Chapter 10
1 S. T. Oyama and G. A. Somorjai, J. Chem. Educ., 65, 765 (1986).
2 V. Haensel and R. L. Burwell, Jr., Sci. Am., 225(10), 46.
3 R. I. Masel, Chemical Kinetics and Catalysis (New York: Wiley Interscience, 2001), p. 741.
4 H. S. Taylor, Proc. R. Soc. London, A108, 105 (1928).
5 R. I. Masel, Principles of Adsorption and Reaction on Solid Surfaces (New York: Wiley, 1996).
6 Named after Irving Langmuir (1881–1957), who first proposed it. He received the Nobel Prize in 1932 for his discoveries in surface chemistry.
7 C. N. Hinshelwood, The Kinetics of Chemical Change (Oxford: Clarendon Press, 1940).
8 O. A. Hougen and K. M. Watson, Ind. Eng. Chem., 35, 529 (1943).
9 From the literature cited in G. A. Somorjai, Introduction to Surface Chemistry and Catalysis (New York: Wiley, 1994), p. 482.
10 Strictly speaking, one should compare the product kAPC with kS and kD.
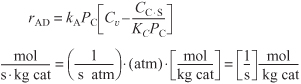
Dividing rAD by kAPC we note ![]() . The reason we do this is that in order to compare terms, the ratios
. The reason we do this is that in order to compare terms, the ratios ![]() ,
, ![]() and
and ![]() must all have the same units
must all have the same units ![]() . Luckily for us, the end result is the same, however.
. Luckily for us, the end result is the same, however.
11 Some prefer to write the surface reaction rate in terms of the fraction of the surface of sites covered (i.e., fA) rather than the number of sites CA · S covered, the difference being the multiplication factor of the total site concentration, Ct. In any event, the final form of the rate law is the same because Ct, KA, kS, and so on, are all lumped into the reaction rate constant, k.
13 R. I. Masel, Principles of Adsorption and Reaction on Solid Surfaces (New York: Wiley, 1996), p. 506, http://www.uiuc.edu/ph/www/r-masel/.
14 G. E. P. Box, W. G. Hunter, and J. S. Hunter, Statistics for Engineers (New York: Wiley, 1978).
15 J. Papp, D. Kallo, and G. Schay, J. Catal., 23, 168 (1971).
17 Ibid.
18 See the Supplementary Reading for a variety of techniques for estimating the rate law parameters.
19 H. Ishii and Y. Takahashi, J. Electrochem. Soc., 135, p. 1539.
20 See G. F. Froment and K. B. Bishoff, Chemical Reaction Analysis and Design, 2nd ed. (New York: Wiley, 1990), p. 96.
21 Scheffe, J.R., J. Li, and A. W. Weimer, “A Spinel Ferrite/Hercynite Water-Splitting Redox Cycle,” International Journal of Hydrogen Energy, 35, 3333-3340 (2010).
