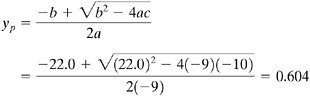13.4. COMPLETE-MIXING MODEL FOR GAS SEPARATION BY MEMBRANES
13.4A. Basic Equations Used
In Fig. 13.4-1, a detailed process flow diagram is shown for complete mixing. When a separator element is operated at a low recovery (i.e., where the permeate flow rate is a small fraction of the entering feed rate), there is a minimal change in composition. Then the results derived using the complete-mixing model provide reasonable estimates of permeate purity. This case was derived by Weller and Steiner (W4).
Figure 13.4-1. Process flow for complete mixing case.

Using nomenclature similar to that for distillation (P7), using the feed and nonpermeate flow rate as L and composition x mole fraction and the permeate flow rate as V and composition y.
The overall material balance (Fig. 13.4-1) is as follows:
Equation 13.4-1
![]()
where Lf is total feed flow rate in cm3 (STP)/s; Lo is outlet reject flow rate, cm3 (STP)/s; and Vp is outlet permeate flow rate, cm3 (STP)/s. The cut or fraction of feed permeated, θ, is given as
Equation 13.4-2
![]()
The rate of diffusion or permeation of species A (in a binary of A and B) is given below by an equation similar to Eq. (13.3-6) but using cm3 (STP)/s as rate of permeation rather than flux in kg mol/s · cm2:
Equation 13.4-3
![]()
where ![]() is permeability of A in the membrane, cm3 (STP) · cm/(s · cm2 · cm Hg); VA is flow rate of A in permeate, cm3 (STP)/s; Am is membrane area, cm2; t is membrane thickness, cm; ph is total pressure in the high-pressure (feed) side, cm Hg; pl is total pressure in the low-pressure or permeate side, cm Hg: xo is mole fraction of A in reject side; xf is mole fraction of A in feed; and yp is mole fraction of A in permeate. Note that phxo is the partial pressure of A in the reject gas phase and plyp is the partial pressure in the permeate side. A similar equation can be written for component B.
is permeability of A in the membrane, cm3 (STP) · cm/(s · cm2 · cm Hg); VA is flow rate of A in permeate, cm3 (STP)/s; Am is membrane area, cm2; t is membrane thickness, cm; ph is total pressure in the high-pressure (feed) side, cm Hg; pl is total pressure in the low-pressure or permeate side, cm Hg: xo is mole fraction of A in reject side; xf is mole fraction of A in feed; and yp is mole fraction of A in permeate. Note that phxo is the partial pressure of A in the reject gas phase and plyp is the partial pressure in the permeate side. A similar equation can be written for component B.
Equation 13.4-4
![]()
Where ![]() is permeability of B, cm3 (STP) · cm/(s · cm2 · cm Hg). The ideal separation factor
is permeability of B, cm3 (STP) · cm/(s · cm2 · cm Hg). The ideal separation factor ![]() is
is
Equation 13.4-5
![]()
Dividing Eq. (13.4-3) by (13.4-4),
Equation 13.4-6
![]()
This equation relates the reject concentration xo to the permeate concentration yp. The concentration in the bulk or reject gas phase is xo. The permeate concentration yp in the bulk gas phase is the same as the gas concentration y' on the lower-pressure side immediately adjacent to the dense-phase membrane or on the low-pressure side of the dense skin layer of the asymmetric membrane, which is called the interface or local concentration.
Equation (13.4-6) is a quadratic equation and its solution is
Equation 13.4-7
![]()
where
Making an overall material balance on component A,
Equation 13.4-8
![]()
Dividing by Lf and solving for the outlet reject composition,
Equation 13.4-9
![]()
Substituting Vp = θLf from Eq. (13.4-2) into Eq. (13.4-3) and solving for the membrane area, Am,
Equation 13.4-10
![]()
13.4B. Solution of Equations for Design of Complete-Mixing Case
For design of a system, there are seven variables in the complete-mixing model (H1), xf, xo, yp, θ,
![]() , pl/ph, and Am, four of which are independent variables. Two commonly occurring cases are considered here.
, pl/ph, and Am, four of which are independent variables. Two commonly occurring cases are considered here.
Case 1
This is the simplest case, where xf, xo,
![]() , and pl/ph are given and yp, θ, and Am are to be determined by solution of the equations. By use of the quadratic equation, Eq. (13.4-7) is solved for the permeate composition yp in terms of xo. Hence, to solve this case, yp is first calculated using Eq. (13.4-7). Then the fraction of feed permeated, θ, is calculated using Eq. (13.4-9) and the membrane area, Am, using Eq. (13.4-10).
, and pl/ph are given and yp, θ, and Am are to be determined by solution of the equations. By use of the quadratic equation, Eq. (13.4-7) is solved for the permeate composition yp in terms of xo. Hence, to solve this case, yp is first calculated using Eq. (13.4-7). Then the fraction of feed permeated, θ, is calculated using Eq. (13.4-9) and the membrane area, Am, using Eq. (13.4-10).
EXAMPLE 13.4-1. Design of a Membrane Unit for Complete MixingA membrane is to be used to separate a gaseous mixture of A and B whose feed flow rate is Lf = 1 × 104 cm3 (STP)/s and feed composition of A is xf = 0.50 mole fraction. The desired composition of the reject is xo = 0.25. The membrane thickness t = 2.54 × 10-3 cm, the pressure on the feed side is ph = 80 cm Hg, and on the permeate side it is pl = 20 cm Hg. The permeabilities are Solution: Substituting into Eq. (13.4-5),
Using Eq. (13.4-7),
Using the material-balance equation (13.4-9),
Solving, θ = 0.706. Also, using Eq. (13.4-10),
|
Case 2
In this case xf, θ,
![]() , and pl/ph are given and yp, xo, and Am are to be determined. Equation (13.4-7) cannot be solved for yp since xo is unknown. Hence, xo from Eq. (13.4-9) is substituted into Eq. (13.4-7) and the resulting equation solved for yp using the quadratic equation, to give
, and pl/ph are given and yp, xo, and Am are to be determined. Equation (13.4-7) cannot be solved for yp since xo is unknown. Hence, xo from Eq. (13.4-9) is substituted into Eq. (13.4-7) and the resulting equation solved for yp using the quadratic equation, to give
Equation 13.4-11
![]()
where

