Chapter 23
Chemical Sensors Based on Microorganisms, Living Cells and Tissues
23.1 Living Material Biosensors: General Principles
Biocatalytic sensors were initially developed using enzymes that have been isolated from biological media or organisms and that catalyze the conversion of a substrate. Signal transduction in enzymatic sensors can be achieved by monitoring the concentration of a reactant or product that is involved in the enzymatic reaction.
Instead of using enzymes that have been isolated it is possible to use microorganisms, living cells or living tissues that contain analyte-specific enzymes. By metabolic processes, the analyte undergoes conversion to particular products, this process involving consumption of oxygen or another reactant. Monitoring of reactant consumption or product formation provides a means of signal transduction in metabolism-based biosensors.
Comprehensive overviews of living material-based biosensors are available in several recent books [1–3] and reviews [4–7]. Mathematical modeling of whole-cell biosensors is discussed in ref. [8].
Due to the similarity of the functioning principles, the design of metabolism-based sensors is essentially similar to that of enzymatic sensors. Thus, a layer containing the selected living material is integrated with a transducer device that indicates the concentration of a reactant or product involved in the metabolism of the sensing entity. In this way, the isolation of the relevant enzyme is avoided and the enzyme functions in its natural environment. The natural environment secures an optimal enzymatic activity and, in addition, provides necessary coenzymes and activators. Moreover, a living entity contains a great number of different enzymes that allows multienzyme sensing schemes to be developed.
Counteracting these advantages, there are several drawbacks that arise from using living materials in sensors. First, reactants and products have to cross the cell membrane and this slows down the diffusion process. Therefore, the response time is longer than that normally observed with sensors based on isolated enzymes. Secondly, the large number of enzymes present in a living entity is a source of interferences as various compounds in the sample may be converted to form various products to which the transducer is sensitive. Methods for improving the selectivity of living-material-based sensors are discussed in Section 23.6.
Various kinds of living material can be used in the design of biosensors. The most common are living cells of various origins. Unicellular organisms such as bacteria, algae [7] or yeast are widely used as sensing elements in microbial biosensors. Also useful are cells originating from multicellular organisms such as molds, lichens, or cells isolated from higher organisms such as plant or mammalian tissues [9]. Cells can be readily obtained by cell culture and can be conveniently immobilized at the transducer surface by various methods presented in Section 23.3.
Higher plant or animal material can be used in the form of thin slices integrated with the transducer device to form tissue biosensors.
In making biosensors, intact and viable biomaterial is normally used. However, because enzymatic activity is preserved for some time after biomaterial becomes nonviable, nonviable biological materials can also be used to construct biosensors.
The discussion above focused on the application of spontaneous metabolic processes that occur when the substrate and certain additional reactants are supplied to the sensing living entity. In such cases, it is the substrate that undergoes metabolic conversion.
There are, however, other sensing mechanisms in which the substrate is not metabolized, but where certain metabolic processes that result in detectable compounds are induced upon cell stimulation by the analyte.
23.2 Sensing Strategies in Living-Material-Based Sensors
23.2.1 Biocatalytic Sensors
Transduction in living material biosensors can be achieved by monitoring the concentration of the product of an enzymatic reaction, such as hydrogen ions, ammonia or carbon dioxide.
If the relevant enzyme in the sensing cell is an oxidase, transduction can be performed by monitoring the consumption of oxygen. Oxygen, which functions as an electron acceptor in the enzymatic reaction, can be replaced by an artificial electron acceptor such as hexacyanoferrate(III) or another redox mediator (see Section 14.2). In this case, transduction is performed by monitoring the reduced form of the electron acceptor.
Oxygen monitoring forms the basis of so-called respiratory sensors. Using microorganisms capable of metabolizing a broad variety of organic compounds, this approach allows water pollution with organic compounds to be assessed. In water chemistry, an indicator of the total content of organic material is called the biological oxygen demand (BOD) [10]. The biosensor approach is a convenient alternative to the standard laboratory method for determining the BOD. Certain vitamins can be assessed using their stimulating effect on the metabolism of the microorganism.
Respiratory sensors are also suitable for assessing the content of enzyme inhibitors such as toxic metal ions, antibiotics or organic pollutants. As inhibition is, as a rule, irreversible, the sensing membrane must be renewed after each assay taking care to preserve the density and metabolic status of the microbial cells.
Multistep sequences of enzymatic reactions can be implemented if a single-enzyme reaction does not provide a detectable compound. For example, a nitrate ion biosensor has been produced using Azotobacter vinelandii that initiates the reaction sequence (23.1)–(23.2), which results in ammonia, which can be detected by various methods:
Multistep reaction sensing can also be performed by associating an isolated enzyme and living cells to form what is called a mixed biosensor.
23.2.2 External-Stimuli-Based Biosensors
Some substances can be assessed by exploiting their effect on the permeability of cellular membranes. The cells used for such assays are usually modified by incorporation of an ion that is not naturally occurring and that acts as a marker. The analyte affects in some way the permeability of the cell membrane for this marker that is monitored after its release into the extracellular environment. Thus, the antibiotic, Nystatin which can act as an ionophore, modifies the permeability of cell membranes to alkali metal ions. Using Rb+ as marker, Nystatin promotes the release of this ion from the yeast cells and allows transduction to be performed by means of an Rb+ sensor.
An alternative approach relies on the breaking down of cells (lysis) under the effect of the analyte. This method has been applied to the assay of the lysozyme enzyme that produces the lysis of polysaccharide present in cell walls of bacteria. Under the effect of this enzyme, an atypical ion incorporated in the microorganism is released and its concentration indicates the lysozyme concentration in the sample.
