Chapter 11. Nonisothermal Reactor Design–The Steady State Energy Balance and Adiabatic PFR Applications
If you can’t stand the heat, get out of the kitchen.
—Harry S Truman
11.1 Rationale
To identify the additional information necessary to design nonisothermal reactors, we consider the following example, in which a highly exothermic reaction is carried out adiabatically in a plug-flow reactor.
Example 11-1. What Additional Information Is Required?
The first order liquid phase reaction
![]()
is carried out in a PFR. The reaction is exothermic and the reactor is operated adiabatically. As a result, the temperature will increase with conversion down the length of the reactor. Because T varies along the length of the reactor, k will also vary, which was not the case for isothermal plug-flow reactors.
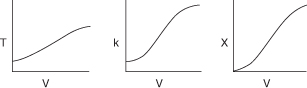
Calculate the reactor volume necessary for 70% conversion.
The same CRE algorithm can be applied to nonisothermal reactions as to isothermal reactions by adding one more step, the energy balance.
- Mole Balance (design equation):
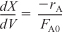
- Rate Law:

Recalling the Arrhenius equation,

we know that k is a function of temperature, T.
- Stoichiometry (liquid phase): υ = υ0
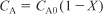
- Combining:
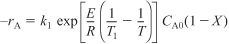
Combining Equations (E11-1.1), (E11-1.2), and (E11-1.4) and canceling the entering concentration, CA0, yields
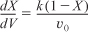
Combining Equations (E11-1.3) and (E11-1.6) gives us
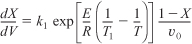
We see that we need another relationship relating X and T or T and V to solve this equation. The energy balance will provide us with this relationship.
So we add another step to our algorithm; this step is the energy balance.
- Energy Balance:
In this step, we will find the appropriate energy balance to relate temperature and conversion or reaction rate. For example, if the reaction is adiabatic, we will show that the temperature-conversion relationship can be written in a form such as

We now have all the equations we need to solve for the conversion and temperature profiles.
Analysis: The purpose of this example was to demonstrate that for non-isothermal chemical reactions we need another step in our CRE algorithm, the energy balance. The energy balance allows us to solve for the reaction temperature, which is necessary in evaluating the specific reaction rate constant k(T).
