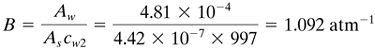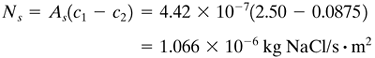13.10. APPLICATIONS, EQUIPMENT, AND MODELS FOR REVERSE OSMOSIS
13.10A. Effects of Operating Variables
In many commercial units, operating pressures in reverse osmosis range from about 1035 up to 10350 kPa (150 up to 1500 psi). Comparison of Eq. (13.9-2) for solvent flux with Eq. (13.9-4) for solute flux shows that the solvent flux Nw depends only on the net pressure difference, while the solute flux Ns depends only on the concentration difference. Hence, as the feed pressure is increased, solvent or water flow through the membrane increases and the solute flow remains approximately constant, giving lower solute concentration in the product solution.
At a constant applied pressure, increasing the feed solute concentration increases the product solute concentration. This is caused by the increase in the feed osmotic pressure, since as more solvent is extracted from the feed solution (as water recovery increases), the solute concentration becomes higher and the water flux decreases. Also, the amount of solute present in the product solution increases because of the higher feed concentration.
If a reverse-osmosis unit has a large membrane area (as in a commercial unit), and the path between the feed inlet and outlet is long, the outlet feed concentration can be considerably higher than the inlet feed c1. Then the salt flux will be greater at the outlet feed as compared to the inlet (K2). Many manufacturers use the feed solute or salt concentration average between inlet and outlet to calculate the solute or salt rejection R in Eq. (13.9-7).
EXAMPLE 13.10-1. Prediction of Performance in a Reverse-Osmosis UnitA reverse-osmosis membrane to be used at 25°C for a NaCl feed solution containing 2.5 g NaCl/L (2.5 kg NaCl/m3, ρ = 999 kg/m3) has a water permeability constant Aw = 4.81 × 10-4 kg/s · m2 · atm and a solute (NaCl) permeability constant As = 4.42 × 10-7 m/s (A1). Calculate the water flux and solute flux through the membrane using ΔP = 27.20 atm and the solute rejection R. Also calculate c2 of the product solution. Solution: In the feed solution, c1 = 2.5 kg NaCl/m3 and ρ1 = 999 kg solution/m3. Hence, for the feed, 999 - 2.5 = 996.5 kg H2O in 1.0 m3 solution: also for the feed, (2.50 × 1000)/(996.5 × 58.45) = 0.04292 g mol NaCl/kg H2O. From Table 13.9-1, π1 = 1.97 atm. Since the product solution c2 is unknown, a value of c2 = 0.1 kg NaCl/m3 will be assumed. Also, since this is quite dilute, ρ2 = 997 kg solution/m3 and Cw2 = 997 kg solvent/m3. Then for the product solution, (0.10 × 1000)/(996.9 × 58.45) = 0.00172 g mol NaCl/kg H2O and π2 = 0.08 atm. Also, Δπ = π1 - π2 = 1.97 - 0.08 = 1.89 atm. Substituting into Eq. (13.9-2),
For calculation of R, substituting first into Eq. (13.9-9),
Next, substituting into Eq. (13.9-8),
Using this value of R in Eq. (13.9-7),
Solving, c2 = 0.0875 kg NaCl/m3 for the product solution. This is close enough to the assumed value of c2 = 0.10 that π2 will not change significantly on a second trial. Hence, the final value of c2 is 0.0875 kg NaCl/m3 (0.0875 g NaCl/L). Substituting into Eq. (13.9-4),
|
13.10B. Concentration Polarization in Reverse-Osmosis Diffusion Model
In desalination, localized concentrations of solute build up at the point where the solvent leaves the solution and enters the membrane. The solute accumulates in a relatively stable boundary layer (Fig. 13.9-3) next to the membrane. Concentration polarization, β, is defined as the ratio of the salt concentration at the membrane surface to the salt concentration in the bulk feed stream c1. Concentration polarization causes the water flux to decrease, since the osmotic pressure π1 increases as the boundary layer concentration increases and the overall driving force (ΔP - Δπ) decreases. Also, the solute flux increases, since the solute concentration increases at the boundary. Hence, often the ΔP must be increased to compensate, which results in higher power costs (K2).
The effect of the concentration polarization β can be included approximately by modifying the value of Δπ in Eqs. (13.9-2) and (13.9-8) as follows (P6):
Equation 13.10-1
![]()
It is assumed that the osmotic pressure π1 is directly proportional to the concentration, which is approximately correct. Also, Eq. (13.9-4) can be modified as
Equation 13.10-2
![]()
The usual concentration polarization ratio (K3) is 1.2 to 2.0, that is, the concentration in the boundary layer is 1.2-2.0 times c1 in the bulk feed solution. This ratio is often difficult to predict. In desalination of seawater, using values of about 1000 psia = ΔP, π1 can be large. Increasing this π1 by a factor of 1.2-2.0 can appreciably reduce the solvent flux. For brackish waters containing 2–10 g/L and using ΔP values of 17–55 atm abs, the value of π1 is low and concentration polarization is not important.
