13.2.2 Case History of a Batch Reactor with Interrupted Isothermal Operation Causing a Runaway Reaction
In Chapters 5 and 6 we discussed the design of reactors operating isothermally. This operation can be achieved by efficient control of a heat exchanger. The following example shows what can happen when the heat exchanger suddenly fails.
Example 13-2. Safety in Chemical Plants with Exothermic Runaway Reactions2
A serious accident occurred at the Monsanto plant in Sauget, Illinois, on August 8 at 12:18 A.M. (see Figure E13-2.1). The blast was heard as far as 10 miles away in Belleville, Illinois, where people were awakened from their sleep. The explosion occurred in a batch reactor that was used to produce nitroaniline from ammonia and o-nitrochlorobenzene (ONCB):

Figure E13-2.1. Aftermath of the explosion.

St. Louis Globe/Democrat photo by Roy Cook. Courtesy of St. Louis Mercantile Library.
This reaction is normally carried out isothermally at 175°C and about 500 psi. The ambient temperature of the cooling water in the heat exchanger is 25°C. By adjusting the coolant rate, the reactor temperature could be maintained at 175°C. At the maximum coolant rate, the ambient temperature is 25°C throughout the heat exchanger.
Let me tell you something about the operation of this reactor. Over the years, the heat exchanger would fail from time to time, but the technicians would be “Johnny on the Spot” and run out and get it up and running in 10 minutes or so, and there was never any problem. It is believed that one day someone looked at the reactor and said, “It looks as if your reactor is only a third full and you still have room to add more reactants and to make more product and more money. How about filling it up to the top so we could triple production?” They did, and you can see what happened in Figure E13-2.1.

On the day of the accident, two changes in normal operation occurred.
- The reactor was charged with 9.044 kmol of ONCB, 33.0 kmol of NH3, and 103.7 kmol of H2O. Normally, the reactor is charged with 3.17 kmol of ONCB, 103.6 kmol of H2O, and 43 kmol of NH3.
- The reaction is normally carried out isothermally at 175°C over a 24-h period. However, approximately 45 min after the reaction was started, cooling to the reactor failed, but only for 10 min and cooling was again up and running at the 55 minute mark. Cooling may have been halted for 10 min or so on previous occasions when the normal charge of 3.17 kmol of ONCB was used and no ill effects occurred.
The reactor had a rupture disk designed to burst when the pressure exceeded approximately 700 psi. If the disk would have ruptured, the pressure in the reactor would have dropped, causing the water to vaporize, and the reaction would have been cooled (quenched) by the latent heat of vaporization.
a. Plot and analyze the temperature–time trajectory up to a period of 120 min after the reactants were mixed and brought up to 175°C (448K).
b. Show that all the following three conditions had to have been present for the explosion to occur: (1) increased ONCB charge, (2) cooling stopped for 10 min at a time early in the reaction, and (3) relief system failure.
Additional information:
Rate law: –rONCB = kCONCBCNH3
with ![]() at 188°C (461K) and E = 11,273 cal/mol
at 188°C (461K) and E = 11,273 cal/mol
The reaction volume for the new charge of 9.0448 kmol of ONCB:
V = 3.265 m3 ONCB/NH3 + 1.854 m3 H2O = 5.119 m3
The reaction volume for the previous charge of 3.17 kmol of ONCB:

Assume that ΔCP ≈ 0:
![]()
![]()
Mole Balance:
![]()
Rate Law:
![]()
Stoichiometry (liquid phase):
![]()
with
![]()
![]()
Combine:
![]()

Substituting our parameter values into Equation (3-21)
![]()
We obtain
![]()
Energy Balance:
![]()
For ΔCP = 0,
Σ NiCPi = NA0CPA + NB0CPB + NWCPW = NCP
Let Qg be the heat generated [i.e., Qg = (rAV)(ΔHRx)] and let Qr be the heat removed [i.e., Qr = UA(T – Ta)]:
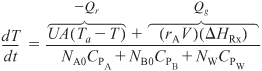
Then

Parameter evaluation for day of explosion:
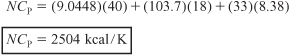
A. Isothermal Operation Up to 45 Minutes
We will first carry out the reaction isothermally at 175°C (448 K) up to the time the cooling was turned off at 45 min. Combining and canceling yields
E13-2.9
![]()
![]()
At 175°C = 448 K, k = 0.0001167m3/kmol · min. Integrating Equation (E13-2.9) gives us
E13-2.10
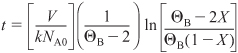
Substituting the parameter values
![]()
Solving for X, we find that at t = 45 min, then X = 0.033.
We will calculate the rate of generation Qg at this temperature and conversion and compare it with the maximum rate of heat removal Qr. The rate of generation Qg is
E13-2.11
![]()

At this time (i.e., t = 45 min, X = 0.033, T = 175°C) we calculate k, then Qr and Qg. At 175°C, k = 0.0001167 m3/min · kmol.

