12. Bioprocess Considerations in Using Animal Cell Cultures
Animal cells have become important catalysts for many bioprocesses. They are capable of making a vast array of important therapeutic proteins. With recombinant DNA techniques, it is possible to achieve significant levels of production of compounds made only in minute amounts in native cells. Animal cells possess the machinery to do complex posttranslational modifications to proteins. This capacity is essential in some cases to the formation of a clinically useful product particularly for therapeutic proteins. Currently, more than 50% of new therapeutic proteins are being produced using mammalian cell tissue culture. Also, the development of stem cell technology opens the possibility that the cells themselves will become the product of interest. In this chapter, we consider some of the important constraints in using these cells.
12.1. Structure and Biochemistry of Animal Cells
Animal cells vary in size (10 to 30 μm) and shape (spherical, ellipsoidal). In terms of intracellular structures, animal cells are typical eucaryotes, and we discussed their cellular structure in Chapter 2, “An Overview of Biological Basics.” Figure 12.1 summarizes the structure of a typical animal cell.

Figure 12.1. Generalized animal cell. The cytosol, endoplasmic reticulum, Golgi apparatus, endosome, nucleus, lysosome, mitochondrion, and peroxisome are distinct compartments isolated from the rest of the cell by selectively permeable membranes.
Animal cells do not have a cell wall but are surrounded by a thin and fragile plasma membrane that is composed of protein, lipid, and carbohydrate. This structure results in significant shear sensitivity. In some cells, a portion of the plasma membrane is modified to form a number of projections called microvilli. The microvilli increase the surface area of the cell and provide more effective passage of materials across the plasma membrane. The composition of plasma membrane is not uniform and varies in different regions of the membrane. The surface of an animal cell is negatively charged, and cells tend to grow on positively charged surfaces, such as Sephadex or collagen (anchorage-dependent cells). Many cells possess specific cell surface receptors that adhere to ligands on the surface. For example, the binding to collagen may be nonspecific or may be mediated by specific cell surface receptors. The degree of cell adhesiveness is usually greater if attachment is receptor mediated. Some animal cells such as hybridomas are nonanchorage dependent and grow in suspension culture.
Inside the cytoplasm of most animal cells is an extensive network of membrane-bounded channels called the endoplasmic reticulum (ER). The membranes of the ER divide the cytoplasm into two phases: the lumenal phase (inside the endoplasmic reticulum) and the cytosol (outside the rough endoplasmic membrane). Ribosomes are usually located on the outer surface of the endoplasmic reticulum. Some ribosomes are located in cytoplasm and may be interconnected by fine filaments. The ER is critical in protein synthesis and initial stages of posttranslational processing (see Chapter 4, “How Cells Work”).
Mitochondria are the powerhouse of cells where respiration takes place and the bulk of the adenosine triphosphates (ATPs) are produced. Mitochondria are independent organelles in the cytoplasm containing DNA and are capable of independent reproduction. Each mitochondrion is surrounded by a double membrane: a smooth outer membrane and a highly folded inner membrane called the cristae. The mitochondrial matrix often contains crystallike inclusions.
Lysosomes are rather small cytoplasmic organelles bound by a single membrane, and they contain various hydrolytic enzymes, such as proteases, nucleases, and esterases. Lysosomes are responsible for the digestion of certain food particles ingested by the cell.
The Golgi body is a cytoplasmic organelle surrounded by a rather irregularly shaped membrane called the cisternae. The cisternae of a Golgi body are often stacked together in parallel rows, called dictyosome. The Golgi apparatus is responsible for the completion of complex glycosylation and for collecting and secreting extracellular proteins or directing intracellular protein traffic to other organelles.
Some cells contain small cytoplasmic organelles called peroxisomes and glyoxysomes. These organelles are bounded by a single membrane and contain a number of enzymes, including peroxidases (hydrolysis of H2O2) and glyoxalases (glyoxylic acid metabolism).
The nucleus is bounded by two nuclear membranes that form a nuclear envelope. At certain positions, the inner and outer membranes of nucleus fuse to form pores. Nuclear pores provide continuity between the cytoplasm and the inner part of the nucleus. The perinuclear space (the space between the two membranes of the nucleus) may have access to the outer cell. The nucleus contains chromosomal DNA and also some dark granular structures called nucleoli, which can be seen under an electron microscope. Nucleoli are not bound by a membrane and appear to be formed by ribosomal material.
Animal cells have a cytoskeleton or system of protein filaments that provide cell mechanical strength, control cell shape, and guide cell movement. These elements are often critical components in controlling cell response to mechanical forces such as from fluid flow or from attachment to surfaces. The three types of filaments are actin filaments, intermediate filaments, and microtubules. Actin filaments are relatively thin, while microtubules have the largest diameter. Microtubules are critical in cell division and cell movement. Cell division and movement require the polymerization and depolymerization of microtubules; the anticancer agent paclitaxel (Taxol) works by preventing depolymerization of microtubules and thus “freezing” the cell in a nondividing step. The centrosome is the primary microtubule-organizing center in the cell and is a small organelle involved in the formation of spindle poles during mitosis. Some animal cells may contain cilia, which are used to transport substrate across the cell surface but not for self-locomotion. Each cilium is covered by a plasma membrane and contains a specific array of microtubules.
The metabolism of nutrients by animal cells in culture is very different from that in other types of cells. A typical laboratory growth medium of an animal cell culture contains glucose, glutamine, nonessential and essential amino acids, serum (horse or calf), and mineral salts (for example, Dulbecco’s modified Eagle’s media, or DMEM). For commercial use of these cells to make therapeutic proteins, a serum-free medium is preferred. The major metabolic pathways for substrate uptake and utilization by animal cells in culture are depicted in Figure 12.2. Glucose is converted to pyruvate by glycolysis and also is utilized for biomass synthesis through the pentose phosphate pathway. Pyruvate is converted partly to CO2 and H2O by the tricarboxylic acid (TCA) cycle, partly to lactic acid and partly to fatty acids. Glucose is used as a carbon and energy source, as is glutamine. Part of the glutamine is deaminated to yield ammonium and glutamate, which is converted to other amino acids for biosynthesis purposes. Glutamine also enters into the TCA cycle to yield carbon skeletons for other amino acids and to yield ATP, CO2, and H2O. Animal cells are also capable of synthesizing glucose from pyruvate by the gluconeogenesis pathway. The release of lactate and ammonia as waste products of metabolism is a major problem in high-cell-density culture systems. Both lactate and ammonia at high levels are toxic to cells, primarily because they alter intracellular and lysosomal pH.