The boundary layer can be reduced by increasing the turbulence by using higher feed-solution velocities. However, this extra flow results in a smaller ratio of product solution to feed. Also, screens can be put in the path to induce turbulence. Equations for predicting the mass-transfer coefficient to the surface and, hence, the concentration polarization, are given for specific geometries such as flow past plates, inside tubes, outside tubes, and so on (H2, N1). Then equations for the flux of water can be used with these mass-transfer coefficients in a manner similar to that for ultrafiltration given in Section 13.11.
13.10C. Permeability Constants for Reverse-Osmosis Membranes
Permeability constants for membranes must be determined experimentally for the particular type of membrane to be used. For cellulose acetate membranes, typical water permeability constants Aw range from about 1 × 10-4 to 5 × 10-4 kg solvent/s · m2 · atm (A1, M3, W1). Values for other types of membranes can differ widely. Generally, the water permeability constant for a particular membrane does not depend upon the solute present. For the solute permeability constants AS of cellulose acetate membranes, some relative typical values are as follows, assuming a value of As = 4 × 10-7 m/s for NaCl: 1.6 × 10-7 m/s (BaCl2), 2.2 × 10-7 (MgCl2), 2.4 × 10-7 (CaCl2), 4.0 × 10-7 (Na2SO4), 6.0 × 10-7 (KC1), 6.0 × 10-7 (NH4C1) (A1).
13.10D. Types of Equipment for Reverse Osmosis
The equipment for reverse osmosis is quite similar to that for gas permeation membrane processes described in Section 13.3C. In the plate-and-frame-type unit, thin plastic support plates with thin grooves are covered on both sides with membranes as in a filter press. Pressurized feed solution flows between the closely spaced membranes (L1). Solvent permeates through the membrane and flows in the grooves to an outlet. In the tubular-type unit, membranes in the form of tubes are inserted inside porous-tube casings, which serve as a pressure vessel. These tubes are then arranged in bundles like a heat exchanger.
In the spiral-wound type, a planar membrane is used and a flat, porous support material is sandwiched between the membranes. Then the membranes, support, and a mesh feed-side spacer are wrapped in a spiral around a tube. In the hollow-fiber type, fibers of 100–200 μm diameter with walls about 25 μm thick are arranged in a bundle similar to a heat exchanger (L1, R1).
13.10E. Complete-Mixing Model for Reverse Osmosis
The process flow diagram for the complete-mixing model is shown in Fig. 13.10-1. The model is a simplified one for use with low concentrations of salt of about 1% or so, such as occur in brackish waters. Also, a relatively low recovery of solvent occurs and the effects of concentration polarization are small. Since the concentration of the permeate is very low, the permeate side acts as though it were completely mixed.
Figure 13.10-1. Process flow for complete-mixing model for reverse osmosis.
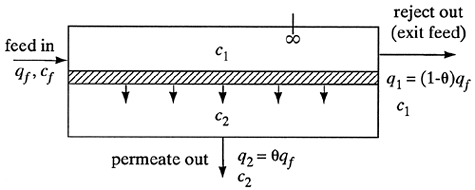
For the overall material balance for dilute solutions,
Equation 13.10-3
![]()
where qf is volumetric flow rate of feed, m3/s; q2 is flow rate of permeate, m3/s; and q1 is flow rate of residue or exit, m3/s. Making a solute balance,
Equation 13.10-4
![]()
Defining the cut or fraction of solvent recovered as θ = q2/qf, Eq. (13.10-4) becomes
Equation 13.10-5
![]()
The equations previously derived for the fluxes and rejection are useful in this case and are as follows:
Equation 13.9-2
![]()
Equation 13.9-4
![]()
Equation 13.9-7
![]()
Equation 13.9-8
![]()
When the cut or fraction recovered, θ, is specified, the solution is trial and error. Since the permeate and reject concentrations c1 and c2 are unknown, a value of c2 is assumed. Then c1 is calculated from Eq. (13.10-5). Next, Nw is obtained from Eq. (13.9-2) and c2 from Eqs. (13.9-7) and (13.9-8). If the calculated value of c2 does not equal the assumed value, the procedure is repeated.
When concentration-polarization effects are present, an estimated value of β can be used to make an approximate correction for these effects. This is used in Eq. (13.10-1) to obtain a value of Δπ for use in Eqs. (13.9-2) and (13.9-8). Also, Eq. (13.10-2) will replace Eq. (13.9-4). A more detailed analysis of this complete mixing model is given by others (H1, K1), in which the mass-transfer coefficient in the concentration-polarization boundary layer is used.
The cross-flow model for reverse osmosis is similar to that for gas separation by membranes which was discussed in Section 13.6. Because of the small solute concentration, the permeate side acts as if completely mixed. Hence, even if the module is designed for countercurrent or cocurrent flow, the cross-flow model is valid. This is discussed in detail elsewhere (H1).