5.5.1 Pressure Drop and the Rate Law
We now focus our attention on accounting for the pressure drop in the rate law. For an ideal gas, we recall Equation (4-25) to write the concentration of reacting species i as
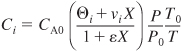
where ![]() , ε = yA0δ, and νi is the stoichiometric coefficient (e.g., νA = –1, νB = –b/a). We now must determine the ratio Pressure (P/P0) as a function of the PFR reactor volume, V, or the PBR catalyst weight, W, to account for pressure drop. We then can combine the concentration, rate law, and design equation. However, whenever accounting for the effects of pressure drop, the differential form of the mole balance (design equation) must be used.
, ε = yA0δ, and νi is the stoichiometric coefficient (e.g., νA = –1, νB = –b/a). We now must determine the ratio Pressure (P/P0) as a function of the PFR reactor volume, V, or the PBR catalyst weight, W, to account for pressure drop. We then can combine the concentration, rate law, and design equation. However, whenever accounting for the effects of pressure drop, the differential form of the mole balance (design equation) must be used.
If, for example, the second-order reaction
![]()
is being carried out in a packed-bed reactor, the differential form of the mole balance equation in terms of catalyst weight is
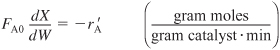
The rate law is
![]()
From stoichiometry for gas-phase reactions (Table 3-5),
![]()
and the rate law can be written as
![]()
Note from Equation (5-20) that the larger the pressure drop (i.e., the smaller P) from frictional losses, the smaller the reaction rate!
Combining Equation (5-20) with the mole balance (2-17) and assuming isothermal operation (T = T0) gives
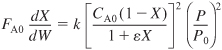
Dividing by FA0 (i.e. υ0 CA0) yields
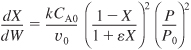
For isothermal operation (T = T0), the right-hand side is a function of only conversion and pressure:
![]()
We now need to relate the pressure drop to the catalyst weight in order to determine the conversion as a function of catalyst weight (i.e., catalyst mass).
