Chapter 22
Microcantilever Sensors
Microcantilevers belong to the class of microelectromechanical devices that encompass all those devices produced by microfabrication except integrated circuits [1]. When integrated with a sensing element, a microcantilever responds to the recognition event by mechanical deformation. An alternative mechanical transduction method utilizes cantilevers that vibrate at the resonance frequency. In this case, the mass change produced by the recognition event induces a change in the resonance frequency.
Microcantilevers with a sharp tip were originally introduced as probes for exploring solid surface topography by atomic force microscopy in the early 1980s. It was soon realized that microcantilevers are sensitive to physical parameter such as temperature and humidity. By the mid-1990s, it was demonstrated that cantilevers modified with chemically sensitive layers respond selectively to particular chemical compounds. This opened the way to the development of a new class of chemical sensor in which the transduction is based on the mechanics of elastic solids. Despite its relatively recent introduction, this field of research has already produced very promising results. Early results obtained in the microcantilever sensor area have been summarized in refs. [2, 3]. A good introduction to the field is given in ref. [4]. The mechanical principles of microcantilever operation are reviewed in [5, 6], while comprehensive overviews of fabrication methods and recent advances are available in refs. [7–9]. Finally, applications of microcantilever sensors in the determination of ions, small molecules and biomacromolecules are surveyed in refs. [9–11].
22.1 Principles of Microcantilever Transduction
22.1.1 The Microcantilever
A microcantilever is a submillimeter beam of solid material, as shown in Figure 22.1A, which displays a rectangular microcantilever. V-shaped, U-shaped or T-shaped microcantilevers have also been employed in developing sensors. Owing to their small size, cantilevers can be integrated in arrays, as demonstrated in Figure 22.1B.
Figure 22.1 The microcantilever. (A) Conformation and geometrical parameters. Typical dimensions: l = 100–350 μm; w = 20–40 μm; h = 0.6–1 μm. (B) Scanning electron micrograph of a cantilever array. (B) Reprinted with permission from [13]. Copyright 2001 Elsevier.
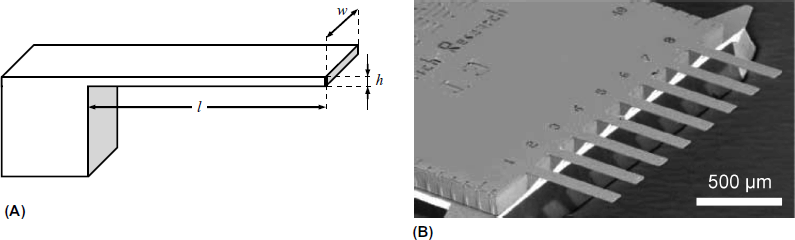
Microcantilevers are produced from metals or silicon materials (e.g., single-crystal silicon, polysilicon or silicon nitride) by micromachining, a technology based on the formation of sequences of thin films by deposition, patterning or etching (Section 5.13.3). Certain polymer materials are also used in microcantilever fabrication, particularly the SU-8 epoxy-based polymer that was originally developed as a negative photoresist mask for the microelectronics industry [12]. Polymer cantilevers are fabricated by injection molding.
Chemical sensing with a microcantilever is achieved by letting the analyte bind to the upper surface. This event induces a surface stress that modifies the plate area. As the length is much greater than the width, the most prominent effect is a modification of the length of the interacting surface which produces a tensile stress. If this stress is not compensated at the other side of a thin beam, the whole structure will bend. Figure 22.2A demonstrates the cantilever bending due to an increase in the surface stress (compressive stress) which causes the cantilever to bend downwards. If analyte interaction with the surface reduces the surface stress, which produces a tensile stress, this results in upward bending of the microcantilever. As the cantilever remains immobile after attaining an equilibrium state, this operational mode is called the static deformation mode.
Figure 22.2 Transduction principles in microcantilever sensors. The cantilever is seen from the side. (A) Static deformation mode. The surface stress induces a slight elongation of the upper surface that results in downward beam bending. (B) Dynamic mode (resonator cantilever); (C) Doubly clamped beam resonator (bridge configuration).
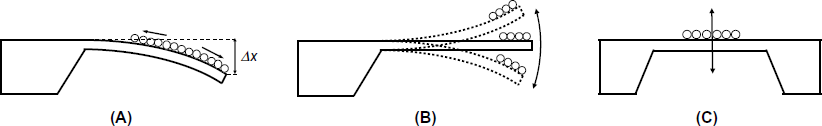
Alternatively, the microcantilever sensor can be operated in the dynamic mode by letting it vibrate at the resonance frequency. An increase in mass results in a change in the resonance frequency, as in the case of the quartz crystal microbalance. By this means, the cantilever resonator functions as an extremely sensitive mass transducer with mass resolution in the picogram region. A resonator microcantilever can be fabricated in the single-clamped (Figure 22.2B) or doubly clamped (bridge) format (Figure 22.2C). Compared with the quartz crystal microbalance, beam resonators may be several orders of magnitude more sensitive. In the bridge format, the beam resonator displays mass resolution well below the picogram level.
There are various techniques for measuring the cantilever deflection, the most common being the optical and the piezoresistive methods. Optical transduction is based on deflection of a light beam at the cantilever surface. The deflected beam is detected by a photoelectric position detector. In the piezoresistive method beam deflection is indicated by the change in the resistance of an incorporated resistor.
