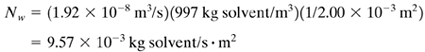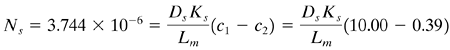13.9. REVERSE-OSMOSIS MEMBRANE PROCESSES
13.9A. Introduction
1. Introduction
To be useful for separation of different species, a membrane must allow passage of certain molecules and exclude or greatly restrict passage of others. In osmosis, a spontaneous transport of solvent occurs from a dilute solute or salt solution to a concentrated solute or salt solution across a semipermeable membrane which allows passage of the solvent but impedes passage of the salt solutes. In Fig. 13.9-1a, the solvent water normally flows through the semipermeable membrane to the salt solution. The levels of both liquids are the same as shown. The solvent flow can be reduced by exerting a pressure on the salt-solution side and membrane, as shown in Fig. 13.9-1b, until at a certain pressure, called the osmotic pressure π of the salt solution, equilibrium is reached and the amount of the solvent passing in opposite directions is equal. The chemical potentials of the solvent on both sides of the membrane are equal. The properties of the solution determine only the value of the osmotic pressure, not the membrane, provided that it is truly semipermeable. To reverse the flow of the water so that it flows from the salt solution to the fresh solvent, as in Fig. 13.9-1c, the pressure is increased above the osmotic pressure on the solution side.
Figure 13.9-1. Osmosis and reverse osmosis: (a) osmosis, (b) osmotic equilibrium, (c) reverse osmosis.

This phenomenon, called reverse osmosis, is used in a number of processes. An important commercial use is in the desalination of seawater or brackish water to produce fresh water. Unlike distillation and freezing processes used to remove solvents, reverse osmosis can operate at ambient temperature without phase change. This process is quite useful for the processing of thermally and chemically unstable products. Applications include concentration of fruit juices and milk, recovery of protein and sugar from cheese whey, and concentration of enzymes.
2. Osmotic pressure of solutions
Experimental data show that the osmotic pressure π of a solution is proportional to the concentration of the solute and temperature T. Van't Hoff originally showed that the relationship is similar to that for pressure of an ideal gas. For example, for dilute water solutions,
Equation 13.9-1
![]()
where n is the number of kg mol of solute, Vm the volume of pure solvent water in m3 associated with n kg mol of solute, R the gas law constant 82.057 × 10-3 m3 · atm/kg mol · K, and T is temperature in K. If a solute exists as two or more ions in solution, n represents the total number of ions. For more concentrated solutions, Eq. (13.9-1) is modified using the osmotic coefficient ϕ, which is the ratio of the actual osmotic pressure π to the ideal π calculated from the equation. For very dilute solutions, ϕ has a value of unity and usually decreases as concentration increases. In Table 13.9-1 some experimental values of π are given for NaCl solutions, sucrose solutions, and seawater solutions (S3, S5).
| Sodium Chloride Solutions | Sea Salt Solutions | Sucrose Solutions | ||||
|---|---|---|---|---|---|---|
| Density (kg/m3) | Osmotic Pressure (atm) | Wt % Salts | Osmotic Pressure (atm) | Solute Mol. Frac. × 103 | Osmotic Pressure (atm) | |
| 0 | 997.0 | 0 | 0 | 0 | 0 | 0 |
| 0.01 | 997.4 | 0.47 | 1.00 | 7.10 | 1.798 | 2.48 |
| 0.10 | 1001.1 | 4.56 | 3.45[*] | 25.02 | 5.375 | 7.48 |
| 0.50 | 1017.2 | 22.55 | 7.50 | 58.43 | 10.69 | 15.31 |
| 1.00 | 1036.2 | 45.80 | 10.00 | 82.12 | 17.70 | 26.33 |
| 2.00 | 1072.3 | 96.2 | ||||
[*] Value for standard seawater.