Another methodology is based on the initiation of metabolism under the influence of the analyte as a stimulus for some physiological process. In this case, the analyte interacts with a specific cell receptor located at the plasma membrane. Formation of the analyte–receptor complex activates a metabolic process that gives rise to a detectable product.
23.3 Immobilization of Living Cells and Microorganisms
Immobilization of microorganisms and cells can be achieved by aggregation–flocculation, adsorption or adhesion, entrapment in polymeric networks or crosslinking with glutaraldehyde [5, 11]. As a rule, gentle techniques should be used in order to preserve the viability of cells. That is why covalent immobilization should be used with caution because of the possible damaging effects of chemical reagents. Covalent immobilization at gold surfaces can be achieved by means of primary self-assembled monolayers containing reactive end groups that allow for conjugation with functional groups present at the cell wall.
Passive immobilization of cells into the pores, or adhesion onto the surface, of cellulose or other materials allows direct contact of the cells with the liquid sample. Therefore, no additional diffusion barrier is formed by immobilization, in contrast to gel entrapment. Adhesion is secured by coating the cells, the support or both with polyethyleneimine. Polyethyleneimine is a cationic polymer that imparts adhesion by electrostatic interaction with the negatively charged outer surface of cells.
Microorganism immobilization can be effected by affinity interaction with lectins (which are sugar-binding proteins). To this end, a lectin layer is first attached to the support in order to form a platform onto which microorganisms are further immobilized by interactions with saccharides present in the cell-wall membrane. As the immobilization process is reversible, a sensor formed in this way can be regenerated by eluting the used microorganism layer in order to load the transducer surface with a fresh batch of the microorganism.
Entrapment in poly(vinyl alcohol) or ionotropic gels (such as alginate) is a suitable method for immobilization of viable cells. A suspension of cells in a sodium alginate solution is added drop wise to a calcium chloride solution. As the alginate macromolecule is a polyanion, the divalent calcium ions promote gelation by forming ionic bridges between the macromolecules. In this way, gel particles several millimeters in size are obtained. The alginate gel is not stable in the presence of Ca2+-binding compounds and certain cations that can substitute Ca2+ in the gel network. Gel stabilization can be achieved by crosslinking.
Other common entrapment matrices used in whole-cell biosensors are silica or organically modified silica (Ormosil) prepared by the sol-gel method [12]. In order to preserve the viability of the microorganism, inorganic precursors are preferred over organic ones that release toxic alcohols as byproducts. A typical entrapment protocol starts with mixing a cell suspension with glycerol. To this mixture is then added hydrochloric acid and sodium silicate solution in order to promote the sol-gel process [13]. In this preparation the incorporation of glycerol leads to the formation of a well-defined cavity around the cell that prevents direct contact between cell and matrix. As a result, cell membrane permeability is not affected. In addition, the cell is not subject to mechanical stress that could impair cell viability.
Whole-cell biosensor arrays are produced for parallel assays of multiple samples by forming individually addressable cell spots of micrometer size. This process, which is commonly known as cell patterning can be conducted in different ways [14]. In such techniques, cells are immobilized in the form of monolayers on solid supports by adhesion. Adhesion is a natural process in which cells bind to an adhesive extracellular matrix. Binding is achieved by the attraction of specific peptide regions in an adhesive extracellular matrix protein (integrin) that forms transmembrane receptors on the plasma membrane. Following this coupling, supramolecular complexes form to provide structural links between the cytoskeleton and the extracellular membrane [15]. In order to promote cell adhesion to inorganic surfaces, a protein layer should be formed by standard protein immobilization methods. This layer then acts as an adhesive extracellular matrix.
Cell patterning can be achieved by elastomer stenciling. In this technique, a micromachined thin sheet (stencil) containing patterned holes is applied over the support. This mask acts as a template during the cell-seeding process.
Microcontact printing (soft lithography) is used to create a pattern of protein spots to which the cells are then allowed to adhere.
Ink-jet patterning is a patterning method that has the advantage of allowing different cell strains to be patterned on the same substrate surface.
An elegant cell-patterning method is based on photolithography coupled with surface-specific chemical reactions [16]. Thus, a gold-electrode array is first formed by photolithography over a silicon dioxide film. This is followed by self-assembly of a long-chain thiocarboxylic acid monolayer over the gold surface. Protein or peptides are finally conjugated by covalent binding through the carboxyl groups in order to form an adhesive extracellular matrix for cell adhesion. In order to prevent cell adhesion to the exposed silicon dioxide insulator film, the silicon dioxide is coated with a protecting layer by silanisation.
23.4 Electrochemical Microbial Biosensors
Electrochemical transduction in whole-cell biosensor exploits the formation or consumption of electrochemically detectable species [17]. Amperometric methods are used for monitoring oxygen consumption in respiratory sensors as well as in sensors based on the inhibition of photosynthetic activity. Amperometry can also be used in conjunction with whole-cell biosensors including an enzyme that converts an artificial substrate into an electrochemically active compound. Ion formation by cell enzymes can be monitored in a selective way by potentiometric ion sensors or in a non-selective way by means of conductance measurements.
23.4.1 Amperometric Microbial Biosensors
The structure of an amperometric microbial biosensor is similar to that of its enzymatic counterpart (Section 14.1). Accordingly, a whole-cell layer is formed at the surface of an amperometric probe that functions as the transducer.
The most extensively studied sensor of this kind is the biological oxygen demand (BOD) sensor. Biochemical oxygen demand represents the amount of dissolved oxygen needed by aerobic biological organisms to break down organic material present in a given water sample at a given temperature over a specific time period. BOD is an indicator of the amount of organic material in the water. The BOD value is most commonly expressed in milligrams of oxygen consumed per liter of sample during 5 days of incubation at 20 °C and is a standard method for the assay of the organic pollution of water.