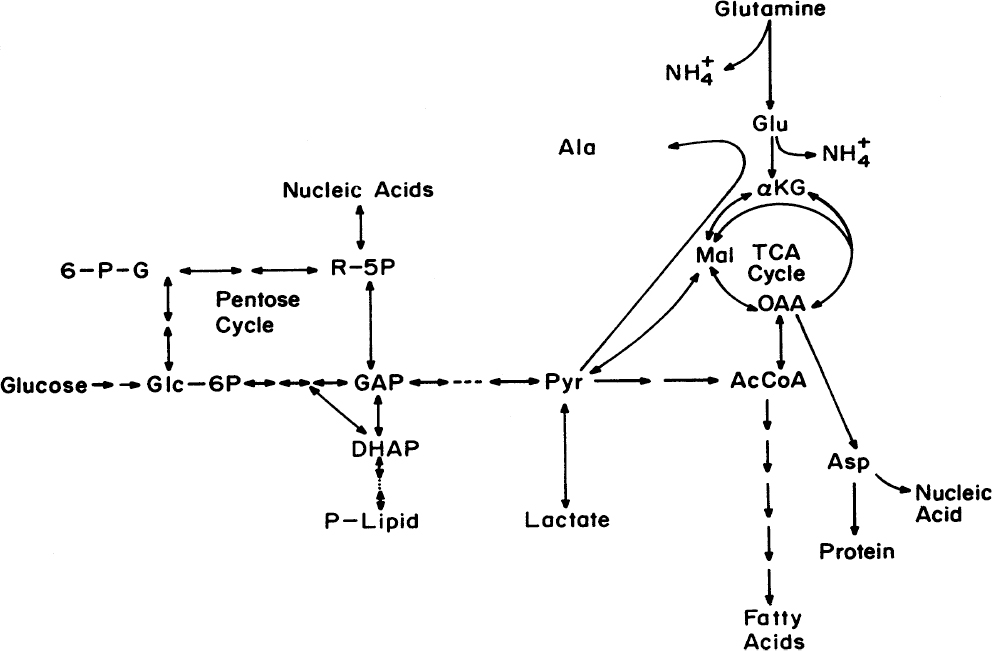
Figure 12.2. Major metabolic pathways in an animal cell. For cultured cells, glucose and glutamine are the major sources of carbon and energy. Lactate and ammonia are the primary waste products, although the release of alanine can be important. Symbols: 6-P-G is 6-P-gluconate; Glc-6P is glucose-6-phosphate; R-5P is ribose-5-phosphate; GAP is glyceraldehyde 3-phosphate; DHAP is dihydroxyacetone phosphate; PYR is pyruvate; AcCoA is acetyl-CoA; OAA is oxaloacetate; Mal is malate; αKG is α-ketoglutarate.
12.2. Methods used for the Cultivation of Animal Cells
The techniques used for cultivation of animal cells differ significantly from those used with bacteria, yeasts, and fungi. In this section, we discuss some of the issues in the application of these techniques and the basic growth dynamics of animal cells in simple culture systems.
12.2.1. Basic Techniques for Animal Cell Culture
Several methods can be used to culture animal cells in the context of bioprocesses. Tissues excised from specific organs of animals, such as lung and kidney, under aseptic conditions are transferred into a growth medium containing serum and small amounts of antibiotics in small T-flasks. These cells form a primary culture. Unlike plant cells, primary mammalian cells do not normally form aggregates but grow in the form of monolayers on support surfaces such as glass surfaces of flasks. Using the proteolytic enzyme trypsin, individual cells in a tissue can be separated to form single-cell cultures.
To start cultures of animal cells, excised tissues are cut into small pieces (~2 mm3) and are placed in an agitated flask containing a dilute solution of trypsin (~0.25% w/v) in buffered saline for 120 min at 37°C. The cell suspension is passed through a presterilized filter to clear the solution, and cells are washed in the centrifuge. The cells are then resuspended in growth medium and placed in T-flasks or roller bottles. Cells usually attach onto the glass surface of the bottle and grow to form a monolayer. The cells growing on support surfaces are known as anchorage-dependent cells. Surface attachment is necessary for these cells to assume the three-dimensional shape necessary for the alignment of internal structures in a manner allowing growth. However, some cells grow in suspension culture and are known to be nonanchorage-dependent cells. Often anchorage-dependent cells can be adapted to growth in suspension culture, which is desirable for industrial processes.
The cells directly derived from excised tissues are known as the primary culture. A cell line obtained from the primary culture is known as the secondary culture. Cells are removed from the surfaces of flasks using a solution of ethylenediaminetetraacetic acid (EDTA), trypsin, collagenase, or pronase. The exposure time for cell removal is 5 to 30 min at 37°C. After cells are removed from surfaces, serum is added to the culture bottle. The serum-containing suspension is centrifuged, washed with buffered isotonic saline solution, and used to inoculate secondary cultures. Many secondary lines can be adapted to grow in suspension and are nonanchorage dependent.
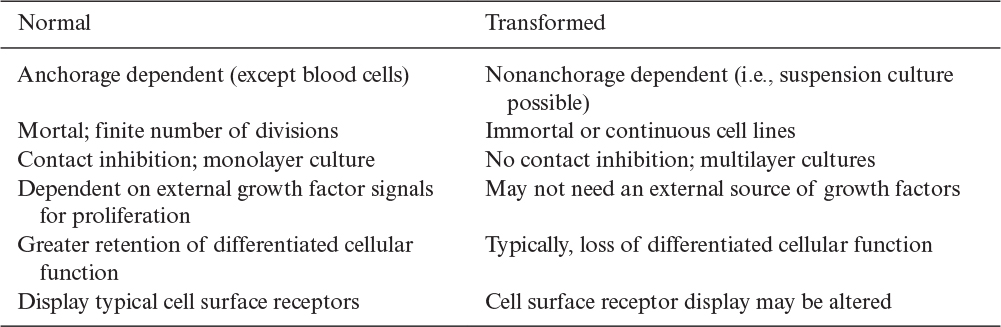
Most differentiated mammalian cell lines (e.g., human fibroblasts such as WI-38 and MRC-5 that are licensed for human vaccine production) are mortal. These cell lines undergo a process called senescence. Essentially, the cells, for reasons that are not completely understood, will divide only for a limited number of generations (e.g., about 30 generations for the MRC-5 cells). Cells that can be propagated indefinitely are called continuous, immortal, or transformed cell lines. Cancer cells are naturally immortal. All cancerous cell lines are transformed, although it is not clear whether all transformed cell lines are cancerous.