In order to promote selective binding of the analyte, the cantilever surface is coated with a recognition receptor film. If the cantilever is to be operated in the static deformation mode, the receptor layer should be formed only on one side, typically on the upper surface. The other surface should be coated with a passive film to prevent any interaction with the analyte. This precaution is necessary because analyte interaction with both surfaces leads to an intricate response. Microcantilever sensors operated in the dynamic mode can be coated on both sides, but if only one side is coated, it is possible to measure both the deflection and the resonance frequency.
Bimaterial cantilevers, obtained by coating a nonmetallic cantilever with a metal film, are utilized as temperature transducers. The difference in the expansion coefficients of the two layers causes the cantilever to bend as a function of the ambient temperature. In this way, the bimaterial cantilever is employed as a microcalorimeter in order to monitor the temperature changes caused by chemical reactions or phase-transition processes occurring at the cantilever surface. Temperature variations down to 10−5 K can be detected in this way.
Microcantilevers can be affected in a spurious way by the thermal drift or by interactions with the environment, particularly when operated in a liquid. In addition, nonspecific binding of sample concomitants or physical adsorption of the analyte may also contribute to the total response. That is why it is essential to use a reference sensor integrated with the working sensor in the same array. The corrected response, which is obtained as the difference between the sensor and reference signals, is much more reliable than the uncorrected response. That is why microcantilever sensors are typically produced and operated in the array format. Operation in an array configuration brings about an additional advantage, namely the possibility of multiplexing or parallelization of the assay.
22.1.2 Static Deformation Transduction
As mentioned above, the static deformation mode relies on beam bending as a result of analyte interaction with the sensor layer. The energy needed to bend the cantilever originates from the change in the Gibbs free energy that accompanies the interaction of the analyte with the upper surface. The energy contribution arises from the analyte binding energy, repulsive or attractive forces between bound molecules, and surface stress induced by direct interaction of an adsorbate with the cantilever surface. Entropy variation in the response to the recognition event can also contribute to the change in the Gibbs surface free energy.
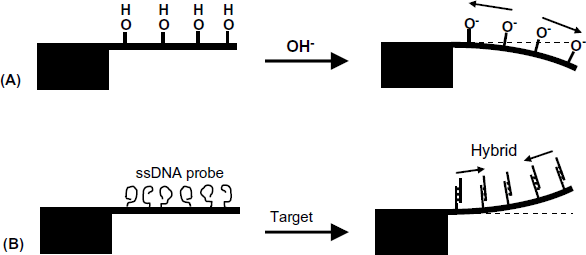
Figure 22.3 demonstrates two types of molecular interactions causing cantilever binding. If the surface interaction generates ionic groups, electrostatic repulsion produces compressive stress and the cantilever bends down (Figure 22.3A). Tensile stress and thereby up bending might be noticed with DNA hybridization sensors (Figure 22.3B). In this case, the single-strand DNA probe assumes a random coiled structure upon immobilization. After hybridization with the target, the stiff double-strand hybrid assumes a near-perpendicular orientation to the surface that relaxes the repulsive interactions and induces tensile deformation.
Figure 22.3 Cantilever deflection in response to surface interactions. (A) Ionization of a surface acidic group; (B) DNA hybridization. Reprinted with permission from [4]. Copyright 2008 The Royal Society of Chemistry.
As already mentioned, the sensor response in the static deformation mode is the cantilever deflection (![]() , Figure 22.2A), which is caused by the difference in surface stress (
, Figure 22.2A), which is caused by the difference in surface stress (![]() ) between the responsive and the passive surfaces of the cantilever. The bending radius of the cantilever (r) depends on the surface stress difference according to Stoney's equation:
) between the responsive and the passive surfaces of the cantilever. The bending radius of the cantilever (r) depends on the surface stress difference according to Stoney's equation:
(22.1) ![]()
where E is the Young modulus (which describes tensile elasticity), h is the thickness of the cantilever and ![]() is the Poisson ratio (which equals the ratio of lateral strain to axial strain). Assuming that
is the Poisson ratio (which equals the ratio of lateral strain to axial strain). Assuming that ![]() (where l is the length of the cantilever), Stoney's equation leads to the following equation that gives the cantilever deflection as a function of the surface stress difference:
(where l is the length of the cantilever), Stoney's equation leads to the following equation that gives the cantilever deflection as a function of the surface stress difference:
Equation (22.2) demonstrates that the sensitivity in terms of ![]() can be enhanced by increasing the length and decreasing the thickness of the cantilever. Sensitivity depends also on the cantilever material through the E and
can be enhanced by increasing the length and decreasing the thickness of the cantilever. Sensitivity depends also on the cantilever material through the E and ![]() constants. The Young's modulus is an indicator of the elasticity of the material and a lower value of the Young modulus brings about a higher sensitivity. For example, the polymer SU-8 has a Young modulus of about 40 times lower than that of silicon and hence can provide a greater sensitivity.
constants. The Young's modulus is an indicator of the elasticity of the material and a lower value of the Young modulus brings about a higher sensitivity. For example, the polymer SU-8 has a Young modulus of about 40 times lower than that of silicon and hence can provide a greater sensitivity.
22.1.3 Resonance-Mode Transduction
A microcantilever that vibrates at its resonance frequency can act as an extremely sensitive mass transducer. Actuation of a microcantilever resonator can be achieved by means of a piezoelectric resonator integrated with the microcantilever. Typically, PZT (Pb (Zr0.52Ti0.48)O3) or aluminum nitride are employed as piezoelectric materials for actuation. Thermal actuation is possible with bimetal-type cantilevers by periodic supply of heat by means of an external laser or an integrated resistive heater. Electrostatic or magnetomotive actuation methods have also been developed.