The above-mentioned BOD determination method (denoted as BOD5) takes five days. The assay duration can be reduced considerably by using BOD amperometric sensors, which are obtained by assembling an amperometric oxygen probe with a film of microorganisms that are able to metabolize organic compounds in water with oxygen consumption (Figure 23.1A). In the absence of organic compounds, dissolved oxygen diffuses through the microorganism layer and is partially consumed in microorganism respiration. The oxygen probe generates a constant response current (![]() ), as shown on the initial portion of the curve in Figure 23.1B. When a sample is added, organic compounds are metabolized by the microorganism and the oxygen consumption increases, leading to a gradual decrease in the response current. A constant, steady-state current (
), as shown on the initial portion of the curve in Figure 23.1B. When a sample is added, organic compounds are metabolized by the microorganism and the oxygen consumption increases, leading to a gradual decrease in the response current. A constant, steady-state current (![]() ) is obtained after about 10 min. The content of organic compound is indicated by the current difference
) is obtained after about 10 min. The content of organic compound is indicated by the current difference ![]() . This difference increases proportionally with the BOD over a certain range (e.g., 5–100 mg O2/L [19]) but levels off at higher BOD values where the limit of the metabolism rate is attained. Clearly, in the BOD assay the water sample should be saturated with air.
. This difference increases proportionally with the BOD over a certain range (e.g., 5–100 mg O2/L [19]) but levels off at higher BOD values where the limit of the metabolism rate is attained. Clearly, in the BOD assay the water sample should be saturated with air.
Figure 23.1 The amperometric BOD sensor. (A) Sensor configuration. (1) Ag/AgCl reference electrode; (2) electrolyte; (3) Pt cathode; (4) dialysis membrane; (5) immobilized whole cell layer; (6) oxygen-permeable membrane. (B) Response curve of an amperometric BOD sensor. Adapted with permission from [18]. Copyright 2002 Elsevier.
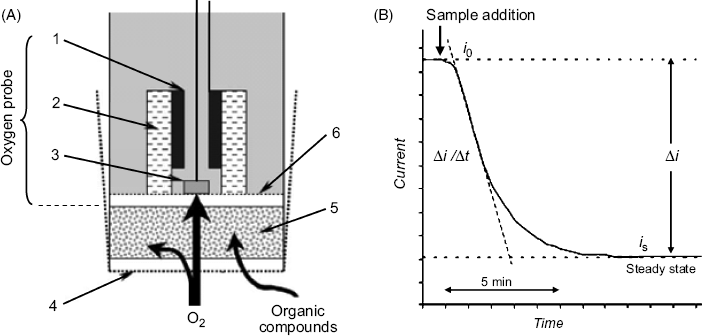
An alternative measurement method utilizes the rate of signal variation with time (![]() ) that is indicated by the slope of the quasilinear portion of the current–time curve, as shown in Figure 23.1B. Compared with the steady-state measurement, the rate-base method provides the response after a shorter time lag.
) that is indicated by the slope of the quasilinear portion of the current–time curve, as shown in Figure 23.1B. Compared with the steady-state measurement, the rate-base method provides the response after a shorter time lag.
In order to be suitable for application in a BOD sensor, the microorganism should be able to metabolize a broad spectrum of substrates and to maintain its metabolic activity even in the presence of adverse factors such as toxic metal ions or a high salt concentration (when used in the testing of sea water).
Various microbial species have been proposed as recognition elements in BOD sensors. The sensor can include a single-strain culture (e.g., Bacillus subtilis or Trichosporon cutaneum), a mixture of two identified microbial strains (e.g., Bacillus subtilis and Bacillus licheniformis) or activated sludge from wastewater processing plants, which include a mixture of bacteria and protozoan microorganisms.
Typically, single-strain BOD sensors display stable characteristics over a reasonable time period but their application is limited by the narrow substrate spectrum of the sensing strain. Good precision and operational stability, as well as a broader substrate spectrum is provided by mixtures of two identifiable strains. Activated sludge responds to a very broad range of substrates but they are not suitable for long-term operation owing to the instability of the composition of the microorganism mixture over time.
Instead of measuring the change in the oxygen concentration, a BOD sensor can be designed to perform transduction by means of a redox mediator such as the ferricyanide ion [20, 21]. The mediator accepts electrons from the microorganism cells and after diffusion to the anode undergoes oxidation, thereby generating the response current. In this way, it is possible to determine the BOD of water samples with low oxygen contents without the need for additional supply of air.
In the amperometric BOD sensor, the current is produced under the influence of a constant negative potential applied to the working electrode. An alternative design is based on the fuel cell principle, as shown in Figure 23.2 [22]. The cell is composed of two compartments separated by a cation-exchanger membrane. The microorganism is selected so as to be able to perform direct electron transfer to the anode. Therefore, organic material is processed by the microbial film and electrons released in the oxidation reactions are released to the anode. Electrons pass through the external circuit and reduce dissolved oxygen supplied to the cathode chamber. The current indicated by the ammeter depends on the content of organic compounds in the sample supplied to the anode chamber. The first BOD sensor that was reported by Karube et al. in 1977 was of the fuel-cell type [23].
Figure 23.2 A microbial fuel cell. OM indicates organic material. Reprinted with permission from [22]. Copyright 2010 Elsevier.
