Chapter 21
Acoustic-Wave Sensors
Mass change is the most straightforward modification caused by the recognition event in a chemical sensor. Mass-change assessment appears therefore as a very advantageous transduction method, particularly as it does not involve labels. However, extremely sensitive mass transducers are required for this purpose as the mass change can be below the μg level. Such ultrasensitive transducers are based on vibrating piezoelectric crystals.
Acoustics is the study of mechanical vibrations in gases, liquids, and solids. As vibrations are perceived as sound, the name acoustic-wave sensor is ascribed to the devices presented in this chapter An acoustic wave can develop either simply at the surface of a vibrating device or it can expand to its whole volume. The first case refers to surface-acoustic waves, whereas the second refers to bulk acoustic waves.
Applications of acoustic waves in chemical sensors rely on the interaction of the analyte with an adjacent recognition layer, with resulting modifications in the wave frequency and other wave parameters.
As the key physical phenomenon in an acoustic-wave sensor is piezoelectricity, the first part of this chapter introduces the basic concept of this physical effect. The second part presents the principles of the thickness–shear mode vibrating piezoelectric crystal that is currently the most widely used mass transducer in chemical sensors. Subsequent sections address a series of applications of this transducer in various kinds of chemical sensors such as gas sensors, affinity sensors and nucleic acid sensors.
Commonly, a transducer or a sensor based on a thickness–shear vibrating piezoelectric crystal is referred to as quartz crystal microbalance (QCM) due to the extreme sensitivity to mass loading. The QCM is a bulk acoustic-wave device. Despite its name, the QCM is much more than a microbalance. It is also a valuable tool for investigating elasticity and viscosity of various materials including synthetic and biological polymers currently employed in the development of chemical sensors. Therefore, the QCM is an unparalleled tool for assessing physicochemical properties of thin layers used in chemical sensors. Rational use of the wide potential of QCM makes available a wealth of information on the physicochemical properties of the sensing element and also allows for rational design and operation of various types of chemical sensors.
Basic terms and definitions related to piezoelectric materials, sensing devices and analytical procedures with piezoelectric chemical sensors are available in ref. [1]. Various aspects of QCM principles, theory and applications are extensively addressed in collective volumes [2, 3] and reviews [4–6].
21.1 The Piezoelectric Effect
The piezoelectric effect, which was discovered in 1880 by the brothers Pierre and Jacques Curie, refers to a particular property of certain crystals that develops electrical polarization under mechanical stress. The term piezoelectric is derived from the Greek piezein that means to press. This effect is noticed with crystals that have unique anisotropic symmetry axis, the most common material of this type being crystalline quartz. Other piezoelectric materials commonly used are zinc oxide, lithium niobate and lithium tantalate.
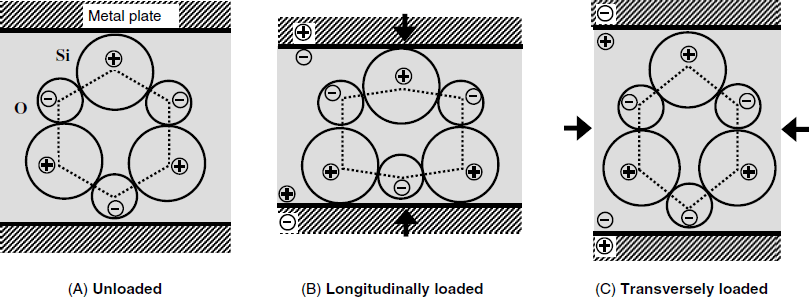
The development of electric polarization in a piezoelectric SiO2 quartz crystal is demonstrated in Figure 21.1. Due to the different electronegativity, each oxygen atom assume a partial negative charge while each silicon atom assume an opposite charge. At equilibrium (unloaded crystal, A) the atoms are positioned such that opposite charges balance each other and no overall charge can be noticed at the macroscopic scale. If the crystal is longitudinally loaded (B), the hexagonal frame is distorted such that an excess negative charge builds up at the upper part, whereas a positive charge appears at the other side. Although the overall charge of the system is still zero, a distinct electric charge appears at each crystal side. In order to detect the charge, the crystal is embedded between two metal plates (electrodes). Electrons at the metal surface are attracted or repelled by electrostatic forces, giving rise to a charge opposite to that at the crystal surface. If the crystal is transversely compressed, the distortion of the crystal lattice results in an opposite electric polarization (C). In this way, crystal distortion along particular directions results in an electrical voltage between the metal plates. This voltage depends on the distortion degree, that is, on the applied stress. The piezoelectric effect is reversible as the voltage vanishes if the loading is removed.
Figure 21.1 Effect of the mechanical stress on charge distribution in a piezoelectric SiO2 crystal.
The above effect is termed the direct piezoelectric effect. It is applied to perform conversion of an acoustic signal into an electrical one in the case of the piezoelectric microphone. A converse piezoelectric effect is equally possible. It consists of crystal distortion under the effect of an electric voltage applied to the metal plates. Ultrasound generators make use of this effect as they are based on the vibration of a piezoelectric crystal under the effect of an applied AC voltage. Therefore, a piezoelectric crystal can perform interconversion of electrical and mechanical energy and can act as a stress–electrical voltage transducer.
21.2 The Thickness–Shear Mode Piezoelectric Resonator
21.2.1 The Quartz Crystal Microbalance
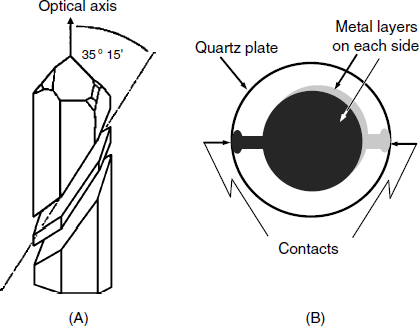
Piezoelectric quartz crystals can be cut from a quartz crystal at various orientations with respect to the crystal optical axis. The most advantageous is the AT cut, depicted in Figure 21.2A because the thermal expansion coefficient of the crystal thus obtained is negligible. A shear-deformation mode crystal is shown in Figure 21.2B. It consists of a very thin (about 0.2 mm) AT-cut quartz crystal disk plated on each side with a metal layer (Au, Ag, Pt or others). The active area is that between the electrodes and this may have a diameter of 5 to 20 mm. Electrical contacts are available at the periphery of the plate.
Figure 21.2 The thickness–shear piezoelectric crystal. (A) AT-cut of a ![]() -quartz wafer. The quartz plate is cut at an angle of 35° 15′ with respect to the optical axis. (B) A thickness–shear mode resonator. The quartz wafer (some 0.2 mm thick and 15–30 mm diameter) is partially sandwiched between two thin metal electrodes fitted with contact pads at the periphery. The vibrating zone is that contained between the electrodes. (A) was reproduced with permission from [6]. Copyright 2000 Wiley-VCH Verlag GmBH & Co. KGaA.
-quartz wafer. The quartz plate is cut at an angle of 35° 15′ with respect to the optical axis. (B) A thickness–shear mode resonator. The quartz wafer (some 0.2 mm thick and 15–30 mm diameter) is partially sandwiched between two thin metal electrodes fitted with contact pads at the periphery. The vibrating zone is that contained between the electrodes. (A) was reproduced with permission from [6]. Copyright 2000 Wiley-VCH Verlag GmBH & Co. KGaA.
An AT-cut quartz wafer undergoes thickness–shear deformation under a vertical load applied to its surface. Figure 21.3 presents the converse piezoelectric effect experienced by such a crystal. When the electrodes are not charged, the plate is at equilibrium (A) but once a voltage is applied, each end of the molecular dipole in the crystal is subject to electrostatic forces that shift laterally the plane in the crystal lattice and distort the crystal as shown in (B). If the voltage is reversed, the crystal is distorted in the opposite direction.
Figure 21.3 The converse piezoelectric effect in the case of the thickness–shear deformation. (A) No voltage applied; (B) DC voltage applied; (C) AC voltage applied. The arrow shows the vibration direction. (A) and (B) were adapted with permission from [4]. Copyright 1992 American Chemical Society.
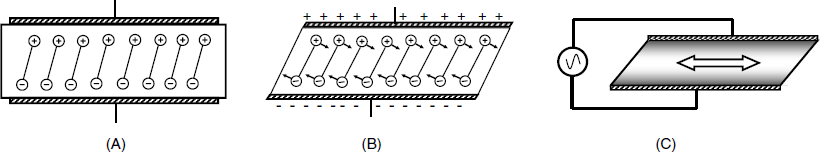
Figure 21.1B shows the shear deformation of a piezoelectric crystal under the effect of an applied DC voltage. The applications of such devices are based on crystal oscillation under the effect of an applied AC voltage so as to form a piezoelectric resonator. If an AC voltage is applied to the device, it is expected to undergo periodic shear-distortion following the voltage oscillation (Figure 21.3C). This actually occurs only within a very narrow frequency range around the specific vibration frequency of the crystal itself. The frequency at which the vibration amplitude reaches a maximum is termed the resonant frequency ![]() . This depends on the crystal thickness and also on the density and elasticity of the piezoelectric material. As the applied voltage increases, the shear-deformation amplitude increases as well. Due to the elastic deformation, potential energy is stored, as is also the case in a compressed spring. A voltage decrease causes the stress to reduce and due to its elasticity the crystal tends to return to the undistorted position. During the opposite half-period, the crystal undergoes distortion in the opposite direction. The resonance occurs when the voltage frequency matches the intrinsic vibration frequency of the crystal; under these conditions, the vibration amplitude is at a maximum. The amplitude of lateral displacement rarely exceeds a nanometer. It is maximum at the center but decreases gradually to zero at the periphery of the active zone.
. This depends on the crystal thickness and also on the density and elasticity of the piezoelectric material. As the applied voltage increases, the shear-deformation amplitude increases as well. Due to the elastic deformation, potential energy is stored, as is also the case in a compressed spring. A voltage decrease causes the stress to reduce and due to its elasticity the crystal tends to return to the undistorted position. During the opposite half-period, the crystal undergoes distortion in the opposite direction. The resonance occurs when the voltage frequency matches the intrinsic vibration frequency of the crystal; under these conditions, the vibration amplitude is at a maximum. The amplitude of lateral displacement rarely exceeds a nanometer. It is maximum at the center but decreases gradually to zero at the periphery of the active zone.
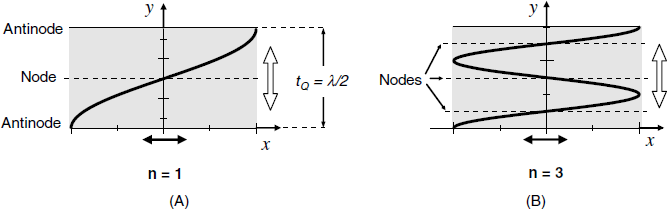
The radial displacement profile at resonance is presented in Figure 21.4A for the so-called fundamental mode. In this case, the wavelength is twice the crystal thickness, ![]() . A standing wave forms at resonance, this implying that there is no displacement at the node plane and that the maximum displacement occurs at the lateral faces of the crystal (
. A standing wave forms at resonance, this implying that there is no displacement at the node plane and that the maximum displacement occurs at the lateral faces of the crystal (![]() and
and ![]() ). The resonators can be operated at a number of overtones (harmonics), typically indexed by the number of nodal planes, n, parallel to the crystal surfaces. Only odd harmonics can be excited (n = 1, 3, 5,...). The vibration amplitude is inversely proportional to the square of the overtone number. The vibration expands over the whole crystal volume; which is why the name bulk acoustic-wave transducer is assigned to such a device. Due to the deformation type, this device is better described as a thickness–shear mode (TSM) piezoelectric crystal.
). The resonators can be operated at a number of overtones (harmonics), typically indexed by the number of nodal planes, n, parallel to the crystal surfaces. Only odd harmonics can be excited (n = 1, 3, 5,...). The vibration amplitude is inversely proportional to the square of the overtone number. The vibration expands over the whole crystal volume; which is why the name bulk acoustic-wave transducer is assigned to such a device. Due to the deformation type, this device is better described as a thickness–shear mode (TSM) piezoelectric crystal.
Figure 21.4 The profile of a standing acoustic wave across the TSM piezoelectric crystal in the fundamental mode (A) and third overtone (B) mode. Black arrows indicate the direction of particle displacement; white arrows indicate the direction of wave propagation. Nodes correspond to zero displacement and antinodes correspond to maximum displacement.
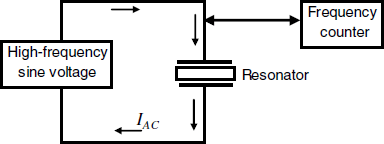
As will be shown below, the key parameter in analytical applications of such devices is the resonant frequency. That is why the resonator is integrated with an excitation AC voltage source and a frequency counter (Figure 21.5). Note that a DC current cannot flow across the piezoelectric crystal because it consists of an insulator material. However, the electrodes–crystal system behaves somewhat as a capacitor and therefore allows an AC current to flow along the left-hand loop. The current amplitude is at a maximum when the AC voltage frequency matches the crystal resonant frequency.
Figure 21.5 Schematics of electrical wiring of the piezoelectric resonator.
21.2.2 The Unperturbed Resonator
In order to understand the functioning principles of QCM sensors it is important to get a clear insight into the physical principle governing the functioning of the unperturbed resonator.
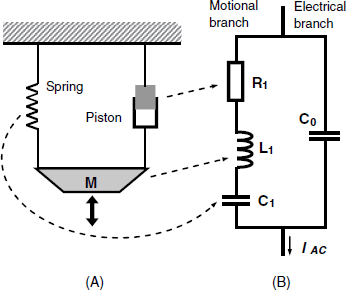
Piezoelectric crystals are electromechanical devices that perform interconversion of electrical and mechanical energy. That is why the resonator can be modeled either by a mechanical layout or an electrical equivalent circuit, as shown in Figure 21.6. The mechanical model (A) consists of a mass M attached to a spring (which indicates energy accumulation by the elastic deformation of the crystal) and a piston that represents energy dissipation by internal friction and damping from the crystal mounting. Once disturbed, this system starts oscillating as shown by the black arrow, but the oscillation dampens due to energy dissipation in the piston. If energy is periodically provided the oscillation is maintained for an undefined time. The spring accumulates energy by extension or compression and its electrical analog is from this standpoint the capacitor C1. Energy dissipation in the equivalent circuit (Figure 21.6B) is accounted for by the resistor R1 and the inertial element M in (A) (which accumulates potential energy) is represented by the inductor L1 in (B). The series combination of R1, C1, and L1 represents the motional (or acoustic) branch of the network because AC current flows along it only when the crystal vibrates at resonance. C0 on the electrical branch is the static capacitance that arises from the electrodes located on the opposite sides of the crystal. A more accurate model includes in addition a parasitic capacitance that arises in the test fixture and is coupled in parallel with C0. Of course, components of the motional branch depend on the geometrical and physical properties of the piezoelectric material, such as thickness, density and the shear stiffness.
Figure 21.6 The mechanical model (A) and the equivalent circuit of the TSM unperturbed resonator (B). The total electrical impedance of the equivalent circuit is similar to that of the resonator itself.
As energy dissipation occurs under crystal vibration, the power loss is compensated by the driving AC voltage supply.
According to Figure 21.6B, the motional impedance of the unloaded resonator is (with ![]() ) [7]:
) [7]:
(21.1) ![]()
Here, ![]() is the angular frequency (
is the angular frequency (![]() )
) ![]() is the resistance,
is the resistance, ![]() is the inductance and
is the inductance and ![]() is the capacitance of the pertinent component in Figure 21.6. The last two terms in the above equation account for reactances in the equivalent circuit. Reactance quantifies the opposition of a circuit element to a change of current, caused by the build-up of electric or magnetic fields in the element. Those fields act to produce a countervoltage that is proportional to either the derivative of the current (capacitive reactance, phase shift, −90°), or the integral of the current (inductive reactance, phase shift, 90°). The absolute value of an inductive reactance is
is the capacitance of the pertinent component in Figure 21.6. The last two terms in the above equation account for reactances in the equivalent circuit. Reactance quantifies the opposition of a circuit element to a change of current, caused by the build-up of electric or magnetic fields in the element. Those fields act to produce a countervoltage that is proportional to either the derivative of the current (capacitive reactance, phase shift, −90°), or the integral of the current (inductive reactance, phase shift, 90°). The absolute value of an inductive reactance is ![]() where L represents the inductance. For a capacitor of capacitance C, the absolute value of the reactance is
where L represents the inductance. For a capacitor of capacitance C, the absolute value of the reactance is ![]() . Note that a reactance introduces a phase shift, in contrast to a pure resistance. Therefore,
. Note that a reactance introduces a phase shift, in contrast to a pure resistance. Therefore, ![]() is the real part of the motional impedance, whereas the imaginary part is
is the real part of the motional impedance, whereas the imaginary part is ![]() .
