10.4.2 Finding a Mechanism Consistent with Experimental Observations
We now propose a mechanism for the hydrodemethylation of toluene. We assume that toluene is adsorbed on the surface and then reacts with hydrogen in the gas phase to produce benzene adsorbed on the surface and methane in the gas phase. Benzene is then desorbed from the surface. Because approximately 75% to 80% of all heterogeneous reaction mechanisms are surface-reaction-limited rather than adsorption- or desorption-limited, we begin by assuming the reaction between adsorbed toluene and gaseous hydrogen to be reaction-rate-limited. Symbolically, this mechanism and associated rate laws for each elementary step are
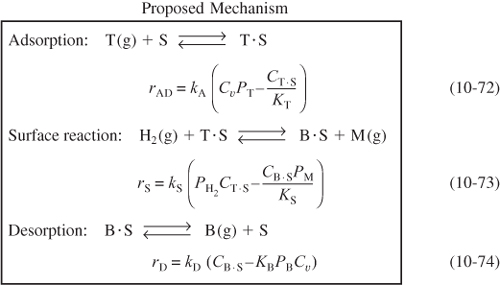
For surface-reaction-limited mechanisms,
![]()
we see that we need to replace CT · S and CB · S in Equation (10-73) by quantities that we can measure.
For surface-reaction-limited mechanisms, we use the adsorption rate Equation (10-72) to obtain CT · S16:
![]()
Then
![]()
and we use the desorption rate Equation (10-74) to obtain CB · S:
![]()
Then
![]()
The total concentration of sites is
![]()
Substituting Equations (10-75) and (10-76) into Equation (10-77) and rearranging, we obtain
![]()
Next, substitute for CT · S and CB · S and then substitute for Cv in Equation (10-73) to obtain the rate law for the case of surface-reaction control:

Neglecting the reverse reaction, we have
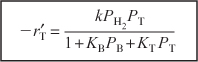
Again we note that the adsorption equilibrium constant of a given species is exactly the reciprocal of the desorption equilibrium constant of that species.