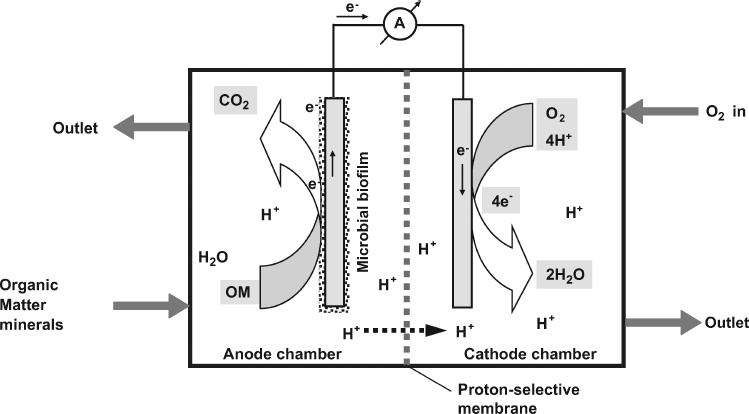
Electrochemically inactive microorganisms can also be used in microbial fuel cells but in such cases an electron-transfer mediator should be present in the anode chamber. As the mediator has to cross the lipophilic cell membrane, it should be itself lipophilic. Suitable lipophilic mediators are menadione and benzoamines. Ferricyanide ion is widely used as the mediator either alone or in conjunction with menadione.
The application of BOD sensors reduces considerably the duration of the assay, from 5 days in the standard method to about 15 min. More advanced BOD sensor designs are based on a planar configuration that is suitable for multiplexing and allows for parallel assay of multiple samples.
Amperometric respiratory sensors have also been developed for various compounds of interest to the food and beverage industry (e.g., ethanol or glucose) or water pollutants such as phenols or surfactants.
Amperometric transduction can be achieved not only by monitoring the respiration of the microorganism but also by selecting a substrate that forms on oxidation an electrochemically active compound. For example, p-nitrophenol can be sensed by means of microorganisms that promote its oxidation to benzoquinone.
Sensors discussed above are based on the metabolic processing of the analyte by microorganisms. An alternative sensing strategy relies on the change of the enzyme activity under the effect of an analyte that act either as an inhibitor or as an activator. In general, enzyme inhibition is a poorly selective process. Improved selectivity can be achieved by means of genetically modified microorganisms in which a selected enzyme is produced in order to impart selectivity to a particular kind of inhibitor.
23.4.2 Potentiometric Microbial Biosensors
In the presence of a selected substrate, microorganism metabolism can result in ions (e.g., H+) or gases (e.g., ammonia or carbon dioxide) that can be detected by a potentiometric sensor integrated with the microbiological sensing element. Thus, the first reported potentiometric microbial sensor was obtained by combining a potentiometric ammonia sensor with a layer of Streptococcus faecium [24]. This microorganism metabolizes the amino acid L-arginine forming ammonia. As a result, the potential-response of the ammonia sensor is a linear function of the logarithm of arginine concentration. Using a similar design, microbial biosensors for various amino acids or pollutants have been developed [25].
In the above approach the signaling ion or gas is produced by the metabolic conversion of the analyte. An alternative sensing mechanism involves the interaction of the analyte with a cell receptor leading to a metabolic process that changes the extracellular ion concentration [26]. The mechanism of induced metabolic response is presented in Figure 23.3. Glucose and oxygen are consumed to produce adenosine triphosphate (ATP) that is the source of energy in various metabolic processes indicated in this figure by the arrows. The products of the metabolic processes are lactic acid and carbon dioxide (or the hydrogen carbonate ion). Excretion of the waste products induces acidification of the extracellular medium that can be detected by a suitable transducer. The rate of these metabolic processes can be enhanced if the analyte is a ligand that interacts with a cell receptor and thereby enhances the metabolic activity. A particular activation mechanism is the activation of the sodium-potassium pump located in the cell membrane. Using energy provided by ATP hydrolysis, this molecular pump pumps sodium and potassium ions against their concentration gradients. This results in an increase of the sodium ion concentration in the extracellular fluid accompanied by a decrease of the potassium ion concentration in the same medium. Monitoring of pH or alkali ion concentration by means of suitable potentiometric sensors provide a response signal that is dependent on the concentration of the ligand-analyte.
Figure 23.3 Schematic presentation of the cellular metabolism and its relationship with cell receptor activation by an external chemical stimulus. Adapted with permission from [26]. Copyright Elsevier 1992.
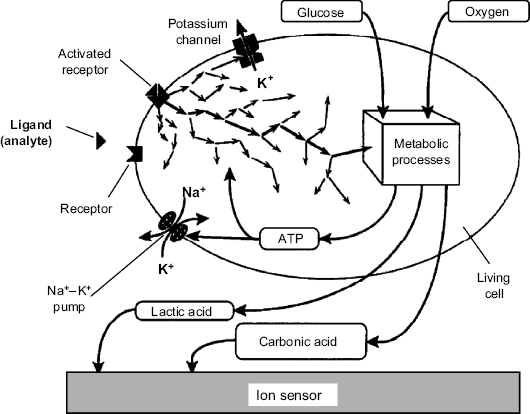
The most straightforward transduction method is based on pH change produced by excreted acidic waste products. In order to obtain a quasilinear response, the pH should be adjusted to a value as close as possible to the pK value of the buffer system. Typically, natural acid-base compound present in the cell environment (such as carbonate or phosphate ions or amino acids) provide a suitable buffer capacity.
Both ion-selective electrodes and ion-selective field effect devices have been used as transducers in whole cell biosensors [27]. The response in each case is linearly dependent on the logarithm of the analyte concentration. Facile miniaturization and compatibility with microfabrication technology makes potentiometric sensors well suited for integration in sensor arrays and microfluidic analytical systems.