Because of the linkage of cancer to cell transformation, the FDA had been reluctant to approve products made from transformed cells. However, transformed cells usually become attachment independent and can be propagated indefinitely in suspension culture, which is highly desirable for large-scale production in bioreactors. In 1986, the FDA approved the first process for production of products, such as the therapeutic protein tissue plasminogen activator, from processes using immortalized cells. The approval of such processes has provided a major impetus for development of bioprocesses based on suspension cultures of animal cells.
Table 12.1 provides a summary of differences between nontransformed and transformed cells. One particular characteristic is contact inhibition. In two-dimensional culture on a surface, nontransformed cells form only a monolayer, as cell division is inhibited when a cell’s surface is in contact with other cells. Transformed cells do not “sense” the presence of other cells and keep on dividing to form multilayer structures.
Although mammalian cell lines have been the primary focus of work in animal cell culture, the two are not synonymous. Insect, fish, and crustacean cell cultures are evolving technologies. In particular, insect cell culture is unusually promising for biotechnological purposes. The baculovirus that infects insect cells is an ideal vector for genetic engineering, because it is nonpathogenic to humans and has a very strong promoter that encodes for a protein that is not essential for virus production in cell culture. The insertion of a gene under the control of this promoter can lead to high expression levels (40% of the total protein as the target protein). Most cell lines are derived from ovaries or embryonic tissue, although at least one differentiated cell line (a BTI-EAA blood cell line) has been maintained in indefinite culture for more than 25 years. Insect cell lines are not transformed but are naturally continuous.
Insect cell cultures can do a significant level of posttranslational processing, but the glycoforms are not authentic with humans. However, the differences can be useful and sometimes trigger a more intense immune response or target specific cells or organs in humans. In these cases, production in insect cells is advantageous. Production of modified insect viruses have been used as insecticides. The baculovirus expression system used with insect cells has been used to make commercial levels of human and veterinary vaccines and a gene therapy agent against familial lipoprotein deficiency.
Another important category is the culture of hybridomas. Hybridoma cells are obtained by fusing lymphocytes (normal blood cells that make antibodies) with myeloma (cancer) cells. Lymphocytes producing antibodies grow slowly and are mortal. After fusion with myeloma cells, hybridomas become immortal, can reproduce indefinitely, and produce antibodies. Using hybridoma cells, highly specific, monoclonal (originating from one cell) antibodies (MAb’s) can be produced against specific antigens.
To produce hybridoma cells, animals (mice) are injected (immunized) with certain antigens (see Figure 12.3). As a reaction to antigens, the animals produce antibodies. The antibody-producing cells (e.g., spleen B lymphocytes) are separated from blood sera and fused with certain tumor cells (e.g., myeloma cells with infinite capacity to proliferate and derived from lymphocytes). The resulting cells are hybrid cells (hybridomas), which secrete highly specific antibodies (MAb’s) against the immunizing antigen.

Figure 12.3. Formation of a hybridoma for making a monoclonal antibody. (a) Antigen is injected into a mouse; (b) lymphocytes in the mouse are activated to produce specific antibodies to the antigen; (c) lymphocytes are collected from the mouse; these lymphocytes grow poorly in tissue culture; (d) myeloma (cancer) cells growing in tissue culture are produced; (e) myeloma cells are fused with lymphocytes; (f) the hybrid cell grows well in tissue culture and make a single monoclonal antibody. Progeny are called hybridomas and can be propagated indefinitely.
Mammalian cell culture is used to produce hundreds of therapeutic proteins using genetically engineered cells. Although many host cells can be used, Chinese hamster ovary (CHO) cells are particularly popular. Most commercial CHO cell lines have been selected to be cultured in liquid suspension, although the original CHO cell lines were anchorage dependent. About 75% of proteins produced commercially from mammalian cell culture use CHO cells. Not only are high product concentrations possible from CHO cells (protein products up to 10 g/l), they are effective excreters of proteins simplifying product recovery. Other popular host cell lines include mouse myelomas (NSO and SP2/0), baby hamster kidney (BHK-21), human embryonic kidney (HEK-293), and human retina–derived (PerC6) cells. Human-based cell lines may have some advantages in terms of more authentic (humanlike) posttranslational processing, such as N-linked glycosylation (see Chapter 4), but there are increased concerns in using human cells due to inadvertent human viral contamination necessitating multiple viral inactivation steps. Also, the overall productivity of the human cell lines is typically somewhat lower than with CHO, but that may change with increased experience, and it is protein dependent.
12.2.2. Growth Media
A typical growth medium for mammalian cells contains serum (5% to 20%), inorganic salts, nitrogen sources, carbon and energy sources, vitamins, trace elements, growth factors, and buffers in water. Serum is a cell-free liquid recovered from blood. Examples are FBS (fetal bovine serum), CS (calf serum), and HS (horse serum). The exact composition of any serum product is not known. However, serum is known to contain amino acids, growth factors, vitamins, certain proteins, hormones, lipids, and minerals. A list of major serum components and their functions is given in Table 12.2. The major functions of serum are as follows:
• To stimulate cell growth and other cell activities by hormones and growth factors
• To enhance cell attachment by certain proteins, such as collagen and fibronectin
• To provide transport proteins carrying hormones, minerals, and lipids
Serum is an expensive medium component ($100 to $500/l) and may cause further complications in the cultivation and downstream separation processes. The demand for serum is increasing rapidly and periodically exceeds supply. The presence of serum proteins and peptides greatly complicates downstream processing. Serum must be filtered and sterilized, and contamination with viruses and possibly mycoplasma (a wall-less bacterium) are potential problems. Contamination by prions (agents that cause diseases such as mad cow disease) has been a real concern, and source animals cannot come from regions known to have contaminated animals. Serum-containing medium foams easily. Perhaps most disturbing from a production perspective is the intrinsic variability in serum. No one batch of serum is ever identical to another. A batch of serum will last only a year before deteriorating. Changes in serum batches require extensive testing.