.
The resonance occurs when the impedance of the motional branch is at a minimum, that is, when ![]() . At frequencies far from the resonant value,
. At frequencies far from the resonant value, ![]() is very high and the AC current flows along the electric branch only. However, near the resonant frequency, the motional impedance becomes very low as a standing acoustic wave develops within the crystal. In this case, the contribution of the motional branch to the total impedance is negligible and current flows mostly along this branch.
is very high and the AC current flows along the electric branch only. However, near the resonant frequency, the motional impedance becomes very low as a standing acoustic wave develops within the crystal. In this case, the contribution of the motional branch to the total impedance is negligible and current flows mostly along this branch.
21.2.3 QCM Loading by a Rigid Overlayer. The Sauerbrey Equation
The most common application of the QCM is microweighing. It is therefore essential to consider the effect of mass loading on the resonance frequency.
The simplest case to be considered is the loading of one of the crystal surfaces by a thin rigid layer with uniform thickness and perfect adherence to the surface. Experiments proved that crystal loading causes the resonant frequency to decrease in a measurable amount, ![]() . This case was first addressed in 1959 by Sauerbrey who derived and tested a relationship between frequency change and the loading mass [8]. The derivation of the Sauerbrey equation is straightforward and will be given next in order to provide a better understanding of the functioning principle of the QCM as well as the limitations of this equation. Referring to Figure 21.4A, it is clear that the standing wave can form only when the wavelength (λ) is twice the crystal thickness (
. This case was first addressed in 1959 by Sauerbrey who derived and tested a relationship between frequency change and the loading mass [8]. The derivation of the Sauerbrey equation is straightforward and will be given next in order to provide a better understanding of the functioning principle of the QCM as well as the limitations of this equation. Referring to Figure 21.4A, it is clear that the standing wave can form only when the wavelength (λ) is twice the crystal thickness (![]() ), that is:
), that is:
(21.2) ![]()
For the unloaded resonator, the wavelength is related to the resonant frequency (f0) as ![]() , where
, where ![]() is the propagation velocity of the transverse wave. Hence, the resonance condition is:
is the propagation velocity of the transverse wave. Hence, the resonance condition is:
Coating with a rigid layer expands the thickness of the vibrating system by ![]() and Equation (21.3) assumes in this case the following form:
and Equation (21.3) assumes in this case the following form:
Assuming that ![]() is negligible with respect to
is negligible with respect to ![]() , the above equations leads to:
, the above equations leads to:
(21.5) ![]()
In order to account for mass variation, the crystal thickness will be expressed as a function of the crystal density, ![]() , volume,
, volume, ![]() , the area, A, and mass of the vibrating zone,
, the area, A, and mass of the vibrating zone, ![]() , as follows:
, as follows:
(21.6) ![]()
Analogously, the thickness of the coating layer is:
(21.7) ![]()
where ![]() and
and ![]() represent the mass and density of the coating layer, respectively. By inserting in Equation (21.4) the above thickness expressions and assuming that
represent the mass and density of the coating layer, respectively. By inserting in Equation (21.4) the above thickness expressions and assuming that ![]() one obtains the Sauerbrey equation in the following form:
one obtains the Sauerbrey equation in the following form:
This equation proves that the frequency decreases proportionally to the mass change. In order to put into evidence the effect of the physical properties of the crystal, ![]() will be substituted by
will be substituted by ![]() . On the other hand, the crystal thickness and the resonant frequency are related by Equation (21.3) that includes the wave velocity. This latter depends on material density (which represents the inertial opposition to displacement) and its shear elastic modulus (
. On the other hand, the crystal thickness and the resonant frequency are related by Equation (21.3) that includes the wave velocity. This latter depends on material density (which represents the inertial opposition to displacement) and its shear elastic modulus (![]() ) that account for the accumulation of potential energy, as is the case for the deformation of a spring. Acoustics theory proves that the wave velocity along the y-axis in Figure 21.4A is:
) that account for the accumulation of potential energy, as is the case for the deformation of a spring. Acoustics theory proves that the wave velocity along the y-axis in Figure 21.4A is:
(21.9) ![]()
Therefore, the thickness–frequency relationship is:
By substituting in Equation (21.8) the pertinent expression for ![]() and then using Equation (21.10) one obtains the Sauerbrey equation in the following widely used form:
and then using Equation (21.10) one obtains the Sauerbrey equation in the following widely used form:
This equation proves that the resonant frequency decreases as the mass loading per surface unit increases. For a particular type of crystal, the mass sensitivity constant ![]() can be introduced. This constant is proportional to the squared resonant frequency. That is why 5- to 10-MHz resonators are commonly employed as mass transducers in order to achieve good mass sensitivity. As the QCM responds to mass loading per surface unit, the sensitivity is independent of the crystal diameter. However, in order to avoid edge effects, resonators with the diameter less than 5 mm are seldom used.
can be introduced. This constant is proportional to the squared resonant frequency. That is why 5- to 10-MHz resonators are commonly employed as mass transducers in order to achieve good mass sensitivity. As the QCM responds to mass loading per surface unit, the sensitivity is independent of the crystal diameter. However, in order to avoid edge effects, resonators with the diameter less than 5 mm are seldom used.
The previous approach addressed only the fundamental frequency case. If a higher overtone is considered (for example, n = 3), then the standing wave develops when the crystal thickness is ![]() (Figure 21.4B). Denoting by
(Figure 21.4B). Denoting by ![]() the overtone frequency (
the overtone frequency (![]() , n = 1, 3, 5...) and using the same reasoning line as before, the Sauerbrey equation is obtained in the following form:
, n = 1, 3, 5...) and using the same reasoning line as before, the Sauerbrey equation is obtained in the following form:
(21.12) ![]()
Because the sensitivity increases as the squared frequency, the sensitivity is enhanced when the resonator is operated at a higher overtone.
In order to illustrate the outstanding mass sensitivity of the QCM, a resonator of 5 MHz resonant frequency operated in the fundamental mode will be considered. Using numerical values of the constants in Equation (21.11) one obtains ![]() Hz cm2 μg−1. That means that the resonant frequency changes by 56.6 Hz when a mass load of 1 μg/cm2 is applied. As the frequency resolution can be as low as 1 Hz, it can be seen that that a mass load of about 20 ng cm−2 can be accurately measured with such a device. The exceptional mass sensitivity of this transducer fully justifies its assigned name (QCM); some authors prefer to call it a quartz crystal nanobalance (QCN).
Hz cm2 μg−1. That means that the resonant frequency changes by 56.6 Hz when a mass load of 1 μg/cm2 is applied. As the frequency resolution can be as low as 1 Hz, it can be seen that that a mass load of about 20 ng cm−2 can be accurately measured with such a device. The exceptional mass sensitivity of this transducer fully justifies its assigned name (QCM); some authors prefer to call it a quartz crystal nanobalance (QCN).
The above derivation of the Sauerbrey equation involved a series of simplifying assumptions that limit to some extent its applicability. In this respect, it should be remembered that the loading film is treated as an extension of crystal thickness. Therefore, this equation applies only to systems in which the following three conditions are met: the deposited mass must be rigid, evenly distributed, and the mass change must be less than 2% of the crystal mass (that is, ![]() ). These conditions are best fulfilled by solid layers such as metals and oxides. Molecularly thin films behave also as a rigid layer since the energy dissipated is negligible [9]. In addition this equation holds accurately only if the resonator is operated in vacuum or in a gaseous atmosphere such that the mechanical energy is not dissipated by friction. Under these conditions, the mass sensitivity of the QCM can be calculated from its resonant frequency and the intrinsic properties of the piezoelectric material. In addition, the mass sensitivity is independent of the physical parameters of the deposited material, except its mass. Therefore, no preliminary calibration is required in gas phase applications provided the deposited material covers evenly the active vibrating area. The Sauerbrey equation has been firmly validated using various solid films and the relative error in the film mass determination by means of this equation did not exceed 2%.
). These conditions are best fulfilled by solid layers such as metals and oxides. Molecularly thin films behave also as a rigid layer since the energy dissipated is negligible [9]. In addition this equation holds accurately only if the resonator is operated in vacuum or in a gaseous atmosphere such that the mechanical energy is not dissipated by friction. Under these conditions, the mass sensitivity of the QCM can be calculated from its resonant frequency and the intrinsic properties of the piezoelectric material. In addition, the mass sensitivity is independent of the physical parameters of the deposited material, except its mass. Therefore, no preliminary calibration is required in gas phase applications provided the deposited material covers evenly the active vibrating area. The Sauerbrey equation has been firmly validated using various solid films and the relative error in the film mass determination by means of this equation did not exceed 2%.
21.2.4 The QCM in Contact with Liquids
The QCM is very often operated in contact with a liquid that is entrained in vibration by the resonator surface. This implies that a part of the mechanical energy is dissipated within the fluid, which brings about drastic changes in the vibration parameters. Vibrational coupling with the fluid depends on the fluid rheological properties that determine its deformation behavior under applied stress. From the rheology standpoint we can distinguish Newtonian liquids (such as water and dilute aqueous solutions) and non-Newtonian fluids (e.g., a dense suspension of corn flour in water). The behavior of Newtonian liquids is illustrated in Figure 21.7 for the case in which it is in contact with a moving plate. Due to friction with the plate wall, the plate movement gives rise to a shear stress Txy (drag) that causes the fluid to move in the same direction. The stress represents the force per unit area along the movement direction. It decreases with the distance y due to the friction between successive liquid layers. As a consequence, the flow rate varies along the vertical axis giving rise to a velocity gradient ![]() . Internal friction is quantified by the dynamic viscosity,
. Internal friction is quantified by the dynamic viscosity, ![]() , which is defined by the following equation:
, which is defined by the following equation:
(21.13) ![]()
Figure 21.7 Drag of a Newtonian liquid by a moving plate. The fluid velocity decreases as the distance from the surface increases.
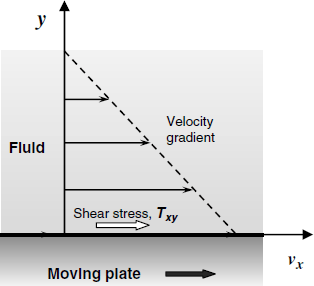
The viscosity of a Newtonian liquid is constant and independent of the rate of the stress application. An elastic material recovers its initial shape after removing the stress, because of the potential energy stored under deformation. Newtonian liquids do not behave like that because the applied energy is dissipated by internal friction.
Non-Newtonian fluids display a more intricate behavior under stress. Among them, the viscoelastic fluids are particularly relevant in this context because biopolymer gels used as sensing layers in a number of QCM biosensor behave as viscoelastic fluids. A viscoelastic fluid displays both viscous and elastic properties. When the stress is removed, they do not return completely to the initial shape (as an elastic material would do) because energy is partially dissipated by internal friction.
21.2.5 The QCM in Contact with a Newtonian Liquid
If a face of the resonator is immersed in a liquid, the liquid breaks the vibration by friction and causes the resonant frequency to lessen. At the same time, the adjacent liquid layer is subject to shear stress due to the crystal vibration and is itself entrained in periodical shear deformations. The stress in the liquid is maximal at the contact with the resonator surface but it reduces quickly with the distance from the surface due to friction within the oscillating liquid. So, under the effect of the resonator oscillation, a damped wave propagates in the liquid, and decays over a very short distance. (Figure 21.8A). The frequency change due to the liquid contact, ![]() , depends on liquid density
, depends on liquid density ![]() (inertial parameter) and viscosity
(inertial parameter) and viscosity ![]() (dissipative parameter) according to the following equation [10]:
(dissipative parameter) according to the following equation [10]:
Figure 21.8 Vibration profile of resonator in contact with liquids. (A) Semi-infinite Newtonian liquid (the liquid thickness is much greater than the wave decay length); (B) finite thickness viscoelastic film. The thickness of the film is exaggerated relative to that of the quartz; ![]() represents the shear displacement. (A) Reproduced with permission from [11]. Copyright 1993 American Chemical Society. (B) Reproduced with permission from [12]. Copyright 2000 American Chemical Society.
represents the shear displacement. (A) Reproduced with permission from [11]. Copyright 1993 American Chemical Society. (B) Reproduced with permission from [12]. Copyright 2000 American Chemical Society.
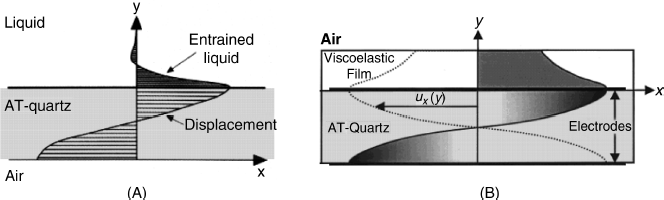
This equation holds when the thickness of the liquid layer is much greater than the wave decay length (![]() ) that is of some 250 nm for a 5 MHz resonator in water. Therefore, the transfer of the QCM from air to a viscous liquid causes the resonant frequency to decrease although no rigid layer forms at its surface. The frequency change depends on liquid density and its viscosity and is independent of the resonator area. It is equivalent to that produced by a hypothetical rigid liquid film of thickness
) that is of some 250 nm for a 5 MHz resonator in water. Therefore, the transfer of the QCM from air to a viscous liquid causes the resonant frequency to decrease although no rigid layer forms at its surface. The frequency change depends on liquid density and its viscosity and is independent of the resonator area. It is equivalent to that produced by a hypothetical rigid liquid film of thickness ![]() .
.
Equation (21.14) has been derived under the assumption that the liquid film does not slip at the contact with the resonator. This is only an approximation; in fact, film slippage does occur and this enhances the energy dissipation. In addition, due to the surface rugosity, a small amount of liquid at the interface vibrates almost like a rigid layer. Consequently, the real frequency change can be much higher than that predicted by Equation (21.14) (that is, some 650 Hz in water). Equation (21.14) has been retrieved as a limiting case of a more general equation including the coefficient of friction between the liquid film and the resonator surface [9].
It is often of interest to measure the mass loading with the QCM immersed in a liquid. A theoretical approach of this problem under nonslip conditions led to the conclusion that the mass loading and the liquid contact produce an additive effect as long as the rigid overlayer film conforms to the Sauerbrey assumptions [13]. Therefore, the total frequency change ![]() can be formulated as:
can be formulated as:
The additive behavior implies that there is no interplay between the vibration of the rigid film and the wave propagating into the liquid except for energy dissipation. Hence, if the density and viscosity of the liquid are invariable, the frequency change reports only on the change in the mass loading.
21.2.6 The QCM in Contact with a Viscoelastic Fluid
From the standpoint of chemical sensor applications, it is of interest to examine the case in which a finite-thickness viscoelastic film is coated on top of the resonator. This is because in many instances, the recognition layer consists of biopolymers that behave as viscoelastic liquids. The vibration profile of such a system is shown in Figure 21.8B which demonstrates that a standing wave also develops in the overlayer. However, it is dampened as a result of power dissipation and, in addition its phase is shifted with respect to the standing wave in the resonator. That is why the frequency shift induced by a viscoelastic film depends on its mass in an intricate way. A more detailed description of this system can be made by considering its complex impedance, as shown in the next section.
