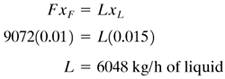8.4. CALCULATION METHODS FOR SINGLE-EFFECT EVAPORATORS
8.4A. Heat and Material Balances for Evaporators
The basic equation for solving for the capacity of a single-effect evaporator is Eq. (8.2-1), which can be written as
Equation 8.4-1
![]()
where ΔTK (°F) is the difference in temperature between the condensing steam and the boiling liquid in the evaporator. In order to solve Eq. (8.4-1) the value of q in W (btu/h) must be determined by making a heat and material balance on the evaporator shown in Fig. 8.4-1. The feed to the evaporator is F kg/h (lbm/h) having a solids content of xF mass fraction, temperature TF, and enthalpy hF J/kg (btu/lbm). Coming out as a liquid is the concentrated liquid L kg/h (lbm/h) having a solids content of xL, temperature T1, and enthalpy hL. The vapor V kg/h (lbm/h) is given off as pure solvent having a solids content of yV = 0, temperature T1, and enthalpy HV. Saturated steam entering is S kg/h (lbm/h) and has a temperature of Ts and enthalpy of HS. The condensed steam leaving of S kg/h is assumed usually to be at Ts, the saturation temperature, with an enthalpy of hS. This means that the steam gives off only its latent heat, λ, where
Equation 8.4-2
![]()
Figure 8.4-1. Heat and mass balance for single-effect evaporator.

Since the vapor V is in equilibrium with the liquid L, the temperatures of vapor and liquid are the same. Also, the pressure P1 is the saturation vapor pressure of the liquid of composition xL at its boiling point T1. (This assumes no boiling-point rise.)
For the material balance, since we are at steady state, the rate of mass in = rate of mass out. Then, for a total balance,
Equation 8.4-3
![]()
For a balance on the solute (solids) alone,
Equation 8.4-4
![]()
For the heat balance, since the total heat entering = total heat leaving,
Equation 8.4-5
![]()
This assumes no heat lost by radiation or convection. Substituting into Eq. (8.4-5),
Equation 8.4-6
![]()
Substituting Eq. (8.4-2) into (8.4-6),
Equation 8.4-7
![]()
The heat q transferred in the evaporator is then
Equation 8.4-8
![]()
In Eq. (8.4-7) the latent heat λ of steam at the saturation temperature Ts can be obtained from the steam tables in Appendix A.2. However, the enthalpies of the feed and products are often not available; these enthalpy-concentration data are available for only a few substances in solution. Hence, some approximations are made in order to make a heat balance. These are as follows:
It can be demonstrated as an approximation that the latent heat of evaporation of 1 kg mass of the water from an aqueous solution can be obtained from the steam tables using the temperature of the boiling solution T1 (exposed surface temperature) rather than the equilibrium temperature for pure water at P1.
EXAMPLE 8.4-1. Heat-Transfer Area in Single-Effect EvaporatorA continuous single-effect evaporator concentrates 9072 kg/h of a 1.0 wt % salt solution entering at 311.0 K (37.8°C) to a final concentration of 1.5 wt %. The vapor space of the evaporator is at 101.325 kPa (1.0 atm abs) and the steam supplied is saturated at 143.3 kPa. The overall coefficient U = 1704 W/m2 · K. Calculate the amounts of vapor and liquid product and the heat-transfer area required. Assume that, since it is dilute, the solution has the same boiling point as water. Solution: The flow diagram is the same as in Fig. 8.4-1. For the material balance, substituting into Eq. (8.4-3), Equation 8.4-3
Substituting into Eq. (8.4-4) and solving, Equation 8.4-4
Substituting into Eq. (8.4-3) and solving,
The heat capacity of the feed is assumed to be The enthalpy of the feed can be calculated from Equation 8.4-9
Substituting into Eq. (8.4-7) with hL = 0, since it is at the datum of 373.2 K,
The heat q transferred through the heating surface area A is, from Eq. (8.4-8), Equation 8.4-8
Substituting into Eq. (8.4-1), where ΔT = Ts – T1,
Solving, A = 149.3 m2. |
8.4B. Effects of Processing Variables on Evaporator Operation
1. Effect of feed temperature
The inlet temperature of the feed has a large effect on the operation of the evaporator. In Example 8.4-1 the feed entering was at a temperature of 311.0 K, cold as compared to the boiling temperature of 373.2 K. About ![]() of the steam used for heating was used to heat the cold feed to the boiling point. Hence, only about
of the steam used for heating was used to heat the cold feed to the boiling point. Hence, only about ![]() of the steam was left for vaporization of the feed. If the feed is under pressure and enters the evaporator at a temperature above the boiling point in the evaporator, additional vaporization is obtained by flashing part of the entering hot feed. Preheating the feed can reduce the size of evaporator heat-transfer area needed.
