10.3.4 Summary of the Cumene Decomposition
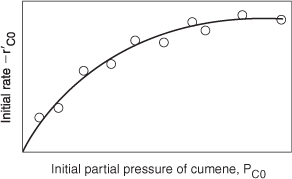
The experimental observations of ![]() as a function of PC0 are shown in Figure 10-18. From the plot in Figure 10-18, we can clearly see that neither adsorption nor desorption is rate-limiting. For the reaction and mechanism given by
as a function of PC0 are shown in Figure 10-18. From the plot in Figure 10-18, we can clearly see that neither adsorption nor desorption is rate-limiting. For the reaction and mechanism given by
![]()
![]()
![]()
the rate law derived by assuming that the surface reaction is rate-limiting agrees with the data.
Figure 10-18. Actual initial rate as a function of partial pressure of cumene.
The rate law for the case of no inerts adsorbing on the surface is
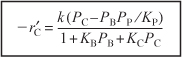
The forward cumene decomposition reaction is a single-site mechanism involving only adsorbed cumene, while the reverse reaction of propylene in the gas phase reacting with adsorbed benzene is an Eley–Rideal mechanism.
If we were to have an adsorbing inert in the feed, the inert would not participate in the reaction but would occupy active sites on the catalyst surface:
![]()
Our site balance is now
![]()
Because the adsorption of the inert is at equilibrium, the concentration of sites occupied by the inert is
![]()
Substituting for the inert sites in the site balance, the rate law for surface reaction control when an adsorbing inert is present is
![]()
One observes that the rate decreases as the partial pressure of inerts increases.