A cantilever behaves as a single-dimensional harmonic oscillator with the fundamental resonance frequency (![]() ) depending on the mass of the beam (m) and on the spring constant of the beam material (k):
) depending on the mass of the beam (m) and on the spring constant of the beam material (k):
where n is a geometrical parameter for the fundamental vibration mode of a rectangular cantilever beam. The product ![]() represents the effective mass of the vibrating system. The elastic properties of the beam material are indicated by the spring constant, which is the quotient of strain and stress under a longitudinally applied stress and depends on the Young's modulus and the geometrical parameters as:
represents the effective mass of the vibrating system. The elastic properties of the beam material are indicated by the spring constant, which is the quotient of strain and stress under a longitudinally applied stress and depends on the Young's modulus and the geometrical parameters as:
Using the above two equations, the resonance frequency of a rectangular silicon microcantilever of 500 × 100 × 1 μm was found to be of 6 kHz [4]. Upon combining Equations (22.3) and (22.4), it can be found that the resonance frequency increases with decrease in the length and increase of the thickness of the cantilever.
The resonance frequency decreases if an additional mass is loaded over the cantilever resonator. If the mass load is uniformly distributed over the entire beam surface, the mass change can be found by means of the following equation in which fm is the frequency of the loaded beam:
(22.5) ![]()
At very small changes in frequency the above equation can be converted into the following approximate form that indicates the mass sensitivity of the cantilever resonator:
According to this equation, mass sensitivity increases with increasing fundamental resonance frequency, which is obtained by having a large Young's modulus, low density and small dimensions. Greater sensitivity is obtained by operating the resonator in a higher vibration mode (overtone).
The above treatment assumes that the spring constant is not affected by the analyte interaction with the cantilever surface. Actually, this constant can vary as a result of fusion of materials (change in the Young's modulus) and change in cantilever thickness. Such complications are avoided if the sensing layer is localized at the terminal end of the beam, as shown in Figure 22.2B. In this case, the changes in resonance frequency can be mostly attributed to mass loading.
Mass sensitivity depends to a large extent on the quality factor of the resonator (Q), which is defined in the following equation:
where ![]() is the stored vibrational energy and
is the stored vibrational energy and ![]() is the energy dissipated per cycle. The minimum detectable frequency shift of a cantilever is proportional to
is the energy dissipated per cycle. The minimum detectable frequency shift of a cantilever is proportional to ![]() . Hence, the minimum detectable frequency, as well as mass sensitivity, increases with increasing quality factor of the cantilever. For proper operation, the quality factor should be at least 100.
. Hence, the minimum detectable frequency, as well as mass sensitivity, increases with increasing quality factor of the cantilever. For proper operation, the quality factor should be at least 100.
As indicated in Equation (22.7) a good quality factor is obtained when energy dissipation is very low. Energy dissipation is produced by intrinsic factors associated with the resonator (internal dissipation) and by energy loss to the ambient medium (external damping). External damping increases with the viscosity and density of the surrounding medium, this effect being much more pronounced in liquids than in gases.
The quality factor can be increased by active feedback, a process in which energy is supplied to the resonator in order to compensate for dissipation. This principle is similar to that employed in the quartz crystal microbalance.
Compared with the quartz crystal microbalance, which typically displays mass resolution in the nanogram (10−9 g) region, the mass resolution of a resonator microcantilever is in the picogram (10−12 g) region, that is, about 100–1000 times better.
Much better mass sensitivity is obtained with doubly clamped slab resonators (Figure 22.2C), as the resonance frequency in the bridge format is about 6 times greater than that of a singly clamped beam of the same dimension. At the same time, the bridge-type resonator shows a higher quality factor. Very high quality factors have been reported for string-like (stretched) doubly clamped beams that allow for mass resolution below the picogram level.
Very high mass sensitivity has been achieved by reducing the size of doubly clamped beams to the nanometer region to produce nanoelectromechanical systems [14, 15]. Nanometer-sized resonators have an exceptionally small mass and thereby a very high resonance frequency, which could be 100 MHz. At the same time, the quality factor of a nanoresonator can attain tremendously great values (up to 105). The combined effect of very low mass and a high quality factor leads to mass resolution in the attogram region (10−18 g) which makes possible the detection of individual macromolecules.
22.2 Measurement of Cantilever Deflection
22.2.1 Optical Measurement of Cantilever Deflection
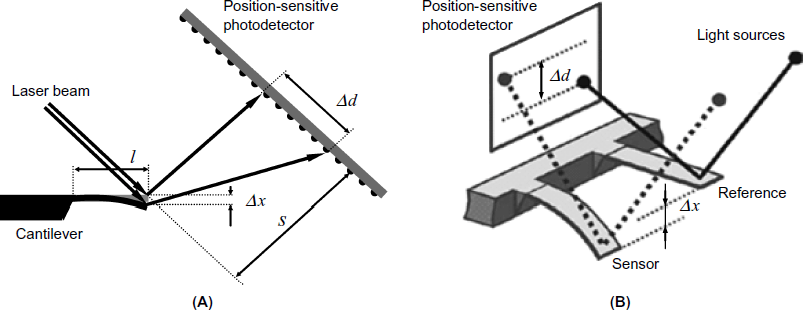
In the optical lever technique, a laser beam is impinged onto the cantilever end and the reflected beam is allowed to fall on an array of light microdetectors that act as a position-sensitive detector (Figure 22.4A). Cantilever bending causes the reflected light spot to shift on the detector screen. Using Stoney's equation, the deflection at the end of the cantilever is obtained as:
Figure 22.4 Optical measurement of beam deflection. (A) Principle of the optical lever technique. A laser beam is reflected by the cantilever and impinges onto the screen of the position-sensitive detector. Beam deflection produces a measurable ![]() shift of the light spot. (B) Measurement of the sensor beam deflection with respect to a reference beam [4]. (B) Reprinted with permission from [4]. Copyright 2008 The Royal Society of Chemistry.
shift of the light spot. (B) Measurement of the sensor beam deflection with respect to a reference beam [4]. (B) Reprinted with permission from [4]. Copyright 2008 The Royal Society of Chemistry.