Ion-selective field effect devices have been used to develop sensor arrays for multiple-parameter monitoring of the cell metabolism. This can be accomplished by measuring the concentration of a series of extruded ions such as H+, K+ and Ca2+ by means of light-addressable potentiometric sensors [28]. Additional sensors for oxygen and organic compounds of physiological relevance can be included in the array in order to provide a more comprehensive assessment of the cell metabolism. Such a device, which is termed a microphysiometer, can be used in fundamental research of cell physiology as well as in the assay of antibiotics or toxic species which affect the cell metabolism.
23.4.3 Conductometric Microbial Sensors
Many microbe-catalyzed reactions result in a change of the local ion concentration, which can be monitored in a nonselective way by conductance measurements. The configuration of conductometric cell biosensors is similar to that of conductometric enzymatic sensors (Section 17.7.4). In short, a living-cell layer is formed between two electrodes and the electric conductance of this layer is monitored by a conductometer. For example, a sensor for acrylonitrile has been developed using the Rhodococcus ruber microorganism that converts acrylonitrile into the ammonium acrylate ion [29]. A compact design is obtained by means of interdigitated electrode pairs.
Carbamates and organophosphorus pesticides can be assessed by inhibition of the acetylcholinesterase enzyme in the Chlorella vulgaris microalgae [30]. Using methylumbelliferylphosphate as substrate, a quaternary ammonium compound forms. The same microorganism contains alkaline phosphatase that is inhibited by Cd2+ and Zn2+ ions. The substrate of this enzyme (p-nitrophenylphosphate) produces by hydrolysis phosphate ions that alter the local conductance. Therefore, this sensor is useful in the assay of water pollution by either pesticides or toxic metal ions.
The response signal in conductometric biosensors is overlapped by a significant background produced by ions in the pH buffer system. Background correction can be performed by subtracting from the overall signal the signal produced by a reference sensor. This sensor includes a strain of inactive microorganism.
23.4.4 Electrical Impedance Transduction
Electrical impedance measurement is suitable for monitoring electrodes coated by living-cell monolayers immobilized by adhesion. Impedance measurements (Sections 17.1 and 17.2) provide a wealth of information about the cell physiology, adhesion propensity, shape, spreading and motility [14, 31]. Typically, mammalian or higher eukaryotic whole cells are used in this kind of sensor in order to substitute cell cultures for whole animals in the investigation of the effects of toxins, drugs, viruses and bacteria on living organisms [32]. Cell adhesion to the electrode is promoted by prior coating the electrode with a specific protein.
Current distribution at a whole-cell monolayer coated on a metal electrode is illustrated in Figure 23.4A. As can be seen, ion current can flow at parts of the electrode exposed to the solution (path 1), by crossing the cells (path 2), or by passing through the solution channels between the cell and the electrode surface (path 3). The equivalent circuit for an electrode coated with a cell monolayer is shown in Figure 23.4B. In this circuit, the Ze impedance corresponds to path 1, while path 3 meets the seal resistance (Rseal) which is the resistance at the cell–substrate contact. The parallel track 2 leads the current across the cell where there are two impedances (Zb and Za) generated by the cell membrane. Each of them is composed of a parallel combination of a capacitor and a resistor. The capacitor accounts for the insulating properties of the membrane whilst the resistor represents the parallel combination of the ion channels available for the transport of various ions through the membrane. The counterelectrode has a large surface area exposed to the solution and thereby its impedance (Zce) is meaningless.
Figure 23.4 Principle of impedimetric assay of whole cells. (A) Current flow at an electrode coated with a cell monolayer. (B) The equivalent circuit of a system formed of a living cell attached to an electrode surface. WE: working electrode; CE: counterelectrode; Ze and Zce: impedances at the direct contact of electrodes with the solution; Rseal: the resistance at the cell-substrate contact; Za and Zb: apical and basal cell membrane impedances, respectively. (A) Adapted with permission from [14]. Copyright 2007 The Royal Society of Chemistry. (B) Adapted with permission from [32]. Copyright 2009 Elsevier.
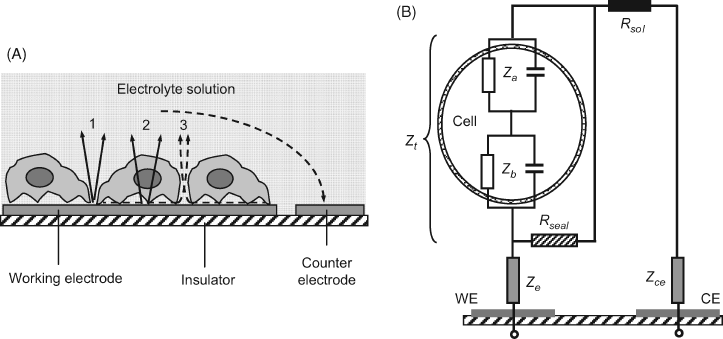
The cell impedance is therefore represented by the series sum of basal and apical cell impedances:
(23.3) ![]()
The total impedance associated with the cell (Zt) includes also the effect of the sealing resistance:
(23.4) ![]()
If ![]() , the total impedance approximates to seal resistance, whilst in the opposite case (
, the total impedance approximates to seal resistance, whilst in the opposite case (![]() ) the total impedance is mostly determined by the cell impedance. The impedance at the electrode–electrolyte surface is of no relevance and should be kept as high as possible by patterning the cells in a tightly bound, well-spread conformation.
) the total impedance is mostly determined by the cell impedance. The impedance at the electrode–electrolyte surface is of no relevance and should be kept as high as possible by patterning the cells in a tightly bound, well-spread conformation.