EXAMPLE 13.9-1. Calculation of Osmotic Pressure of Salt SolutionCalculate the osmotic pressure of a solution containing 0.10 g mol NaCl/1000 g H2O at 25°C. Solution: From Table A.2-3, the density of water = 997.0 kg/m3. Then, n = 2 × 0.10 × 10-3 = 2.00 × 10-4 kg mol (NaCl gives two ions). Also, the volume of the pure solvent water Vm = 1.00 kg/(997.0 kg/m3). Substituting into Eq. (13.9-1),
This compares with the experimental value in Table 13.9-1 of 4.56 atm. |
3. Types of membranes for reverse osmosis
One of the more important membranes for reverse-osmosis desalination and many other reverse-osmosis processes is the cellulose acetate membrane. The asymmetric membrane is made as a composite film in which a thin, dense layer about 0.1–10 μm thick of extremely fine pores is supported upon a much thicker (50–125 μm) layer of microporous sponge with little resistance to permeation. The thin, dense layer has the ability to block the passage of quite small solute molecules. In desalination the membrane rejects the salt solute and allows the solvent water to pass through. Solutes which are most effectively excluded by the cellulose acetate membrane are the salts NaCl, NaBr, CaCl2, and Na2SO4; sucrose; and tetralkyl ammonium salts. The main limitations of the cellulose acetate membrane are that for the most part it can only be used in aqueous solutions and that it must be used below about 60°C.
Another important membrane useful for seawater, wastewater, nickel-plating rinse solutions, and other solutes is the synthetic aromatic polyamide membrane "Permasep," made in the form of very fine, hollow fibers (L1, P3). When used industrially this type of membrane withstands continued operation at pH values of 10 to 11 (S4). Many other anisotropic membranes have also been synthesized from synthetic polymers, some of which can be used in organic solvents, at higher temperatures, and at high or low pH (M2, Rl).
13.9B. Flux Equations for Reverse Osmosis
1. Basic models for membrane processes
There are two basic types of mass-transport mechanisms which can take place in membranes. In the first basic type, using tight membranes, which are capable of retaining solutes of about 10 ![]() in size or less, diffusion-type transport mainly occurs. Both the solute and the solvent migrate by molecular or Fickian diffusion in the polymer, driven by concentration gradients set up in the membrane by the applied pressure difference. In the second basic type, using loose, microporous membranes which retain particles larger than 10
in size or less, diffusion-type transport mainly occurs. Both the solute and the solvent migrate by molecular or Fickian diffusion in the polymer, driven by concentration gradients set up in the membrane by the applied pressure difference. In the second basic type, using loose, microporous membranes which retain particles larger than 10 ![]() , a sieve-type mechanism occurs, where the solvent moves through the micropores in essentially viscous flow and the solute molecules small enough to pass through the pores are carried by convection with the solvent. For details of this second type of mechanism, see (M2, W1).
, a sieve-type mechanism occurs, where the solvent moves through the micropores in essentially viscous flow and the solute molecules small enough to pass through the pores are carried by convection with the solvent. For details of this second type of mechanism, see (M2, W1).
2. Diffusion-type model
For diffusion-type membranes, the steady-state equations governing the transport of solvent and solute are to a first approximation as follows (M2, M3). For the diffusion of the solvent through the membrane, as shown in Fig. 13.9-2,
Equation 13.9-2
![]()
Equation 13.9-3
Figure 13.9-2. Concentrations and fluxes in reverse-osmosis process.
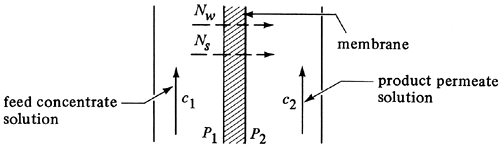
where Nw is the solvent (water) flux in kg/s · m2; Pw the solvent membrane permeability, kg solvent/s · m · atm; Lm the membrane thickness, m; Aw the solvent permeability constant, kg solvent/s · m2 · atm; ΔP = P1 - P2 (hydrostatic pressure difference with P1 pressure exerted on feed and P2 on product solution), atm; and Δπ = π1 - π2 (osmotic pressure of feed solution — osmotic pressure of product solution), atm. Note that subscript 1 is the feed or upstream side of the membrane and 2 the product or downstream side of the membrane.
For the diffusion of the solute through the membrane, an approximation for the flux of the solute is (Cl, Ml)
Equation 13.9-4
![]()
Equation 13.9-5
![]()
where Ns is the solute (salt) flux in kg solute/s · m2; Ds the diffusivity of solute in membrane, m2/s; Ks = cm/c (distribution coefficient), concentration of solute in membrane/concentration of solute in solution; As is the solute permeability constant, m/s; c1 the solute concentration in upstream or feed (concentrate) solution, kg solute/m3; and c2 the solute concentration in downstream or product (permeate) solution, kg solute/m3. The distribution coefficient Ks is approximately constant over the membrane.
