Patterning of viable B35 neuroblasts by using triazene polymer intermediate absorbing layer (Doraiswamy et al., 2006) | − | |||
| Table Continued | |||||||

| Availability | Bioprinter Name | Country | Bioprinting Mechanism | University/Institution | Use | Dual Bioink Printability | |
| Custom-built laser-based bioprinter based on ArF excimer laser | USA | MAPLE-DW | US Naval Research Laboratory and University of North Carolina at Chapel Hill | Codeposition of hydroxyapatite and viable MG 63 osteoblast-like cells (Doraiswamy et al., 2007) | − | ||
| Custom-built laser-based bioprinter based on ArF excimer laser | USA | MAPLE-DW | Clemson University, University of Florida, Rensselaer Polytechnic Institute, and Tulane University | Laser fluence (energy) impact on yeast (Lin et al., 2009) and human colon cancer cells viability (Lin et al., 2010), alginate long tubes and annular constructs fabrication (Yan et al., 2013), alginate gelation impact on bioprinted NIH 3T3 cell viability (Gudapati et al., 2014) and bifurcated hollow tubular tissue (NIH 3T3 cells and alginate) constructs (Xiong et al., 2015a) | − | ||
| Custom-built laser-based bioprinter based on Nd:YAG laser | Germany | LIFT | Laser Zentrum Hannover e.V., Hannover Medical School, and Helmholtz Institute of the RWTH Aachen University | 3D cell arrays comprising endothelial colony forming cells (ECFCs) and adipose-derived stem cells (ASCs) to study cell-to-cell interactions in 3D environments (Gruene et al., 2011a,b), skin tissue consisting of NIH-3T3 fibroblasts, human keratinocyte cells and collagen (Koch et al., 2012), and LaBP process impact on adipogenic stem cell proliferation and differentiation (Gruene et al., 2011a,b) | − | ||
| Custom-built laser-based bioprinter based on KrF excimer laser | USA and Hungary | LIFT | Hungarian Academy of Sciences and University of Szeged, University of Szeged, and US Naval Research Laboratory | AFA–LIFT impact on viability and proliferation of bioprinted rat Schwann and astroglial cells and pig lens epithelial cells (Hopp et al., 2005) | − |