21.2.7 Modeling the Loaded TSM Resonator
21.2.7.1 Basic Principles
The basic physical process in TSM resonators is the propagation of acoustic waves induced and sustained by an applied AC voltage. Therefore, such a system has a composite character as it includes both mechanical and electrical components. The behavior of this system can be analyzed in a more practical way if it is represented only by electrical circuit elements such as resistors, capacitors, and inductors. Such an approach is also used to model electrochemical cells and has been introduced in the chapter dealing with electrochemical impedance spectrometry (Chapter 17) where more details on electrical modeling are available. The advantage of the electrical modeling is twofold. First, it has a sound theoretical basis developed in the frame of the theory of electric network. Secondly, it relies on electrical measurements only and makes use of standard instruments for the analysis of electrical networks. Such instruments are known as network analyzers.
Electrical modeling of acoustic-wave devices is of a particular interest when a deeper insight into the functioning principles of acoustic-wave sensors is sought. Such sensors includes, as a rule, a sensing layer that can consist of synthetic polymers or biopolymers that display an intricate behavior under the mechanical stress induced by vibration. Hence, a careful investigation of such properties is essential in the design and operation of acoustic-wave sensors. On the other hand, modeling may suggest particular data processing algorithms that enhances the analytical power of the sensor.
Impedance analysis reveals the effect of the vibrating frequency on the electric impedance of the system. The impedance Z represents the opposition of the system to current flow and is a complex quantity. It is therefore represented by two numbers: the real part Z′ and the imaginary part Z″. The complex impedance is hence represented as:
(21.16) ![]()
An important parameter associated with the impedance is the phase angle ![]() ; it represents the delay of a periodical quantity with respect to a reference periodic signal. In the case of acoustic-wave sensors, the phase angle quantifies the loss of energy by internal friction.
; it represents the delay of a periodical quantity with respect to a reference periodic signal. In the case of acoustic-wave sensors, the phase angle quantifies the loss of energy by internal friction.
An acoustic-wave device vibrates with maximum amplitude at the resonant frequency and the amplitude decays abruptly if the applied frequency deviates from this value. That is why impedance analysis of such devices is confined to a very narrow frequency range (typically 1 kHz) around the resonant conditions.
In order to perform impedance analysis an equivalent circuit of the device is inferred either intuitively or by a theoretical approach. Impedance data are then fitted to this model using dedicated software and finally the fit quality is checked. A good fit is not always a sound indication of the correctness of the model. It should be combined with a careful test of the physical fit of the model to the physical system.
Two types of electrical models of the acoustic-wave devices are commonly employed: the transmission line model (Mason) and the lumped element model often quoted as the Butterworth–Van Dyke model (Figure 21.6). The second model is less accurate but more handy.
In the transmission line model a composite resonator is represented as an arrangement of the respective number of transmission lines in series [7, 14]. Energy supply is represented by an electrical transformer with the secondary coil connected to the electrical port at A and B. Both the transformer turn ratio ![]() and the element jX depend only on the physical and geometrical parameters of the resonator. Each transmission line has two acoustic ports (EF and GH) each representing one face of the resonator (Figure 21.9). The acoustic ports are connected to the electrical ports by a transmission line that represents the phase shift and the power loss experienced by an acoustic wave propagating across the resonator thickness. Mechanical loading of the resonator is represented by the mechanical impedance Zs that is by definition the ratio of the surface stress to the particle velocity at the resonator surface (Figure 21.7):
and the element jX depend only on the physical and geometrical parameters of the resonator. Each transmission line has two acoustic ports (EF and GH) each representing one face of the resonator (Figure 21.9). The acoustic ports are connected to the electrical ports by a transmission line that represents the phase shift and the power loss experienced by an acoustic wave propagating across the resonator thickness. Mechanical loading of the resonator is represented by the mechanical impedance Zs that is by definition the ratio of the surface stress to the particle velocity at the resonator surface (Figure 21.7):
(21.17) ![]()
Figure 21.9 Mason transmission line model of the TSM piezoelectric resonator.
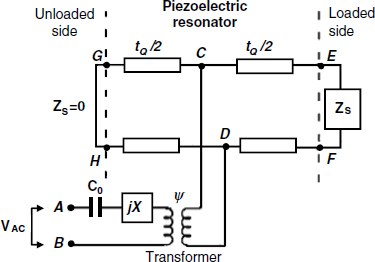
For an unloaded resonator, both sides can be considered as stress-free edges that give ![]() , as in the case of a short circuit at both acoustic ports. Commonly, in sensor applications one of the sides is unloaded (
, as in the case of a short circuit at both acoustic ports. Commonly, in sensor applications one of the sides is unloaded (![]() ) whereas the loading layer is represented by a nonzero mechanical impedance
) whereas the loading layer is represented by a nonzero mechanical impedance ![]() (Figure 21.9). The total impedance can be represented by the motional impedance Zm in parallel with the static capacitance C0.
(Figure 21.9). The total impedance can be represented by the motional impedance Zm in parallel with the static capacitance C0.
Although the transmission line model is very accurate the less-accurate lumped-element model is often preferred because it is easier to visualize and often allows for an easier derivation of characteristic parameters. Theoretically, if the ![]() ratio does not exceed 0.1, the second model predicts a response that is very close to that given by the first. The approach in next Section is based on the lumped-element model.
ratio does not exceed 0.1, the second model predicts a response that is very close to that given by the first. The approach in next Section is based on the lumped-element model.
21.2.7.2 The Unloaded Resonator
As shown in Figure 21.6B the unloaded resonator can be modeled by an equivalent circuit composed of a resistor, a capacitor and an inductor that account, respectively, for energy dissipation, energy storage and inertial behavior. The impedance of this series combination yields the motional impedance of the unloaded resonator ![]() that, for AT-cut quartz, has an absolute value of 8.8 × 106 kg m−2 s−1.
that, for AT-cut quartz, has an absolute value of 8.8 × 106 kg m−2 s−1.
When dealing with equivalent-circuit analysis, one makes a distinction between the series and the parallel resonant frequency. The first is the resonance frequency of the motional branch alone, whereas the second applies to the parallel combination of both branches and is reported by the measuring instrument.
21.2.7.3 The Loaded Resonator
Loading effect be represented by adding the surface motional impedance ![]() in series with the motional impedance of the unperturbed resonator (Figure 21.10A). For the case in which the coating layer is much thinner than the crystal, the additional impedance consists of a resistor R2 and an inductor L2 in series. The first element accounts for energy dissipation in the overlayer, while the second represents the mass load. Assuming that the coating is rigid and does not experience any shear deformation under vibration, energy dispersion in this layer is negligible (
in series with the motional impedance of the unperturbed resonator (Figure 21.10A). For the case in which the coating layer is much thinner than the crystal, the additional impedance consists of a resistor R2 and an inductor L2 in series. The first element accounts for energy dissipation in the overlayer, while the second represents the mass load. Assuming that the coating is rigid and does not experience any shear deformation under vibration, energy dispersion in this layer is negligible (![]() ), whereas the reactance of the inductive element L2 is proportional to the density of the coating layer. The overall mass of the vibrating system is therefore increased to some extent, which causes the resonant frequency to decrease as:
), whereas the reactance of the inductive element L2 is proportional to the density of the coating layer. The overall mass of the vibrating system is therefore increased to some extent, which causes the resonant frequency to decrease as:
Figure 21.10 Effect of mass loading on the piezoelectric TSM resonator. (A) The equivalent circuit; (B) Conductance spectra of the unloaded and loaded resonator with a resonant frequency of 12 MHz. Conductance is the real part of the complex admittance.
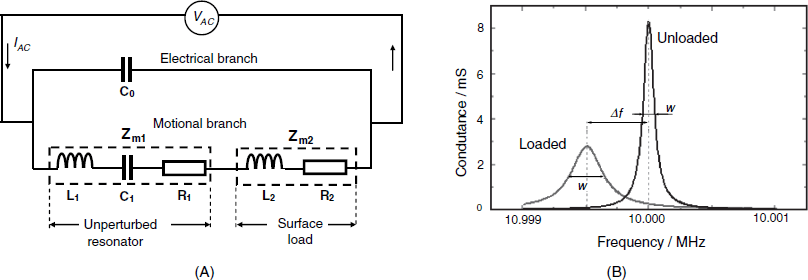
Here, ![]() is the frequency change,
is the frequency change, ![]() is the resonant frequency of the unloaded crystal,
is the resonant frequency of the unloaded crystal, ![]() is the mass of the coating (evenly distributed), A is the area of the active surface, and
is the mass of the coating (evenly distributed), A is the area of the active surface, and ![]() is a constant determined by the physical properties of the piezoelectric resonator. Equation (21.18) is in fact an alternative form of the Sauerbrey equation.
is a constant determined by the physical properties of the piezoelectric resonator. Equation (21.18) is in fact an alternative form of the Sauerbrey equation.
According to this model, the total motional impedance ![]() of the loaded resonator is:
of the loaded resonator is:
(21.19) ![]()
Impedance is a complex number that can be separated into a real and an imaginary component. The imaginary component displays a zero phase shift with respect to the applied AC voltage and its reciprocal is termed conductance. If the conductance is plotted against the frequency around the resonant frequency, one obtains a sharp peak, as shown in Figure 21.10B, for the unloaded resonator. The peak frequency is the series resonant frequency. The sharpness of the peak is quantified by the full width at half-maximum w. A key parameter related to the bandwidth is the quality factor Q that is by definition the quotient of the stored electrical energy and the energy dissipated in one period:
(21.20) ![]()
The ![]() factor is included for convenience. For very high Q values, Q is related to the bandwidth as follows:
factor is included for convenience. For very high Q values, Q is related to the bandwidth as follows:
(21.21) ![]()
For the unloaded resonator, the quality factor is:
(21.22) ![]()
As ![]() is very low, the quality factor of the unloaded resonator is about 105 and the bandwidth is very low. Consequently, the resonant frequency can be measured very accurately.
is very low, the quality factor of the unloaded resonator is about 105 and the bandwidth is very low. Consequently, the resonant frequency can be measured very accurately.
If a load is applied, it can bring about supplementary dissipation in addition to the inertial contribution. Therefore, the resonant frequency shifts to lower values but, at the same time, the bandwidth increases and the quality factor decreases (Figure 21.10B). The quality factor is in relation to the phase shift ![]() of the acoustic wave, that is, the phase difference of the acoustic wave between the crystal surface and the outer surface of the overlayer. When the quality factor becomes sufficiently low, the AC generator circuit can no longer sustain the oscillation and the QCM ceases to function.
of the acoustic wave, that is, the phase difference of the acoustic wave between the crystal surface and the outer surface of the overlayer. When the quality factor becomes sufficiently low, the AC generator circuit can no longer sustain the oscillation and the QCM ceases to function.
Generally, the overlayer motional impedance is a complex quantity:
(21.23) ![]()
The imaginary part ![]() determines the change in the series resonant frequency
determines the change in the series resonant frequency ![]() as follows:
as follows:
(21.24) ![]()
At the same time, the real part ![]() describes the energy loss within the layer:
describes the energy loss within the layer:
(21.25) ![]()
A clear description of the resonator response to loading can be formulated in terms of two factors: the mass factor M and the acoustic (vibrational) factor V[15]:
where ![]() and
and ![]() are, respectively, the density and the thickness of the overlayer, and
are, respectively, the density and the thickness of the overlayer, and ![]() is the phase shift. With these parameters, the motional impedance associated with the overlayer is:
is the phase shift. With these parameters, the motional impedance associated with the overlayer is:
(21.27) ![]()
It is apparent from the previous discussion that the QCM parameters could vary in response to both mass loading (the M factor) and energy loss in the overlayer (the V factor). Therefore, the QCM is not only a microbalance but also provides information on other physical parameters of the loading layer. Accordingly, one can distinguish various functioning regimes of the QCM according to the properties of the overlayer as shown in Table 21.1[15].
Table 21.1 Functioning regimes of the QCM sensors. M and V are defined in Equation (21.26); ![]() and
and ![]() are, respectively, the change in the M and V factors induced by resonator loading.
are, respectively, the change in the M and V factors induced by resonator loading.

In the first case in this table, the QCM functions as a true gravimetric sensor and reports on the mass change in the overlayer. In the second cases, the response is not purely gravimetric. Case 2 corresponds to a composite response that depends on both mass change and changes in the viscoelastic properties of the overlayer. In the third case, the QCM responds only to the change of the viscoelastic properties of the overlayer under constant-mass conditions. An example of this type is represented by the QCM in contact with a liquid.
21.2.7.4 The Ideal Mass Loading
This is represented by a rigid overlayer that moves synchronously with the resonator surface. The transverse wave propagates across this layer with a negligible phase shift. In this limiting case, no energy dissipation occurs and hence ![]() . The reactance
. The reactance ![]() (
(![]() ) of the inductive element represents the imaginary component of
) of the inductive element represents the imaginary component of ![]() and in the fundamental mode it is [12]:
and in the fundamental mode it is [12]:
(21.28) ![]()
This is the case 1 in Table 21.1, that is, the case in which the QCM elicits a pure gravimetric response and obeys the Sauerbrey equation. Both the phase shift and the motional resistance ![]() are negligible, which implies an absence of energy dissipation in the overlayer.
are negligible, which implies an absence of energy dissipation in the overlayer.
21.2.7.5 Loading by a Newtonian Liquid
If one side of the resonator is in contact with a thick Newtonian liquid layer, ![]() and
and ![]() in Figure 21.10A represent, respectively, the energy dissipated into the contacting liquid and the kinetic energy of the entrained liquid layer. In this case, equal amounts of power are distributed to each component, as shown by the following equation that applies under resonance conditions [7]:
in Figure 21.10A represent, respectively, the energy dissipated into the contacting liquid and the kinetic energy of the entrained liquid layer. In this case, equal amounts of power are distributed to each component, as shown by the following equation that applies under resonance conditions [7]:
The liquid contact translates the peak of the admittance magnitude to lower frequency (in accordance with Equation (21.14)) and, at the same time, reduces the quality factor due to power dissipation (Figure 21.10B). The identity of ![]() and
and ![]() (and implicitly a phase shift of
(and implicitly a phase shift of ![]() ) proves that the liquid contacting the resonator is a Newtonian one. It should be kept in mind that Equation (21.29) has been derived under the assumption that no film slip occurs. If this is not the case, some deviations from the above conclusions would occur.
) proves that the liquid contacting the resonator is a Newtonian one. It should be kept in mind that Equation (21.29) has been derived under the assumption that no film slip occurs. If this is not the case, some deviations from the above conclusions would occur.
21.2.7.6 Viscoelastic Overlayer
The next discussion addresses the case of a finite-thickness viscoelastic film deposited on top of the resonator and in contact with air on the opposite side [12]. Therefore, no power dissipation occurs at the external surface of this system.
A viscoelastic material exhibits both elastic and viscous behavior. That is why the shear modulus G is a complex quantity:
(21.30) ![]()
The real part ![]() represents the stress component in phase with strain, giving rise to energy storage (elastic component). The imaginary part
represents the stress component in phase with strain, giving rise to energy storage (elastic component). The imaginary part ![]() represents the stress component
represents the stress component ![]() radians out of phase, which causes power dissipation and accounts for the viscosity effect. The film can vibrate using power transferred from the resonator. That is why the resonator–viscoelastic film system can be treated as two coupled resonators [16]. A standing wave develops across the film due to the interference of the direct wave and the wave reflected at the film/air surface. As both elastic and viscous properties are manifest, the acoustic wave experiences both a phase shift and attenuation while traversing the film. When the phase shift reaches
radians out of phase, which causes power dissipation and accounts for the viscosity effect. The film can vibrate using power transferred from the resonator. That is why the resonator–viscoelastic film system can be treated as two coupled resonators [16]. A standing wave develops across the film due to the interference of the direct wave and the wave reflected at the film/air surface. As both elastic and viscous properties are manifest, the acoustic wave experiences both a phase shift and attenuation while traversing the film. When the phase shift reaches ![]() (where
(where ![]() is an odd integer) interference becomes constructive, resulting in enhanced absorption of the acoustic power by the film and leading to maximum damping of the crystal resonance. Under such conditions, the film experiences resonance vibration. For a given resonant frequency of the resonator, film resonance is obtained at a specific film thickness.
is an odd integer) interference becomes constructive, resulting in enhanced absorption of the acoustic power by the film and leading to maximum damping of the crystal resonance. Under such conditions, the film experiences resonance vibration. For a given resonant frequency of the resonator, film resonance is obtained at a specific film thickness.
