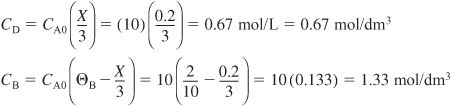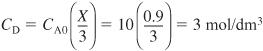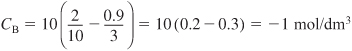4.1.1 Equations for Batch Concentrations
The concentration of A is the number of moles of A per unit volume:
![]()
After writing similar equations for B, C, and D, we use the stoichiometric table to express the concentration of each component in terms of the conversion X:
![]()
![]()
![]()
![]()
Because almost all batch reactors are solid vessels, the reactor volume is constant, so we can take V = V0, then
![]()
![]()
We will soon see that Equation (4-6) also applies to liquid systems.
We further simplify these equations by defining the parameter Θi, which allows us to factor out NA0 in each of the expressions for concentration:

![]()
![]()
with ![]()
For an equilmolar feed ΘB = 1 and for a stoichiometric feed ΘB = b/a.
Continuing for species C and D
![]()
![]()
with ![]()
![]()
![]()
with ![]()
For constant volume batch reactors, V = V0, we now have concentration as a function of conversion. If we know the rate law, we can now obtain –rA = f (X) to couple with the differential mole balance in terms of conversion in order to solve for the reaction time, t.
For liquid-phase reactions taking place in solution, the solvent usually dominates the situation. For example, most liquid-phase organic reactions do not change density during the course of the reaction and represent still another case for which the constant-volume simplifications apply. As a result, changes in the density of the solute do not affect the overall density of the solution significantly and therefore it is essentially a constant-volume reaction process, V = V0 and υ = υ0. Consequently, Equations (4-6) through (4-9) can be used for liquid-phase reactions as well. An important exception to this general rule exists for polymerization processes.
To summarize for constant volume batch systems and for liquid-phase reactions, we can use a rate law for reaction (2-2) such as –rA = kACACB to obtain –rA = f(X), that is,
![]()

Substituting for the given parameters k, CA0, and ΘB, we can now use the techniques in Chapter 2 to size the CSTRs and PFRs for liquid-phase reactions.
Example 4-1. Expressing Cj = hj (X) for a Liquid-Phase Batch Reaction
Soap consists of the sodium and potassium salts of various fatty acids, such as oleic, stearic, palmitic, lauric, and myristic acids. The saponification for the formation of soap from aqueous caustic soda and glyceryl stearate is
![]()
Letting X represent the conversion of sodium hydroxide (the moles of sodium hydroxide reacted per mole of sodium hydroxide initially present), set up a stoichiometric table expressing the concentration of each species in terms of its initial concentration and the conversion X.
Because we have taken sodium hydroxide as our basis of calculation, we divide through by the stoichiometric coefficient of sodium hydroxide to put the reaction expression in the form
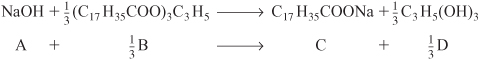
We may then perform the calculations shown in Table E4-1.1. Because this is a liquid-phase reaction, the density ρ is considered to be constant; therefore, V = V0.

Table E4-1.1. Stoichiometric Table for Liquid-Phase Soap Reaction
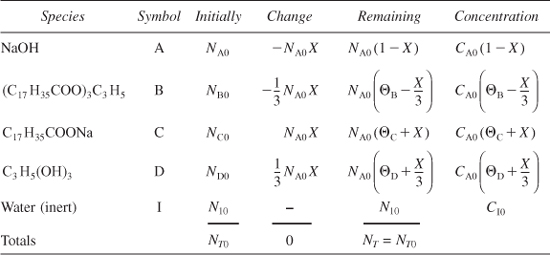
Analysis: The purpose of this example was to show how the generic reaction in Table 4-1 is applied to a real reaction.
Example 4-2. What Is the Limiting Reactant?
Having set up the stoichiometric table in Example 4-1, one can now readily use it to calculate the concentrations at a given conversion. If the initial mixture consists of sodium hydroxide at a concentration of 10 mol/dm3 (i.e., 10 mol/L or 10 kmol/m3) and glyceryl stearate at a concentration of 2 mol/dm3, what are the concentrations of glycerol stearate, B, and of glycerine, D, when the conversion of sodium hydroxide is (a) 20% and (b) 90%?
Analysis: We chose the wrong basis of calculation! Ninety percent conversion of NaOH is not possible because glyceryl stearate is the limiting reactant and is used up before 90% of the NaOH can be reacted. Glyceryl stearate should have been our basis of calculation and therefore we should not have divided the reaction as written by the stoichiometric coefficient of 3.
4.2 Flow Systems
The form of the stoichiometric table for a continuous-flow system (see Figure 4-2) is virtually identical to that for a batch system (Table 4-1) except that we replace Nj0 by Fj0 and Nj by Fj (Table 4-2). Again taking A as the basis, we divide Equation (2-1) through by the stoichiometric coefficient of A to obtain
![]()
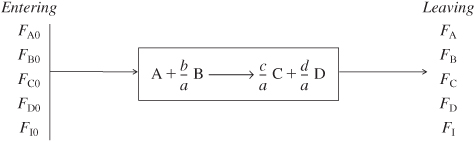
Table 4-2. Stoichiometric Table for a Flow System
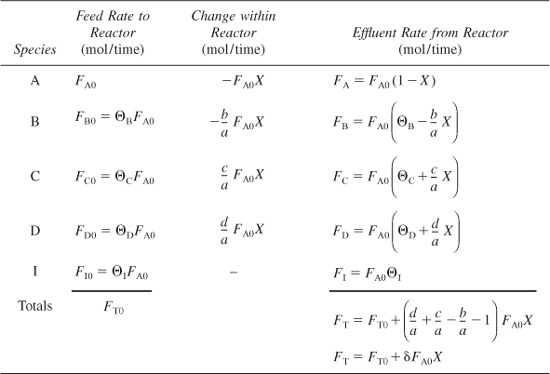
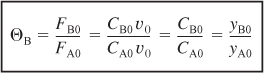
and ΘC, ΘD, and Θ1 are defined similarly
and where