The corresponding maximum cooling rate is
E13-2.12

Therefore
E13-2.13
![]()
The reaction can be controlled. There would have been no explosion had the cooling not failed.
B. Adiabatic Operation for 10 Minutes
Unexpectedly the cooling was off from 45 to 55 min after the reaction was started. We will now use the conditions at the end of the period of isothermal operation as our initial conditions for the adiabatic operation period between 45 and 55 min:
t = 45 min X = 0.033 T = 448 K
Between t = 45 and t = 55 min, Qr = 0. The Polymath program was modified to account for the time of adiabatic operation by using an “if statement” for Qr in the program, i.e., Qr = if (t > 45 and t < 55) then (0) else (UA(T – 298)). A similar “if statement” is used for isothermal operation, i.e., (dT/dt) = 0.
For the 45- to 55-min period without cooling, the temperature rose from 448 K to 468 K, and the conversion increased from 0.033 to 0.0424. Using this temperature and conversion in Equation (E13-2.11), we calculate the rate of generation Qg at 55 min as
Qg = 6591 kcal/min
The maximum rate of cooling at this reactor temperature is found from Equation (E13-2.12) to be
Qr = 6093 kcal/min
Here we see that at the end of the 10-minute down time, the heat exchange system is now operating again, but now
E13-2.14
![]()
and the temperature will continue to increase. We have a Runaway Reaction!! The point of no return has been passed and the temperature will continue to increase, as will the rate of reaction until the explosion occurs.
C. Batch Operation with Heat Exchange
Return of the cooling occurs at 55 min after startup. The values at the end of the period of adiabatic operation (T = 468 K, X = 0.0423) become the initial conditions for the period of restored operation with the heat exchange. The cooling is turned on at its maximum capacity, Qr = UA(T – 298), at 55 min. Table E13-2.1 gives the Polymath program to determine the temperature–time trajectory. [Note that one can change NA0 and NB0 to 3.17 and 43 kmol in the program and show that, if the cooling is shut off for 10 min, at the end of that 10 min, Qr will still be greater than Qg and no explosion will occur.]
The complete temperature–time trajectory is shown in Figure E13-2.2. One notes the long plateau after the cooling is turned back on. Using the values of Qg and Qr at 55 min and substituting into Equation (E13-2.8), we find that
![]()

Table E13-2.1. Polymath Program
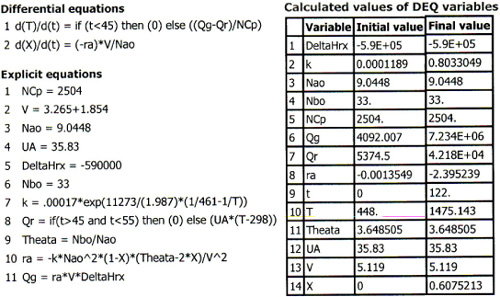
Figure E13-2.2. Temperature–time trajectory.

Consequently, even though dT/dt is positive, the temperature increases very slowly at first, 0.2°C/min. By 11:45, the temperature has reached 240°C and is beginning to increase more rapidly. The reaction is running away! One observes in Figure E13-2.2 that 119 min after the batch was started, the temperature increases sharply and the reactor explodes at approximately midnight. If the mass and heat capacity of the stirrer and reaction vessel had been included, the NCp term would have increased by about 5% and extended the time until the explosion occurred by 15 or so minutes, which would predict the actual time the explosion occurred, at 12:18 A.M.
When the temperature reached 300°C, a secondary reaction, the decomposition of nitroaniline to noncondensable gases such as CO, N2, and NO2, occurred, releasing even more energy. The total energy released was estimated to be 6.8 × 109 J, which is enough energy to lift the entire 2500-ton building 300 m (the length of three football fields) straight up.
D. Disk Rupture
We note that the pressure relief disk should have ruptured when the temperature reached 265°C (ca. 700 psi) but did not and the temperature continued to rise. If it had ruptured and all the water had vaporized, 106 kcal would have been drawn from the reacting solution, thereby lowering its temperature and quenching the runaway reaction.
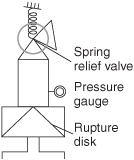
If the disk had ruptured at 265°C (700 psi), we know from fluid mechanics that the maximum mass flow rate, ![]() , out of the 2-in. orifice to the atmosphere (1 atm) would have been 830 kg/min at the time of rupture.
, out of the 2-in. orifice to the atmosphere (1 atm) would have been 830 kg/min at the time of rupture.