Viscoelastic coatings could behave as in the cases 2 and 3 in Table 21.1, that is, the QCM response is not purely gravimetric. In case 2, which is the most common, the response is affected by both mass change and energy dissipation. In case 3, the response can change even if the mass does not change appreciably. Hence, the response modification is due mostly to certain changes in the structural properties of the film leading to enhanced dissipation.
An equivalent circuit consisting of a parallel R2, L2, C2 network has been advanced for modeling the motional impedance of a viscoelastic film of finite thickness coated atop of a resonator oscillating in air [12] (Figure 21.11).
Figure 21.11 Equivalent circuit of a TSM resonator coated with a viscoelastic film.
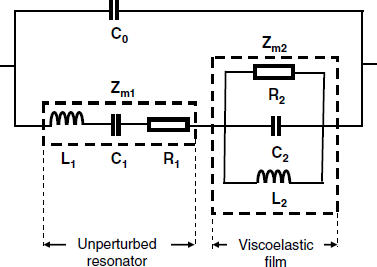
Near the resonant frequency, the components of the equivalent circuit are related to the physical properties of the film as follows [12]:
(21.31) ![]()
(21.32) ![]()
(21.33) ![]()
where ![]() is the coating film thickness and
is the coating film thickness and ![]() is the film mass. This model suggests that near-resonance impedance analysis allows inferring the mass of the viscoelastic film from the reactance of the L2 element.
is the film mass. This model suggests that near-resonance impedance analysis allows inferring the mass of the viscoelastic film from the reactance of the L2 element.
In a more advanced approach the resonant frequency is considered as a complex quantity ![]() [17, 18]. In this case, the real part of the complex frequency represents the resonant frequency of the series equivalent circuit, whereas the imaginary part is the half-band-half-width
[17, 18]. In this case, the real part of the complex frequency represents the resonant frequency of the series equivalent circuit, whereas the imaginary part is the half-band-half-width ![]() (
(![]() , Figure 21.10B). Therefore, the complex frequency is:
, Figure 21.10B). Therefore, the complex frequency is:
and the complex frequency change is:
(21.35) ![]()
The change in the complex resonant frequency is related to the load impedance by the following basic equation:
(21.36) ![]()
where ![]() is a parameter approximately equal to the resonant frequency of the fundamental mode and
is a parameter approximately equal to the resonant frequency of the fundamental mode and ![]() is the areal mass density of the crystal (that is, mass per surface area unit). This equation applies only if the frequency change is very small compared with the resonant frequency.
is the areal mass density of the crystal (that is, mass per surface area unit). This equation applies only if the frequency change is very small compared with the resonant frequency.
For a viscoelastic film, the above equation assumes the following form:
(21.37) ![]()
where ![]() is the areal mass density of the film. This equation shows that the acoustic properties of the overlayer are fully specified by two parameters: its acoustic impedance and its mass per unit area. Therefore, it is not possible to derive simultaneously the thickness, density and viscoelastic parameters of a film from acoustic impedance measurements alone.
is the areal mass density of the film. This equation shows that the acoustic properties of the overlayer are fully specified by two parameters: its acoustic impedance and its mass per unit area. Therefore, it is not possible to derive simultaneously the thickness, density and viscoelastic parameters of a film from acoustic impedance measurements alone.
21.2.7.7 Multiple Loading
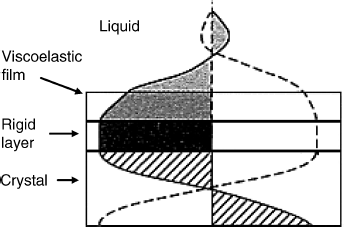
In many applications of the QCM, the surface is coated by multiple layers. Thus, a thioalkane chemisorbed film can serve for immobilization of a protein layer by crosslinking in order to obtain the sensing layer. The first layer could behave as an ideal mass load, whereas the second layer could display viscoelastic properties. Under these conditions, a linear combination of film impedances is a reasonable approximation. This implies that no power dissipation occurs across the first layer and the second one experiences at its lower side the same stress as in the case in which it was coated directly on the resonator. Simple addition of individual motional impedances is also applicable in the case of an ideal mass load in contact with a Newtonian fluid. In fact, this property led to Equation (21.15). However, in general, multiple layers do not add algebraically but combine in a nonlinear way. The most common nonlinear system is that of a viscoelastic film with a Newtonian fluid on top (Figure 21.12). As the wave propagates away from the rigid layer, it experiences a considerable decay. Typical decay lengths are 0, 2 and 0.2 μm for the rigid layer, the viscoelastic film and the aqueous phase, respectively. Due to the phase shift across the viscoelastic film the total motional impedance does not equal the sum of individual impedances of each layer. Instead, the total motional impedance depends in a more intricate fashion on the properties of each layer [7].
Figure 21.12 Propagation of the acoustic shear wave launch induced by a TSM resonator coated with a viscoelastic layer exposed to a liquid. Adapted with permission from [19]. Copyright 2003 Wiley-VCH Verlag GmBH & Co. KGaA.
The application of the concept of complex frequency brought about a more accurate picture of the TSM resonator with both viscoelastic and Newtonian liquid loading [17, 18].
21.2.8 The Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
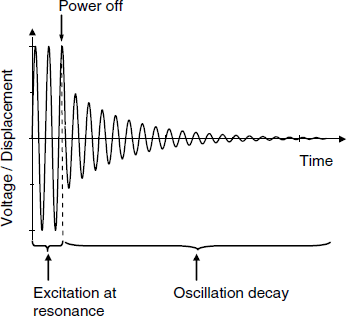
Previous sections proved that in the case of a soft material loading, both the resonance frequency and the quality factor change. Both these parameters can be inferred from impedance spectrometry data by a relatively laborious approach. A particular method of operating the QCM allows straightforward and fast extraction of both parameters [20]. This method, known as the quartz crystal microbalance with dissipation monitoring (QCM-D, [20]), proved to be very practical and is widely used currently particularly for investigating thin soft material films and biorecognition processes [21]. In this method, the resonator is excited at the resonant frequency for about 10 ms, then the power supply is disconnected and the system is left to oscillate freely (Figure 21.13). Due to energy dissipation, the oscillation amplitude decays exponentially as:
Figure 21.13 Principle of the QCM-D method. An excitation voltage is applied for about 10 ms, then the driving power supply is switched off and the decaying amplitude of the free-oscillating system is monitored. Typically, the decay time is of a few ms.
(21.38) ![]()
where ![]() is the decay time constant and the time, t, is measured from the moment when power is switched off. The duration of the overall run, including data transfer is about 25 ms. Moreover, the system can be operated at multiple overtones in order to assess the effect of the frequency. Data processing allows the extraction of two parameters: the resonant frequency and the decay time constant. The decay time constant is related to the quality factor as:
is the decay time constant and the time, t, is measured from the moment when power is switched off. The duration of the overall run, including data transfer is about 25 ms. Moreover, the system can be operated at multiple overtones in order to assess the effect of the frequency. Data processing allows the extraction of two parameters: the resonant frequency and the decay time constant. The decay time constant is related to the quality factor as:
(21.39) ![]()
By definition, the dissipation is the reciprocal of the quality factor:
(21.40) ![]()
As the dissipation is related to the bandwidth, it results that this method reports on both terms of the complex frequency included in Equation (21.34).
The QCM-D method has found various applications in fundamental research of thin soft materials layers including biomaterials. Thus, it allows straightforward assessment of the validity of the Sauerbrey equation for particular systems. If the load is purely inertial (rigid overlayer) then the dispersion is very low. As a soft material overlayer can undergo hydration and swelling, the QCM-D method affords facile appraisal of such phenomena and provides insight into structural changes.
21.2.9 Operation of QCM Sensors
As an analytical device, the QCM can be used with either gas or liquid samples. In the second case, the QCM is used as a transducer in affinity sensors. In order to avoid electrical shorting via electrolyte solutions, the resonator is mounted such that only one face is exposed to the sample.
The resonant frequency depends on the temperature; that is why it is recommended to operate the sensor under thermostated conditions. In order to avoid interference due to ambient electromagnetic noise, the QCM should be installed in a Faraday cage.
As demonstrated before, the solution contact itself brings about a considerable change in the resonance frequency. That is why it is preferred in some applications to resort to the “dip-and-dry” operation method. In this method, the resonant frequency of the unaltered sensor is first measured in air and then the sensor is dipped into the sample and left to attain the equilibrium state indicated by an invariable frequency. Next, the sensor is removed from the sample, carefully washed to remove nonspecifically bound sample components and gently dried (e.g., by a stream of nitrogen). Finally, the frequency is measured again with the sensor in air. The frequency change reports on the mass change during the interaction with the analyte in the sample.
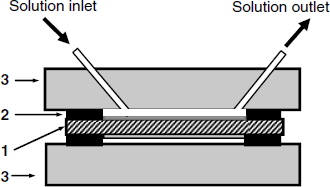
Affinity assays require a series of washing steps and also sensor regeneration by means of a solution containing immunocomplex-disrupting reagents. That is why it is convenient to install the sensor in a flow cell integrated with an automatic flow-analysis system. Such a cell is presented in Figure 21.14. The quartz crystal is fastened between two plastic material blocks by means of an elastic O-ring. Solutions are pumped through the lower chamber with a volume of about 50 μL and put in contact with the receptor-modified gold layer. Note that only one side of the resonator is exposed to the aqueous sample. In order to avoid gas bubble accumulation at the sensor surface, preliminary sample degassing is recommended.
Figure 21.14 Flow cell for QCM sensors. (1) TSM resonator with the sensing layer at the upper side; (2) elastic O-ring; (3) plastic plate.
A QCM flow-analysis system is shown in Figure 21.15. It includes a pump and a series of computer-controlled valves that allow flushing of the sensor with various solutions such as the sample, the regeneration solution and the washing buffer. Data supplied by the frequency counter are stored by the computer for further processing.
Figure 21.15 A semiautomatic setup for measurements with piezoelectric immunosensors.
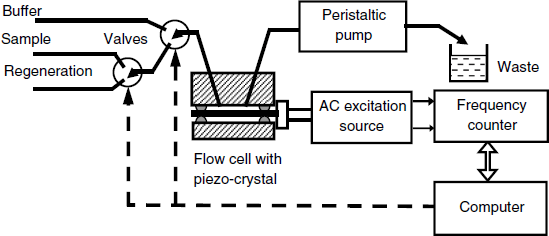
The flow-analysis system can be operated in two modes. In the flow-through mode, the fluid sample crosses the cell steadily at constant flow rate until the recognition process attains equilibrium. The frequency change is then read out and the valves are switched such as to allow the regeneration solution and then the washing buffer to flow along the sensor surface.
Alternatively, the analyzer can be operated under the flow-injection analysis regime. In this case, a stream of buffer solution flows steadily through the cell. A minute amount of sample is inserted in this stream by means of a sample-introduction valve and is carried as a plug to the cell. The frequency changes first in response to the recognition reaction but decays back to the background level as the sample plug is flushed out from the cell. Therefore, the response has a transient character and the maximum frequency change is used to derive the analyte concentration. This method is faster, but the contact time is relatively short and the recognition process cannot always reach the equilibrium state. This can in some instances impair the sensitivity and also the reliability of the results.
21.2.10 Calibration of the QCM
In many analytical applications the frequency change is correlated with the analyte concentration by a calibration graph and the absolute mass of the overlayer is overlooked. However, it is often of interest to assess the loading mass of a loading layer. Even if many workers make use of the Sauerbrey constant derived from quartz properties, it could be useful to perform QCM calibration in solution in order to account for various effects of the liquid contact. Thus, in addition to energy loss by wave propagation in the liquid, deviations might occur due to surface roughness, and sensor sealing.
The calibration can be performed by recording simultaneously the current and the frequency change during the electrodeposition of silver on one of the crystal side under cyclic voltammetry conditions. The mass change due to silver deposition/dissolution can be calculated from the associated electric charge by means of the Faraday electrolysis law. The electric charge is obtained as the time integral of the current. A plot of the recorded ![]() vs. charge yields a straight line and the Cf constant can be derived from the line slope [19, 22].
vs. charge yields a straight line and the Cf constant can be derived from the line slope [19, 22].
21.2.11 Outlook
The QCM was initially developed as an ultrasensitive mass transducer. This feature has been exploited for developing chemical sensors based on mass change in response to the recognition effect. The label-free character of these sensors made them very attractive for various applications in both gas and liquid sample analysis. Such sensors are manufactured by coating a piezoelectric resonator with a thin recognition layer.
However, the QCM is a true mass transducer only under severely limited conditions. The main limitation is the absence of acoustic energy dissipation within the vibrating system. Hence, the coating layer should be very thin and, to a good approximation it should behave as a rigid layer, both before and after the recognition event. This condition is met by solid overlayers and by molecularly thin layers of organic compounds. More generally, the Sauerbrey equation holds whenever the overlayer thickness is negligible with respect to the wavelength of the acoustic wave. QCM gas sensors fulfill this condition and generally obey the Sauerbrey equation.
If the QCM is put in contact with a Newtonian liquid, acoustic energy is dissipated into this liquid that results in an appreciable change of the resonant frequency and a reduction of the quality factor. However, if the overlayer has a rigid character, its mass can be assessed as the liquid contact effect is constant at constant viscosity and density of the liquid.
The situation is much more intricate when the overlayer is a non-Newtonian liquid. In contact with such a material, the resonator experiences both resonant frequency change and large energy dissipation. The frequency change in this case is no longer a simple result of the mass change but is also influenced to a great extent by the viscoelastic properties of the coating. This results in a mass sensitivity much lower that that predicted by the Sauerbrey equation. Such a situation is typical for affinity sensor based on biomacromolecules as receptors. In many instances, such a sensor cannot achieve the expected sensitivity and signal amplification by postrecognition expansion of the mass of layer is needed. Although this procedure proved successful, it requires additional reagents and expands the duration of the assay.
The QCM is more than just a microbalance but is also a valuable method of investigation of viscoelastic properties of soft materials. In fact, the term “balance” makes sense even for nongravimetric applications if it is understood in the sense of a force balance. This terminology is justified by the fact that the force exerted by the crystal upon the overlayer is balanced by a force originating from the shear gradient inside this layer.
Sensor applications of the QCM still rely mostly on measurement of frequency change. However, recognition methods involving macromolecules or cells bring about many other effects in addition to the mass change, such as modification of the layer thickness and shear modulus. Mass variation may result not only from the addition of supplementary material by the recognition event. This event can be accompanied by water exclusion/inclusion or similar processes involving hydrated ions. That is why a more detailed picture is obtained by impedance measurements. Despite the cost and intricacy of this method, possible applications in sensor transduction may be expected. The QCM-D method, which provides essentially the same kind of information, appears as a convenient substitute for impedance analysis.
21.3 QCM Gas and Vapor Sensors
The gas phase provides the most convenient operating conditions for a QCM. It is therefore not surprising that gas analysis has been among the first applications of QCM sensors [23–25]. Such sensors can be manufactured by coating a recognition layer on one or both sides of the resonator. The sensor can be exposed directly to the gas sample but in some instances, when the sample contains dust or aerosol, it is better to install the sensor in a gas flow cell to which the sample is pumped through a filter.
In general, QCM gas and vapor sensors elicit good reversibility. Due to the analyte volatility, QCM gas sensors can be regenerated by flushing with a pure gas, eventually accompanied by heating.
The simplest applications rely on using the metal layer on the resonator as the sensing element. Thus, mercury vapor interacts with the surface of gold electrodes forming amalgams that allows the determination of mercury vapors in atmosphere down to 1 ng/L [26]. Sensor regeneration can be achieved by heating in a pure gas atmosphere. As mercury vapors accumulate steadily under contact with the gas, this device can function as a dosimeter for assessing the mercury vapor exposure.
Palladium is known to absorb strongly hydrogen and deuterium. Hence, a QCM with palladium electrodes can function as a sensor for both of the above gases or for assessing the deuterium content in a hydrogen–deuterium gas mixture [27].
