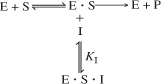9.3.2 Uncompetitive Inhibition
Here the inhibitor has no affinity for the enzyme by itself and thus does not compete with the substrate for the enzyme; instead it ties up the enzyme–substrate complex by forming an inhibitor–enzyme–substrate complex, (I · E · S), which is inactive. In uncompetitive inhibition, the inhibitor reversibly ties up enzyme–substrate complex after it has been formed.
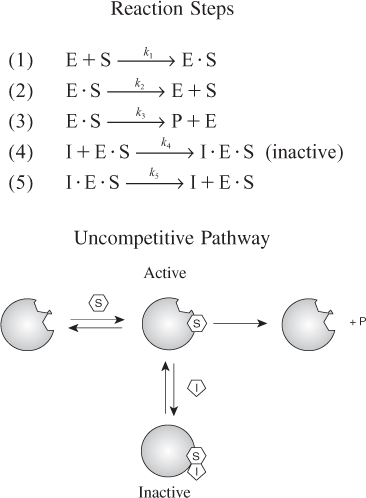
As with competitive inhibition, two additional reaction steps are added to the Michaelis–Menten kinetics for uncompetitive inhibition, as shown in reaction steps 4 and 5 in Figure 9-11.
Figure 9-11. Steps in uncompetitive enzyme inhibition.
Starting with the equation for the rate of formation of product, Equation (9-34), and then applying the pseudo-steady-state hypothesis to the intermediate (I · E · S), we arrive at the rate law for uncompetitive inhibition
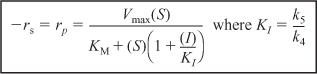
The intermediate steps are shown in the Chapter 9 Summary Notes on the DVD-ROM and on the Web. Rearranging Equation (9-40)

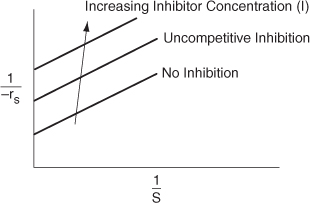
The Lineweaver–Burk plot is shown in Figure 9-12 for different inhibitor concentrations. The slope (KM/Vmax) remains the same as the inhibitor (I) concentration is increased, while the intercept [(1/Vmax)(1 + (I)/KI)] increases.
Figure 9-12. Lineweaver–Burk plot for uncompetitive inhibition.