Making a material balance at steady state for the solute, the solute diffusing through the membrane must equal the amount of solute leaving in the downstream or product (permeate) solution:
Equation 13.9-6

where cw2 is the concentration of solvent in stream 2 (permeate), kg solvent/m3. If the stream 2 is dilute in solute, cw2 is approximately the density of the solvent. In reverse osmosis, the solute rejection R is defined as the ratio concentration difference across the membrane divided by the bulk concentration on the feed or concentrate side (fraction of solute remaining in the feed stream):
Equation 13.9-7
![]()
This can be related to the flux equations as follows, by first substituting Eqs. (13.9-2) and (13.9-4) into (13.9-6) to eliminate Nw and Ns in Eq. (13.9-6). Then, solving for c2/c1 and substituting this result into Eq. (13.9-7),
Equation 13.9-8
![]()
Equation 13.9-9
![]()
where B is in atm-1. Note that B is composed of the various physical properties Pw, Ds, and Ks of the membrane and must be determined experimentally for each membrane. Usually it is the product DsKs that is determined, not the values of Ds and Ks separately. Also, many of the data reported in the literature give values of (Pw/Lm) or Aw in kg solvent/s · m2 · atm and (DsKs/Lm) or As in m/s and not separate values of Lm, Pw, and so on.
EXAMPLE 13.9-2. Experimental Determination of Membrane PermeabilityExperiments at 25°C were performed to determine the permeabilities of a cellulose acetate membrane (A1, W1). The laboratory test section shown in Fig. 13.9-3 has membrane area A = 2.00 × 10-3 m2. The inlet feed solution concentration of NaCl is c1 = 10.0 kg NaCl/m3 solution (10.0 g NaCl/L, ρ1 = 1004 kg solution/m3). The water recovery is assumed low so that the concentration c1 in the entering feed solution flowing past the membrane and the concentration of the exit feed solution are essentially equal. The product solution contains c2 = 0.39 kg NaCl/m3 solution (ρ2 = 997 kg solution/m3) and its measured flow rate is 1.92 × 10-8 m3 solution/s. A pressure differential of 5514 kPa (54.42 atm) is used. Calculate the permeability constants of the membrane and the solute rejection R. Figure 13.9-3. Process flow diagram of experimental reverse-osmosis laboratory unit.
Solution: Since c2 is very low (dilute solution), the value of cw2 can be assumed as the density of water (Table 13.9-1), or cw2 = 997 kg solvent/m3. To convert the product flow rate to water flux, Nw, using an area of 2.00 × 10-3 m2,
Substituting into Eq. (13.9-6),
To determine the osmotic pressures from Table 13.9-1, the concentrations are converted as follows. For c1, 10 kg NaCl is in 1004 kg solution/m3 (ρ1 = 1004). Then, 1004 - 10 = 994 kg H2O in 1 m3 solution. Hence, in the feed solution, where the molecular weight of NaCl = 58.45, (10.00 × 1000)/ (994 × 58.45) = 0.1721 g mol NaCl/kg H2O. From Table 13.9-1, π1 = 7.80 atm by linear interpolation. Substituting into Eq. (13.9-1), the predicted π1 = 8.39 atm, which is higher than the experimental value. For the product solution, 997 - 0.39 = 996.6 kg H2O. Hence, (0.39 × 1000)/(996.6 × 58.45) = 0.00670 g mol NaCl/kg H2O. From Table 13.9-1, π2 = 0.32 atm. Then, Δπ = π1 - π2 = 7.80 - 0.32 = 7.48 atm and ΔP = 54.42 atm. Substituting into Eq. (13.9-2),
Solving, (Pw/Lm) = Aw = 2.039 × 10-4 kg solvent/s · m2 · atm. Substituting into Eq. (13.9-4),
Solving, (DsKs/Lm) = As = 3.896 × 10-7 m/s. To calculate the solute rejection R by substituting into Eq. (13.9-7),
Also, substituting into Eq. (13.9-9) and then Eq. (13.9-8),
|