Chapter 9. Reaction Mechanisms, Pathways, Bioreactions, and Bioreactors
The next best thing to knowing something is knowing where to find it.
—Samuel Johnson (1709–1784)
9.1 Active Intermediates and Nonelementary Rate Laws
In Chapter 3 a number of simple power law models, e.g.,
![]()
were presented, where n was an integer of 0, 1, or 2 corresponding to a zero-, first-, and second-order reaction. However, for a large number of reactions, the orders are either noninteger, such as the decomposition of acetaldehyde at 500°C
CH3CHO → CH4 + CO
where the rate law developed in problem P9-5B(b) is
![]()
The rate law could also have concentration terms in both the numerator and denominator such as the formation of HBr from hydrogen and bromine
H2 + Br2 → 2HBr
where the rate law developed in problem P9-5B(c) is
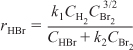
Rate laws of this form usually involve a number of elementary reactions and at least one active intermediate. An active intermediate is a high-energy molecule that reacts virtually as fast as it is formed. As a result, it is present in very small concentrations. Active intermediates (e.g., A*) can be formed by collision or interaction with other molecules.
A + M → A* + M
Here the activation occurs when translational kinetic energy is transferred into internal energy i.e., vibrational and rotational energy.1 An unstable molecule (i.e., active intermediate) is not formed solely as a consequence of the molecule moving at a high velocity (high translational kinetic energy). The energy must be absorbed into the chemical bonds, where high-amplitude oscillations will lead to bond ruptures, molecular rearrangement, and decomposition. In the absence of photochemical effects or similar phenomena, the transfer of translational energy to vibrational energy to produce an active intermediate can occur only as a consequence of molecular collision or interaction. Collision theory is discussed in the Professional Reference Shelf in Chapter 3. Other types of active intermediates that can be formed are free radicals (one or more unpaired electrons, e.g., CH3•), ionic intermediates (e.g., carbonium ion), and enzyme-substrate complexes, to mention a few.
The idea of an active intermediate was first postulated in 1922 by F. A. Lindemann2 who used it to explain changes in reaction order with changes in reactant concentrations. Because the active intermediates were so short lived and present in such low concentrations, their existence was not really definitively confirmed until the work of Ahmed Zewail who received the Nobel Prize in 1999 for femtosecond spectroscopy.3 His work on cyclobutane showed that the reaction to form two ethylene molecules did not proceed directly, as shown in Figure 9-1(a), but formed the active intermediate shown in the small trough at the top of the energy barrier on the reaction-coordinate diagram in Figure 9-1(b). As discussed in Chapter 3, an estimation of the barrier height, E, can be obtained using computational software packages such as Spartan, Cerius2, or Gaussian as discussed in the Molecular Modeling Web Module in Chapter 3.
![]()
Figure 9-1. Reaction coordinate. Courtesy Science News, 156, 247 (1999).

