9.4.2 Rate Laws
While many laws exist for the cell growth rate of new cells, that is,
![]()
the most commonly used expression is the Monod equation for exponential growth:
![]()
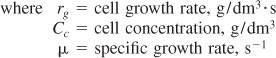
The cell concentration is often given in terms of weight (g) of dry cells per liquid volume and is specified “grams dry weight per dm3,” i.e., (gdw/dm3).
The specific cell growth rate can be expressed as
![]()
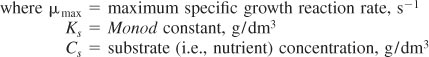
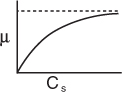
Figure 9-23. Specific cell growth rate, μ, as a function of substrate concentration Cs.
Representative values of μmax and Ks are 1.3 h–1 and 2.2 × 10–5 g/dm3, respectively, which are the parameter values for the E. coli growth on glucose. Combining Equations (9-51) and (9-52), we arrive at the Monod equation for bacterial cell growth rate
![]()
For a number of different bacteria, the constant Ks is very small, with regard to typical substrate concentrations, in which case the rate law reduces to
![]()
The growth rate, rg, often depends on more than one nutrient concentration; however, the nutrient that is limiting is usually the one used in Equation (9-53).
In many systems the product inhibits the rate of growth. A classic example of this inhibition is in wine-making, where the fermentation of glucose to produce ethanol is inhibited by the product ethanol. There are a number of different equations to account for inhibition; one such rate law takes the empirical form
![]()
where
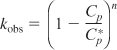
with
Cp = product concentration (g/dm3)
![]() = product concentration at which all metabolism ceases, g/dm3
= product concentration at which all metabolism ceases, g/dm3
n = empirical constant
For the glucose-to-ethanol fermentation, typical inhibition parameters are
![]()
In addition to the Monod equation, two other equations are also commonly used to describe the cell growth rate; they are the Tessier equation,
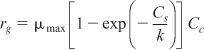
![]()
where λ and k are empirical constants determined by a best fit of the data. The Moser and Tessier growth laws are often used because they have been found to better fit experimental data at the beginning or end of fermentation. Other growth equations can be found in Dean.21
The cell death rate is a result of harsh environments, mixing shear forces, local depletion of nutrients, and the presence of toxic substances. The rate law is
![]()
where Ct is the concentration of a substance toxic to the cell. The specific death rate constants kd and kt refer to the natural death and death due to a toxic substance, respectively. Representative values of kd range from 0.1 h–1 to less than 0.0005 h–1. The value of kt depends on the nature of the toxin.
Microbial growth rates are measured in terms of doubling times. Doubling time is the time required for a mass of an organism to double. Typical doubling times for bacteria range from 45 minutes to 1 hour but can be as fast as 15 minutes. Doubling times for simple eukaryotes, such as yeast, range from 1.5 to 2 hours but may be as fast as 45 minutes.
Effect of Temperature
As with enzymes (cf. Figure 9-8), there is an optimum in growth rate with temperature, owing to the competition of increased rates with increasing temperature and enzyme denaturation at high temperatures. An empirical law that describes this functionality is given in Aiba et al.22 and is of the form
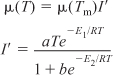
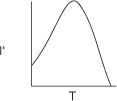
where I′ is the fraction of the maximum growth rate, Tm is the temperature at which the maximum growth occurs, and μ(Tm) is the growth rate at this temperature. For the rate of oxygen uptake of Rhizobium trifollic, the equation takes the form
![]()
The maximum growth of Rhizobium trifollic occurs at 310K.
