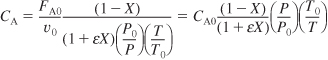Chapter 4. Stoichiometry
If you think you can, you can.
If you think you can’t, you can’t.
You are right either way.
Now that we have shown how the rate law can be expressed as a function of concentrations, we need only express concentration as a function of conversion in order to carry out calculations similar to those presented in Chapter 2 to size reactors. If the rate law depends on more than one species, we must relate the concentrations of the different species to each other. This relationship is most easily established with the aid of a stoichiometric table. This table presents the stoichiometric relationships between reacting molecules for a single reaction. That is, it tells us how many molecules of one species will be formed during a chemical reaction when a given number of molecules of another species disappears. These relationships will be developed for the general reaction
![]()
Recall that we have already used stoichiometry to relate the relative rates of reaction for Equation (2-1):
![]()
This stoichiometric relationship relating reaction rates will be used in Chapters 6 and 8.
In formulating our stoichiometric table, we shall take species A as our basis of calculation (i.e., the limiting reactant) and then divide through by the stoichiometric coefficient of A,
![]()
in order to put everything on a basis of “per mole of A.”
Next, we develop the stoichiometric relationships for reacting species that give the change in the number of moles of each species (i.e., A, B, C, and D).
4.1 Batch Systems
Batch reactors are primarily used for the production of specialty chemicals and to obtain reaction rate data in order to determine reaction rate laws and rate law parameters such as k, the specific reaction rate.
Figure 4-1 shows a starving artist’s rendition of a batch system in which we will carry out the reaction given by Equation (2-2). At time t = 0, we will open the reactor and place a number of moles of species A, B, C, and D, and inerts I (NA0, NB0, NC0, ND0, and NI0, respectively) into the reactor.
Figure 4-1. Batch reactor. (Schematic with special permission by Renwahr.)
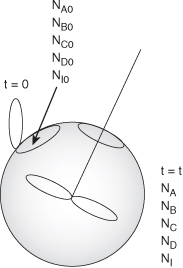
Species A is our basis of calculation, and NA0 is the number of moles of A initially present in the reactor. After a time t, NA0X moles of A are consumed in the system as a result of the chemical reaction, leaving (NA0 – NA0X) moles of A in the system. That is, the number of moles of A remaining in the reactor after a conversion X has been achieved is
NA = NA0 – NA0X = NA0(1 – X)
We now will use conversion in this fashion to express the number of moles of B, C, and D in terms of conversion.
To determine the number of moles of each species remaining after NA0X moles of A have reacted, we form the stoichiometric table (Table 4-1). This stoichiometric table presents the following information:
Table 4-1. Stoichiometric Table for a Batch System
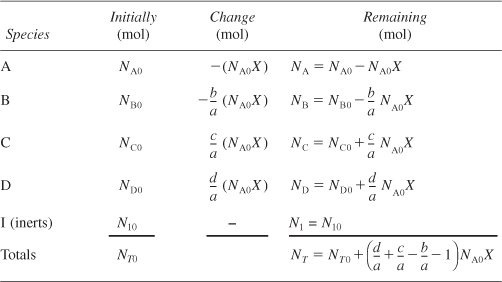
Column 1: the species in the reaction
Column 2: the number of moles of each species initially present
Column 3: the change in the number of moles brought about by reaction
Column 4: the number of moles remaining in the system at time t
To calculate the number of moles of species B remaining at time t, we recall that at time t the number of moles of A that have reacted is NA0X. For every mole of A that reacts, b/a moles of B must react; therefore, the total number of moles of B that have reacted is
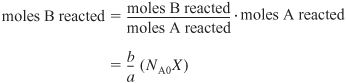
Because B is disappearing from the system, the sign of the “change” is negative. NB0 is the number of moles of B initially in the system. Therefore, the number of moles of B remaining in the system, NB, at a time t, is given in the last column of Table 4-1 as
![]()
The complete stoichiometric table delineated in Table 4-1 is for all species in the general reaction
![]()
Let’s take a look at the totals in the last column of Table 4-1. The stoichiometric coefficients in parentheses (d/a + c/a – b/a – 1) represent the change in the total number of moles per mole of A reacted. Because this term occurs so often in our calculations, it is given the symbol δ:
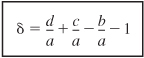
The parameter δ tells us the change in the total number of moles per mole of A reacted. The total number of moles can now be calculated from the equation
NT = NT0 + δNA0X
We recall from Chapter 1 and Chapter 3 that the kinetic rate law (e.g., ![]() ) is a function solely of the intensive properties of the reacting system (e.g., temperature, pressure, concentration, and catalysts, if any). The reaction rate, –rA, usually depends on the concentration of the reacting species raised to some power. Consequently, to determine the reaction rate as a function of conversion X, we need to know the concentrations of the reacting species as a function of conversion, X.
) is a function solely of the intensive properties of the reacting system (e.g., temperature, pressure, concentration, and catalysts, if any). The reaction rate, –rA, usually depends on the concentration of the reacting species raised to some power. Consequently, to determine the reaction rate as a function of conversion X, we need to know the concentrations of the reacting species as a function of conversion, X.