Cell impedance measurements are utilized in cell biology to assess in real time the cell response to physical or chemical stimuli, such as temperature, pH and various chemical compounds. Electrical impedance whole-cell biosensors have been developed for the assay of organic or inorganic toxic species. Arrays of such sensors have been produced for parallel toxicity assays of multiple water samples.
23.5 Optical Whole-Cell Sensors
23.5.1 Optical Respiratory Biosensors
Microorganism respiration can be monitored by means of optical oxygen sensors. Hence, optical analogs of the amperometric microbial sensor (Section 23.4.1) have been developed.
Fluorescent BOD sensors have been constructed by forming a culture of microorganisms at the end surface of a fiber optic oxygen sensor (Figure 23.5). As oxygen sensing is based on fluorescence quenching, the fluorescence response intensity increases with the oxygen consumption rate and the response function is similar to that of an amperometric BOD sensor. Trichosporon cutaneum or Bacillus subtilis microorganisms have been used in the sensing layer.
Figure 23.5 Cross section of an optical BOD sensor. (1) porous polycarbonate cover; (2) layer of yeast (Trichosporon cutaneum) immobilized in poly (vinyl alcohol); (3) 1 μm layer of charcoal (optical insulator); (4) oxygen-sensitive fluorescent layer; (5) inert and gas-impermeable polyester layer; (6) optical fiber bundle. Redrawn with permission from [34]. Copyright 1994 American Chemical Society.
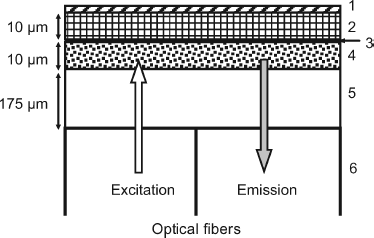
As lower selectivity of the microorganism layer allows the monitoring of more possible pollutants, an optical BOD sensor has been constructed using a combination of three microorganisms (Bacillus licheniformis, Dietzia maris and Marinobacter marinus) [33]. Compared with the sensor that makes use of Bacillus licheniformis only, the three-microorganism sensor allows the limit of detection to be decreased from 0.9 to 0.3 mg/L and the response time from 30 to 3.2 min.
Optical respiratory biosensors are also useful in the assay of toxic chemical ionic species that cause respiratory activity to decrease.
23.5.2 External-Stimuli-Based Optical Sensors
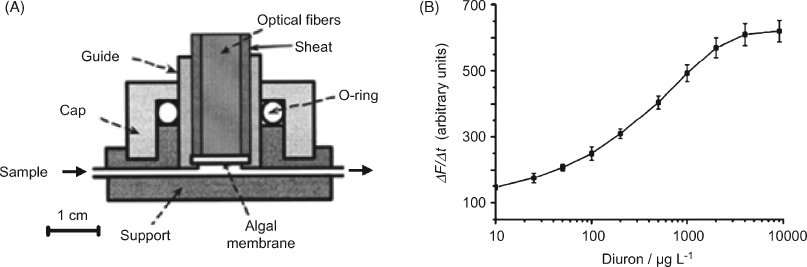
In addition to the metabolic function, other biological processes in whole cells can be exploited in the design of optical sensors. For example, photosynthesis, which is an essential process in plant cells, has been used to develop herbicide or pesticide sensors based on the inhibition of an electron-transfer step occurring during photosynthesis [13, 35]. As a result, the chlorophyll concentration in the cell increases and this modification can be detected by means of chlorophyll fluorescence at 682 nm. A sensor for the herbicide diuron based on photosynthesis inhibition is shown in Figure 23.6A. The sensing membrane in this sensor has been obtained by entrapment of the Chlorella vulgaris alga in a silica matrix prepared by sol-gel chemistry. The membrane was positioned in a flow-through cell and a bifurcated optical fiber cable was used to provide excitation and collect the fluorescent light. The response is obtained as the time derivative of the fluorescence intensity measured 5 min after sample introduction. A typical response curve is presented in Figure 23.6B. The detection limit of this sensor is 1 μg L−1 diuron, which is better than the value reported for high-performance liquid chromatography.
Figure 23.6 Optical alga biosensor for the herbicide Diuron. (A) Sensor configuration; (B) response function. Adapted with permission from [35]. Copyright 2007 Elsevier.
23.5.3 Bioreporters
A very promising approach in biosensing is represented by induced synthesis of certain proteins that can be detected by means of luminescence. Living cells performing this function are known as bioreporters.
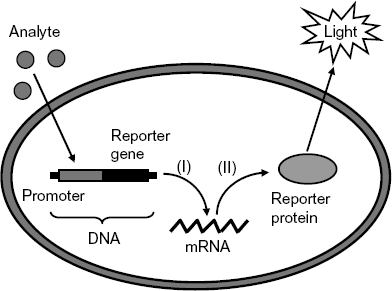
Bioreporters are intact living cells that produce a measurable signal in response to a specific chemical or physical agent in their environment [36–38]. Typically, genetically modified organisms are suitable for such applications. Bioreporter cells contain two specific genetic elements, a promoter sequence (which promotes the gene transcription into RNA) and a reporter gene (Figure 23.7). In a genetically modified organism, the reporter gene is substituted for a natural gene that is involved in cell regulatory functions. When the analyte (or one of its metabolites) is recognized by specific receptors (regulatory protein), a natural regulatory process is triggered. In this process, the reporter gene code is first transcribed into messenger RNA (mRNA) and then translated into a luminescent reporter protein that can be detected (Figure 23.7). In a cell culture, the amount of reporter protein depends on the analyte concentration in the sample.
