8.3.2 Maximizing the Desired Product for One Reactant
In this section, we examine ways to maximize the instantaneous selectivity, SD/U, for different reaction orders of the desired and undesired products.
Case 1: α1>α2· The reaction order of the desired product, α1, is greater than the reaction order of the undesired product, α2. Let a be a positive number that is the difference between these reaction orders (a > 0):
α1 – α2 = a
Then, upon substitution into Equation (8-10), we obtain
![]()
To make this ratio as large as possible, we want to carry out the reaction in a manner that will keep the concentration of reactant A as high as possible during the reaction. If the reaction is carried out in the gas phase, we should run it without inerts and at high pressures to keep CA high. If the reaction is in the liquid phase, the use of diluents should be kept to a minimum.1
A batch or plug-flow reactor should be used in this case because, in these two reactors, the concentration of A starts at a high value and drops progressively during the course of the reaction. In a perfectly mixed CSTR, the concentration of reactant within the reactor is always at its lowest value (i.e., that of the outlet concentration) and therefore the CSTR should not be chosen under these circumstances.
Case 2: α2>α1· The reaction order of the undesired product is greater than that of the desired product. Let b = α2 – α1, where b is a positive number; then
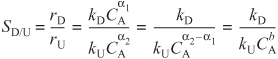
For the ratio γD/γU to be high, the concentration of A should be as low as possible.
This low concentration may be accomplished by diluting the feed with inerts and running the reactor at low concentrations of species A. A CSTR should be used because the concentrations of reactants are maintained at a low level. A recycle reactor in which the product stream acts as a diluent could be used to maintain the entering concentrations of A at a low value.
Because the activation energies of the two reactions in cases 1 and 2 are not given, it cannot be determined whether the reaction should be run at high or low temperatures. The sensitivity of the rate selectivity parameter to temperature can be determined from the ratio of the specific reaction rates,
![]()
where A is the frequency factor, and E the activation energy, where the subscripts D and U refer to desired and undesired product, respectively.
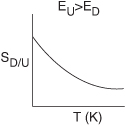
Case 3: ED>EU· In this case, the specific reaction rate of the desired reaction kD (and therefore the overall rate rD) increases more rapidly with increasing temperature, T, than does the specific rate of the undesired reaction kU. Consequently, the reaction system should be operated at the highest possible temperature to maximize SD/U.

Case 4: EU>ED· In this case, the reaction should be carried out at a low temperature to maximize SD/U, but not so low that the desired reaction does not proceed to any significant extent.
Example 8-1. Maximizing the Selectivity for the Famous Trambouze Reactions
Reactant A decomposes by three simultaneous reactions to form three products, one that is desired, B, and two that are undesired, X and Y. These gas-phase reactions, along with the appropriate rate laws, are called the Trambouze reactions [AIChE J., 5, 384].
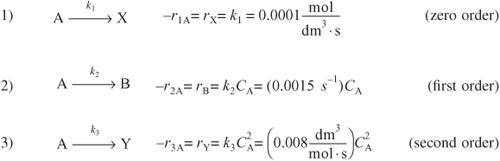
The specific reaction rates are given at 300 K and the activation energies for reactions (1), (2), and (3) are E1 = 10,000 kcal/mole, E2 = 15,000 kcal/mole, and E3 = 20,000 kcal/mole.
a. How and under what conditions (e.g., reactor type(s), temperature, concentrations) should the reaction be carried out to maximize the selectivity of species B for an entering concentration of species A of 0.4 M and a volumetric flow rate of 2.0 dm3/s?
b. How could the conversion of B be increased and still keep selectivity relatively high?
Part (a)
The instantaneous selectivity of species B with respect to species X and Y is

Plotting SB/XY vs. CA, we see that there is a maximum, as shown in Figure E8-1.1.
Figure E8-1.1. Selectivity as a function of the concentration of A.
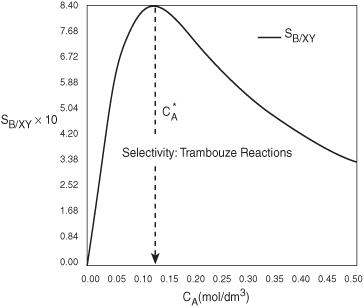
As we can see, the selectivity reaches a maximum at a concentration ![]() . Because the concentration changes down the length of a PFR, we cannot operate at this maximum. Consequently, we will use a CSTR and design it to operate at this maximum. To find the maximum,
. Because the concentration changes down the length of a PFR, we cannot operate at this maximum. Consequently, we will use a CSTR and design it to operate at this maximum. To find the maximum, ![]() , we differentiate SB/XY with respect to CA, set the derivative to zero, and solve for
, we differentiate SB/XY with respect to CA, set the derivative to zero, and solve for ![]() . That is,
. That is,
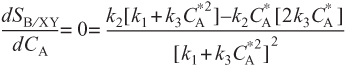
Solving for ![]() ,
,