of the steam was left for vaporization of the feed. If the feed is under pressure and enters the evaporator at a temperature above the boiling point in the evaporator, additional vaporization is obtained by flashing part of the entering hot feed. Preheating the feed can reduce the size of evaporator heat-transfer area needed.
2. Effect of pressure
In Example 8.4-1 a pressure of 101.32 kPa abs was used in the vapor space of the evaporator. This set the boiling point of the solution at 373.2 K and gave a ΔT for use in Eq. (8.4-1) of 383.2 – 373.2, or 10 K. In many cases a larger ΔT is desirable, since, as ΔT increases, the heating-surface area A and cost of the evaporator decrease. To reduce the pressure below 101.32 kPa, that is, to be under vacuum, a condenser and vacuum pump can be used. For example, if the pressure were reduced to 41.4 kPa, the boiling point of water would be 349.9 K and the new ΔT would be 383.2 – 349.9, or 33.3 K. A large decrease in heating-surface area would be obtained.
3. Effect of steam pressure
Using higher-pressure, saturated steam increases ΔT, which decreases the size and cost of the evaporator. However, high-pressure steam is more costly as well as often being more valuable as a source of power elsewhere. Hence, overall economic balances are really needed to determine the optimum steam pressures.
8.4C. Boiling-Point Rise of Solutions
In the majority of cases in evaporation, the solutions are not dilute solutions such as those considered in Example 8.4-1. In most cases, the thermal properties of the solution being evaporated may differ considerably from those of water. The concentrations of the solutions are high enough that the heat capacity and boiling point are quite different from those for water.
For strong solutions of dissolved solutes the boiling-point rise due to the solutes in the solution usually cannot be predicted. However, a useful empirical law known as Dühring's rule can be applied. According to this rule, a straight line is obtained if the boiling point of a solution in °C or °F is plotted against the boiling point of pure water at the same pressure for a given concentration at different pressures. A different straight line is obtained for each given concentration. In Fig. 8.4-2 such a Dühring-line chart is given for solutions of sodium hydroxide in water. It is necessary to know the boiling point of a given solution at only two pressures to determine a line.
Figure 8.4-2. Dühring lines for aqueous solutions of sodium hydroxide.

EXAMPLE 8.4-2. Use of Dühring Chart for Boiling-Point RiseAs an example of use of the chart, the pressure in an evaporator is given as 25.6 kPa (3.72 psia) and a solution of 30% NaOH is being boiled. Determine the boiling temperature of the NaOH solution and the boiling-point rise BPR of the solution over that of water at the same pressure. Solution: From the steam tables in Appendix A.2, the boiling point of water at 25.6 kPa is 65.6°C. From Fig. 8.4-2 for 65.6°C (150°F) and 30% NaOH, the boiling point of the NaOH solution is 79.5°C (175°F). The boiling-point rise is 79.5 – 65.6 = 13.9°C (25°F). |
In Perry and Green (P1) a chart is given for estimating the boiling-point rise of a large number of common aqueous solutions used in chemical and biological processes. In addition to the common salts and solutes, such as NaNO3, NaOH, NaCl, and H2SO4, the biological solutes sucrose, citric acid, kraft solution, and glycerol are given. These biological solutes have quite small boiling-point-rise values compared to those of common salts.