Figure 23.7 Mechanism of bioreporter response to a chemical stimulus. Step (I): transcription; step (II): translation.
In order to perform signal transduction by fluorescence, the reporter gene is selected such as to be expressed into green fluorescent protein (GFP) that is encoded in the gfp gene. GFP can be modified to emit light at other wavelengths (cyan, red, and yellow), which allows for multianalyte detection. Alternatively, bioluminescence can be produced if the reporter protein is an enzyme of the luciferase class, which is encoded in a lux gene. Firefly luciferase is particularly suitable owing to its high quantum yield but bacterial luciferase is also utilized. In order for bioluminescence to occur, an exogenous substrate (luciferin) should be supplied.
In the above mechanism, the light signal is enhanced under the effect of the analyte. An alternative sensing mechanism is based on the reduction of the light signal upon expression of a repressor protein that restrains the expression of the reporter gene.
Bioreporters can be designed for various applications in environment pollution control. These applications address determinations of organic compounds, metal and nonmetal toxic species, and the assay of sample toxicity or stress conditions. Of particular interest is the utilization of bioreporters in the assay of bioavailable toxic metal ions such as Co2+ and Ni2+. As the toxic effect is exerted only by bioavailable ion species, the concentration of these species is more enlightening than the total ion concentration. As the response of a bioreporter cell depends on intricate biochemical reactions, the response time is relatively long and can vary between 30 min to 5 h.
23.6 Improving the Selectivity of Microorganism Biosensors
Biosensors based on living cells are reputed to be poorly selective for various reasons. Typical interferences arise from the presence of a high number of different enzymes in the same cell. Hence, various metabolic pathways are possible and some of them can give rise to the same signaling product starting from different substrates. Therefore, the sensor response would be higher than that expected for the target analyte. Another source of interferences in microbial sensor is represented by contamination with other bacterial species that give rise by metabolism to products that interfere with the transduction process.
Selective microbial sensors can be obtained if the analyte is essential for the growth of the selected microorganism. This approach has been applied in the development of biosensors for vitamins.
Selectivity can also be achieved by proper selection of the operating conditions such as the pH or presence or absence of oxygen. In certain cases, the interfering metabolic pathway can be repressed by means of enzyme inhibitors.
Good selectivity can be obtained upon combining an isolated enzyme with a microorganism. Thus, urea determination can be selectively performed by a hybrid sensor composed of urease and nitrifying bacteria. Ammonia produced by the enzymatic reaction is further metabolized by the bacteria with oxygen consumption and the measurement of the decrease in the oxygen specific signal is correlated with the substrate concentration. The selectivity of this hybrid sensor originates from the fact that nitrifying bacteria can use only ammonia as an energy source.
It should be pointed that in certain applications, poor selectivity is an advantage rather than a drawback. This applies to BOD sensors or sensors designed for toxicity assay.
23.7 Conclusions
Living cell-based biosensor utilize living biological cells and monitor physiological modifications produced by chemical stimuli. There are two particular strategies for biosensing with living cells. The first relies on the metabolic conversion of the target species accompanied by consumption or production of a detectable chemical species. From this standpoint, the whole cell biosensor functions as an enzymatic sensor in which the active enzyme functions in its natural environment. The second alternative relies on alteration of the cell physiology or morphology in response to a chemical stimulus.
As the receptor species are included in the cell's natural environment, they are not exposed to degradation or inactivation. This feature brings about a reduced manufacturing cost since the isolation of recognition receptors is avoided.
In general, metabolism-based biosensors display rather poor selectivity owing to the occurrence of multiple metabolic pathways. At first sight, the poor selectivity of such sensors appears to be a drawback, particularly when a specific chemical species is targeted. However, the poor selectivity becomes an advantage when a whole class of target compounds (such as dissolved organic material or toxic species) is being addressed.
Although tissues of higher organisms are suitable for certain applications, whole-cell biosensors are commonly preferred owing to easier fabrication and the high degree of control over the characteristics of the biological material. Whole-cell biosensors make use of either microorganisms or higher-organism cell cultures.
The application of whole-cell biosensors encompasses a broad area, including environment monitoring, medicine and clinical chemistry [28, 39], the pharmaceutical industry, food and fermentation industries, and biosecurity. Of great interest is the capacity of whole-cell biosensors to respond to various kinds of toxic species. This characteristic is employed in the assay of ecotoxicity [21, 32] and the detection of chemical-warfare agents.
1. Racek, J. (1995) Cell-Based Biosensors, Technomic Publishing Company, Inc., Lancaster.
2. Zourob, M., Elwary, S., and Turner, A. (eds) (2008) Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems, Springer, New York.
3. Wang, P. and Liu, Q. (eds) (2010) Cell-Based Biosensors: Principles and Applications, Artech House, Norwood.
4. Nakamura, H., Shimomura-Shimizu, M., and Karube, I. (2008) Development of microbial sensors and their application. Adv. Biochem. Eng./Biotechnol., 109, 351–394.
5. D'Souza, S.F. (2001) Microbial biosensors. Biosens. Bioelect., 16, 337–353.
6. Su, L., Jia, W., Hou, C. et al. (2011) Microbial biosensors: A review. Biosen. Bioelec., 26, 1788–1799.
7. Lei, Y., Chen, W., and Mulchandani, A. (2006) Microbial biosensors. Anal. Chim. Acta., 568, 200–210.
8. Baronas, R., Kulys, J., and Ivanauskas, F. (2009) Mathematical Modeling of Biosensors: An Introduction for Chemists and Mathematicians, Springer Netherlands, Dordrecht.
9. Rechnitz, G.A. (1988) Bioselective membrane electrodes using tissue materials as biocatalysts. Method Enzymol., 137, 138–152.
