10.3. SINGLE AND MULTIPLE EQUILIBRIUM CONTACT STAGES
10.3A. Single-Stage Equilibrium Contact
In many operations of the chemical and other process industries, the transfer of mass from one phase to another occurs, usually accompanied by a separation of the components of the mixture, since one component will be transferred to a larger extent than will another component.
A single-stage process can be defined as one in which two different phases are brought into intimate contact with each other and then are separated. During the time of contact, intimate mixing occurs and the various components diffuse and redistribute themselves between the two phases. If mixing time is long enough, the components are essentially at equilibrium in the two phases after separation and the process is considered a single equilibrium stage.
A single equilibrium stage can be represented as in Fig. 10.3-1. The two entering phases, L0 and V2, of known amounts and compositions, enter the stage; mixing and equilibration occur; and the two exit streams, L1 and V1, leave in equilibrium with each other. Making a total mass balance,
Equation 10.3-1
![]()
Figure 10.3-1. Single-stage equilibrium process.

where L is kg (lbm), V is kg, and M is total kg.
Assuming that three components, A, B and C, are present in the streams and making a balance on A and C,
Equation 10.3-2
![]()
Equation 10.3-3
![]()
An equation for B is not needed since xA + xB + xC = 1.0. The mass fraction of A in the L stream is xA and is yA in the V stream. The mass fraction of A in the M stream is xAM.
To solve the three equations, the equilibrium relations between the components must be known. In Section 10.3B, this will be done for a gas–liquid system and in Chapter 11 for a vapor–liquid system. Note that Eqs. (10.3-1)–(10.3-3) can also be written using mole units, with L and V having units of moles and xA and yA units of mole fraction.
10.3B. Single-Stage Equilibrium Contact for Gas–Liquid System
In the usual gas–liquid system the solute A is in the gas phase V, along with inert air B, and in the liquid phase L, along with inert water C. Assuming that air is essentially insoluble in the water phase and that water does not vaporize to the gas phase, the gas phase is a binary A–B and the liquid phase is a binary A–C. Using moles and mole fraction units, Eq. (10.3-1) holds for a single-stage process for the total material balance. Since component A is the only component that redistributes between the two phases, a balance on A can be written as follows:
Equation 10.3-4
![]()
where L' is moles inert water C and V' is moles inert air B. Both L' and V' are constant and usually known.
To solve Eq. (10.3-4), the relation between yA1 and xA1 in equilibrium is given by Henry's law:
Equation 10.3-5
![]()
If the solution is not dilute, equilibrium data in the form of a plot of pA or yA versus xA must be available, as in Fig. 10.2-1.
EXAMPLE 10.3-1. Equilibrium Stage Contact for CO2–Air–WaterA gas mixture at 1.0 atm pressure abs containing air and CO2 is contacted in a single-stage mixer continuously with pure water at 293 K. The two exit gas and liquid streams reach equilibrium. The inlet gas flow rate is 100 kg mol/h, with a mole fraction of CO2 of yA2 = 0.20. The liquid flow rate entering is 300 kg mol water/h. Calculate the amounts and compositions of the two outlet phases. Assume that water does not vaporize to the gas phase. Solution: The flow diagram is the same as given in Fig. 10.3-1. The inert water flow is L' = L0 = 300 kg mol/h. The inert air flow V' is obtained from Eq. (10.3-6): Equation 10.3-6
Hence, the inert air flow is V' = V2(1 − yA2) = 100(1 − 0.20) = 80 kg mol/h. Substituting into Eq. (10.3-4) to make a balance on CO2 (A), Equation 10.3-7
At 293 K, the Henry's law constant from Appendix A.3 is H = 0.142 × 104 atm/mol frac. Then H' = H/P = 0.142 × 104/1.0 = 0.142 × 104 mol frac gas/mol frac liquid. Substituting into Eq. (10.3-5), Equation 10.3-8
Substituting Eq. (10.3-8) into (10.3-7) and solving, xA1 = 1.41 × 10−4 and yA1 = 0.20. To calculate the total flow rates leaving,
In this case, since the liquid solution is so dilute, L0 ≅ L1. |
10.3C. Countercurrent Multiple-Contact Stages
1. Derivation of general equation
In Section 10.3A we used single-stage contact to transfer the solute A between the V and L phases. In order to transfer more solute from, say, the V1 stream, the single-stage contact can be repeated by again contacting the V1 stream leaving the first stage with fresh L0. This can be repeated using multiple stages. However, this is wasteful of the L0 stream and gives a dilute product in the outlet L1 streams. To conserve use of the L0 stream and to get a more concentrated product, countercurrent multiple-stage contacting is generally used. This is somewhat similar to countercurrent heat transfer in a heat exchanger, where the outlet heated stream approaches more closely the temperature of the inlet hot stream.