(22.8) ![]()
where l is the length of the cantilever, s is the distance between the cantilever and the screen of the position-sensitive detector, and ![]() is the spot displacement. Figure 22.4B demonstrates the measurement of a sensor cantilever deflection with respect to a reference cantilever.
is the spot displacement. Figure 22.4B demonstrates the measurement of a sensor cantilever deflection with respect to a reference cantilever.
The optical lever technique allows a vertical deflection of 0.1 nm to be measured routinely. The mean disadvantage of this read-out technique is that it requires frequent optical alignment and calibration. In addition, the optical read-out is susceptible to errors due to light absorption or scattering in the sensor environment. When applied to cantilever arrays, an individual light source should be available for each cantilever. In a simpler configuration, a single laser beam is scanned sequentially across all the beams in the array. Another possibility in array operation is to illuminate all the cantilevers with a single collimated beam. Individual reflected beams are projected onto the image plane of a charge-coupled device camera to assess the deflection of each cantilever.
The optical lever techniques allow for simultaneous measurement of beam deflection, resonance frequency and vibration amplitude.
Optical measurement of cantilever deflection can also be performed by means of light interference. In this technique, coherent light waves reflected by a reference and a measuring cantilever interfere when there is a difference in deflection amplitude. Interference is caused by the difference between the path lengths of each wave (see Section 18.6.2). The deflection difference is indicated by the change in the intensity of the selected diffracted order. This technique is extremely sensitive as it is capable of detecting deflections as small as 0.01 Å, but the technique has a very narrow dynamic range.
A more compact optical read-out system has been developed by forming a SiO2 grating over a silicon cantilever [16]. By means of the grating, light is coupled in the cantilever, which acts as a waveguide. A second waveguide, which is placed in front of the cantilever, collects guided light. Any displacement of the cantilever disturbs the alignment of the above waveguides and thereby modifies the intensity of the collected light. This system needs no preliminary adjustment except for a relatively rough alignment for light coupling.
22.2.2 Electrical Measurement of Cantilever Deflection
The second most common transduction technique in microcantilever sensors is based on the piezoresistive effect that represents the change in the electrical resistance of a conductor under the effect of mechanical strain. As the electrical resistance is proportional to the resistor length, any change in length results in a modification of the resistance. The strain sensitivity of a piezoresistive transducer is indicated by the gauge factor (GF) defined as:
(22.9) ![]()
where ![]() is the change in resistance, R is the initial resistance,
is the change in resistance, R is the initial resistance, ![]() is the change in the length and l is the initial length.
is the change in the length and l is the initial length.
Piezoresistive gauges can be made of metal or semiconductor materials and can be included in the cantilever or attached to its surface. A semiconductor strain gauge is smaller, less expensive, and more sensitive than a metal film gauge. On the other hand, semiconductor gauges are sensitive to temperature variations and are susceptible to drift.
The configuration of a piezoresistive microcantilever transducer is shown in Figure 22.5A. It is formed of two cantilevers, one of them acting as reference. On each cantilever, a gold resistor is formed by vapor deposition in the form of a meander track, as shown in the close-up view in Figure 22.5B. Two resistors are formed in the same way on the chip and connections are provided so as to form a Wheatstone bridge along with cantilever resistors. The sensing process induces unequal variations in beam resistance and thereby an inbalance of the Wheatstone bridge. The output voltage of the bridge indicates the deflection difference.
Figure 22.5 Principle of piezoresistive measurement of cantilever deflection using metal-film piezoresistors. (A) Device configuration. Cantilevers are produced from SU-8 photoresist polymer. Two resistors on the chip along with the resistors on each cantilever form a Wheatstone bridge. (B) A close-up view of the cantilever with integrated meander-type gold resistor. Reprinted with permission from [17]. Copyright 2002 IOP Publishing Ltd.
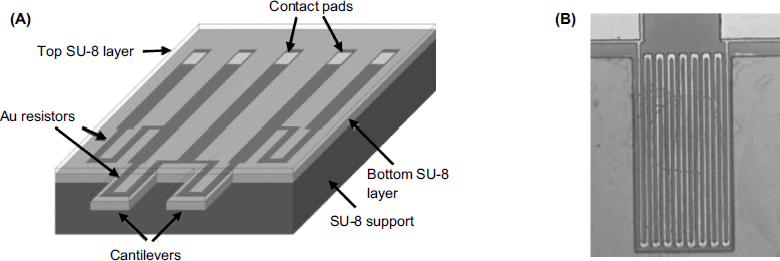
When operated in liquid samples, all resistors should be coated with an insulator layer in order to prevent short circuiting.
An analysis of the response of the piezoresistive microcantilever led to the conclusion that the sensitivity increases with a decrease in the total thickness of the device [18]. The width and the length of the device also affect the sensitivity, although to a lesser extent.
