10.3.1 Is the Adsorption of Cumene Rate-Limiting?
To answer this question we shall assume that the adsorption of cumene is indeed rate-limiting, derive the corresponding rate law, and then check to see if it is consistent with experimental observation. By postulating that this (or any other) step is rate-limiting, we are assuming that the reaction rate constant of this step (in this case kA) is small with respect to the specific rates of the other steps (in this case kS and kD).10 The rate of adsorption is

Because we can measure neither Cv or CC · S, we must replace these variables in the rate law with measurable quantities for the equation to be meaningful.
For steady-state operation we have
![]()
For adsorption-limited reactions, kA is very small and kS and kD are very large. Consequently, the ratios rS/kS and rD/kD are very small (approximately zero), whereas the ratio rAD/kA is relatively large.
The surface reaction rate law is

Again, for adsorption-limited reactions, the surface-specific reaction rate kS is large by comparison, and we can set
![]()
and solve Equation (10-31) for CC · S:
![]()
To be able to express CC · S solely in terms of the partial pressures of the species present, we must evaluate CB · S. The rate of desorption of benzene is
![]()
However, for adsorption-limited reactions, kD is large by comparison, and we can set
![]()
and then solve Equation (10-29) for CB · S:
![]()
After combining Equations (10-33) and (10-35), we have
![]()
Replacing CC · S in the rate equation by Equation (10-36) and then factoring Cv, we obtain

We observe that at equilibrium that rAD = 0 and Equation (10-37) rearranges to
![]()
We also know from thermodynamics (Appendix C) that for the reaction
![]()
also at equilibrium ![]() we have the following relationship for partial pressure equilibrium constant KP:
we have the following relationship for partial pressure equilibrium constant KP:
![]()
Consequently, the following relationship must hold

The equilibrium constant can be determined from thermodynamic data and is related to the change in the Gibbs free energy, ΔG°, by the equation (see Appendix C)
![]()
where R is the ideal gas constant and T is the absolute temperature.
The concentration of vacant sites, Cv, can now be eliminated from Equation (10-37) by utilizing the site balance to give the total concentration of sites, Ct, which is assumed constant11:
Total sites = Vacant sites + Occupied sites
Because cumene and benzene are adsorbed on the surface, the concentration of occupied sites is (CC · S + CB · S), and the total concentration of sites is
![]()
Substituting Equations (10-35) and (10-36) into Equation (10-40), we have
![]()
Solving for Cυ, we have
![]()
Combining Equations (10-41) and (10-37), we find that the rate law for the catalytic decomposition of cumene, assuming that the adsorption of cumene is the rate-limiting step, is

We now wish to sketch a plot of the initial rate of reaction as a function of the partial pressure of cumene, PC0. Initially, no products are present; consequently, PP = PB = 0. The initial rate is given by
![]()
If the cumene decomposition is adsorption rate limited, then the initial rate will be linear with the initial partial pressure of cumene, as shown in Figure 10-15.
Figure 10-15. Adsorption-limited reaction.
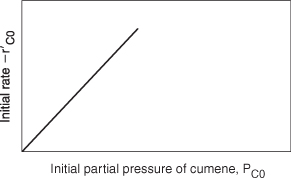
Before checking to see if Figure 10-15 is consistent with experimental observation, we shall derive the corresponding rate laws for the other possible rate-limiting steps and then develop initial rate plots for the case when the surface reaction is rate-limiting and then for the case when the desorption of benzene is rate-limiting.