The process flow diagram for a countercurrent stage process is shown in Fig. 10.3-2. The inlet L stream is L0 and the inlet V stream is VN+1 instead of V2 as for a single-stage in Fig. 10.3-1. The outlet product streams are V1 and LN and the total number of stages is N. The component A is being exchanged between the V and L streams. The V stream is composed mainly of component B and the L stream of component C. Components B and C may or may not be somewhat miscible in each other. The two-phase system can be gas–liquid, vapor–liquid, liquid–liquid, or other.
Figure 10.3-2. Countercurrent multiple-stage process.

Making a total overall balance on all stages,
Equation 10.3-9
![]()
where VN+1 is mol/h entering LN is mol/h leaving the process, and M is the total flow. Note in Fig. 10.3-2 that any two streams leaving a stage are in equilibrium with each other. For example, in stage n, Vn and Ln are in equilibrium. For an overall component balance on A, B, or C,
Equation 10.3-10
![]()
where x and y are mole fractions. Flows in kg/h (lbm/h) and mass fraction can also be used in these equations.
Making a total balance over the first n stages,
Equation 10.3-11
![]()
Making a component balance over the first n stages,
Equation 10.3-12
![]()
Solving for yn+1 in Eq. (10.3-12),
Equation 10.3-13
![]()
This is an important material-balance equation, often called an operating line. It relates the concentration yn+1 in the V stream with xn in the L stream passing it. The terms V1, y1, L0, and x0 are constant and are usually known or can be determined from Eqs. (10.3-9)–(10.3-12).
2. Countercurrent contact with immiscible streams
An important case in which the solute A is being transferred occurs when the solvent stream V contains components A and B with no C and the solvent stream L contains A and C with no B. The two streams L and V are immiscible in each other, with only A being transferred. When Eq. (10.3-13) is plotted on an x-y plot (xA and yA of component A) such as Fig. 10.3-3, it is often curved, since the slope Ln/Vn+1 of the operating line varies if the L and V streams vary from stage to stage.
Figure 10.3-3. Number of stages in a countercurrent multiple-stage contact process.

In Fig. 10.3-3 the equilibrium line that relates the compositions of two streams leaving a stage in equilibrium with each other is plotted. To determine the number of ideal stages required to bring about a given separation or reduction of the concentration of A from yN+1 to y1, the calculation is often done graphically. Starting at stage 1, y1 and x0 are on the operating line, Eq. (10.3-13), plotted in the figure. The vapor y1 leaving is in equilibrium with the leaving x1 and both compositions are on the equilibrium line. Then y2 and x1 are on the operating line and y2 is in equilibrium with x2, and so on. Each stage is represented by a step drawn on Fig. 10.3-3. The steps are continued on the graph until yN+1 is reached. Alternatively, we can start at yN+1 and draw the steps going to y1.
If the streams L and V are dilute in component A, the streams are approximately constant and the slope Ln/Vn+1 of Eq. (10.3-13) is nearly constant. Hence, the operating line is essentially a straight line on an x-y plot. In distillation, where only components A and B are present, Eq. (10.3-13) also holds for the operating line; this will be covered in Chapter 11. Cases where A, B, and C are appreciably soluble in each other often occur in liquid–liquid extraction and will be discussed in Chapter 12.
Example 10.3-2. Absorption of Acetone in a Countercurrent Stage TowerIt is desired to absorb 90% of the acetone in a gas containing 1.0 mol % acetone in air in a countercurrent stage tower. The total inlet gas flow to the tower is 30.0 kg mol/h, and the total inlet pure water flow to be used to absorb the acetone is 90 kg mol H2O/h. The process is to operate isothermally at 300 K and a total pressure of 101.3 kPa. The equilibrium relation for the acetone (A) in the gas–liquid is yA = 2.53xA. Determine the number of theoretical stages required for this separation. Solution: The process flow diagram is similar to Fig. 10.3-3. Given values are yAN+1 = 0.01, xA0 = 0, VN+1 = 30.0 kg mol/h, and L0 = 90.0 kg mol/h. Making an acetone material balance,
Since the flow of liquid varies only slightly from L0 = 90.0 at the inlet to LN = 90.27 at the outlet and V from 30.0 to 29.73, the slope Ln/Vn+1 of the operating line in Eq. (10.3-13) is essentially constant. This line is plotted in Fig. 10.3-4 together with the equilibrium relation yA = 2.53xA. Starting at point yA1, xA0, the stages are drawn as shown. About 5.2 theoretical stages are required. Figure 10.3-4. Theoretical stages for countercurrent absorption in Example 10.3-2.
|
10.3D. Analytical Equations for Countercurrent Stage Contact
When the flow rates V and L in a countercurrent process are essentially constant, the operating-line equation (10.3-13) becomes straight. If the equilibrium line is also a straight line over the concentration range, simplified analytical expressions can be derived for the number of equilibrium stages in a countercurrent stage process.