Compared with optical transduction, electrical transduction is advantageous in that optical components are not required and neither is laser alignment. The response is not affected by optical properties of the surrounding medium. In addition, the read-out electronics can be integrated on the same chip to form a self-contained sensing–measuring device.
One additional advantage results from the possibility of using the resistor on the cantilever as a heating element to control the temperature of the sensing layer. This characteristic can be employed for thermal breaking of the target–probe duplex in DNA sensors in order to perform sensor regeneration after a DNA assay. Heating can also be used to sustain polymerase chain reactions at the cantilever surface.
An electrostatic transduction method is based on capacitance measurements. In this method, a capacitor is formed of a metalized cantilever and a fixed electrode placed underneath. As the cantilever deflects, the distance between the two elements changes and thereby modifies the capacitance of the system. Sensitivity in capacitive transduction is enhanced by forming a square pad on the end of the cantilever beam because the capacitance is proportional to the surface area of the movable plate. Capacitive transduction has been employed in gas sensors, since in such cases there is no risk of the ambient fluid short circuiting the plates. This method is advantageous due to the simplicity of the associated electronics and its low power consumption.
22.3 Functionalization of Microcantilevers
In order to impart selectivity, recognition receptors are attached to the cantilever surface. Receptor immobilization can be conducted on silicon material surfaces upon activation to form hydroxyl groups, followed by silanization. Gold-coated cantilevers can be functionalized by forming first a thiocarboxylic acid self-assembled monolayer. In each case, the first layer acts as a linker to which recognition receptor proteins are grafted by covalent immobilization. Thiolated nucleotides can be attached directly to the gold film via thiol chemisorption. Electrochemical polymerization can also be utilized to form a polymer layer containing functional groups to which the receptor is further attached.
The inactive area of the microcantilever should be passivated by coating with a material that does not interact with the analyte or other solution components. Typically, the hydrophilic polymer polyethylene glycol is utilized for passivation against biomolecules.
The reproducibility in the fabrication of the sensing layer is poor at the cantilever edge; therefore, a greater width of the cantilever beam brings about an improvement in batch reproducibility.
As microcantilevers are commonly used in the array format, it is important to utilize techniques that allow the modifying reagents to be delivered sequentially or in parallel to each individual cantilever [19]. Drop deposition can be performed by inkjet printing or by microcontact printing. Such methods achieve modification of the upper side of the cantilever only. If both sides have to be modified, arrays of glass microcapillaries are utilized. Each cantilever is introduced in a microcapillary that guides the solution of the needed reagent.
22.4 Microcantilever Gas and Vapor Sensors
In order to detect gas or vapors, a microcantilever is coated with a thin layer of material that interacts more or less selectively with the analyte [20]. In general, gas-sensitive coatings are similar to those used in other types of gas sensor.
Metal coatings allow certain gases and vapors to be sensed. For example, a palladium coating is used in hydrogen sensing whilst a gold coating is used for trace mercury determination. Gold coating also imparts specificity to mercaptans that adsorb on gold via the thiol group.
Water vapors can be sensed by cantilevers coated with hydrophilic polymers such as polyvinyl alcohol, carboxymethyl cellulose or polyvinylpyrrolidone.
Detection of organic vapors is achieved by means of polymer coatings. Common polymers such as polystyrene, polyurethane, poly(methyl methacrylate) and their blends can be used as coatings in organic vapor sensors. Because the selectivity of such coatings is poor, they are used to fabricate arrays of cross-sensitive sensors for applications in artificial olfaction. As is typical in this technique, each cantilever in the array is coated with a different polymer material so that it responds with a particular sensitivity to each component of the gas sample.
Selectivity to particular classes of organic vapors is imparted by chromatographic stationary phase beads (about 0.1 μm diameter) incorporated in a gel at the cantilever surface. More advanced selectivity to particular analytes is obtained by means of supramolecular chemistry receptors such as cyclodextrins.
Owing to the exceptional sensitivity of microcantilever sensors, detection of the vapors of explosives in the search for explosives is a promising application in the fight against terrorism. Explosive vapors can be detected by adsorption at a silicon surface, or, more generally, by means of specific sensing coatings. TNT (2,4,6-trinitrotoluene) can be detected by deflagration on a heated cantilever, the deflagration producing a bump in the cantilever bending signal [21].
22.5 Microcantilever Affinity Sensors
22.5.1 General Aspects
Microcantilever devices are suitable platforms for developing label-free sensors based on various kinds of affinity reactions. Most of the reported applications in this area make use of antibodies as recognition receptors. Such applications encompass determination of proteins, haptene molecules, bacteria or viruses [10]. An immunosensor can be obtained by coating the cantilever with a selected antibody layer (Figure 22.6) that functions as a selective receptor for the analyte. Analyte binding induces compressive stress that leads to beam bending in proportion with the analyte concentration.
Figure 22.6 Functioning principle of a microcantilever affinity sensor. (A) Before analyte binding; (B) after analyte binding. Reproduced with permission from [22]. Copyright 2006 Elsevier.
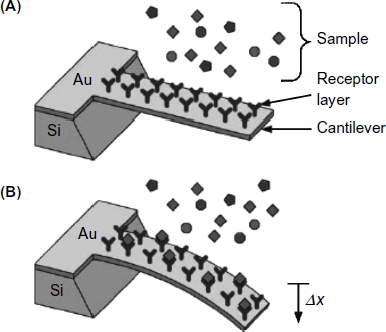
The response of the microcantilever immunosensor can be amplified if a second antibody is attached to the initial immunocomplex to form a sandwich ternary complex [23]. When operating the sensor in the static deformation mode, formation of the ternary complex produces additional surface stress that enhances the beam deflection. With a sensor operated as a resonator, the frequency shift is enhanced if the second antibody is conjugated with silicon nanoparticles that increase the overall mass of the cantilever.