Coating with a specific sensing layer permits the determination of various gas compounds. For example, aliphatic amine coatings allow detecting acid gases such as hydrochloric acid or sulfur dioxide. Aromatic hydrocarbons in a hydrocarbon mixture can be detected by means of certain metal complexes that form coordinate bonds with ![]() -electrons in aromatics but that do not interact with aliphatic compounds.
-electrons in aromatics but that do not interact with aliphatic compounds.
Coating with a specific biomaterial receptor allows selective determination of certain organic compounds. Thus, a formaldehyde vapor QCM sensor has been manufactured by coating the resonator with formaldehyde dehydrogenase and its cofactors (NAD+ and reduced glutathione) [28]. The products of the enzymatic reaction accumulate at the sensor surface and cause the resonant frequency to decrease proportionally to the formaldehyde concentration. Parathion in water samples has been determined in the gas phase by means of an antiparathion antibody-coated QCM [29]. The sample is flushed by a gas stream that carries out the volatile analyte to a QCM gas flow cell.
Better sensitivity in gas analysis can be achieved by means of surface-acoustic wave transducers (Section 21.6)that, in addition, allow for miniaturization and microarraying.
21.4 QCM Affinity Sensors
In principle, any kind of recognition process based on affinity interactions can be monitored by means of a QCM if a suitable receptor layer is formed over the surface of the resonator crystal. Much research effort has been devoted to the development of QCM immunosensors owing to the label-free character of this approach [30–32]. However, the viscoelastic characteristic of the sensing layer causes most of the vibrational energy to be dissipated, and the response can be very small despite the large mass change produced by the recognition event. That is why it is often best to resort to signal amplification by postrecognition modification with high-mass particles.
In addition to immunocompounds, great interest is also shown in using synthetic materials for the preparation of sensing layers in affinity QCM sensors.
21.4.1 QCM Immunosensors
QCM immunosensors are best operated under flow-analysis conditions so as to automate the process. The main steps in a flow-analysis QCM immunoassay are reviewed in Figure 21.16, which refers to the case in which an antibody is determined by means of an antigen-modified quartz crystal. Before sample introduction, it is important to wait until the frequency stabilizes. After sample introduction, the receptor–analyte binding gradually lowers the frequency and when the frequency reaches a stable value the sample is washed out by a buffer solution in order to remove nonspecifically bound compounds. Then, the signal is recorded and the sensor is regenerated by disrupting the analyte–receptor complex.
Figure 21.16 Operational sequence in a quartz crystal sensor immunoassay.
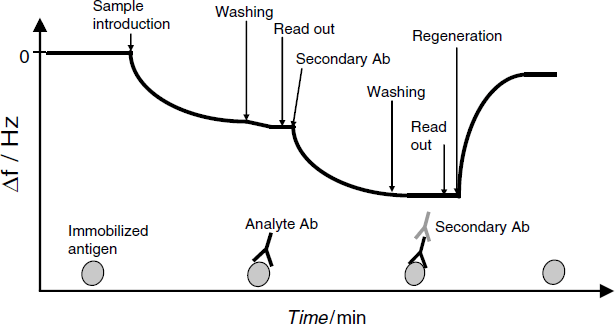
If amplification is sought, a secondary antibody is added after the washing step, as shown in Figure 21.16. Formation of a ternary complex brings about an additional frequency shift.
The previous scheme represents a direct assay, and the amplification by a secondary antibody can be viewed as sandwich format labeling. Competitive immunoassay by QCM immunosensors is also achievable. In this case, an analyte-analog is added to the solution along with the analyte itself. If the mass of the analyte-analog is much lower than that of the analyte, frequency decreases with increasing analyte concentration.
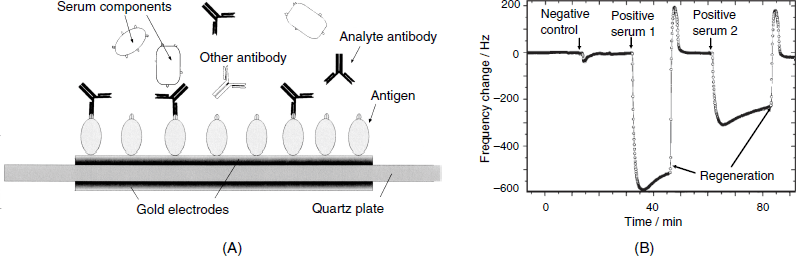
An example of a QCM immunosensor developed for the diagnosis of the viral infection caused by the African Swine Fever disease (ASF) is shown in Figure 21.17A. The ASF-specific antibody can be detected by means of a sensing layer formed from a protein that is found in the outer membrane of the virus and that functions as an antigen. This protein has been immobilized by irreversible adsorption on a gold-electrode surface. The course of the assay is shown in Figure 21.17B. First, a negative control sample is applied, and then the unknown serum is assessed. No effect due to the blank serum is observed but large frequency shifts are caused by the sera containing the ASV antibody secreted by the organism in response to infection (positive sera). Subsequent regenerations by a pH 11 buffer solution is successful as demonstrated by the return of frequency to the baseline. Such a sensor proved to be reliable for 10 successive runs.
Figure 21.17 (A) Configuration of a piezoelectric immunosensor for the diagnostic of African Swine Fever disease; (B) The course of an immunoassay test with the sensor in (A). Each sample was diluted 10-fold with the pH buffer solution. Adapted with permission from [33]. Copyright 1998 Elsevier.
21.4.2 Amplification in QCM Immunosensors
The amplification principles and benefits are demonstrated next by the case of the prostate specific antigen (PSA) determination (Figure 21.18). The PSA sensor has been obtained by amine coupling of the specific antibody to a thiocarboxylic acid initially assembled on the gold electrode surface. As shown in Figure 21.18A, when in contact with the analyte solution, the frequency attains a stable value after a certain time interval that depends on the velocity of the binding interaction and, hence, on the analyte concentration. The effect of nonspecific interactions have been tested with a control sensor formed by the immobilization of a nonspecific antibody. As demonstrated by curve 5, which was recorded with the control sensor in the presence of a large analyte concentration, nonspecific binding is negligible. However, the working range of this sensor is well above the normal PSA level in blood serum (2 ng/mL). Therefore, signal amplification is needed. Line 1 in Figure 21.18B shows the sensor response after amplification by postrecognition binding of a secondary antibody as demonstrated in Figure 21.16. Although the response is enhanced in this way, the working range is still above the normal PSA level. A sufficiently large signal has been obtained by using a secondary antibody conjugated with gold nanoparticles (line 2). The large mass of the nanoparticle brings about a considerable change in frequency and allows reliable determinations of PSA to be performed within the normal concentration range.
Figure 21.18 Prostate-specific antigen determination in 75% human serum by a QCM immunosensor. (A) Sensor response to various PSA concentrations (in ng/mL): (1) 5000; (2) 312; (3) 78; (4) 21.8. Curve 5 represents the response of the control sensor to 5000 ng/mL PSA. (B) The calibration graph for PSA determination with signal amplification. (1) Amplification by a secondary antibody; (2) amplification by gold nanoparticles. Adapted with permission from [34]. Copyright 2010 Elsevier.
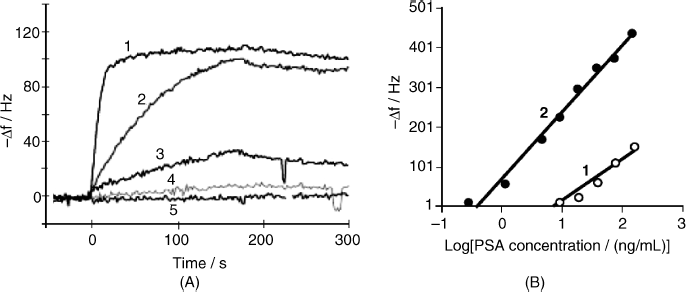
Further signal enhancement is possible by growing the nanoparticles attached to the ternary complex formed at the sensor surface. As shown in Figure 21.19, this can be achieved by the chemical reduction of HAuCl4 under the action of hydroxylamine as the reducing agent. The initial, small gold nanoparticle functions as a crystallization center and grows considerably by silver deposition thus enhancing the mass load. Particle growth can also be accomplished by chemical deposition of silver.
Figure 21.19 Signal amplification by means of gold nanoparticles in QCM immunoassay of human lung carcinoma cell in the sandwich format. The secondary antibody is tagged with gold nanoparticles that are further grown by chemical deposition of gold [35]. Reproduced with permission of The Royal Society of Chemistry (RSC) for the Center National de la Recherche Scientifique.
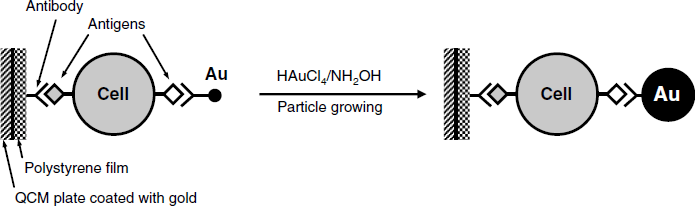
A substantial simplification of sensor manufacturing and test protocol is obtained if the assay is conducted in the solution phase using magnetic nanoparticles conjugated with the immunocomplex [36, 37]. This allows for simple accumulation of this complex at the transducer surface by means of a magnetic field, thus avoiding the laborious immobilization step. Regeneration of the immunosensor in this case can be accomplished by removing the magnetic field.
21.4.3 Determination of Small Molecules Using Natural Receptors
Direct determination of small organic molecules (haptens) by properly tailored antibodies is also achievable by means of QCM sensors. However, the low molecular mass, combined with the viscoelastic effect in the immobilized antibody layer can limit severely the sensitivity of such a sensor. A more convenient approach relies on the competitive assay in which the sensing layer is formed from an analyte-analog (X) immobilized at the resonator surface. To the sample containing the analyte A, a heavy receptor R, which can bind to both the analyte and the analog, is added. Hence, competition between the analyte and an immobilized analog for binding to the receptor R will occur as follows:
(21.41) 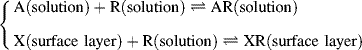
The mass variation is therefore an inverse function of the analyte concentration.
This principle has been applied for the detection of organophosphorus pesticides (A) in water using the cholinesterase enzyme as receptor (R) [38]. The catalytic activity is not addressed at all; instead one makes use of the property of the enzyme to bind reversibly to certain molecules that normally act as inhibitors. Therefore, the recognition interaction in this sensor is of the affinity type. The sensing layer consists of a cocaine derivative (X) immobilized at the surface of the gold electrode. This sensor was shown to respond to pesticide concentrations below the accepted limit for drinking water.
21.4.4 QCM Sensors Based on Molecularly Imprinted Polymers
Among synthetic receptors, molecularly imprinted polymers are the most frequently applied to developing piezoelectric sensors [39]. Most of the published work in this area addresses the detection of small molecules [41]. Despite the low molecular mass, such compounds can lead to clear mass variation due to their capacity to diffuse inside the pores of the imprinted polymer. In this way, the analyte will be accumulated in a sufficiently large amount. Therefore, bulk accumulation occurs in this case, in contrast to the immunosensors that are characterized by surface-localized analyte–receptor binding.
An example of an imprinted polymer application is the piezoelectric bilirubin sensor described in [40]. Bilirubin content in urine is an indicator of liver malfunction. In order to produce the sensor, the gold electrode surface has been coated with allyl mercaptan (2-propene-1-thiol) in the form of a self-assembly monolayer, and benzophenone, as the polymerization initiator, has been then deposited over it. Polymerization has been effected next by UV irradiation in a solution layer over the previously formed film using 4-vinylpyridine as monomer, divinyl benzene as crosslinker and bilirubin as template. After completing the polymerization (about 150 nm film thickness), the template is washed out with 10 mM NaOH. This manufacturing method demonstrated good batch-to-batch reproducibility.
Electropolymerization is another practical method for including receptor sites in a coating layer. Thus, a piezoelectric dopamine sensor has been obtained by electrochemical copolymerization of two pyrrole derivative monomers shown in Figure 21.20A. The first monomer includes a macrocyclic polyether that binds to the positively charge ammonium group in dopamine by ion–dipole interactions. The second monomer imparts to the imprinted site hydroxyl groups that can form hydrogen bonds with the analyte. This sensor has been operated under flow-injection conditions. In this method, a sample plug is carried by a buffer solution stream and flows across the sensor surface. As shown in Figure 21.20B, the response displays a transient character, with a maximum corresponding to the moment when the plug center reaches the sensor. The calibration graph is obtained by plotting the peak response as a function of the analyte concentration. Dopamine concentrations between 0.1 and 130 mM have been determined with negligible interference from compounds such as ascorbic acid, histamine or 2-phenylethylamine. The particular feature of this approach is the inclusion of a host receptor (here, a macrocyclic ether) within the imprinted site.
Figure 21.20 A dopamine piezoelectric senor based on an electrochemically polymerized imprinted polymer. (A) Structure of dopamine and the monomers used in the synthesis of the molecularly imprinted polymer; (B) Flow-injection analysis response in 0.2 M phosphate buffer (pH = 7.0); sample volume, 100 μL. The analyte concentration is shown on each curve. (B) was adapted with permission from [42]. Copyright 2010 Elsevier B.V.
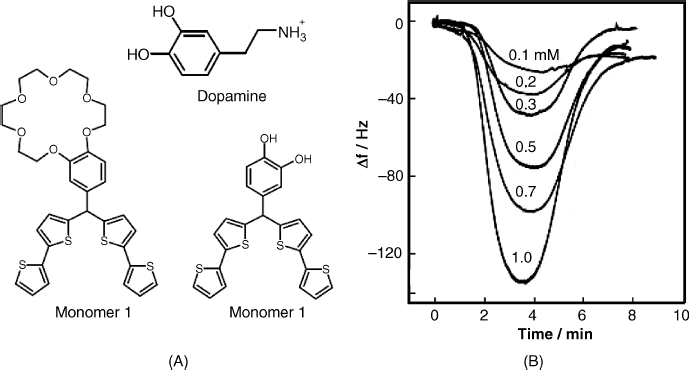
Molecular imprinting technology has also been applied to the development of piezoelectric sensors for bulky species such as cells, viruses and enzymes [43–45]. However, such species cannot diffuse into the polymer pores and therefore the imprinting technology should be designed so as to produce surface-imprinted polymers. This can be achieved by imprinting the template on a pretreated polymer mixture as shown in Figure 21.21. The polymer mixture is first deposited on the QCM surface and then the printing is performed by means of a stamp with micro-organisms attached to its surface. After polymer curing, the stamp is removed leaving the surface imprinted with a honeycomb-like structure as shown in Figure 21.22A.
Figure 21.21 A molecularly imprinted polymer as recognition element in a piezoelectric yeast sensor. Adapted with permission from [44]. Copyright 2001 Wiley-VCH Verlag GmBH & Co. KGaA.
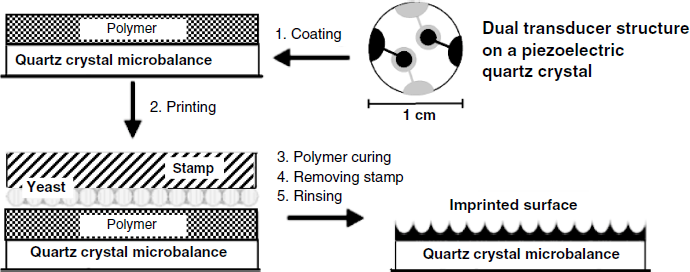
Figure 21.22 (A) Atomic force microscopy image of a yeast surface imprinted polymer. (B) The response of the control sensor (nonimprinted polymer, curve 1) and the working sensor (curve 2) to 0.1 mg/ml yeast (Saccharomyces cerevisiae). (A) Adapted from [46]. (B) Adapted with permission from [44]. Copyright 2001 Wiley-VCH Verlag GmBH & Co. KGaA.