After solving for yp, the value of xo is calculated from Eq. (13.4-9) and Am from Eq. (13.4-10).
EXAMPLE 13.4-2. Membrane Design for Separation of AirIt is desired to determine the membrane area needed to separate an air stream using a membrane 1 mil thick with an oxygen permeability of Solution: Using Eq. (13.4-11) for a feed composition of xf = 0.209,
Substituting into Eq. (13.4-9),
Finally, using Eq. (13.4-10) to find the area,
|
13.4C. Minimum Concentration of Reject Stream
If all of the feed is permeated, then θ = 1 and the feed composition xf = yp. For all values of θ < 1, the permeate composition yp > xf (H1). Substituting the value xf = yp into Eq. (13.4-7) and solving, the minimum reject composition xoM for a given xf value is obtained as
Equation 13.4-12

Hence, a feed of xf concentration cannot be stripped lower than a value of xoM even with an infinitely large membrane area for a completely mixed system. To strip beyond this limiting value, a cascade-type system could be used. However, a single unit which is not completely mixed but is designed for plug flow could also be used.
EXAMPLE 13.4-3. Effect of Feed Composition on Minimum Reject ConcentrationCalculate the minimum reject concentration for Example 13.4-1 where the feed concentration is xf = 0.50. Also, what is the effect of raising the feed purity to xf = 0.65? Solution: Substituting xf = 0.50 into Eq. (13.4-12),
For xf = 0.65,
|