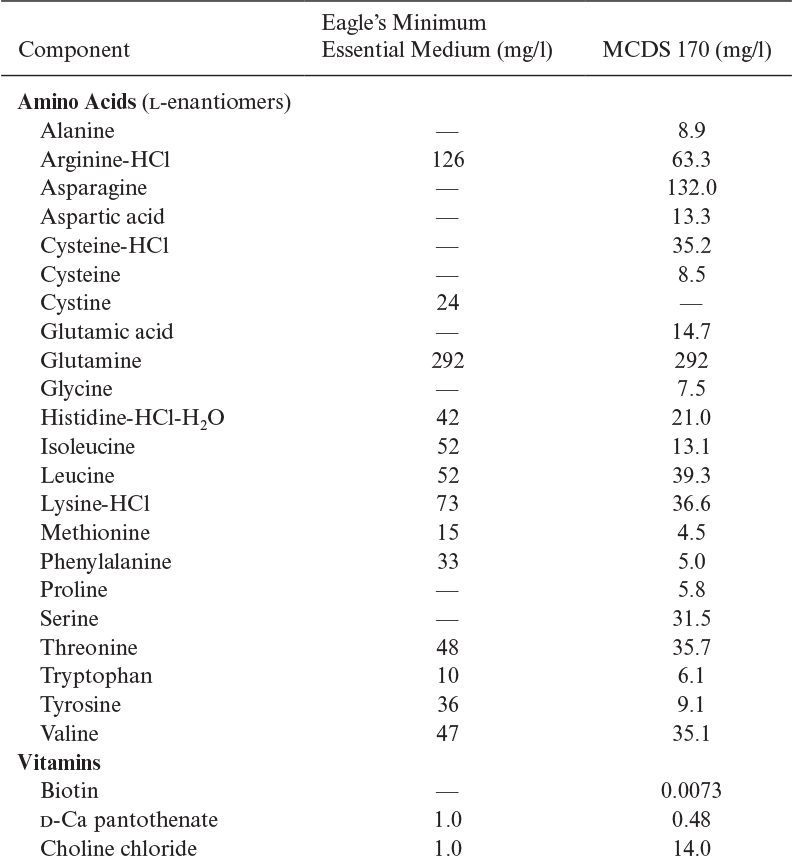
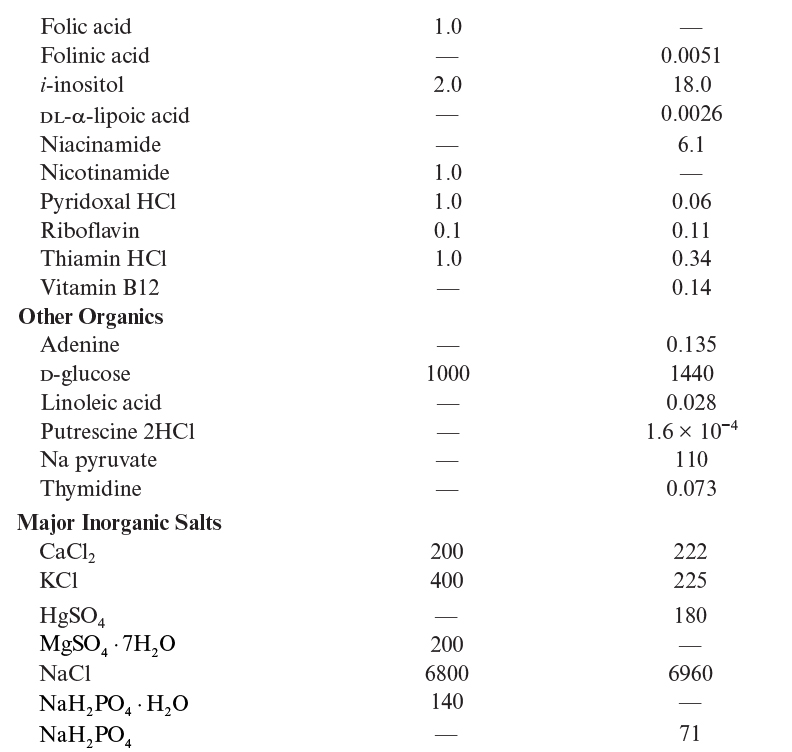
Because of the aforementioned disadvantages of serum-containing media, low-serum or serum-free media have been developed. Typical compositions of two defined media are listed in Table 12.3. Serum-free medium contains basal salts (inorganic salts, carbon, nitrogen compounds), vitamins, growth factors, and hormones (e.g., insulin, transferrin, hydrocortisone, progesterone, fibronectin). By using a serum-free medium, we may reduce the cost of media, eliminate some potential problems in product purification, reduce the chance of contamination, and most importantly improve the reproducibility of results. Different cell lines require different serum-free media composition. Not all cell lines have been adapted to serum-free media. In some cases, a serum-free, animal-protein-free medium is advantageous, but such media can be quite challenging to formulate.
A number of defined media have been developed. Eagle’s minimal essential medium (MEM), DMEM, and rather complex media, such as Ham’s F12, CMRL 1066, and RPMI 1640, are commonly used. Media such as Eagle’s MEM are often supplemented with 5% to 10% (by volume) of serum when used. For serum-free media formulations, often a 1:1 (v/v) mixture of DMEM (nutrient rich) and F12 (rich in trace elements and vitamins) is used. More specialized media (e.g., MCDB 170MDS) may be used for serum-free growth of specific cell lines. A simpler serum-free medium may contain insulin, transferrin, and selenium as serum replacement components, in addition to glucose, glutamine, other amino acids, and salts. Filtered whole lymph from a cow has been used as a growth medium for some mammalian cells.
12.2.3. Growth Dynamics for Animal Cells
Mammalian cells grow at 37°C and pH ≈7.3. Typical doubling times are 12 to 20 h. Usually, 5% CO2-enriched air is used to buffer the medium pH. A carbonate buffer ![]() is used to control pH around 7.3. Since bicarbonate is used up by the cells, CO2-enriched air is provided to balance carbonate equilibrium to keep pH ≈7.3. The culture medium needs to be gently aerated and agitated. Other buffers such as HEPES (N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]) are also used to keep pH at desired level. Insect cells grow best at about 28°C and a pH of about 6.2. Fish cell lines tolerate a wide range of temperature, although temperatures below 37°C are usually preferred (25° to 35°C). Values for pH typical of mammalian cell cultures are usually satisfactory for fish cells; pH values between 7.0 and 7.5 usually give good growth.
is used to control pH around 7.3. Since bicarbonate is used up by the cells, CO2-enriched air is provided to balance carbonate equilibrium to keep pH ≈7.3. The culture medium needs to be gently aerated and agitated. Other buffers such as HEPES (N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]) are also used to keep pH at desired level. Insect cells grow best at about 28°C and a pH of about 6.2. Fish cell lines tolerate a wide range of temperature, although temperatures below 37°C are usually preferred (25° to 35°C). Values for pH typical of mammalian cell cultures are usually satisfactory for fish cells; pH values between 7.0 and 7.5 usually give good growth.