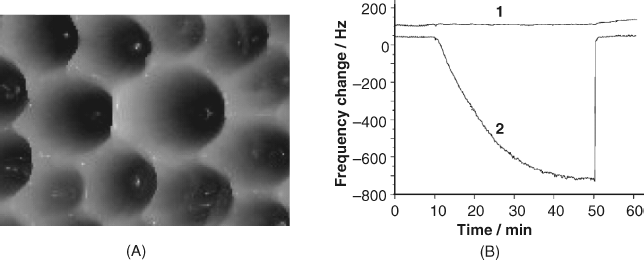
In order to assess the specificity of cell recognition two sensors have been assembled on the same piezoelectric crystal (Figure 21.21). One of them, which functions as the working sensor has been coated with the imprinted polymer, whereas the second one (the control sensor) has been covered by the same polymer in the nonimprinted form. The response of each sensor is plotted in Figure 21.22B, which demonstrates the excellent selectivity of the sensing layer.
Similar technology has been used to produce piezoelectric sensors for bacterial pathogens, viruses, and proteins. This opens the way to the development of straightforward and inexpensive methods for the determination of such species without the need to use biochemical reagents and procedures.
21.4.5 QCM Sensors Based on Small Synthetic Receptors
Among the receptors in this class, calixarenes have aroused much interest due to the broad range of their interactions with small molecules and the great flexibility of the immobilization methods. An application of a calixarene to mass-sensitive sensors is illustrated by a dopamine sensor that is based on calix-4-arene functionalized with long-chain thiol moieties at the lower rim [47]. This allows for immobilization along with a long-chain thioalkane by sulfur chemisorption at the gold-electrode surface. The thioalkane layer provides surface protection against contact with the solution. The recognition occurs by the electrostatic interaction of the dopamine cation (Figure 21.20) with anionic carboxylate groups on the upper rim of the calixarene. Despite the low molecular mass of the analyte, track A in Figure 21.23 demonstrates a clear frequency change in response to dopamine addition. This effect has been studied further by recording also the change in the motional impedance that is shown by the track B in Figure 21.23. As the change in the resonant frequency is accompanied by a proportional variation of the motional impedance, it shows that the viscoelastic effect alone is responsible for the sensor response. It is thus proved that small molecules that cannot induce detectable mass variations can still be determined by means of the modification in the viscosity of the surface layer (see also Table 21.1).
Figure 21.23 The response of a dopamine piezoelectric sensor to a sequential increase in analyte concentration. (A) Resonance frequency; (B) motional impedance. Arrows indicate dopamine addition (DA), and washing buffer addition (BF). Dopamine concentration (in nM): (1) 0.053; (2) 0.28; (3) 0.53; (4) 2.8; (5) 5.3. Reproduced by permission from [47]. Copyright 2010 Elsevier B.V.
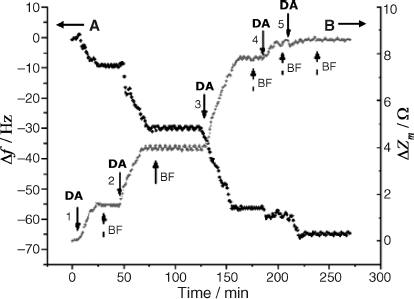
Various types of sugar sensors rely on phenylboronic acid derivatives as receptors. At a suitable pH, phenylboronic acid forms spontaneously and reversibly a cyclic ester by interacting with hydroxyl groups in sugar molecules, as shown in Figure 21.24. This kind of interaction has been employed to devise a piezoelectric sensor for a glycated hemoglobin, a sugar derivative of hemoglobin, which is of interest in diabetes mellitus monitoring [48, 49]. 3-Aminophenylboronic acid has been appended to an inert protein layer previously formed at the surface of the gold electrode, thus providing a high density of binding groups. After each run, sensor regeneration is carried out with 0.2 M HCl and is completed in only 2 min. The sensitivity remained unchanged after a sequence of 15 successive runs. Such a sensor responds, in principle, to any sugar in the sample, including glucose. However, glucose does not affect the response because its mass is negligible with respect to that of the analyte. Glucose competition in the analyte binding can be made negligible by securing a high density of binding sites at the sensor surface.
Figure 21.24 The cyclic ester product of phenylboronic interaction with a sugar molecule.
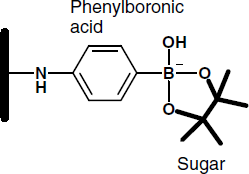
21.4.6 Outlook
The label-free character of QCM transduction makes it a very attractive method for the development of affinity sensors based on either immunocompounds or synthetic receptors. When very large molecules are involved, QCM transduction is impaired by the viscoelastic effect that reduces the sensitivity. This problem can be mitigated by resorting to postrecognition amplification, which can enhance considerably the sensitivity. However, amplification techniques involve processes similar to that in label-based methods.
Very promising perspectives are opened up by application of synthetic receptors that eliminate the use of biochemical compounds and biochemical operations. Thus, molecularly imprinted polymers prove to be effective in the determination of both small molecules and large entities such as proteins, viruses and micro-organisms. Molecular synthetic receptors are also of great interest.
21.5 QCM Nucleic Acid Sensors
21.5.1 Hybridization Sensors
The label-free character of the piezoelectric transduction has proved advantageous for constructing DNA hybridization sensors [50, 51].
Probe immobilization should be performed so as to obtain an optimum probe density, avoid nonspecific interactions at the sensor surface, and secure good stability and resilience in the regeneration step. Immobilization can be achieved simply by chemisorption of a thiol-tethered oligonucleotide to the gold coating over the quartz wafer. More elaborate methods, such as avidin–biotin-based linking, impart superior characteristics to the recognition layer [52].
A piezoelectric sensor for the detection of genetically modified organisms (GMOs) has been fabricated using a 25-unit oligomer DNA probe that is complementary to a characteristic sequence in the GMO's DNA [53]. The biotinylated probe was immobilized by affinity coupling to the gold layer that was modified first with a mercaptoalcohol (![]() ) and streptavidin-modified dextran. The readout was adjusted to zero with the sensor immersed in the hybridization buffer and then the sample was added (see Figure 21.25). After 20 min incubation, the frequency reached a constant value that was recorded. Finally sensor was washed with the buffer solution in order to remove unbound oligonucleotides. The analytical signal
) and streptavidin-modified dextran. The readout was adjusted to zero with the sensor immersed in the hybridization buffer and then the sample was added (see Figure 21.25). After 20 min incubation, the frequency reached a constant value that was recorded. Finally sensor was washed with the buffer solution in order to remove unbound oligonucleotides. The analytical signal ![]() is the frequency difference between levels A and B, as shown in Figure 21.25.
is the frequency difference between levels A and B, as shown in Figure 21.25.
Figure 21.25 Time evolution of ![]() during a hybridization assay using a piezoelectric DNA sensor. Adapted with permission from [53]. Copyright 2001 Springer Science+Business Media B.V.
during a hybridization assay using a piezoelectric DNA sensor. Adapted with permission from [53]. Copyright 2001 Springer Science+Business Media B.V.
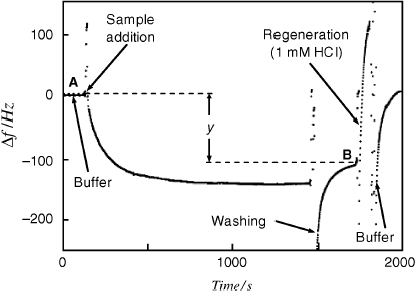
In order to perform the next assay, the single-strand probe was regenerated by a one-minute treatment with dilute hydrochloric acid. Regeneration could be performed satisfactorily up to 10 times.
An assay with a noncomplementary oligonucleotide yielded no measurable frequency shift proving the absence of non-specific interactions. Tests with PCR-amplified DNA samples from a transgenic soya-bean flour certified reference material gave an almost linear response from 0.01 to 20 μM target concentration.
Similarly, detection of bacterial pathogens in environmental samples has been effected by means of sensing layers including capture probes specific to a particular sequence in the DNA of the target micro-organism [54].
The sensitivity of a direct piezoelectric sensor is too low for detecting the target in a biological sample and amplification by PCR should be performed prior to the determination. This time-consuming step can be avoided by means of gold nanoparticle attached to the primary hybridization product [55, 56]. This principle is illustrated in Figure 21.26. First, the target is hybridized with the capture probe attached to the piezocrystal surface to form the primary hybrid. Next, a gold nanoparticle modified with a sequence complementary to the overhanging moiety of the target is attached to the primary hybrid in a sandwich arrangement. Subsequent amplification can be achieved by growing a dendrimer aggregate starting from this structure and using nanoparticles modified with suitable sequences. Mass amplification by this technique allows the determination of target concentrations down to 10−10 M.
Figure 21.26 Amplification of the piezoelectric sensor response by attaching gold nanoparticles to the probe–target hybrid.
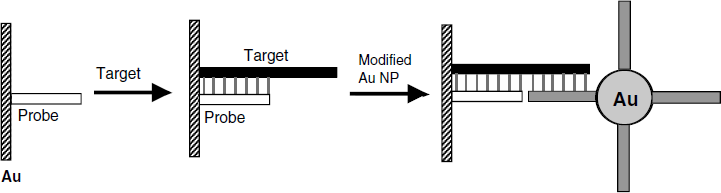
Mass amplification can be further enhanced by chemical deposition of a metal on nanoparticles previously attached to the probe–target hybrid. As indicated before, in the chemical reduction of ![]() to gold by hydroxylamine, gold nanoparticle act as crystallization centers and their mass is augmented by gold deposition. This allowed an outstanding detection limit close to 10−15 M to be reached.
to gold by hydroxylamine, gold nanoparticle act as crystallization centers and their mass is augmented by gold deposition. This allowed an outstanding detection limit close to 10−15 M to be reached.
Signal amplification by means of gold nanoparticles is intricate and time consuming but this drawback is compensated by the possibility of detecting the target in the sample with no prior DNA amplification.
21.5.2 Piezoelectric Aptasensors
Various strategies for protein recognition in piezoelectric aptasensors have been elaborated, as demonstrated in Figure 21.27. In this figure, BF represents a simple aptamer that can bind to one specific site in the target protein. BFA is similar to BF as far as the recognition behavior is concerned. However, in order to keep the aptamer position as far as possible from the surface, the linkage fragment in BFA is composed of a rigid double strand. BFF is composed of two aptamers similar to BF joined together by means of complementary fragments. This receptor, which is an aptabody, includes two similar binding sites and yields a greater response because each receptor binds two analyte molecules. BFH is also a composite receptor but each aptamer in its structure binds to a different site in the analyte molecule.
Figure 21.27 Various types of aptamers used for thrombin recognition in QCM sensors. BF: conventional single-stranded aptamer; BFA: similar to BF but containing a double-strand linker fragment that provides a more rigid orientation; BFF: two BF aptamers connected through complementary fragments; BFH: two aptamers, each of them being specific to a particular site in the thrombin molecule [57]. Reproduced with permission of the Royal Society of Chemistry.
A piezoelectric aptasensor for human ![]() -thrombin has been described in ref. [58]. The recognition layer consists of a mixture of carbon nanotubes and positively charged methylene blue deposited on the gold surface. Aptamers were attached to this primer by electrostatic interaction. Two versions of this sensor have been tested. In the first one, the receptor was a thrombin aptamer. In the second one, an aptabody, formed by conjugation of two aptamers with complementary oligonucleotide tails (like BFF in Figure 21.27) has been used. The aptabody sensor yields a detection limit of 0.3 nM that is three times better that that demonstrated by the single-aptamer version. A common protein (human serum albumin, HSA) had almost no effect on the sensor response, proving the absence of nonspecific interactions. The absence of interferences was also demonstrated by the similarity of calibration curves obtained with thrombin in pure buffer, in a 3 mg/ml HSA containing buffer and in 10 times diluted blood serum.
-thrombin has been described in ref. [58]. The recognition layer consists of a mixture of carbon nanotubes and positively charged methylene blue deposited on the gold surface. Aptamers were attached to this primer by electrostatic interaction. Two versions of this sensor have been tested. In the first one, the receptor was a thrombin aptamer. In the second one, an aptabody, formed by conjugation of two aptamers with complementary oligonucleotide tails (like BFF in Figure 21.27) has been used. The aptabody sensor yields a detection limit of 0.3 nM that is three times better that that demonstrated by the single-aptamer version. A common protein (human serum albumin, HSA) had almost no effect on the sensor response, proving the absence of nonspecific interactions. The absence of interferences was also demonstrated by the similarity of calibration curves obtained with thrombin in pure buffer, in a 3 mg/ml HSA containing buffer and in 10 times diluted blood serum.
As is common with large protein layers, the viscoelastic effect contributes to the overall frequency shift, and, therefore, the resonance frequency and the motional resistance needs to be extracted from the total response [57].
A similar aptamer sensor for the determination of prion (an infectious agent composed of protein in a misfolded form) has also been developed [59].
Considerable improvement in detection limit has been achieved by means of postrecognition attachment of gold nanoparticles conjugated with a second aptamer to form a sandwich ternary complex [60]. Further amplification has been obtained by growing the nanoparticles by gold depositions from a ![]() solution containing 1,4-dihydronicotinamide adenine dinucleotide (NADH) as reducing agent.
solution containing 1,4-dihydronicotinamide adenine dinucleotide (NADH) as reducing agent.
21.5.3 Outlook
The QCM is a versatile transduction technique in nucleic acid sensors owing to its label-free character. Superior sensitivity can be achieved by means of posthybridization treatment aimed at increasing the overall mass load by appending gold nanoparticles. Further sensitivity enhancement is achieved by chemical deposition of gold on the appended particles.
Aptamer application in QCM protein sensors represents a promising alternative to the QCM immunoassay.
21.6 Surface-Launched Acoustic-Wave Sensors
21.6.1 Principles
The quartz crystal microbalance, which was covered in the previous sections, relies on thickness shear vibration induced by electrodes placed on each side of the piezoelectric wafer. In contrast, surface-acoustic-wave devices are based on acoustic excitation by means of two electrodes placed on the same surface. The electrodes are configured as an interdigitated transducer (IDT). The acoustic wave induced by an IDT is propagated in an extremely thin layer at the surface of the piezoelectric wafer. The wave motion is detected by the same or an additional transducer on the same surface. This configuration is typical of surface-launched acoustic-wave devices(SLAW).
Commonly used SLAW devices are shown in Figure 21.28. Such devices are utilized as transducers in chemical sensors for gas or liquid phase analysis. SLAW functioning principles and sensor applications have been reviewed in refs. [11, 62–64]. This section introduces first the physical principles of SLAW devices and next presents their sensor applications.
Figure 21.28 SLAW devices and acoustic wave propagation modes. Black arrows indicate particle displacement; white arrows indicate wave propagation. For each type, the typical material is further indicated. (A) SAW: surface acoustic wave or Rayleigh wave (ST-cut quartz). (B) SH-APM: shear horizontal acoustic plate mode (AT-quartz; lithium niobate, LiNbO3). (C) FPW: flexural plate-wave device – Lamb wave (ZnO on silicon nitride). (D) STW: surface transverse wave device – Love wave (AT-cut quartz; lithium tantalate (LiTaO3)). Adapted with permission from [61]. Copyright 2000 Springer Science+Business Media B.V.
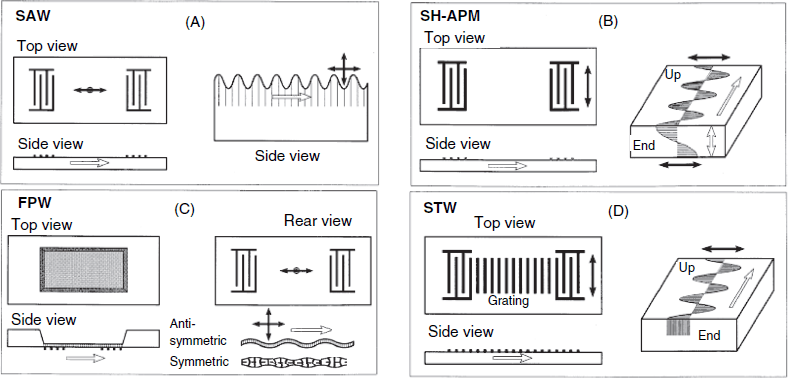
21.6.2 The Surface Acoustic Wave
The surface acoustic wave (SAW) also known as the Rayleigh wave (Figure 21.28A) involves particle displacement along two directions: a longitudinal-compressional component (that is, back and forth, parallel to the surface) and shear vertical component (that is, up and down). The superposition of these two components gives rise to particle trajectories that follow an elliptical path around their rest positions. The thickness of the layer involved in vibration is of one or two acoustic wavelengths.