Exceptional sensitivity has been reported for microchannel resonators operating in high vacuum [24, 25]. In this approach, the microcantilever includes a microchannel through which the sample is allowed to flow in order to perform recognition by a receptor layer at the microchannel surface. Vacuum operation brings about a very high quality factor, which, in combination with the very low mass of the resonator, allows a single biomolecule or a single cell to be detected.
Microcantilever immunosensors have been developed for various types of target analytes such as proteins, viruses and bacteria. An overview of the underlying design principles is given in ref. [18].
22.5.2 Microcantilever Protein Sensors
An example of a protein assay by a microcantilever sensor is the determination of creatine kinase and myoglobin in blood [27]. These proteins are biomarkers that help diagnose or rule out a heart attack. Microcantilever sensors for each of the above proteins have been obtained by using the corresponding antibody layer over a microcantilever. A very sensitive resonator microcantilever myoglobin sensor has been produced using antibody immobilization by biotin–streptavidin interaction [28]. If the antibody orientation at the surface is random, a fraction of the present antibodies is not accessible to the analyte. In order to maximize the availability of the antibody to analyte binding, antibody biotinylation has been conducted so as to let all binding sites be exposed to the sample analyte. This immobilization method results in a 10-fold greater sensitivity compared with a randomly oriented antibody layer.
Another biomarker of interest in clinical chemistry is the prostate-specific antigen (PSA), a glycoprotein that is used for the detection of prostate cancer. Using an anti-PSA antibody, a cantilever operated in the static deformation mode allows free-PSA to be detected at concentration from 0.2 ng mL−1 to 60 μg mL−1, which includes the clinical relevant PSA concentration range.
Among chemical warfare agents there are certain protein toxins that could be detected by microcantilever immunosensors. An example of this kind is ricin, a potent cytotoxin that prevents living cells from producing essential proteins. A microcantilever sensor including a ricin antibody has allowed a limit of detection of 60 ppt of ricin to be attained [28].
22.5.3 Microcantilever Pathogen Sensors
Detection of pathogenic organisms such as viruses and bacteria has also been approached by microcantilever immunosensors. Pathogen recognition is achieved by means of a corresponding antibody grafted to the microcantilever surface. Identification of bacteria spores is of great importance to the food and beverage industry as well as in the assessment of drinking water quality. Accordingly, sensors for spores of Bacillus anthracis, which is the pathogen of the acute Anthrax disease, have been developed. A microcantilever sensor for the spores of the potential biological warfare agent Francisella tularensis (which causes tularemia disease) has been developed using a commercial antibody [28]. The reported limit of detection of Francisella tularensis is 1000 organisms per ml when the sensor signal is corrected by the signal of a reference cantilever.
Detection of viruses with antibody-modified microcantilevers has also been demonstrated.
22.5.4 Microcantilever Affinity Sensors Based on Other Recognition Receptors
Besides immunosensors, microcantilever sensors based on other molecular receptors have also been developed. For example, a short peptide with a selected amino acid sequence can act as the receptor for the identification of Bacillus subtilis spores [29]. Detection of fungal spores has been demonstrated with Aspergillus niger that is a spoilage agent and a pathogen to humans. The Concanavalin A protein, which recognizes saccharides in the cell wall, has been used as a recognition receptor [30].
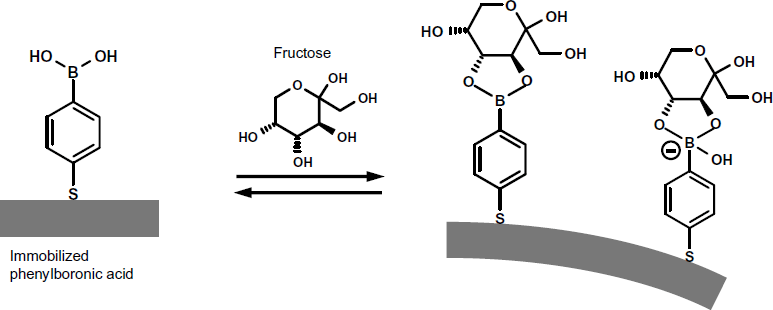
Phenylboronic acid is a suitable receptor for saccharides. In order to perform fructose determination, a thiolated phenylboronic acid derivative was immobilized over a gold-coated microcantilever [31]. Phenylboronic acid interacts with two vicinal hydroxyl groups in fructose to form a boronate compound (Figure 22.7). Depending on the pH, this boronate compound can be present as a neutral or negatively charged species. Thereby, beam deflection has been ascribed to the electrostatic repulsion of charged molecules in the surface molecular layer. As the molar fraction of the charged form increases with the pH, the response signal also increases with this parameter. When operated in the static deformation mode at pH 7, the surface stress was linear in analyte concentration over the 0–25 mM range, allowing fructose to be readily detected at physiologically meaningful concentrations.
Figure 22.7 Fructose determination by means of a phenylboronic acid coated cantilever. Phenylboronic acid is immobilized by thiol chemisorption on a gold coating. Adapted with permission from [31]. Copyright 2008 American Chemical Society.