8.4D. Enthalpy-Concentration Charts of Solutions
If the heat of solution of the aqueous solution being concentrated in the evaporator is large, neglecting it could cause errors in the heat balances. This heat-of-solution phenomenon can be explained as follows. If pellets of NaOH are dissolved in a given amount of water, it is found that a considerable temperature rise occurs; that is, heat is evolved, called heat of solution. The amount of heat evolved depends on the type of substance and the amount of water used. Also, if a strong solution of NaOH is diluted to a lower concentration, heat is liberated. Conversely, if a solution is concentrated from a low to a high concentration, heat must be added.
In Fig. 8.4-3 an enthalpy-concentration chart for NaOH is given (M1), where the enthalpy is in kJ/kg (btu/lbm) solution, temperature in °C (°F), and concentration in weight fraction NaOH in solution. Such enthalpy-concentration charts are usually not made for solutions having negligible heats of solution, since the heat capacities can be easily used to calculate enthalpies. Also, such charts are available for only a few solutions.
Figure 8.4-3. Enthalpy-concentration chart for the system NaOH-water. [Reference state liquid water at 0°C (273 K) or 32°F.] [From W. L. McCabe, Trans. A.I.Ch.E., 31, 129 (1935). With permission.]

The enthalpy of the liquid water in Fig. 8.4-3 is referred to the same datum or reference state as in the steam tables, that is, liquid water at 0°C (273 K). This means that enthalpies from the figure can be used with those in the steam tables. In Eq. (8.4-7) values for hF and hL can be taken from Fig. 8.4-3 and values for λ and HV from the steam tables. The uses of Fig. 8.4-3 will be better understood from the following example.
EXAMPLE 8.4-3. Evaporation of an NaOH SolutionAn evaporator is used to concentrate 4536 kg/h (10 000 lbm/h) of a 20% solution of NaOH in water entering at 60°C (140°F) to a product of 50% solids. The pressure of the saturated steam used is 172.4 kPa (25 psia) and the pressure in the vapor space of the evaporator is 11.7 kPa (1.7 psia). The overall heat-transfer coefficient is 1560 W/m2 · K (275 btu/h · ft2 · °F). Calculate the steam used, the steam economy in kg vaporized/kg steam used, and the heating surface area in m2. Solution: The process flow diagram and nomenclature are the same as in Fig. 8.4-1. The given variables are F = 4536 kg/h, xF = 0.20 wt fraction, TF = 60°C, P1 = 11.7 kPa, steam pressure = 172.4 kPa, and xL = 0.50 wt fraction. For the overall material balance, substituting into Eq. (8.4-3), Equation 8.4-3
Substituting into Eq. (8.4-4) and solving (8.4-3) and (8.4-4) simultaneously, Equation 8.4-4
To determine the boiling point T1 of the 50% concentrated solution, we first obtain the boiling point of pure water at 11.7 kPa from the steam tables, Appendix A.2, as 48.9°C (120°F). From the Dühring chart, Fig. 8.4-2, for a boiling point of water of 48.9°C and 50% NaOH, the boiling point of the solution is T1 = 89.5°C (193°F). Hence,
From the enthalpy–concentration chart (Fig. 8.4-3), for 20% NaOH at 60°C (140°F), hf = 214 kJ/kg (92 btu/lbm). For 50% NaOH at 89.5°C (193°F), hL = 505 kJ/kg (217 btu/lbm). For the superheated vapor V at 89.5°C (193°F) and 11.7 kPa [superheated 40.6°C (73°F) since the boiling point of water is 48.9°C (120°F) at 11.7 kPa], from the steam tables, HV = 2667 kJ/kg (1147 btu/lbm). An alternative method for calculating the HV is first to obtain the enthalpy of saturated vapor at 48.9°C (120°F) and 11.7 kPa of 2590 kJ/kg (1113.5 btu/lbm). Then, using a heat capacity of 1.884 kJ/kg · K for superheated steam with the superheat of (89.5 – 48.9)°C = (89.5 – 48.9) K,
For the saturated steam at 172.4 kPa, the saturation temperature from the steam tables is 115.6°C (240°F) and the latent heat is λ = 2214 kJ/kg (952 btu/lbm). Substituting into Eq. (8.4-7) and solving for S, Equation 8.4-7
Substituting into Eq. (8.4-8),
Substituting into Eq. (8.4-1) and solving,
Hence, A = 49.2 m2. Also, steam economy = 2722/3255 = 0.836. |