10. Nomura, Y., Chee, G.J., and Karube, I. (1998) Biosensor technology for determination of BOD. Field Anal. Chem. Technol., 2, 333–340.
11. Buchholz, K., Kasche, V., and Bornscheuer, U.T. (2005) Biocatalysts and Enzyme Technology, Wiley-VCH, Weinheim.
12. Depagne, C., Roux, C.C., and Coradin, T. (2011) How to design cell-based biosensors using the sole-gel process. Anal. Bioanal. Chem., 400, 965–976.
13. Pena-Vazquez, E., Maneiro, E., Perez-Conde, C. et al. (2009) Microalgae fiber optic biosensors for herbicide monitoring using sol-gel technology. Biosens. Bioelectron., 24, 3538–3543.
14. Asphahani, F. and Zhang, M. (2007) Cellular impedance biosensors for drug screening and toxin detection. Analyst., 132, 835–841.
15. Garcia, A.J. (2006) Interfaces to control cell-biomaterial adhesive interactions, in Polymers for Regenerative Medicine (ed. C. Werner), Springer, Berlin, pp. 171–190.
16. Veiseh, M., Zareie, M.H., and Zhang, M.Q. (2002) Highly selective protein patterning on gold-silicon substrates for biosensor applications. Langmuir., 18, 6671–6678.
17. Lagarde, F. and Jaffrezic-Renault, N. (2011) Cell-based electrochemical biosensors for water quality assessment. Anal. Bioanal. Chem., 400, 947–964.
18. Liu, J. and Mattiasson, B. (2002) Microbial BOD sensors for wastewater analysis. Water Res., 36, 3786–3802.
19. Riedel, K., Lange, K.P., Stein, H.J. et al. (1990) A microbial sensor for BOD. Water Res., 24, 883–887.
20. Yoshida, N., Yano, K., Morita, T. et al. (2000) A mediator-type biosensor as a new approach to biochemical oxygen demand estimation. Analyst., 125, 2280–2284.
21. Pasco, N.F., Weld, R.J., Hay, J.M. et al. (2011) Development and applications of whole cell biosensors for ecotoxicity testing. Anal. Bioanal. Chem., 400, 931–945.
22. Namour, P. and Jaffrezic-Renault, N. (2010) Sensors for measuring biodegradable and total organic matter in water. TrAC-Trends Anal. Chem., 29, 848–857.
23. Karube, I., Matsunaga, T., Mitsuda, S. et al. (1977) Microbial electrode BOD sensors. Biotechnol. Bioeng., 19, 1535–1547.
24. Rechnitz, G.A., Kobos, R.K., Riechel, S.J. et al. (1977) Bio-selective membrane electrode prepared with living bacterial cells. Anal. Chim. Acta., 94, 357–365.
25. Corcoran, C.A. and Rechnitz, G.A. (1985) Cell based biosensors. Trends Biotechnol., 3, 92–96.
26. Owicki, J.C. and Wallace Parce, J. (1992) Biosensors based on the energy metabolism of living cells: The physical chemistry and cell biology of extracellular acidification. Biosens. Bioelect., 7, 255–272.
27. Poghossian, A., Ingebrandt, S., Offenhausser, A. et al. (2009) Field-effect devices for detecting cellular signals. Semin. Cell Dev. Biol., 20, 41–48.
28. Wang, P., Xu, G.X., Qin, L.F. et al. (2005) Cell-based biosensors and its application in biomedicine. Sens. Actuators B-Chem., 108, 576–584.
29. Roach, P.C.J., Ramsden, D.K., Hughes, J. et al. (2003) Development of a conductimetric biosensor using immobilised Rhodococcus ruber whole cells for the detection and quantification of acrylonitrile. Biosens. Bioelectron., 19, 73–78.
30. Chouteau, C., Dzyadevych, S., Durrieu, C. et al. (2005) A bi-enzymatic whole cell conductometric biosensor for heavy metal ions and pesticides detection in water samples. Biosens. Bioelectron., 21, 273–281.
31. Gu, W.W. and Zhao, Y. (2010) Cellular electrical impedance spectroscopy: an emerging technology of microscale biosensors. Expert Rev. Med. Devices., 7, 767–779.
32. Banerjee, P. and Bhunia, A.K. (2009) Mammalian cell-based biosensors for pathogens and toxins. Trends Biotechnol., 27, 179–188.
33. Lin, L., Xiao, L.L., Huang, S. et al. (2006) Novel BOD optical fiber biosensor based on co-immobilized microorganisms in ormosils matrix. Biosens. Bioelectron., 21, 1703–1709.
34. Preininger, C., Klimant, I., and Wolfbeis, O.S. (1994) Optical fiber sensor for biological oxygen demand. Anal. Chem., 66, 1841–1846.
35. Nguyen-Ngoc, H. and Tran-Minh, C. (2007) Fluorescent biosensor using whole cells in an inorganic translucent matrix. Anal. Chim. Acta., 583, 161–165.
36. van der Meer, J.R., Tropel, D., and Jaspers, M. (2004) Illuminating the detection chain of bacterial bioreporters. Environ. Microbiol., 6, 1005–1020.
37. Belkin, S. (2003) Microbial whole-cell sensing systems of environmental pollutants. Curr. Opin. Microbiol., 6, 206–212.
38. Van der Meer, J.R. (2011) Bacterial Sensors: Synthetic Design and Application Principles, Morgan & Claypool, San Francisco.
39. Kintzios, S.E. (2007) Cell-based biosensors in clinical chemistry. Mini-Rev. Med. Chem., 7, 1019–1026.