The configuration of SAW devices is shown in Figure 21.29. Version A is a two-port delay line; its name is in accordance with its function in electronic equipment. Two IDTs are formed on the surface of the piezoelectric wafer. The first one (transmitter) is connected to a high-frequency AC voltage supply and excites surface vibration by the converse piezoelectric effect. The acoustic wave is detected by the second IDT (receiver) that generates an AC voltage proportional to the wave amplitude. The time delay ![]() is given by the
is given by the ![]() ratio where l is the distance between the IDTs and
ratio where l is the distance between the IDTs and ![]() is the wave velocity. The maximum energy transfer between IDTs occurs at a characteristic frequency
is the wave velocity. The maximum energy transfer between IDTs occurs at a characteristic frequency ![]() , where
, where ![]() is the corresponding wavelength. As the first IDT radiates in both directions, there is significant energy loss. However, if the delay line is integrated in the feedback of an electronic oscillator network, the losses can be compensated and the device performs as a resonator based on a resonant traveling wave. As in the case of a vibrating string, there are many close resonance states with closely spaced resonant frequencies that may complicate the device operation.
is the corresponding wavelength. As the first IDT radiates in both directions, there is significant energy loss. However, if the delay line is integrated in the feedback of an electronic oscillator network, the losses can be compensated and the device performs as a resonator based on a resonant traveling wave. As in the case of a vibrating string, there are many close resonance states with closely spaced resonant frequencies that may complicate the device operation.
Figure 21.29 Two alternative types of SLAW device. (A) Two-port delay-line SLAW device; (B) one-port SAW device. The gratings (R1 and R2) are totally reflecting at the resonant frequency. The wafer is laterally cut slightly off the long axis to prevent interference of the back-reflected wave with the main wave. Alternatively, this problem can be mitigated by coating the ridges with a wave-absorber material. Adapted with permission from [63]. Copyright 1995 Elsevier Science S.A.
The device in Figure 21.29B is a single-port resonator. In this case, the vibration is excited by an IDT placed between two gratings that are totally reflecting at the characteristic frequency. A standing wave develops between the reflectors giving rise to a resonator that vibrates at various overtones with frequencies given by ![]() , where n is the overtone number. The resonant frequencies depend on the properties of the piezoelectric material (density and elasticity) and on the angle of cut. However, the quality factor is much lower than in the case of the QCM.
, where n is the overtone number. The resonant frequencies depend on the properties of the piezoelectric material (density and elasticity) and on the angle of cut. However, the quality factor is much lower than in the case of the QCM.
The wavelength (and hence the operating frequency) is determined by the spacing between the electrodes. Using this parameter the frequency can be adjusted between 150 and 400 MHz. Quartz is a suitable piezoelectric material for such devices, but LiNbO3 displays better electromechanical coupling. As the SAW is strongly damped by liquids its analytical application is limited to gas sensors.
21.6.3 Plate-Mode SLAW Devices
In the case of the SAW device presented before, the vibration is localized at the surface of a piezoelectric plate with the thickness much greater than the wavelength. This section introduces a series of SLAW devices in which the vibration expands over the whole plate thickness although it is excited on only one side. In such devices, the vibrating plate is thinner than the acoustic wavelength.
21.6.4 SLAW Gas and Vapor Sensors
Among the various available SLAW devices, the SAW one has been mostly employed in gas-phase sensing [65, 66]. Surface-wave interaction with the sample alters various wave parameters such as the amplitude, the phase angle, the velocity, and the harmonic content. The two-port SAW delay line is used as the transducer in SAW devices by coating a recognition layer over the vibrating area (Figure 21.30). Therefore, the wave parameters are modified in accordance with the effect of analyte sorption. Most often, the sensor response is represented by the frequency change produced by the recognition event. The frequency change (![]() ) decreases proportionally to the mass load per unit area [11]. However, this simple relationship holds only if the analyte sorption does not produce modifications in the physical properties of the sensing layer. As the resonant frequency is much higher than that of a typical QCM, much better sensitivity is achieved by SAW sensors.
) decreases proportionally to the mass load per unit area [11]. However, this simple relationship holds only if the analyte sorption does not produce modifications in the physical properties of the sensing layer. As the resonant frequency is much higher than that of a typical QCM, much better sensitivity is achieved by SAW sensors.
Figure 21.30 Side view of a SAW sensor showing three distinct stages: (1) excitation of the acoustic wave; (2) wave propagation along the coated surface; (3) transduction of the acoustic signal into an electrical one by the receiver. Adapted with permission from [67]. Copyright 2004 Springer Science+Business Media B.V.
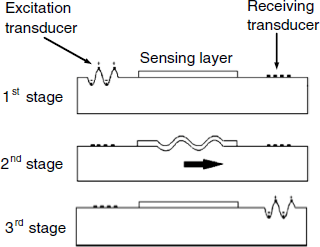
Besides frequency monitoring, the response can be assessed by measuring the variation of the wave amplitude and the phase angle using a vector voltmeter connected between the two IDTs. The phase variation depends on to the mass load and also on other types of modifications in the sensing layer.
The most advanced monitoring method for SAW sensors is based on network analyzers that reports on both the magnitude and the phase angle of the electrical impedance as a function of frequency.
The sensing layer is selected such as to interact with the analyte either by physical or chemical sorption. Although the SAW device is capable of detecting monolayer adsorption, greater sensitivity can be obtained by means of a thicker film that allows the analyte to diffuse through and interact with a larger number of sorption sites.
Sensing by chemical sorption involves relatively strong interaction and provides reasonable selectivity. However, such a sensor is irreversible under normal conditions and will be most useful in dosimetry applications. The dosimeter provides an integrated signal related to the concentration and time of exposure to the target compound. The large dynamic range and low cost of SAW devices renders this alternative a practical one.
Physical sorption-based recognition has the advantage of being a reversible process that allows for easy regeneration of the sensor. However, the selectivity in this case is rather poor. This problem is alleviated by operating arrays consisting of sparingly selective sensors. In view of the small size and low cost of the device, this approach is very practical.
Sensor response depends mainly on the mechanism of the analyte–receptor interaction (Figure 21.31). The simplest case is represented by physical adsorption (A) that causes an abrupt change in the signal initially, when only adsorption at the surface is taking place. Subsequently, the analyte diffuses into the sensing layer giving rise to a further slower, signal change until the equilibrium with the gas phase is attained. As this sensor is reversible, regeneration can be achieved by flushing it with a pure gas. Such a sensor is temperature dependent as both the diffusion rate and the equilibrium concentration of the adsorbed analyte are affected by temperature. At higher temperatures the diffusion is faster, producing a shorter response time. On the other hand, a higher temperature reduces the analyte concentration in the sensing layer. Hence, sensitivity can be enhanced by lowering the device temperature but only at the expense of a longer response time.
Figure 21.31 Possible response profiles of a SAW vapor sensor. (A) Ideal response; (B) Irreversible chemisorption; (C) combined mass and viscoelastic effects; (D) Large change in polymer properties for a small loading mass. Adapted with permission from [62] Copyright 1997 John Wiley & Sons, Inc.
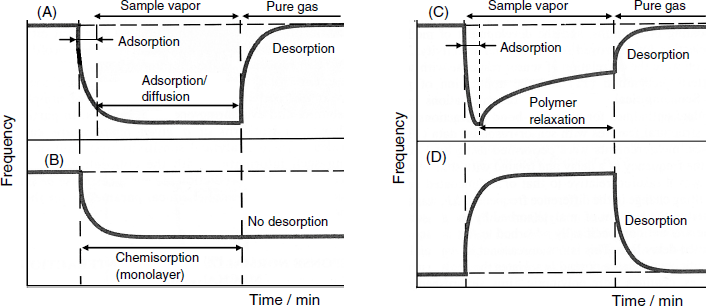
If the analyte undergoes chemical absorption, the response is irreversible and the original value of the signal will not be restored by purging with a pure gas (Figure 21.31B).
A more intricate behavior can be noted when the properties of the sensing layer are altered by analyte sorption. Thus, Figure 21.31C shows a two-step response evolution. Fast sorption during the first step gives rise to a sudden frequency change due to the mass loading, Further changes in the properties of the analyte-permeated sensing layer causes modifications of the viscoelastic properties that also affect the response signal. As shown in Figure 21.31D, the second effect can in some cases be the more dominant and an increase (or decrease) in the resonant frequency could occur due mostly to modifications of the film properties in response to analyte sorption. In the case of a polymer film, sorption of the analyte in the polymer causes a slow conformational rearrangement until a thermodynamically stable conformation is attained. Relaxation of the polymer matrix can lead to modifications in the viscoelastic properties, the dielectric constant or the electric conductance, each of these effects causing a modification of the response signal.
By far the most common gas sensing materials are synthetic polymers deposited by solvent evaporation. The Langmuir–Blodgett method is also convenient for sensing-layer preparation. Another method exploits the presence of hydroxyl groups at the quartz surface in order to prepare self-assembled monolayers capable of interacting with the analyte. A number of particular applications rely on metal coatings such as palladium in hydrogen and platinum in ammonia sensors.
It should be noted that SAW devices are sensitive to temperature and pressure variations. That is why it is practical to combine the sensor itself with a reference sensor that does not responds to the analyte. The differential signal obtained in this way is free from effects arising from spurious influences. Both the working and reference sensor can be formed on the same piezoelectric wafer.
The limit of detection of SAW sensors is determined as usually by the signal-to-noise ratio. As the noise increases proportionally with frequency whereas the signal increases with the square of the frequency the signal-to-noise ratio will increase proportionately with frequency. Noise reduction can also be achieved by reducing the length of the delay line.
The design and manufacturing of a sensor for a particular analyte in a complex gas sample is a difficult task. However, in certain applications it is not necessary to detect each component but to detect together (as one) a complex set of compounds that impart the mixture a particular property such as odor or aroma. Such a task can be performed by an assembly of sensors fabricated such that each of them responds to a number of compounds in the sample. Although none of the sensors is ideally specific, data processing by statistical methods allows a set of numerical parameters to be ascribed to the mixture that allows it to be characterized sufficiently. This approach forms the physical basis of the artificial nose [67] (see also Section 1.7). The low cost and easy fabrication technology of SLAWs makes them very suitable for such applications [68].
21.6.5 Liquid-Phase SLAW Sensing
The most suitable device for liquid-phase sensing is the STW (Love) device due to its enhanced sensitivity to environment effects and to its high quality factor, high frequency (over 100 MHz), robustness and small size [69, 70]. As the device surface is coated with a wave-guiding layer, this surface can function as a support for receptor immobilization.
A typical configuration of a STW device sensor is shown in Figure 21.32. A sensing layer including suitable recognition receptors is deposited on top of the guiding layer. The transmitter IDT that is connected to a high-frequency voltage supply excites the thickness–shear vibration and the guided wave propagates along the device surface. Due to total internal reflection, the wave energy can cross the guiding layer along the vertical direction as an evanescent wave. Acoustic energy is thus transferred to the recognition material on top of the guiding layer. As shown by the graph inserted into Figure 21.32, the wave decays exponentially with distance and its effect is mostly confined to the thin recognition layer. The parameters of the wave reaching the receiver IDT are therefore affected by wave interaction with the sensing film. In order to compensate for spurious effects, a reference sensor is constructed on the same piezoelectric plate and the response is corrected by subtracting the reference signal from that of the working sensor. As in the case of the QCMs, STWs are best operated when incorporated in a flow-analysis system. In order to avoid perturbation caused by pressure variation, gravitational flow or syringe pumps are suitable for delivering the sample.
Figure 21.32 Configuration of a SLAW biosensor. The inset graph demonstrates the exponential decay of the wave amplitude along the vertical direction. Adapted with permission from [70]. Copyright 2007 Elsevier B.V.
Figure 21.33 presents the time evolution of the response of a STW xylene sensor to the analyte concentration in water. As can be noted, two different response signals are available: the frequency change and the variation of the dissipated power. Power loss (![]() , in decibels, dB) can be determined by the following formula in which
, in decibels, dB) can be determined by the following formula in which ![]() means power,
means power, ![]() is the wave amplitude, and the subscripts “out” and “in” indicate output and input value, respectively:
is the wave amplitude, and the subscripts “out” and “in” indicate output and input value, respectively:
Figure 21.33 Flow-analysis response of a STW xylene sensor to increasing analyte concentration between 10 and 60 ppm. (A) Frequency change response; (B) wave power loss response. The sensing layer was a 0.8-μm thick poly(isobutylene) film. After each sample, the sensor was flushed with pure water. Reproduced with permission from [71]. Copyright 2005 American Chemical Society.

(21.42) ![]()
Similar information can be derived from phase-angle measurements.
The measured frequency change response in Figure 21.33A reflects both mass loading and energy dissipation in the viscoelastic polymer film. Energy dissipation is, however, unambiguously revealed by data in Figure 21.33B. In both cases, the response is in a linear dependence on the analyte concentration over a limited range that is narrower in the case of loss measurements. So, two distinct response signals can be generated by a SLAW sensor. This feature is of relevance to the design and data processing in sensor arrays.
The previous example refers to the sensing of small molecules by sorption into a polymer coating. However, the field of application also includes the sensing of large biological entities such as proteins, nucleic acids, bacterial toxins, micro-organisms, and whole cells [70]. Sensors for proteins or pathogens make use of antibodies as recognition reagents. Application of aptamers as recognition receptors for proteins has also been demonstrated. Separation of mass loading and viscoelastic effects can be useful for performing transduction even in the absence of significant mass variation.
An important feature of the STW sensor is the possibility of adapting the functioning mode to the size of the detected entity by tuning the penetration depth of the evanescent wave into the sensing overlayer. Thus, a high frequency results in a small penetration depth that is suitable for sensing small molecules. The opposite case fits best the requirements imposed by the detection of large entities such as proteins or cells.
It was recently pointed out that the number of reported applications of SLAW devices in biosensors is relatively low despite the great potential of such devices [70]. This is most probably due to the intricacy of the theoretical modeling and the complexity of readout procedures that require interdisciplinary teams. Recent advances in this area are surveyed in ref. [72].
21.6.6 Outlook
Currently, various SLAW devices with well-established manufacturing technologies and fairly good theoretical descriptions are available. The broadest field of sensing application is still the development of gas sensors, but the advent of the STW (Love) device has opened up very promising perspectives to applications in liquid-phase sensing.
It is important to note that both SLAW and QCM devices provide essentially the same kind of information, namely the mass load and the viscoelastic properties of the coating layer. The advantage of the QCM is its easy fabrication, the small temperature sensitivity, and the low noise level. On the other hand, SAW sensors use higher frequencies that lead to enhanced mass sensitivity. In addition, the small size of the SLAW sensors, which is in the millimeter range, makes them very suitable for developing sensor arrays.
The performances of piezoelectric sensors (including both QCMs and SLAWs) are often compared with that of an optical method for interface sensing based on surface plasmon resonance (SPR, see Section 18.6.1). SPR is generally more sensitive than the QCM but SLAW sensors approach the sensitivity of the SPR method. However, due to device characteristics, the immobilization support in the SPR is restricted to gold. In contrast, the STW (Love) devices provide many more alternatives as the guiding layer (which acts as an immobilization support) can be fabricated from various organic or inorganic materials. Moreover, the SPR method provides no information on the viscoelastic properties of the sensing layer, as the acoustic-wave devices do.
21.7 Summary
Acoustic-wave transduction is based on coupling the solid device vibration with a recognition layer whose characteristics change in response to the interaction with the analyte. As a consequence of this interaction, the vibration frequency is altered as a function of the analyte concentration.
Vibrating devices are made of piezoelectric materials and the vibration is maintained by means of an AC voltage applied through metal electrodes. A piezoelectric oscillator is characterized by its resonant frequency which depends on geometrical factors (such as device thickness) and the physical properties of the piezoelectric materials (such as density and elasticity).
Ideally, the change in resonance frequency is determined only by the additional mass loaded through the recognition event. This occurs when the added film adheres firmly to the resonator surface, is rigid, and much thinner than the wavelength of the acoustic wave.