The pattern for the kinetics of the growth of mammalian cells is similar to microbial growth. Usually, the stationary phase is relatively short, and the concentration of viable cells drops sharply thereafter, as a result of the accumulation of toxic metabolic products such as lactate and ammonium. A high level of ammonium is usually the result of glutamine metabolism, and lactate is usually a product of glucose metabolism. Controlled feeding of these nutrients to maintain a constant but lower level of nutrients, such as in fed batch, can reduce this problem. Cell concentration reaches a peak value within three to five days. However, product formation, such as MAb formation by hybridoma cells, can continue under nongrowth conditions. Most of the products of mammalian cell cultures are mixed-growth associated, and product formation takes place both during the growth phase and after growth ceases. Figure 12.4 depicts a typical variation of growth, product (MAb) formation, and glucose utilization by hybridoma cells.
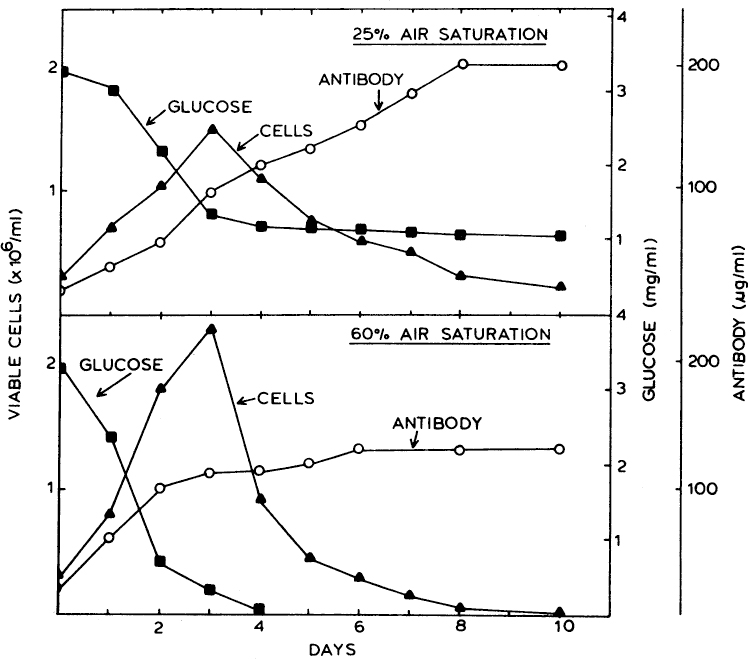
Figure 12.4. Time trajectories of hybridoma growth and monoclonal antibody production at different dissolved-oxygen concentrations in a 3-1 fermenter. (From S. Reuveay, D. Velez, J. D. Macmillian, and L. Miller, J. Factors Affecting Cell Growth and Monoclonal Antibody Production in Stirred Reactors. Journal of Immunological Methods, Volume 86, Issue 1, January 22, 1986, pp. 53–59. With permission from Elsevier.)
The kinetics of product formation (e.g., MAb formation by hybridoma cells) can be described by a Luedeking–Piret equation:
where μ is the specific growth rate. The first term in equation 6.18 is for growth-associated production, and the second term is nongrowth associated. The doubling time of mammalian cells varies between 10 and 50 h, and a typical value is 20 h. As expected, the growth rate varies depending on cell type, medium composition (including growth factors), and other environmental conditions (dissolved oxygen, carbon dioxide levels, pH, ionic strength).
Animal cells require 0.06 to 0.2 × 10–12 mol O2/h-cell, which is about five times less than the oxygen requirements of many plant cells and much lower than for microbial cells. Typical animal cell concentration in suspension culture media is 0.1 to 1 g/l (5 × 105 to 5 × 106 cells/ml) for suspension cultures in simple batch growth. For a cell culture density of 106 cells/ml, the oxygen requirement is on the order of 0.1 to 0.6 mmol O2/l-h. For shallow culture depths of 2 to 5 cm, oxygen supplied by air in the head space of the vessel should meet the oxygen requirements of cells. Forced aeration is required for submerged cultivation of denser cultures. However, animal cells are very shear sensitive, and rising air bubbles may cause shear damage to cells, particularly at the point of bubble rupture. Very small bubbles are less damaging. For this reason, special aeration and agitation systems are designed for animal cell cultures. Techniques that decouple agitation (and shear) from oxygenation are particularly attractive. Chemicals (e.g., Pluronic F-68) can be added to provide shear protection. Since the oxygen requirements of animal cells are relatively low, the oxygen transfer coefficient need not be as high as for bacterial fermentations. Typical values of kLa for suspension cultures (106 cells/ml) are 5 to 25 h–1. At small scales animal cells are usually cultivated in spinner flasks of 5 to 1000 ml in the laboratory. Spinner flasks contain a magnetically driven impeller or spoonlike agitators that operate at 10 to 60 rpm. Aeration is usually by surface aeration using 5% CO2-enriched and filtered air for mammalian cell lines. Spinner flasks are set on a magnetic stirrer plate in a CO2 incubator. However, modern commercial scale extended batch systems with medium replacement can reach cell densities of >107 cell/l. In those cases, oxygen transfer must be augmented; a typical strategy is to use pure oxygen rather than air.
Although cell lysis is the most obvious result of excess shear, sublytic levels of shear can be important. For a variety of cell lines, it has been shown that attached cells respond to shear by elongating and reorienting themselves to minimize shear stress. Also, the distribution and numbers of cell-surface receptors can be altered. These receptors interact directly with components in the medium, such as growth factors, that regulate cell metabolism. Alterations in the production of specific proteins and the rates of DNA synthesis have been observed as responses to shear. This response to sublytic shear complicates scale-up, since the same cells exposed to the same medium, but at different levels of shear, may produce the product of interest at different rates. Also processes, such as N-linked glycosylation, can be altered by shear, resulting in protein products that differ potentially in therapeutic value.
12.3. Bioreactor Considerations for Animal cell Culture
Because mammalian cells are large in size (10 to 20 μm in diameter), slow growing, and shear sensitive, bioreactors must be constructed that are “gently” agitated and aerated. In cases where anchorage-dependent cells are used, as in microcarrier cultures, additional constraints on agitation need to be considered due to liquid shear and particle collisions. Other reactor schemes use entrapped or attached systems where the medium is perfused past the cells. The properties of these cells sets certain constraints on the design of animal cell bioreactors. Following are constraints on these reactors:
• The reactor should be gently aerated and agitated. Some mechanically agitated reactors operating at higher agitation speeds and bubble-column and airlift reactors operating at high aeration rates (and large gas bubbles) may cause shear damage to cells. Shear sensitivity is strain dependent, and some cell lines are fairly shear tolerant.