22.6 Enzyme Assay by Microcantilever Sensors
Enzyme assays aim at measuring enzymatic activity in biological specimens. In principle, an enzyme activity cantilever sensor is obtained upon immobilization of an enzyme substrate on the microcantilever [32]. Substrate conversion under the catalytic effect of the enzyme leads to changes in mass, conformation, and charge that modify the cantilever deflection and allow the enzyme activity to be determined. This approach is also suitable for inhibitor determination, with the enzyme–inhibitor interaction proceeding in the solution phase.
Several methods for signal amplification in enzyme activity sensors have been advanced. In one approach, the immobilized substrate is conjugated with microbeads at its upper end [33]. Under the effect of the target enzyme, the substrate molecule is split, leading to the removal of the bead and, thereby, to a considerable loss of resonator mass.
Signal amplification in static deformation enzyme sensors has been achieved by using an immobilized substrate conjugated with magnetic nanoparticles [34]. Microcantilever deflection is produced in this state by an external magnetic field. The target enzyme breaks down the substrate and the nanoparticle-tagged fragment is disconnected from the cantilever. As a result, cantilever deflection decreases and the deflection variation is in relation to the enzyme activity in the sample.
22.7 Microcantilever Nucleic Acid Sensors
Application of microcantilevers as transducers in DNA hybridization sensors has been demonstrated in ref. [35] using cantilever arrays operated in the static deformation mode. Single-base mismatch detection was possible in this way. Cantilever deflection upon hybridization depends on a series of factors such as surface coverage with the capture probe, degree of disorder at the probe-modified surface, degree of hydration and ionic strength. Depending on the experimental conditions, either compressive or tensile stress can be produced by hybridization.
Detection of nucleic acid hybridization requires reference cantilevers functionalized with an oligonucleotide that is not complementary to the target DNA.
Appreciable signal amplification in microcantilever DNA sensors has been obtained by incorporation of various kinds of nanoparticles.
Magnetic nanoparticles incorporated in the sensing layer upon recognition have been utilized to amplify the microcantilever response in the static deformation mode [36]. The cantilever deflection is enhanced by an external magnetic field that allows determination of DNA with an outstanding limit of detection.
As demonstrated in ref. [37], signal amplification in resonator-type DNA sensors can be obtained by means of metal nanoparticles incorporated into the sensing layer. In this method, the target polynucleotide is hybridized with a shorter capture probe. Next, a complementary probe tagged with gold nanoparticles is hybridized with the overhanging tail of the target. Nanoparticles contribute substantially to the mass increase upon recognition. Additional response enhancement is obtained by chemical deposition of silver onto gold nanoparticles. This is a transposition of a similar amplification protocol originally developed with the quartz crystal microbalance. The above microcantilever sensor produced a linear response in terms of frequency shift vs. logarithm of target concentration over a concentration rage extending from 0.05 to 10 mM.
22.8 Outlook
Microcantilever sensors represent a viable approach to the development of label-free sensors for various kinds of species including gases, small molecules, biomacromolecules and pathogens. From the standpoint of the application field, microcantilever sensors are in competition with well-established sensing methods such as the quartz crystal microbalance, surface acoustic wave and surface plasmon resonance devices. In order to achieve performances comparable with the above techniques, resonant microcantilevers should be produced so as to display a very high resonance frequency, preferably over 1 MHz. Alternatively, high sensitivity can be obtained by operating conventional beam resonators at higher overtones.
Nanoparticle application has opened up a new way to sensitivity enhancement for both static and dynamic mode microcantilever sensors. However, nanoparticles act as a kind of label and the inclusion of the nanoparticle into the sensing layer complicates the assay protocol.
Further progress in this field is expected to be resulting in increased sensitivity and reduced response time. Incorporation of microcantilever arrays into microfluidic cartridges would be one way of shortening the response time and increasing throughput.
1. Judy, J.W. (2001) Microelectromechanical systems (MEMS): fabrication, design and applications. Smart Mater. Struct., 10, 1115–1134.
2. Raiteri, R., Grattarola, M., Butt, H.J. et al. (2001) Micromechanical cantilever-based biosensors. Sens. Actuators B-Chem., 79, 115–126.
3. Sepaniak, M., Datskos, P., Lavrik, N. et al. (2002) Microcantilever transducers: A new approach to sensor technology. Anal. Chem., 74, 568A–575A.
4. Fritz, J. (2008) Cantilever biosensors. Analyst., 133, 855–863.
5. Ziegler, C. (2004) Cantilever-based biosensors. Anal. Bioanal. Chem., 379, 946–959.
6. Lang, H.P. and Gerber, C. (2008) Microcantilever sensors. Top. Curr. Chem., 285, 1–27.
7. Boisen, A., Dohn, S., Keller, S.S. et al. (2011) Cantilever-like micromechanical sensors. Rep. Prog. Phys., 74, Article Nr. 036101.
8. Alvarez, M. and Lechuga, L.M. (2010) Microcantilever-based platforms as biosensing tools. Analyst., 135, 827–836.
9. Goeders, K.M., Colton, J.S., and Bottomley, L.A. (2008) Microcantilevers: Sensing chemical interactions via mechanical motion. Chem. Rev., 108, 522–542.
10. Buchapudi, K.R., Huang, X., Yang, X. et al. (2011) Microcantilever biosensors for chemicals and bioorganisms. Analyst., 136, 1539–1556.
11. Wang, C.Y., Wang, D.Y., Mao, Y.D. et al. (2007) Ultrasensitive biochemical sensors based on microcantilevers of atomic force microscope. Anal. Biochem., 363, 1–11.
