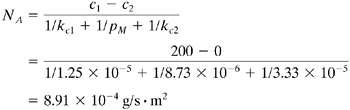13.2. LIQUID PERMEATION MEMBRANE PROCESSES OR DIALYSIS
13.2A. Series Resistances in Membrane Processes
In membrane processes with liquids, the solute molecules must first be transported or diffuse through the liquid film of the first liquid phase on one side of the solid membrane, then through the membrane itself, and finally through the film of the second liquid phase. This is shown in Fig. 13.2-1a, where c1 is the bulk liquid-phase concentration of the diffusing solute A in kg mol A/m3, c1i is the concentration of A in the fluid just adjacent to the solid, and c1iS is the concentration of A in the solid at the surface and is in equilibrium with C1i. The mass-transfer coefficients are kc1 and kc2 in m/s. The equilibrium distribution coefficient K' is defined as
Equation 13.2-1
![]()
Figure 13.2-1. Concentration profiles for membrane processes: (a) two liquid films and a solid, (b) two gas films and a solid.

Note that K' is the inverse of K defined in Eq. (7.1-16).
The flux equations through each phase are all equal to each other at steady state and are as follows:
Equation 13.2-2
![]()
Substituting c1iS = K'c1i and c2iS = K'c2i into Eq. (13.2-2),
Equation 13.2-3
![]()
Equation 13.2-4
![]()
where pM is the permeance in the solid in m/s, L is the thickness in m, and DAB is the diffusivity of A in the solid in m2/s. Note that the permeance pM in Eq. (13.2-4) is different from the permeability PM defined in Eq. (6.5-9). Also, the value of pM is inversely proportional to the thickness L. Instead of determining DAB and K' in two separate experiments, it is more convenient to determine pM in one separate diffusion experiment. Solving each of the parts of Eq. (13.2-3) for the concentration difference,
Equation 13.2-5
![]()
Adding the equations, the internal concentrations c1i and c2i drop out, and the final equation is
Equation 13.2-6
![]()
In some cases, the resistances in the two liquid films are quite small compared to the membrane resistance, which controls the permeation rate.
EXAMPLE 13.2-1. Membrane Diffusion and Liquid Film ResistancesA liquid containing dilute solute A at a concentration c1 = 3 × 10-2 kg mol/m3 is flowing rapidly past a membrane of thickness L = 3.0 × 10-5 m. The distribution coefficient K' = 1.5 and DAB = 7.0 × 10-11 m2/s in the membrane. The solute diffuses through the membrane, and its concentration on the other side is c2 = 0.50 × 10-2 kg mol/m3. The mass-transfer coefficient kc1 is large and can be considered as infinite, and kc2 = 2.02 × 10-5 m/s.
Solution: For part (a), the sketch is shown in Fig. 13.2-2. Note that the concentration profile on the left side is flat (kc1 = ∞) and c1 = c1i. The derivation is the same as for Eq. (13.2-6), but 1/kc1 = 0 to give Equation 13.2-7
Figure 13.2-2. Concentrations for Example 13.2-1.
For part (b), to calculate the flux using Eqs. (13.2-4) and (13.2-7),
To calculate c2i,
Solving, c2i = 0.869 × 10-2 kg mol/m3. Also, using Eq. (13.2-1),
Solving, c2is = 1.304 × 10-2 kg mol/m3. |
13.2B. Dialysis Processes
Dialysis uses a semipermeable membrane to separate species by virtue of their different diffusion rates in the membrane. The feed solution or dialyzate, which contains the solutes to be separated, flows on one side of the membrane and the solvent or diffusate stream on the other side. Some solvent may also diffuse across the membrane in the opposite direction, which reduces performance by diluting the dialyzate.
In practice dialysis is used to separate species which differ appreciably in size and thus have a reasonably large difference in diffusion rates. Solute fluxes depend on the concentration gradient in the membrane. Hence, dialysis is characterized by low flux rates in comparison to other membrane processes, such as reverse osmosis and ultrafiltration, which depend on applied pressure.
In general, dialysis is used with aqueous solutions on both sides of the membrane. The film resistances can be appreciable compared to the membrane resistance. Applications include recovery of sodium hydroxide in cellulose processing, recovery of acids from metallurgical liquors, removal of products from a culture solution in fermentation, desalting of cheese whey solids, and reduction of alcohol content of beer. Many small-scale applications occur in the pharmaceutical industry.
13.2C. Types of Equipment for Dialysis
Various geometrical configurations are used in liquid membrane processes. A common one is similar to a filter press, where the membrane is a flat plate. Vertical solid membranes are placed in between alternating liquor and solvent feed frames, with the liquor to be dialyzed being fed to the bottom and the solvent to the top of these frames. The dialyzate and the diffusate are removed through channels located at the top and bottom of the frames, respectively. The most important type consists of many small tubes or very fine hollow fibers arranged in a bundle, like a heat exchanger. This type of unit has a very high ratio of membrane area to volume of the unit.
13.2D. Hemodialysis in Artificial Kidney
An important example of the liquid permeation process is dialysis with an artificial kidney in the biomedical field. In this application for purifying human blood, the principal solutes removed are the small solutes urea, uric acid, creatinine, phosphates, and excess amounts of chloride. A typical membrane used is cellophane about 0.025 mm thick, which allows small solutes to diffuse but retains the large proteins in the blood. During the hemodialysis, blood is passed on one side of the membrane while an aqueous dialyzing fluid flows on the other side. Solutes such as urea, uric acid, NaCl, and so on, which have elevated concentrations in the blood, diffuse across the membrane to the dialyzing aqueous solution, which contains certain concentrations of solutes such as potassium salts, and so on, to ensure that concentrations in the blood do not drop below certain levels. In one configuration the membranes are stacked in the form of a multilayered sandwich, with blood flowing past one side of the membrane and dialyzing fluid past the other side. The hollow fiber type is used quite often.
EXAMPLE 13.2-2. Dialysis to Remove Urea from BloodCalculate the flux and the rate of removal of urea at steady state in g/h from blood in a cuprophane (cellophane) membrane dialyzer at 37°C. The membrane is 0.025 mm thick and has an area of 2.0 m2. The mass-transfer coefficient on the blood side is estimated as kc1 = 1.25 × 10-5 m/s and that on the aqueous side is 3.33 × 10-5 m/s. The permeance of the membrane is 8.73 × 10-6 m/s (B2). The concentration of urea in the blood is 0.02 g urea/100 mL and that in the dialyzing fluid will be assumed as 0. Solution: The concentration c1 = 0.02/100 = 2.0 × 10-4 g/mL = 200 g/m3 and c2 = 0. Substituting into Eq. (13.2-6),
For a time of 1 h and an area of 2.0 m2,
|