• Well-controlled homogeneous environmental conditions (T, pH, DO, redox potential) and a supply of CO2-enriched oxygen or air need to be provided.
• A large support material surface–volume ratio needs to be provided for anchorage-dependent cells.
• The removal of toxic products of metabolism, such as lactic acid and ammonium, and the concentration of high-value products, such as MAb’s, vaccines, and lymphokines, need to be accomplished during cell cultivation.
The laboratory-scale cultivation of animal cells is carried out in T-flasks (25 ml to 100 ml) for anchorage-dependent cell lines and for shallow suspension cultures, spinner flasks (100 ml to 1 l) with paddle-type magnetic agitators, roller bottles (50 ml to 5 l) rotating at about 1 to 5 rpm, and trays containing shallow liquid suspension culture. These laboratory-scale reactors are placed in a carbon dioxide incubator at 37°C (5% CO2-containing air) for the cultivation of mammalian cells. Different steps need to be taken for anchorage-dependent and suspension cells when scaling up animal cell cultures. Reactors with a high surface–volume ratio (microcarrier systems, hollow-fiber reactors, ceramic matrix systems, weighted porous beads) are used for anchorage-dependent cells. However, modified stirred reactors and airlift or bubble-column reactors can be used for suspension cultures. Membrane bioreactors and microencapsulation methods have been developed for simultaneous cell cultivation, product concentration, and toxic product removal. Many of these systems behave as perfusion reactors (see Chapter 9, “Operating Considerations for Bioreactors for Suspension and Immobilized Cultures”). In essence, a perfusion system exists when cells are retained in the reactor, medium is added continuously or semicontinuously, and spent medium is removed. The removal of spent medium removes toxic metabolites. We can break these reactors into two major types: nonstirred and stirred systems.
12.4. Bioreactor Systems for Animal Cell Culture
Here we describe the range of reactor systems used for animal cell cultures based on cell immobilization and type of agitation. First we look at the use of immobilized cell reactors (as described in Chapter 9). When such reactors are operated with continuous flow and cell retention, they are examples of perfusion systems, and long-term operation (several months) is possible. Several successful commercial processes are based on these techniques, as animal cell culture is quite adaptable to such systems. Standard stirred-tank systems at large scale are used extensively for production of proteins from suspension-adapted animal cells. We explore briefly these options.
12.4.1. Nonstirred Reactor Systems
The classic method for animal cell culture has been roller bottles, and they have been important components in the scale-up of processes using anchorage-dependent cells. With roller bottles, the liquid covers about 25% of the surface area. Bottles are rotated about the long axis (1 to 5 rpm). Cells adhere to the walls of the bottle and are exposed to liquid 25% of the time and to gas (e.g., 5% CO2 and 95% air) 75% of the time. When exposed to the liquid, nutrients can be transported into the cell, and when in the gas phase, aeration takes place. The roller-bottle system has an advantage over T-flasks because of increased surface area, agitation of the liquid, and better aeration. Roller bottles are not typically used for large-scale production because of high labor requirements and bottle-to-bottle variability. However, a highly automated facility making extensive use of robots uses roller bottles for commercial production of erythropoietin (a therapeutic protein). Roller bottles are also used for production of some vaccines.
Hollow-fiber reactors have also been used to provide a high growth surface–volume ratio and, therefore, high cell concentrations. They are examples of single-use technology and are available at scales equivalent to order of a 1000 l stirred-tank system. Cells are immobilized on the external surfaces of hollow fibers, and nutrients pass through the tubes. Cell concentrations comparable to those found in tissues can be reached in hollow-fiber reactors. However, the control of microenvironmental conditions inside the reactor where cells are immobilized on fiber surfaces is very difficult because there is almost no mixing (i.e., a heterogeneous environment). Also, the quantification of growth and product formation is difficult in such a system. However, it is possible to construct the reactor using fibers of known molecular weight (MW) cutoff to control the flux into the effluent stream of products with different MWs. The accumulation of some toxic products, such as lactate and ammonium, in the fiber reactors may cause high levels of inhibition. Selection of fiber material (MW cutoff) and flow regime are critical factors for the rapid removal of toxic metabolic products. Hollow-fiber reactors have been used for the production of extracellular proteins such as MAb’s from hybridoma cells. Antibody concentrations on the order of 50 mg/ml have been obtained with this reactor, since product is retained by the membrane while medium flows in through the membrane and lower molecular wastes flow out of the cell compartment. To overcome some of the difficulties with axial-flow hollow-fiber reactors, radial-flow or cross-flow hollow-fiber units have been developed. Hollow-fiber reactors are well suited to perfusion operation (see Section 9.2.5) with continuous feed. MAb production from hybridomas can be sustained for extended periods (e.g., 100 days), as stationary-phase cells still synthesize product.
Single-use disposable systems using multiple plates for growth of adherent cells operate conceptually much like roller-bottle systems, but they provide high surface areas for culture of such cells. These have generally been used more to generate cells (e.g., mesenchymal stem cells) than proteins because of difficulties in environmental control, resulting in a heterogeneous environment when scaled up to larger sizes. However, at least one commercial system has added an integrated control system for cell expansion to provide improved environmental control. Systems with surface areas greater than 10 m2 are available.
A tubular ceramic matrix has been used for the immobilization and cultivation of mammalian cells. High cell, and therefore high extracellular, protein concentrations can be obtained using such systems. The quantification of cell concentration and the control of microenvironmental conditions within the heterogeneous cell culture in ceramic matrix are some of the major difficulties, although these matrices do provide well-defined flow channels. Scale-up of a ceramic matrix reactor may be difficult because of the heterogeneous nature of the system when long tubes are used, although operating with multiple shorter tubes in parallel is an option. These systems are also often operated in perfusion mode.
12.4.2. Systems for Entrapped Cells in Stirred Reactors
The immobilization of mammalian cells in gel beads (agar, alginate, collagen, polyacrylamide) and the use of such systems in a packed- or fluidized-bed configuration are possible. Such immobilization methods and reactor configurations will reduce or eliminate the shear effects on cells. High cell densities make high volumetric productivities possible. However, the control of microenvironmental conditions inside bead particles and the accumulation of toxic metabolic products in beads are potential problems.
