11.5.1 Equilibrium Conversion
Exothermic Reactions
Figure 11-3(a) shows the variation of the concentration equilibrium constant as a function of temperature for an exothermic reaction (see Appendix C), and Figure 11-3(b) shows the corresponding equilibrium conversion Xe as a function of temperature. In Example 11-3, we saw that for a first-order reaction the equilibrium conversion could be calculated using Equation (E11-3.13)
![]()
Figure 11-3. Variation of equilibrium constant and conversion with temperature for an exothermic reaction.
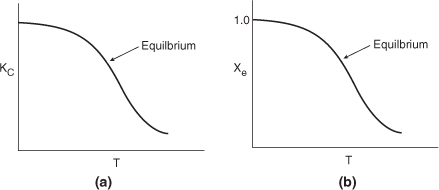
Consequently, Xe can be calculated directly using Figure 11-3(a).
We note that the shape of the Xe versus T curve in Figure 11-3(b) will be similar for reactions that are other than first order.
To determine the maximum conversion that can be achieved in an exothermic reaction carried out adiabatically, we find the intersection of the equilibrium conversion as a function of temperature [Figure 11-3(b)] with temperature–conversion relationships from the energy balance (Figure 11-2 and Equation (T11-1.A)), as shown in Figure 11-4.
![]()
Figure 11-4. Graphical solution of equilibrium and energy balance equations to obtain the adiabatic temperature and the adiabatic equilibrium conversion Xe.
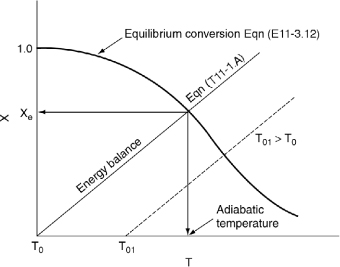
This intersection of the XEB line with the Xe curve gives the adiabatic equilibrium conversion and temperature for an entering temperature T0.
If the entering temperature is increased from T0 to T01, the energy balance line will be shifted to the right and be parallel to the original line, as shown by the dashed line. Note that as the inlet temperature increases, the adiabatic equilibrium conversion decreases.
Example 11-4. Calculating the Adiabatic Equilibrium Temperature
For the elementary solid-catayzed liquid-phase reaction
![]()
make a plot of equilibrium conversion as a function of temperature.
Determine the adiabatic equilibrium temperature and conversion when pure A is fed to the reactor at a temperature of 300 K.
Additional information:
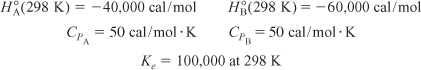
- Rate Law:

- Equilibrium: –rA = 0; so
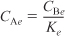

- Stoichiometry: (υ = υ0) yields
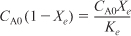
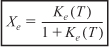
- Equilibrium Constant: Calculate ΔCP, then Ke(T)
ΔCP = CPB – CPA = 50 – 50 = 0 cal/mol · K
For ΔCP = 0, the equilibrium constant varies with temperature according to the relation
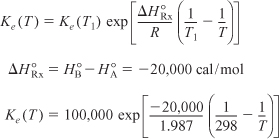
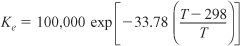
Substituting Equation (E11-4.4) into (E11-4.2), we can calculate the equilibrium conversion as a function of temperature:
- Equilibrium Conversion from Thermodynamics:
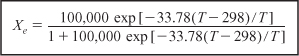
The calculations are shown in Table E11-4.1.
Table E11-4.1. Equilibrium Conversion as a Function of Temperature
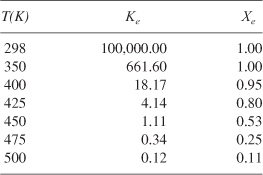
- Energy Balance:
For a reaction carried out adiabatically, the energy balance (Equation (T11-1.A)) reduces to
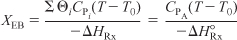
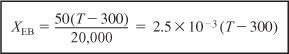
Data from Table E11-6.1 and the following data are plotted in Figure E11-4.1.
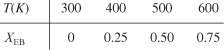
The intersection of XEB(T) and Xe(T) gives Xe= 0.42 and Te = 465 K
For a feed temperature of 300 K, the adiabatic equilibrium temperature is 465 K and the corresponding adiabatic equilibrium conversion is only 0.42.
Figure E11-4.1. Finding the adiabatic equilibrium temperature (Te) and conversion (Xe).
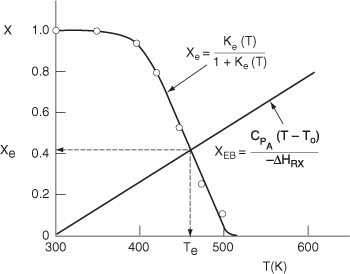
Analysis: The purpose of this example is to introduce the concept of the adiabatic equilibrium conversion and temperature. The adiabatic equilibrium conversion, Xe, is one of the first things to determine when carrying out an analysis involving reversible reactions. It is the maximum conversion one can achieve for a given entering temperature, T0, and feed composition. If Xe is too low to be economical, try lowering the feed temperature and/or adding inerts. From Equation (E11-4.6) we observe that changing the flow rate has no effect on the equilibrium conversion. For exothermic reactions, the adiabatic conversion decreases with increasing entering temperature T0, and for endothermic reactions the conversion increases with increasing entering T0. One can easily generate Figure E11-4.1 using Polymath with Equations (E11-4.5) and (E11-4.7).
If adding inerts or lowering the entering temperature is not feasible then one should consider reactor staging.
