12.5.2 Heat-Generated Term, G(T)
The heat-generated term, Equation (12-29), can be written in terms of conversion. [Recall: X = –rAV/FA0.]
![]()
To obtain a plot of heat generated, G(T), as a function of temperature, we must solve for X as a function of T using the CSTR mole balance, the rate law, and stoichiometry. For example, for a first-order liquid-phase reaction, the CSTR mole balance becomes
![]()
![]()
Substituting for X in Equation (12-31), we obtain
![]()
Finally, substituting for k in terms of the Arrhenius equation, we obtain

Note that equations analogous to Equation (12-33) for G(T) can be derived for other reaction orders and for reversible reactions simply by solving the CSTR mole balance for X. For example, for the second-order liquid-phase reaction
![]()
the corresponding heat generated term is

Let’s now examine the behavior of the G(T) curve. At very low temperatures, the second term in the denominator of Equation (12-33) for the first-order reaction can be neglected, so that G(T) varies as
![]()
[Recall that ![]() means that the standard heat of reaction is evaluated at TR.]
means that the standard heat of reaction is evaluated at TR.]
At very high temperatures, the second term in the denominator dominates, and G(T) is reduced to
![]()
G(T) is shown as a function of T for two different activation energies, E, in Figure 12-9. If the flow rate is decreased or the reactor volume increased so as to increase τ, the heat generated term, G(T), changes, as shown in Figure 12-10.
Figure 12-9. Variation of G(T) curve with activation energy
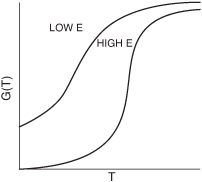
Figure 12-10. Variation of G(T) curve with space-time
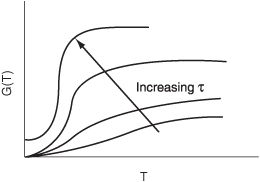
Can you combine Figures 12-10 and 12-8 to explain why a Bunsen burner goes out when you turn up the gas flow rate to a very high rate?