We see from Figure E8-1.1 that the selectivity is indeed a maximum at ![]()
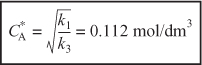
Therefore, to maximize the selectivity SB/XY, we want to carry out our reaction in such a manner that the CSTR concentration of A is always at ![]() . The corresponding selectivity at
. The corresponding selectivity at ![]() is
is

We now calculate the CSTR volume and conversion. The net rate of formation of A from reactions (1), (2), and (3) is
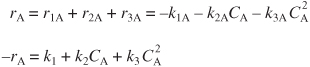
Using Equation (E8-1.5) in the mole balance on a CSTR for this liquid-phase reaction (υ = υ0),
![]()
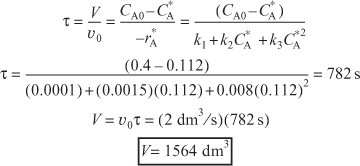
For an entering volumetric flow rate of 2 dm3/s, we must have a CSTR volume of 1564 dm3 to maximize the selectivity, SB/XY.
Maximize the selectivity with respect to temperature
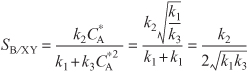
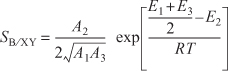
Case 1: 
Case 2: ![]()
For the activation energies given in this example
![]()
So the selectivity for this combination of activation energies is independent of temperature!
What is the conversion of A in the CSTR?

Part (b)
If greater than 72% conversion of A is required, say 90%, then the CSTR operated with a reactor concentration of 0.112 mol/dm3 should be followed by a PFR because the conversion will increase continuously as we move down the PFR [see Figure E8-1.2(b)]. However, as can be seen in Figure E8-1.2, the concentration will decrease continuously from ![]() , as will the selectivity SB/XY as we move down the PFR to an exit concentration CAf. Hence the system
, as will the selectivity SB/XY as we move down the PFR to an exit concentration CAf. Hence the system
![]()
would give the highest selectivity and least total reactor volume while forming more of the desired product B, beyond what was formed at ![]() in a CSTR.
in a CSTR.
Figure E8-1.2. Effect of adding a PFR to increase conversion. (a) Reactor arrangement; (b) Selectivity and conversion trajectories.
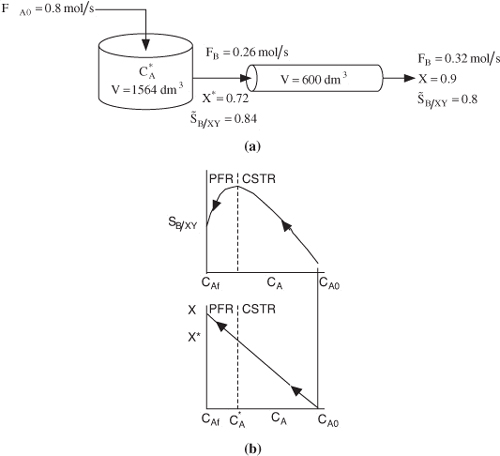
Figure E8-1.2 illustrates how as the conversion is increased above X* by adding the PFR reactor volume, however, the selectivity decreases.
This calculation for the PFR is carried out on the DVD-ROM in Example 8-1. The results of this calculation show that at the exit of the PFR, the molar flow rates are FX = 0.22 mol/s, FB = 0.32 mol/s, and FY = 0.18 mol/s corresponding to a conversion of X = 0.9. The corresponding selectivity at a conversion of 90% is
![]()
Analysis: One now has to make a decision as to whether adding the PFR to increase the conversion of A from 0.72 to 0.9 and the molar flow rate of B from 0.26 to 0.32 mol/s is worth not only the added cost of the PFR, but also the decrease in selectivity from 0.84 to 0.8. In this example we used the Trambouze reactions to show how to optimize the selectivity to species B in a CSTR. Here, we found the optimal exit conditions (CA = 0.112 mol/dm3), conversion (X = 0.72) and selectivity, (SB/XY = 0.84). The corresponding CSTR volume was V = 1564 dm3. If we wanted to increase the conversion to 90%, we could use a PFR to follow the CSTR, and find that the selectivity decreased.