This value of Qr is much greater than Qg (Qg = 27,460 kcal/min), so that the reaction could have been easily quenched.
Analysis: Runaway reactions are the most deadly in the chemical industry. Elaborate safety measures are usually installed to prevent them from occurring. However, as we show in this example, the back-up plan failed. If any one of the following three things had not occurred, the explosion would not have happened.
- Tripled production
- Heat exchanger failure for 10 minutes
- Failure of the relieving device (rupture disk)
In other words, all the above had to happen to cause the explosion. If the relief valve had operated properly, it would not have prevented reaction runaway but it could have prevented the explosion. In addition to using rupture disks as relieving devices, one can also use pressure relief valves. In many cases, sufficient care is not taken to obtain data for the reaction at hand and to use it to properly size the relief device. This data can be obtained using a specially designed batch reactor called the Advanced Reactor Safety Screening Tool (ARSST), as shown in Chapter 13 PRS R13.1.

13.3 Semibatch Reactors with a Heat Exchanger
In our past discussions of reactors with heat exchangers, we assumed that the ambient temperature Ta was spatially uniform throughout the exchangers. This assumption is true if the system is a tubular reactor with the external pipe surface exposed to the atmosphere or if the system is a CSTR or batch where the coolant flow rate through the exchanger is so rapid that the coolant temperatures entering and leaving the exchanger are virtually the same.
We now consider the case where the coolant temperature varies along the length of the exchanger while the temperature in the reactor is spatially uniform. The coolant enters the exchanger at a mass flow rate ![]() at a temperature Ta1 and leaves at a temperature Ta2 (see Figure 13-1). As a first approximation, we assume a quasi-steady state for the coolant flow and neglect the accumulation term (i.e., dTa/dt = 0). As a result, Equation (12-19) will give the rate of heat transfer from the exchanger to the reactor:
at a temperature Ta1 and leaves at a temperature Ta2 (see Figure 13-1). As a first approximation, we assume a quasi-steady state for the coolant flow and neglect the accumulation term (i.e., dTa/dt = 0). As a result, Equation (12-19) will give the rate of heat transfer from the exchanger to the reactor:
![]()
Figure 13-1. Tank reactor with heat exchanger.
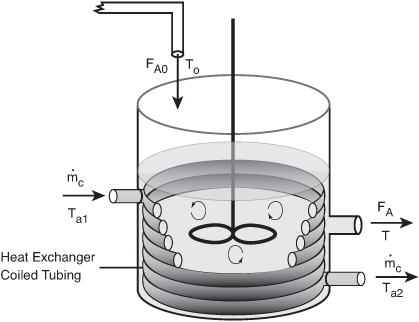
Using Equation (12-19) to substitute for ![]() in Equation (13-9), we obtain
in Equation (13-9), we obtain
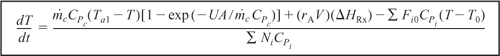
Recall that at steady state (dT/dt = 0), Equation (13-15) can be solved for the conversion X as a function of reaction temperature by recalling that
FA0X = –rAV
and
Σ Fi0CPi (T – T0) = FA0 Σ ΘiCPi (T – T0)
and neglecting ΔCP and then rearranging Equation (13-15) to obtain
Example 13-3. Heat Effects in a Semibatch Reactor
The second-order saponification of ethyl acetate is to be carried out in a semibatch reactor shown schematically below in Figure E13-3.1.
![]()

Figure E13-3.1. Semibatch reactor with heat exchange.