Two kinds of acoustic-wave device can be distinguished, according to the quotient of the device thickness and the depth of penetration of the acoustic wave into the vibrating material. Bulk acoustic-wave devices are characterized by the fact that the vibration encompasses the whole volume of the piezoelectric material. In contrast, in surface-launched acoustic-wave devices, the vibration is limited to a very thin layer at the device surface.
The typical bulk acoustic-wave device is the quartz crystal microbalance, in which the piezoelectric crystal vibrates in the thickness–shear mode. For thin, rigid layers, the frequency change is proportional to the mass variation according to the Sauerbrey equation. Owing to the absence of energy dissipation, the QCM gas sensor functions close to these conditions. When the QCM is operated in a liquid phase, energy dissipation into the liquid brings about a constant frequency change that can be subtracted from the overall response However, when macromolecules are involved, energy dissipation gives rise to strong deviations from the Sauerbrey equation and causes the frequency variation to be much lower than that expected from the mass change. This drawback can be mitigated by signal amplification based on postrecognition modification of the surface layer with gold nanoparticles.
Compared with the QCM, surface-launched acoustic-wave devices are characterized by a much higher resonance frequency, and, hence, provide a better sensitivity. The small size of SLAW devices allows for miniaturization and the fabrication of sensor arrays. SLAW devices in which the vibration is oriented perpendicularly to the resonator surface are useful in gas and vapor sensors. For application in liquid-phase sensing, devices in which the vibration is parallel to the resonator surface are very suitable owing to the very low energy dissipation.
In addition to chemical-sensing applications, acoustic-wave devices are valuable tools for investigating thin layers as far as viscoelastic properties are concerned, providing information that is useful in the development of various sensors based on thin layers attached to metal surfaces.
1. Buck, R.P., Lindner, E., Kutner, W., and Inzelt, G. (2004) Piezoelectric chemical sensors - (IUPAC Technical Report). Pure Appl. Chem., 76, 1139–1160.
2. Steinem, C. and Janshoff, A. (2007) Piezoelectric Sensors, Springer-Verlag, Berlin.
3. Ballantine, D.S., Martin, S.J., Ricco, A.J. et al. (1997) Acoustic Wave Sensors: Theory, Design, and Physico-Chemical Applications, Academic Press, San Diego.
4. Buttry, D.A. and Ward, M.D. (1992) Measurement of interfacial processes at electrode surfaces with the electrochemical quartz crystal microbalance. Chem. Rev., 92, 1355–1379.
5. Marx, K.A. (2003) Quartz crystal microbalance: A useful tool for studying thin polymer films and complex biomolecular systems at the solution-surface interface. Biomacromolecules, 4, 1099–1120.
6. Janshoff, A., Galla, H.J., and Steinem, C. (2000) Piezoelectric mass-sensing devices as biosensors - An alternative to optical biosensors? Angew. Chem. Int. Edit., 39, 4004–4032.
7. Bandey, H.L., Martin, S.J., Cernosek, R.W., and Hillman, A.R. (1999) Modeling the responses of thickness-shear mode resonators under various loading conditions. Anal. Chem., 71, 2205–2214.
8. Sauerbrey, G. (1959) Use of vibrating quartz for thin film weighing and microweighing (in German). Z. Phys., 155, 206–222.
9. Rodahl, M. and Kasemo, B. (1996) On the measurement of thin liquid overlayers with the quartz-crystal microbalance. Sens. Actuators A-Phys., 54, 448–456.
10. Kanazawa, K.K. and Gordon, J.G. (1985) The oscillation frequency of a quartz resonator in contact with a liquid. Anal. Chim. Acta, 175, 99–105.
11. Grate, J.W., Martin, S.J., and White, R.M. (1993) Acoustic-wave microsensors.1. Anal. Chem., 65, A940–A948.
12. Martin, S.J., Bandey, H.L., Cernosek, R.W. et al. (2000) Equivalent-circuit model for the thickness-shear mode resonator with a viscoelastic film near film resonance. Anal. Chem., 72, 141–149.
13. Martin, S.J., Granstaff, V.E., and Frye, G.C. (1991) Characterization of a quartz crystal microbalance with simultaneous mass and liquid loading. Anal. Chem., 63, 2272–2281.
14. Lucklum, R., Behling, C., Cernosek, R.W., and Martin, S.J. (1997) Determination of complex shear modulus with thickness shear mode resonators. J. Phys. D-Appl. Phys., 30, 346–356.
15. Lucklum, R. and Hauptmann, P. (2006) Acoustic microsensors-the challenge behind microgravimmetry. Anal. Bioanal. Chem., 384, 667–682.
16. Mecea, V.M. (1994) Loaded vibrating quartz sensors. Sens. Actuators A-Phys., 40, 1–27.
17. Johannsmann, D. (2008) Viscoelastic, mechanical, and dielectric measurements on complex samples with the quartz crystal microbalance. Phys. Chem. Chem. Phys., 10, 4516–4534.
18. Johannsmann, D. (2007) Studies of viscoelasticity with the QCM, in Piezoelectric Sensors (eds C. Steinem and A. Janshoff), Springer-Verlag, Berlin, pp. 49–109.
19. Hillman, A.R. (2003) The electrochemical quartz crystal microbalance, in Encyclopedia of Electrochemistry (eds A.J. Bard, M. Stratmann, and P.R. Unwin), Wiley-VCH, Weinheim, pp. 230–289.
20. Rodahl, M. and Kasemo, B. (1996) A simple setup to simultaneously measure the resonant frequency and the absolute dissipation factor of a quartz crystal microbalance. Rev. Sci. Instrum., 67, 3238–3241.
21. Höök, F. and Kasemo, B. (2007) The QCM-D technique for probing biomacromolecular recognition reactions, in Piezoelectric Sensors (eds C. Steinem and A. Janshoff) Springer-Verlag, Berlin, pp. 425–447.
22. Hepel, M. (1999) Electrode-solution interface studied with electrochemical quartz crystal nanobalance, in Interfacial Electrochemistry: Theory, Experiment, and Applications (ed. A. Wieckowski) Marcel Dekker, New York, pp. 599–630.
23. Ngeh-Ngwainbi, J., Suleiman, A.A., and Guilbault, G.G. (1990) Piezoelectric crystal biosensors. Biosens. Bioelectron., 5, 13–26.
24. Guilbault, G.G. and Jordan, J.M. (1988) Analytical uses of piezoelectric crystals - a review. CRC Crit. Rev. Anal. Chem., 19, 1–28.
25. Alder, J.F. and McCallum, J.J. (1983) Piezoelectric crystals for mass and chemical measurements - a review. Analyst, 108, 1169–1189.
26. Mirsky, M.M. and Vasjari, M. (2007) Chemical sensors for mercury vapours, in Electrochemical Sensor Analysis (eds S. Alegret and A. Merkoçi), Elsevier, Amsterdam, pp. 235–251.
27. Bucur, R.V. (1974) Piezoelectric quartz crystal microbalance used as detector for gaseous hydrogen (deuterium). Rev. Roum. Phys., 19, 779–786.
28. Guilbault, G.G. (1983) Determination of formaldehyde with an enzyme-coated piezoelectric crystal detector. Anal. Chem., 55, 1682–1684.
29. Ngehngwainbi, J., Foley, P.H., Kuan, S.S., and Guilbault, G.G. (1986) Parathion antibodies on piezoelectric crystals. J. Am. Chem. Soc., 108, 5444–5447.
30. Mascini, M., Minunni, M., Guilbault, G., and Carr, R. (1998) Immunosensors based on piezoelectric crystal device, in Affinity Biosensors: Techniques and Protocols (eds K.R. Rogers and A. Mulchandani), Humana Press, Totowa, pp. 55–76.
31. Skladal, P. (2003) Piezoelectric quartz crystal sensors applied for bioanalytical assays and characterization of affinity interactions. J. Braz. Chem. Soc., 14, 491–502.
32. Vaughan, R.D. and Guilbault, G.G. (2007) Piezoelectric immunosensors, in Piezoelectric Sensors (eds C. Steinem and A. Janshoff), Springer-Verlag, Berlin, pp. 237–280.
33. Uttenthaler, E., Kosslinger, C., and Drost, S. (1998) Quartz crystal biosensor for detection of the African Swine Fever disease. Anal. Chim. Acta, 362, 91–100.
34. Uludag, Y. and Tothill, I.E. (2010) Development of a sensitive detection method of cancer biomarkers in human serum (75%) using a quartz crystal microbalance sensor and nanoparticles amplification system. Talanta, 82, 277–282.
35. Ma, Z.F., Wu, J.L., Zhou, T.H. et al. (2002) Detection of human lung carcinoma cell using quartz crystal microbalance amplified by enlarging Au nanoparticles in vitro. New J. Chem., 26, 1795–1798.
36. Li, J., He, X., Wu, Z. et al. (2003) Piezoelectric immunosensor based on magnetic nanoparticles with simple immobilization procedures. Anal. Chim. Acta, 481, 191–198.
37. Zhang, Y., Wang, H., Yan, B. et al. (2008) A reusable piezoelectric immunosensor using antibody-adsorbed magnetic nanocomposite. J. Immunol. Methods, 332, 103–111.
38. Halamek, J., Pribyl, J., Makower, A. et al. (2005) Sensitive detection of organophosphates in river water by means of a piezoelectric biosensor. Anal. Bioanal. Chem., 382, 1904–1911.
39. Uludag, Y., Piletsky, S.A., Turner, A.P.F., and Cooper, M.A. (2007) Piezoelectric sensors based on molecular imprinted polymers for detection of low molecular mass analytes. FEBS J., 274, 5471–5480.
40. Avila, M., Zougagh, M., Escarpa, A., and Rios, A. (2008) Molecularly imprinted polymers for selective piezoelectric sensing of small molecules. TrAC-Trends Anal. Chem., 27, 54–65.
41. Wu, A.H. and Syu, M.J. (2006) Synthesis of bilirubin imprinted polymer thin film for the continuous detection of bilirubin in an MIP/QCM/FIA system. Biosens. Bioelectron., 21, 2345–2353.
42. Pietrzyk, A., Suriyanarayanan, S., Kutner, W. et al. (2010) Molecularly imprinted poly[bis(2,2′-bithienyl)methane] film with built-in molecular recognition sites for a piezoelectric microgravimetry chemosensor for selective determination of dopamine. Bioelectrochemistry, 80, 62–72.
43. Dickert, F.L. and Lieberzeit, P.A. (2007) Imprinted polymers in chemical recognition for mass-sensitive devices, in Piezoelectric Sensors (eds C. Steinem and A. Janshoff), Springer-Verlag, Berlin, pp. 173–210.
44. Hayden, O. and Dickert, F.L. (2001) Selective microorganism detection with cell surface imprinted polymers. Adv. Mater., 13, 1480–1483.
45. Dickert, F.L., Lieberzeit, P.A., and Hayden, O. (2004) Molecularly imprinted polymers for mass sensitive sensors – from cells to viruses and enzymes, in Molecular Imprinting of Polymers (eds S. Piletsky and A.P.F. Turner), Landes Bioscience, Austin, pp. 50–64.
46. Dickert, F.L., Lieberzeit, P.A., Hayden, O. et al. (2003) Chemical sensors - from molecules, complex mixtures to cells - supramolecular imprinting strategies. Sensors, 3, 381–392.
47. Snejdarkova, M., Poturnayova, A., Rybar, P. et al. (2010) High sensitive calixarene-based sensor for detection of dopamine by electrochemical and acoustic method. Bioelectrochemistry, 80, 55–61.
48. Pribyl, J. and Skladal, P. (2005) Quartz crystal biosensor for detection of sugars and glycated hemoglobin. Anal. Chim. Acta, 530, 75–84.
49. Pribyl, J. and Skladal, P. (2006) Development of a combined setup for simultaneous detection of total and glycated haemoglobin content in blood samples. Biosens. Bioelectron., 21, 1952–1959.
50. Teles, F.R.R. and Fonseca, L.R. (2008) Trends in DNA biosensors. Talanta, 77, 606–623.
51. Sassolas, A., Leca-Bouvier, B.D., and Blum, L.J. (2008) DNA biosensors and microarrays. Chem. Rev., 108, 109–139.
52. Tombelli, S. and Mascini, M. (2000) Piezoelectric quartz crystal biosensors: Recent immobilisation schemes. Anal. Lett., 33, 2129–2151.
53. Minunni, M., Tombelli, S., Mariotti, E. et al. (2001) Biosensors as new analytical tool for detection of Genetically Modified Organisms (GMOs). Fresenius J. Anal. Chem., 369, 589–593.
54. Tombelli, S., Mascini, M., Sacco, C., and Turner, A.P.F. (2000) A DNA piezoelectric biosensor assay coupled with a polymerase chain reaction for bacterial toxicity determination in environmental samples. Anal. Chim. Acta, 418, 1–9.
55. Willner, I., Shlyahovsky, B., Willner, B., and Zayatz, M. (2009) Amplified DNA biosensors, in Functional Nucleic Acids for Analytical Applications (eds Y. Li and Y. Lu), Springer, New York, pp. 199–252.
56. Patolsky, F., Ranjit, K.T., Lichtenstein, A., and Willner, I. (2000) Dendritic amplification of DNA analysis by oligonucleotide-functionalized Au-nanoparticles. Chem. Commun., 1025–1026.
57. Hianik, T., Grman, I., and Karpisova, I. (2009) The effect of DNA aptamer configuration on the sensitivity of detection thrombin at surface by acoustic method. Chem. Commun., 6303–6305.
58. Hianik, T., Porfireva, A., Grman, I., and Evtugyn, G. (2008) Aptabodies - New type of artificial receptors for detection proteins. Protein Pept. Lett., 15, 799–805.
59. Hianik, T., Porfireva, A., Grman, I., and Evtugyn, G. (2009) EQCM biosensors based on DNA aptamers and antibodies for rapid detection of prions. Protein Pept. Lett., 16, 363–367.
60. Willner, I. and Zayats, M. (2007) Electronic aptamer-based sensors. Angew. Chem. Int. Edit., 46, 6408–6418.
61. Kaspar, M., Stadler, H., Weiss, T., and Ziegler, C. (2000) Thickness shear mode resonators (“mass-sensitive devices”) in bioanalysis. Fresenius J. Anal. Chem., 366, 602–610.
62. Thompson, M. and Stone, D.C. (1997) Surface-Launched Acoustic Wave Sensors: Chemical Sensing and Thin-Film Characterization, Wiley, New York.
63. Benes, E., Groschl, M., Burger, W., and Schmid, M. (1995) Sensors based on piezoelectric resonators. Sens. Actuators A-Phys., 48, 1–21.
64. Grate, J.W., Martin, S.J., and White, R.M. (1993) Acoustic-wave microsensors. 2. Anal. Chem., 65, A987–A996.
65. Caliendo, C., Verardi, P., Verona, E. et al. (1997) Advances in SAW-based gas sensors. Smart Mater. Struct., 6, 689–699.
66. Wohltjen, H. (1984) Mechanism of operation and design considerations for surface acoustic-wave device vapor sensors. Sens. Actuators, 5, 307–325.
67. James, D., Scott, S.M., Ali, Z., and O'Hare, W.T. (2005) Chemical sensors for electronic nose systems. Microchim. Acta, 149, 1–17.
68. Grate, J.W. (2000) Acoustic wave microsensor arrays for vapor sensing. Chem. Rev., 100, 2627–2648.
69. Hossenlopp, J.M. (2006) Applications of acoustic wave devices for sensing in liquid environments. Appl. Spectrosc. Rev., 41, 151–164.
70. Gronewold, T.M.A. (2007) Surface acoustic wave sensors in the bioanalytical field: Recent trends and challenges. Anal. Chim. Acta, 603, 119–128.
71. Li, Z.H., Jones, Y., Hossenlopp, J. et al. (2005) Analysis of liquid-phase chemical detection using guided shear horizontal-surface acoustic wave sensors. Anal. Chem., 77, 4595–4603.
72. Lange, K., Rapp, B.E., and Rapp, M. (2008) Surface acoustic wave biosensors: a review. Anal. Bioanal. Chem., 391, 1509–1519.