12. Nordstrom, M., Keller, S., Lillemose, M. et al. (2008) SU-8 cantilevers for bio/chemical sensing; Fabrication, characterisation and development of novel read-out methods. Sensors, 8, 1595–1612.
13. Battiston, F.M., Ramseyer, J.P., Lang, H.P. et al. (2001) A chemical sensor based on a microfabricated cantilever array with simultaneous resonance-frequency and bending readout. Sens. Actuators B-Chem., 77, 122–131.
14. Ekinci, K.L. and Roukes, M.L. (2005) Nanoelectromechanical systems. Rev. Sci. Instrum., 76, Article Nr. 061101.
15. Eom, K., Park, H.S., Yoon, D.S. et al. (2011) Nanomechanical resonators and their applications in biological/chemical detection: Nanomechanics principles. Phys. Rep.-Rev. Sec. Phys. Lett., 503, 115–163.
16. Zinoviev, K., Dominguez, C., Plaza, J.A. et al. (2006) A novel optical waveguide microcantilever sensor for the detection of nanomechanical forces. J. Lightwave Technol., 24, 2132–2138.
17. Thaysen, J., Yalcinkaya, A.D., Vettiger, P. et al. (2002) Polymer-based stress sensor with integrated readout. J. Phys. D-Appl. Phys., 35, 2698–2703.
18. Joshi, M., Gandhi, P.S., Lal, R. et al. (2011) Modeling, simulation, and design guidelines for piezoresistive affinity cantilevers. J. Microelectromech. S., 20, 774–784.
19. Bietsch, A., Zhang, J.Y., Hegner, M. et al. (2004) Rapid functionalization of cantilever array sensors by inkjet printing. Nanotechnology., 15, 873–880.
20. Lang, H.P. (2009) Cantilever-based gas sensing, in Solid State Gas Sensing (eds E. Comini, G. Faglia, and G. Sberveglieri), Springer Science+Business Media, LLC, Boston, MA, pp. 305–328.
21. Pinnaduwage, L.A., Wig, A., Hedden, D.L. et al. (2004) Detection of trinitrotoluene via deflagration on a microcantilever. J. Appl. Phys., 95, 5871–5875.
22. Lim, S.H., Raorane, D., Satyanarayana, S. et al. (2006) Nano-chemo-mechanical sensor array platform for high-throughput chemical analysis. Sens. Actuators B-Chem., 119, 466–474.
23. Lee, S.M., Hwang, K.S., Yoon, H.J. et al. (2009) Sensitivity enhancement of a dynamic mode microcantilever by stress inducer and mass inducer to detect PSA at low picogram levels. Lab Chip., 9, 2683–2690.
24. Burg, T.P., Mirza, A.R., Milovic, N. et al. (2006) Vacuum-packaged suspended microchannel resonant mass sensor for biomolecular detection. J. Microelectromech. S., 15, 1466–1476.
25. Burg, T.P., Godin, M., Knudsen, S.M. et al. (2007) Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature., 446, 1066–1069.
26. Arntz, Y., Seelig, J.D., Lang, H.P. et al. (2003) Label-free protein assay based on a nanomechanical cantilever array. Nanotechnology, 14, 86–90.
27. Grogan, C., Raiteri, R., O'Connor, G.M. et al. (2002) Characterisation of an antibody coated microcantilever as a potential immuno-based biosensor. Biosens. Bioelectron., 17, 201–207.
28. Ji, H.F., Yan, X.D., Zhang, J. et al. (2004) Molecular recognition of biowarfare agents using micromechanical sensors. Expert Rev. Mol. Diagn., 4, 859–866.
29. Dhayal, B., Henne, W.A., Doorneweerd, D.D. et al. (2006) Detection of Bacillus subtilis spores using peptide-functionalized cantilever arrays. J. Am. Chem. Soc., 128, 3716–3721.
30. Nugaeva, N., Gfeller, K.Y., Backmann, N. et al. (2005) Micromechanical cantilever array sensors for selective fungal immobilization and fast growth detection. Biosens. Bioelectron., 21, 849–856.
31. Baker, G.A., Desikan, R., and Thundat, T. (2008) Label-free sugar detection using phenylboronic acid-functionalized piezoresistive microcantilevers. Anal. Chem., 80, 4860–4865.
32. Bottomley, L.A., Ghosh, M., Shen, S. et al. (2006) Microcantilever apparatus and methods for detection of enzymes, enzyme substrates, and enzyme effectors. U.S. Patent 7,141,385.
33. Liu, W., Montana, V., Chapman, E.R. et al. (2003) Botulinum toxin type B micromechanosensor. Proc. Natl. Acad. Sci. USA, 100, 13621–13625.
34. Weizmann, Y., Elnathan, R., Lioubashevski, O. et al. (2005) Magnetomechanical detection of the specific activities of endonucleases by cantilevers. Nano Lett., 5, 741–744.
35. Fritz, J., Baller, M.K., Lang, H.P. et al. (2000) Translating biomolecular recognition into nanomechanics. Science., 288, 316–318.
36. Weizmann, Y., Patolsky, F., Lioubashevski, O. et al. (2004) Magneto-mechanical detection of nucleic acids and telomerase activity in cancer cells. J. Am. Chem. Soc., 126, 1073–1080.
37. Su, M., Li, S.U., and Dravid, V.P. (2003) Microcantilever resonance-based DNA detection with nanoparticle probes. Appl. Phys. Lett., 82, 3562–3564.