Aqueous sodium hydroxide is to be fed at a concentration of 1 kmol/m3, a temperature of 300 K, and a volumetric rate of 0.004 m3/s to an initial volume of 0.2 m3 of water and ethyl acetate. The concentration of water in the feed, CW0, is 55 kmol/m3. The initial concentrations of ethyl acetate and water are 5 kmol/m3 and 30.7 kmol/m3, respectively. The reaction is exothermic, and it is necessary to add a heat exchanger to keep its temperature below 315 K. A heat exchanger with UA = 3000 J/s · K is available for use. The coolant enters at a rate of 100 kg/s and a temperature of 285 K.
Is the heat exchanger and coolant flow rate adequate to keep the reactor temperature below 315 K? Plot temperature, CA, CB, and CC as a function of time.
Additional information3:
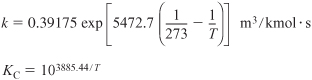
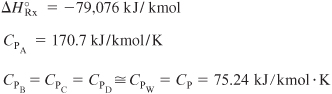

Mole Balances: [See Chapter 6, page 230.]
![]()
![]()

![]()
Initially,
NWi = ViCW0 = (0.2 m3)(30.7 kmol/m3) = 6.14 kmol
Rate Law:
![]()
Stoichiometry:
![]()
![]()
![]()

Energy Balance: Next we replace ![]() in Equation (13-9). Because only B and water continually flow into the reactor
in Equation (13-9). Because only B and water continually flow into the reactor
![]()
However, CPB = CPW:
![]()
where
![]()
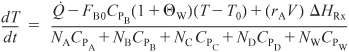
![]()
![]()
Recalling Equation (12-17) for the outlet temperature of the fluid in the heat exchanger

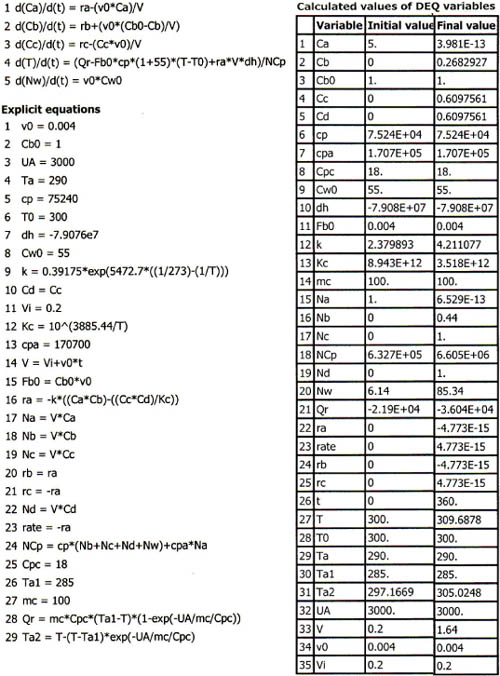
The Polymath program is given in Table E13-3.1. The solution results are shown in Figures E13-3.2 and E13-3.3.
Figure E13-3.2. Temperature–time trajectories in a semibatch reactor.
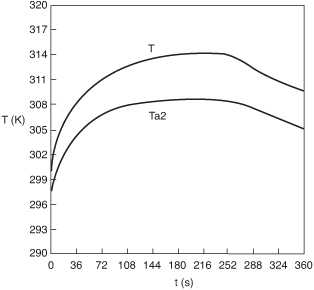
Figure E13-3.3. Concentration–time trajectories in a semibatch reactor.
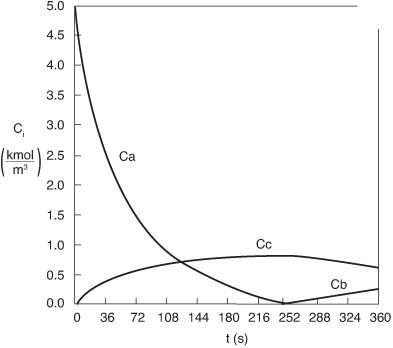
Table E13-3.1. Polymath Program and Output for Semibatch Reactor

Analysis: From Figure E13-3.3 we see that the concentration of species B is virtually zero, owing to the fact that it is consumed virtually as fast as it enters the reactor up to a time of 252s. By the time we reach 252s, all species A has been consumed, and the reaction rate is virtually zero and no more of species C or D are produced and no more B is consumed. Because species B continues to enter the reactor at a volumetric flow rate υ0, after 252 minutes, the fluid volume continues to increase and the concentrations of C and D are diluted. The figure shows that before 252s, Qg > Qr, and the reactor temperature and the coolant temperature increase. However, after 252s, the reaction rate, and hence Qg, are virtually zero so that Qr > Qg and the temperature decreases. See Problem P13-2B (c) to reflect on the time of 252s.