The use of microcarriers for the cultivation of anchorage-dependent mammalian cells is an attractive approach. For example, microcarriers such as DEAE-Sephadex beads, which provide large surface per unit volume of reactor (~70,000 cm2/l), allow high cell concentrations in the medium (>107 cells/ml). The microcarrier beads with cells are suspended in a stirred bioreactor. Other microcarrier beads, such as polyacrylamide, polystyrene, cellulose fibers, hollow glass, and gelatin-coated dextran beads, have been developed. At present, dextran- and DEAE-based (DEAE-Sephadex, DEAE-polyacrylamide) microcarriers are the most commonly used. The surfaces of these microcarrier beads can be modified by addition of compounds, such as collagen, to promote cell adhesion and enhance cell function. Cells grow on the surfaces of microcarriers, usually in the form of monolayers and sometimes as multilayers. Microcarrier culture methods provide a large surface area for growth and a rather homogeneous growth environment. Microcarrier beads can be placed in a gently agitated reactor vessel, in a fluidized bed, or in an airlift (bubble-column) fermenter. Bead-to-bead contact and abrasion of the surfaces can be a problem, and the nonporous nature of these carriers limits the available surface area and, consequently, the maximum cell numbers achievable. Agitation of large-scale vessels with microcarrier cultures is difficult owing to balancing the needs for aeration and mixing against the shear sensitivity of cells attached to the surface of microcarriers. Shear forces are more harmful to cells attached to microcarriers than to suspended cells because attached cells cannot “tumble,” and shear tends to “rip” them off the carrier surface. Macroporous microcarriers can be used; cells that enter the pores are shielded from shear effects. However, diffusional limitations and heterogeneous growth conditions may cause undesirable results. Because cells are easily retained in the bioreactor, microcarrier cultures are well suited for perfusion operation. Sometimes, weighted particles are used, particularly in fluidized bed reactors, to allow more rapid perfusion without loss of the cell-containing particles.
Instead of using a microcarrier bead, cells may be microencapsuled. Animal cells are encapsulated with a porous membrane. Typical capsule size is 300 to 500 μm, and the MW cutoff of these capsule membranes is 60 to 70 kDa. Microcapsules operate like small membrane bioreactors, in which very high cell concentrations can be reached (~108 cells/ml). Using the right capsule membrane with a desired MW cutoff, toxic products such as lactate and ammonium can be removed from intracapsule culture media, and high-MW products such as MAb’s or lymphokines can be concentrated inside capsules. Moreover, cells are protected from hydrodynamic shear by encapsulation, providing a suitable environment for direct aeration and facilitating O2 delivery and CO2 removal. However, the growth and product-formation rates inside capsules may be limited by the diffusion of nutrients such as dissolved oxygen or glucose, especially at large capsule sizes. The reduction of capsule size to eliminate mass-transfer limitations (down to 200 μm) and the control of pore size to concentrate desired products within the capsule are two promising approaches to solve some of the problems associated with encapsulated cells. By varying the concentration and average MW of poly-L-lysine (PLL), the pore size of the capsule membrane can be controlled within certain limits (30 to 80 kDa). Monitoring and control of microenvironmental conditions within capsules (pH, DO level, nutrient–product gradient) are major problems that impose limitations on growth and product formation inside capsules. The fragile nature of the microcapsule also limits the scale-up potential of this system. However, for smaller-scale systems, there is evidence that microencapsulation of CHO cells with recombinant DNA can produce about fivefold higher levels of a target protein than with suspension cultures.
12.4.3. Suspended Cultures
Although specialized bioreactors are used for mammalian cell culture, the traditional stirred-tank reactor described in Chapter 10, “Selection, Scale-Up, Operation, and Control of Bioreactors,” predominates at large scale for culture of mammalian and insect cells. Many shear-protecting agents (such as serum or Pluronic F-68) work by preventing cells from accumulating at the gas–liquid interface. Gas exchange can be accomplished with membranes or silicone rubber tubing, but sparging of gas is the simplest method and is acceptable with many cell lines, particularly when shear protectants are used. Cell lines differ in shear sensitivity; CHO cells, widely used for protein production, are relatively resistant to shear damage. Gross shear damage where cells lyse (necrosis) is obvious, but lower levels of shear can alter cell physiology. More subtle effects of shear include changes in physiology and, possibly, induction of apoptosis, or programmed cell death. These more subtle changes can alter apparent growth rates, productivity, and product quality (e.g., due to release of degradative enzymes into the medium).
Conventional stirred-tank bioreactors have been modified to reduce shear rates on cells in suspension. Sail-type and axial-flow hydrofoil agitators have been developed and used for suspension cultures. The agitation rate in these reactors is on the order of 10 to 40 rpm, providing low shear rate. Some animal cells can be cultured as suspensions in bioreactors up to 20,000 l in size. Because cells are significantly smaller than the turbulent eddies in these bioreactors, cell lysis is minimized. Only cells that are at the interface of an eddy and another eddy or another surface (e.g., reactor wall) are likely to experience damage. Cells at the gas–liquid interface are particularly prone to damage. The breakage of air bubbles is particularly destructive to cells that accumulate at the interface of a gas bubble and medium.
In addition to the use of traditional stainless-steel reactors, a number of single-use, disposable bioreactors have been developed for use with animal cells. We discussed these in Chapter 10 (Section 10.1.4) and divided the reactor types on the basis of external or internal agitation. Both types are used extensively with animal cells and provide effective mixing with minimal cell damage at moderate scales (100 to 2000 l). While typically operated in fed-batch mode, operation in perfusion mode is possible by coupling with cell-separation techniques.
Overall, there are a large number of options for reactors for animal cells reflecting the diversity of constraints on these cellular production systems.
12.5. Products of Animal Cell Cultures
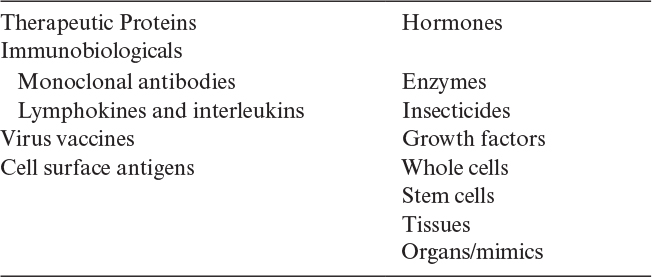
The number of commercial products made from animal cell culture, predominately from mammalian systems, exceeds 200. Table 12.4 lists the major categories of these products. Most of these products are biopharmaceuticals and generally extracellular proteins. Although originally made at very low levels, titers above 5 g/l have been obtained. About 40% of the products are monoclonal therapeutics. Technical improvements allow increased recirculation times in the body, making some of these protein products more cost effective than the originally formulated products. Many of them are targeted to the treatment of autoimmune diseases and for chemotherapy.