Referring again to Fig. 10.3-2, Eq. (10.3-14) is an overall component balance on component A:
Equation 10.3-14
![]()
Rearranging,
Equation 10.3-15
![]()
Making a component balance for A on the first n stages,
Equation 10.3-16
![]()
Rearranging,
Equation 10.3-17
![]()
Equating Eq. (10.3-15) to (10.3-17),
Equation 10.3-18
![]()
Since the molar flows are constant, Ln = LN = constant = L and Vn+1 = VN+1 = constant = V. Then Eq. (10.3-18) becomes
Equation 10.3-19
![]()
Since yn+1 and xn+1 are in equilibrium and the equilibrium line is straight, yn+1 = mxn+1. Also, yN+1 = mxN+1. Substituting mxn+1 for yn+1 and calling A = L/mV, Eq. (10.3-19) becomes
Equation 10.3-20
![]()
where A is an absorption factor and is constant.
All factors on the right-hand side of Eq. (10.3-20) are constant. This equation is a linear first-order difference equation and can be solved by the calculus of finite-difference methods (G1, M1). The final derived equations are as follows.
For transfer of solute A from phase L to V (stripping),
Equation 10.3-21
![]()
Equation 10.3-22

Equation 10.3-23
![]()
For transfer of solute A from phase V to L (absorption),
Equation 10.3-24
![]()
Equation 10.3-25
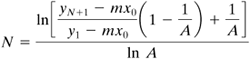
When A = 1,
Equation 10.3-26
![]()
The term A is often called the absorption factor and S the stripping factor, where S = 1/A. These equations can be used with any consistent set of units such as mass flow and mass fraction or molar flow and mole fraction. Such series of equations are often called Kremser equations and are convenient to use.
If the equilibrium line is not straight but curved somewhat, the slope will vary and, hence, m and A = L/mV will vary. For absorption (referring to Fig. 10.3-3) at the concentrated end, the slope mN or tangent of the equilibrium line at the concentrations yN,
xN leaving this bottom stage N is used. This mN is at point xN on the equilibrium line. For the top or dilute end at stage 1 of the tower, the slope of the equilibrium line m1 at the concentrations y1,
x1 leaving this stage is employed. This m1 is at point y1 on the equilibrium line. Then AN = LN/mNVN+1 and A1 = L0/m1V1. The geometric average is used, where ![]() (P1, T3). Also, the dilute m1 is used in Eqs. (10.3-24)–(10.3-26).
(P1, T3). Also, the dilute m1 is used in Eqs. (10.3-24)–(10.3-26).
For stripping at the top or concentrated stage, the slope m1 or tangent to the equilibrium line at the concentrations y1, x1 leaving this stage is used. This m1 is at point y1 on the equilibrium line. At the bottom stage or dilute end of the tower, the slope mN of the equilibrium line at the points yN,
xN is used. This mN is at xN on the equilibrium line. Then, AN = LN/mNVN+1, A1 = L0/m1V1, and ![]() . Again the dilute mN is used in Eqs. (10.3-21)–(10.3-23). Sometimes only the values of A and m at the dilute end are used since more of the stages are in this region.
. Again the dilute mN is used in Eqs. (10.3-21)–(10.3-23). Sometimes only the values of A and m at the dilute end are used since more of the stages are in this region.
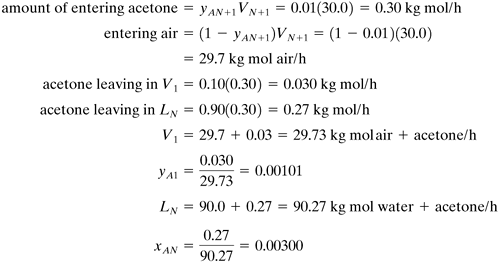
Example 10.3-3. Number of Stages by Analytical Equation.Repeat Example 10.3-2 but use the Kremser analytical equations for countercurrent stage processes. Solution: At one end of the process at stage 1, V1 = 29.73 kg mol/h, yA1 = 0.00101, L0 = 90.0, and xA0 = 0. Also, the equilibrium relation is yA = 2.53xA where m = 2.53. Then,
At stage N, VN+1 = 30.0, yAN+1 = 0.01, LN = 90.27, and xAN = 0.00300.
The geometric average The acetone solute is transferred from the V to the L phase (absorption). Substituting into Eq. (10.3-25),
This compares closely with 5.2 stages obtained using the graphical method. |