9.1.2 Searching for a Mechanism
In many instances the rate data are correlated before a mechanism is found. It is a normal procedure to reduce the additive constant in the denominator to 1. We therefore divide the numerator and denominator of Equation (9-9) by k3 to obtain
![]()
General Considerations
The rules of thumb listed in Table 9-2 may be of some help in the development of a mechanism that is consistent with the experimental rate law.

Table 9-2. Rules of Thumb for Development of a Mechanism
Upon application of Table 9-2 to the azomethane example just discussed, we see the following from rate Equation (9-12):
- The active intermediate, AZO*, collides with azomethane, AZO [Reaction 2], resulting in the concentration of AZO in the denominator.
- AZO* decomposes spontaneously [Reaction 3], resulting in a constant in the denominator of the rate expression.
- The appearance of AZO in the numerator suggests that the active intermediate AZO* is formed from AZO. Referring to [Reaction 1], we see that this case is indeed true.
Example 9-1. The Stern–Volmer Equation
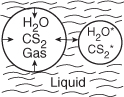
Light is given off when a high-intensity ultrasonic wave is applied to water.6 This light results from microsize gas bubbles (0.1 mm) being formed by the ultrasonic wave and then being compressed by it. During the compression stage of the wave, the contents of the bubble (e.g., water and whatever else is dissolved in the water, e.g., CS2, O2, N2) are compressed adiabatically.
This compression gives rise to high temperatures and kinetic energies of the gas molecules, which through molecular collisions generate active intermediates and cause chemical reactions to occur in the bubble.
![]()
The intensity of the light given off, I, is proportional to the rate of deactivation of an activated water molecule that has been formed in the microbubble.
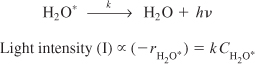
An order-of-magnitude increase in the intensity of sonoluminescence is observed when either carbon disulfide or carbon tetrachloride is added to the water. The intensity of luminescence, I, for the reaction
![]()
is
![]()
A similar result exists for CCl4.
However, when an aliphatic alcohol, X, is added to the solution, the intensity decreases with increasing concentration of alcohol. The data are usually reported in terms of a Stern–Volmer plot in which relative intensity is given as a function of alcohol concentration, CX. (See Figure E9-1.1, where I0 is the sonoluminescence intensity in the absence of alcohol and I is the sonoluminescence intensity in the presence of alcohol.)
a. Suggest a mechanism consistent with experimental observation.
b. Derive a rate law consistent with Figure E9-1.1.
Figure E9-1.1. Ratio of luminescence intensities as a function of scavenger concentration.
a. Mechanism
From the linear plot we know that
E9-1.1
![]()
where CX ≡ (X). Inverting yields
E9-1.2
![]()
From rule 1 of Table 9-2, the denominator suggests that alcohol (X) collides with the active intermediate:
E9-1.3
![]()
The alcohol acts as what is called a scavenger to deactivate the active intermediate. The fact that the addition of CCl4 or CS2 increases the intensity of the luminescence,
E9-1.4
![]()
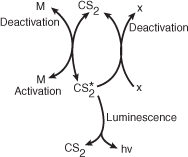
leads us to postulate (rule 3 of Table 9-2) that the active intermediate was probably formed from CS2:
E9-1.5
![]()
where M is a third body (CS2, H2O, N2, etc.).
We also know that deactivation can occur by the reverse of reaction (E9-1.5). Combining this information, we have as our mechanism:
E9-1.5
![]()
E9-1.6
![]()
E9-1.3
![]()
E9-1.7
![]()
E9-1.8
![]()
Using the PSSH on ![]() in each of the above elementry reactions yields
in each of the above elementry reactions yields
![]()
Solving for (![]() ) and substituting into Equation (E9-1.8) gives us
) and substituting into Equation (E9-1.8) gives us
![]()
In the absence of alcohol,
![]()
For constant concentrations of CS2 and the third body, M, we take a ratio of Equation (E9-1.10) to (E9-1.9):
![]()
which is of the same form as that suggested by Figure E9-1.1. Equation (E9-1.11) and similar equations involving scavengers are called Stern–Volmer equations.
Analysis: This example showed how to use the Rules of Thumb (Table 9-2) to develop a mechanism. Each step in the mechanism is assumed to follow an elementary rate law. The PSSH was applied to the net rate of reaction for the active intermediate in order to find the concentration of the active intermediate. This concentration was then substituted in to the rate law for the rate of formation of product to give the rate law. The rate law from the mechanism was found to be consistent with experimental data.

A discussion of luminescence is continued on the DVD-ROM Web Module, Glow Sticks. Here, the PSSH is applied to glow sticks. First, a mechanism for the reactions and luminescence is developed. Next, mole balance equations are written on each species and coupled with the rate law obtained using the PSSH; the resulting equations are solved and compared with experimental data.