Some other products include clotting factors (for treatment of hemophilia), hormones, and enzymes (e.g., replacement therapy to treat diseases such as type l Gaucher disease and cystic fibrosis) and cytokines (e.g., for anticancer chemotherapy and treatment of multiple sclerosis). Most of these products are proteins that require humanlike posttranslational processing to be effective therapeutically.
Vaccines represent another major type of product that can be made from mammalian and nonmammalian cell cultures. Insecticides, typically viruses made in insect cell culture, represent another type of product. Mab’s for use as diagnostics are a well-established product, and posttranslational processing is less important than for therapeutic uses.
Stem cell technology has great promise. The techniques described here have great potential for the effective large-scale production of such cells that may be used for replacement therapies and as surrogates for drug testing and development. The production of stem cells, particularly as replacement in the body, is demanding because of the need to ensure that the cell physiology is essentially identical from batch to batch. Cells are complex, and the appropriate analytical techniques to ensure equivalency are numerous and often expensive to implement.
In the last 30 years, animal cell culture has grown from a novel, small component of the bioprocess industries into a major production technique, and its growth is likely to continue.
12.6. Summary
Animal cells are well suited for the production of proteins requiring extensive and accurate posttranslational processing, organized tissues (artificial skin), certain vaccines, and viruses that can be used as pesticides. Animal cells are more complex and fragile than bacterial, fungal, or yeast cultures. These characteristics require the development of unique strategies for cultivation.
Critical distinctions are primary cell lines (established directly from tissue) and secondary cell lines, which are established from primary cell lines. Normal cell lines are mortal; they can divide only a finite number of times. Other cell lines are transformed or immortal and divide indefinitely. Many cell lines will grow only when attached to a surface and are thus anchorage dependent. Some cells, particularly transformed cell lines, can grow in suspension. One particularly important type of suspension cell is the hybridoma. This cell is a hybrid of a mortal antibody-producing cell with a transformed cell, which results in an immortal, easily cultured cell line that produces a single type of antibody, or a monoclonal antibody.
The media used to grow animal cells must provide a variety of growth factors, glucose, glutamine, amino acids, specific salts, and vitamins. In some cases, serum from animals (especially mammals) is used. However, serum is expensive, increases the probability of contamination, is undefined in composition, is variable from lot to lot, and complicates the downstream recovery of protein products. In many cases, our knowledge of cellular nutrition has allowed the development of serum-free (and animal protein–free, in some cases) media, and the use of such media is of increasing importance.
Many potential bioreactor configurations are commercially available for use with animal cells. The reactor design problem centers on providing sufficient oxygen and nutrients and (for anchorage-dependent cells) surface area to achieve high cell densities while minimizing exposure to liquid shear. Also, complete medium utilization and high product concentrations are sought. Designs must minimize the formation of toxic by-products (e.g., lactate and ammonium) or effectively remove such metabolites from the microenvironment of the cells.
Suggestions for Further Reading
AL-RUBEAI, M., Animal Cell Culture, Springer International, Switzerland, 2015.
BANDARANAYAKE, A. D., AND S. C. ALMO, “Recent Advances in Mammalian Protein Production,” FEBS Letters 588: 253–260, 2014.
CHALMERS, J. J., “Animal Cell Culture: Effects of Agitation and Aeration on Cell Adaption,” in Encyclopedia of Cell Technology, R. Spier, J. B. Griffiths, and A. H. Seragg, eds., Wiley, New York, 2000. (See also other related articles in this book.)
DUMONT, J., D. EUWART, B. MEI, S. ESTES, AND R. KSHIRSAGAR, “Human Cell Lines for Biopharmaceutical Manufacturing: History, Status, and Future Perspectives,” Crit. Rev. Biotechnol. 36(6): 1110–1122, 2015.
KIM, J. Y., Y. G. KIM, AND M. LEE, “CHO Cells in Biotechnology for Production of Recombinant Proteins: Current State and Further Potential,” Appl. Microbiol. Biotechnol. 93(3): 917–930, 2012.
NIENOW, A. W., “Reactor Engineering in Large Scale Animal Cell Culture,” Cytotechnol. 50: 9–31, 2006.
VAN OERS, M. M., G. P. PIJLMAN, AND J. M. VLAK, “Thirty Years of Baculovirus-Insect Cell Protein Expression: From Dark Horse to Mainstream Technology,” J. Gen. Virol. 96: 6–23, 2015.
WURM, F. M., “Production of Recombinant Protein Therapeutics in Cultivated Mammalian Cells,” Nat. Biotechnol. 11: 1393–1398, 2004.
ZHU, J., “Mammalian Cell Protein Expression for Biopharmaceutical Production,” Biotechnol. Adv. 30: 1158–1170, 2012.
Problems
12.1. Cite the major differences between animal, plant (see Chapter 13), and bacterial cells in terms of cell structure and physiology.
12.2. Describe the role of glutamine in animal cell metabolism.
12.3. Compare serum-containing and serum-free media in terms of their advantages and disadvantages.
12.4. What are the roles of CO2 provided in air to animal cell cultures?
12.5. What are the common features of animal cell bioreactors?
12.6. Compare the following immobilization methods used for animal cells in terms of their relative advantages and disadvantages: microcarrier (surface) culture, porous beads, encapsulation, and gel entrapment.
12.7. What do you think is the most suitable reactor type, mode of operation (batch, continuous perfusion), and cultivation method (suspended, immobilized) for animal cell cultures in general? Compare your choice with the other alternatives.
12.8. Compare various methods for the aeration of animal cell cultures. Explain the advantages and disadvantages of each method.
12.9. Describe the process for formation of hybridomas.
12.10. What value of kLa must be achieved to sustain a population of 1 × 10–7 cells/ml when the oxygen consumption is 0.1 × 10–12 mol O2/h-cell?
12.11. Hybridoma cells immobilized on surfaces of Sephadex beads are used in a packed column for production of monoclonal antibodies (MAb). Hybridoma concentration is approximately X = 5 g/l in the bed. The flow rate of the synthetic medium and glucose concentration are Q = 2 l/h and S0 = 40 g/l, respectively. The rate constant for MAb formation is k = 1 gX/l-d. Assume that there are no diffusion limitations and glucose is the rate-limiting nutrient.
a. Determine the volume and the height of the packed bed for 95% glucose conversion. Bed diameter is D0 = 0.2 m. Neglect the growth of the hybridomas and assume first order kinetics.
b. If Yp/s is 4 mg MAb/g glucose, determine the effluent MAb concentration and the productivity of the system.