8.1.1 Types of Reactions
There are four basic types of multiple reactions: series, parallel, complex, and independent. These types of multiple reactions can occur by themselves, in pairs, or all together. When there is a combination of parallel and series reactions, they are often referred to as complex reactions.
Parallel reactions (also called competing reactions) are reactions where the reactant is consumed by two different reaction pathways to form different products:

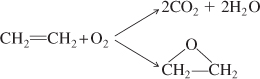
An example of an industrially significant parallel reaction is the oxidation of ethylene to ethylene oxide while avoiding complete combustion to carbon dioxide and water.
Series reactions (also called consecutive reactions) are reactions where the reactant forms an intermediate product, which reacts further to form another product:
![]()
An example of a series reaction is the reaction of ethylene oxide (EO) with ammonia to form mono-, di-, and triethanolamine:

In recent years the shift has been toward the production of diethanolamine as the desired product rather than triethanolamine.
Independent reactions are reactions that occur at the same time but neither the products nor reactants react with themselves or one another.
![]()
An example is the cracking of crude oil to form gasoline, where two of the many reactions occurring are
![]()
Complex reactions are multiple reactions that involve combinations of series and independent parallel reactions, such as
![]()
An example of a combination of parallel and series reactions is the formation of butadiene from ethanol:

