13. Bioprocess Considerations in Using Plant Cell Cultures
The use of plant cell cultures for production of useful compounds is an intriguing possibility, and examples of commercial processes using plant cells have emerged in the last 15 years. Effective bioreactor preparation of elite plants is another application of bioprocesses technology employed with plants. Understanding the response of plant cells and organized tissues to bioprocess choices is critical to the commercial use of plant cell tissue culture.
13.1. Why Plant Cell Cultures?
The plant kingdom is very diverse. Many thousands (>200,000) of chemicals are produced only in plants, and certain chemicals are produced only by a single plant species. The biosynthetic capabilities of plant cells have been little explored. Only a small percentage of the world’s plants have been scientifically named or described, and only a small fraction of these have been screened for the production of novel and useful compounds. The great genetic potential of plants to produce compounds of use to humans has been little exploited. In fact, the rapid destruction of forests worldwide is leading to the extinction of many plants without preservation of their genomes.
Even the small number of characterized plants has yielded many important compounds (e.g., over 120 prescription drugs). In the Western world, over 25% of pharmaceuticals are derived from extraction from whole plants, and a much greater fraction of medicinals are plant-derived in Asia and the rest of the world. In addition to uses as medicinals, plant products are of interest as dyes, food colors, food flavors, fragrances, insecticides, and herbicides. Peptides and proteins such as miraculin, thaumatin, and monellin are also important targets for plant cell culture technology. Some compounds of potential commercial interest are listed in Table 13.1 and shown in Figure 13.1.
These compounds are chemically complex and are generally nonproteins. They are known as natural products, secondary metabolites (not required for growth), or specialized metabolites, and their function is critical to the plant’s adaptation, defense, and survival in a changing environment. The compounds most likely to be of commercial interest are those that are not synthesized by microbes and are of sufficient complexity that chemical synthesis is not a reasonable alternative. Also, these nonprotein products are often made by the condensation of intermediates derived from primary metabolism (Chapter 5, “Major Metabolic Pathways”) and then modified by pathways unique to that particular plant species. Plant genetics and physiology are poorly understood in comparison to animal and bacterial systems. Because of the difficulty of finding and recovering genes from unrelated pathways, genetic engineering using more easily grown organisms, such as yeast or bacteria, to make these products is not currently an attractive alternative to plant cell culture. However, there are emerging examples in which genetically engineered plants are useful. The enzyme human glucocerebrosidase, produced in genetically engineered carrot cells using bioreactors, is an FDA-approved product (trade name Elelyso) for treating Gaucher disease. This plant cell–derived product sells for less than the standard treatment, is said to be more reliable to produce, and avoids problems of misfolded proteins from bacteria. As the cost of transcriptomic platforms is decreasing, it will be possible to elucidate the entire biosynthetic pathway of a specialized metabolite. Such knowledge may make production in yeast and bacteria possible, although those genetic insights can be applied directly to improving plant cell cultures themselves. Plant cell tissue cultures have low volumetric productivities, so higher-value products (≥$500/kg) are desirable targets. Thus, the chemicals of greatest commercial interest must be of intermediate to high value and unique to the plant kingdom.
A good example of bioprocess development using plant cell cultures is for the production of the important anticancer agent paclitaxel (Taxol). Supply problems greatly impeded the clinical development of this drug. Initially, paclitaxel was produced by extraction from the bark of the Pacific yew tree (Taxus brevifolia). It required three 100-year-old trees to supply enough paclitaxel to treat one patient. This tree is relatively uncommon, and its harvestation had significant adverse environmental impacts. Harvestation of whole trees has been replaced by a method of semisynthesis and by plant cell culture. Needles and branches of more common yews can be collected, a precursor compound extracted, and the precursor chemically converted to paclitaxel. The collection of needles and branches can be done in a way to prevent death of the plant. However, there are environmental concerns with both needle collection and disposal of solvents from the chemical processing. Production of paclitaxel in large-scale bioreactors provides a product with fewer contaminants, is highly controllable and reproducible, and is environmentally friendly. Commercial production of paclitaxel using large-scale bioreactors is now a reality. Suspension cultures in stirred-tank vessels of up to 75,000 l have been used.
The factory production of chemicals from plant cell tissue culture offers a number of important advantages:
• Control of supply of product is independent of the availability of the plant itself.
• Cultivation is under controlled and optimized conditions.
• Strain is improved with programs analogous to those used for microbial systems.
• With the feeding of compounds analogous to natural substrates, novel compounds not present in nature can be synthesized.
The production of the dye shikonin further illustrates these advantages. Japan could not grow sufficient Lithospermum to supply its needs, nor was it assured of a stable supply to import. The plant had to grow for at least 2 to 3 years before reaching harvestable size; at harvest, about 1% to 2% of the dry weight was shikonin. Using a combination of strain selection and optimization of reactor conditions, a commercial process that could produce cells with 14 wt% shikonin in a 3-week batch cultivation period was established.
However, the role of bioreactors in plant cell tissue is not limited to chemical production. Transgenic plant cell cultures also can be used to produce proteins such as vaccines. An example is the production of glucocerebrosidase from carrot cells. The production of propagules or of artificial seeds may be economically important. The use of efficient submerged cultivation devices to generate elite plants may replace the current labor-intensive processes for the micropropagation of plants.
Whether the product is a chemical or a new plant, the bioprocess engineer must become familiar with some basic characteristics of plants and their implications for reactor design.
13.2. Plant cells in Culture Compared to Microbes
Plant cells in culture are not microbes in disguise. Aspects of plant cell structure and physiology were discussed in Chapter 2, “An Overview of Biological Basics.” See Figure 13.2 for a summary of plant cell structure. The primary difference between plant cells and microbes is the ability of the cells to undergo differentiation and organization even after extended culture in the undifferentiated state. The capacity to regenerate whole plants from undifferentiated cells under appropriate environmental conditions is called totipotency. This capacity is essential to plant micropropagation and is often associated with secondary metabolite formation. If we could do the same for animals as we can for many plants, we could remove a cell from a person’s tongue and generate millions of nearly identical clones of that person’s whole body!
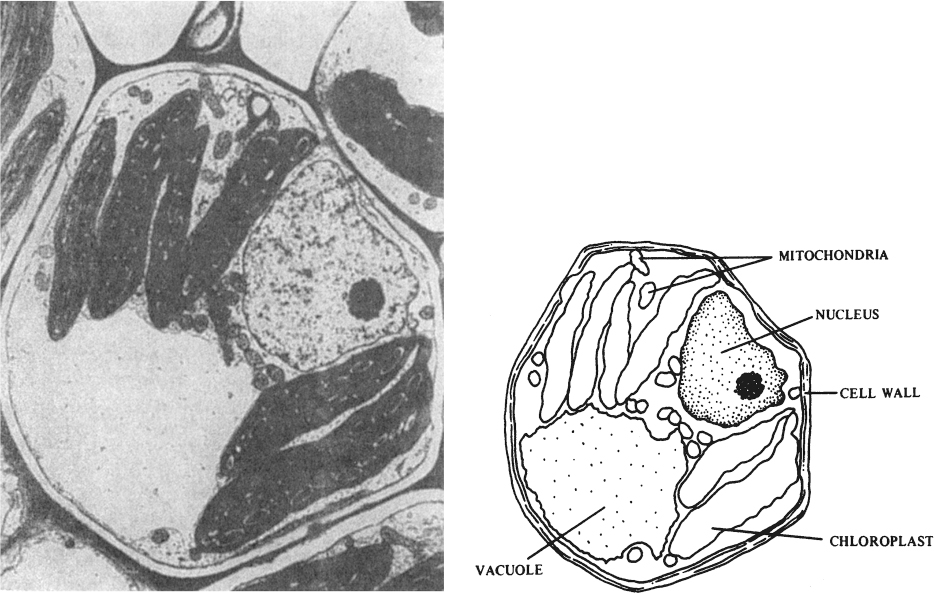
Figure 13.2. (Left) Cross section of a plant cell enlarged about 9000 times its actual size by an electron microscope. (Right) The labeled drawing identifies some of the cell’s structures and organelles, those specialized cell parts that resemble and function as organs. This particular cell is a bundle sheath cell from the leaf of a Zea mays plant. Cells like this one are part of the veins of the leaf and completely surround the water- and food-conducting tissues (xylem and phloem) of the veins. (With permission, from Michael A. Walsh, Utah State University.)
Let us begin by considering the establishment of cell cultures for producing a particular chemical (see Figure 13.3). Callus and suspension cultures have been established from hundreds of different plants. A callus can be formed from any portion of the whole plant that contains dividing cells (Figure 13.4). The excised plant material is placed on solidified medium containing nutrients and hormones that promote rapid cell differentiation. The callus that forms can be quite large (>1 cm across and high) and has no organized structure. Although we speak of a callus as being dedifferentiated tissue, it contains a mixture of cell types. In addition to the environment conditions (e.g., plant hormones), the source and age of the initial tissue (root, shoot, and so on) can make a difference and may result in inheritable changes attributed to dedifferentiation. If we are interested in maximizing the formation of a particular compound, it is advisable to initiate the callus from a plant that is known to be a high producer. Even from the same batch of seeds, there may be considerable variation from plant to plant in their biosynthetic capacity.

Figure 13.3. Suspension cultures can be established from many plants by establishing first callus culture and then suspension cultures; alternatively, organ cultures can be produced from callus culture. For some plants, the process can be reversed to generate whole plants from suspensions on organ cultures.
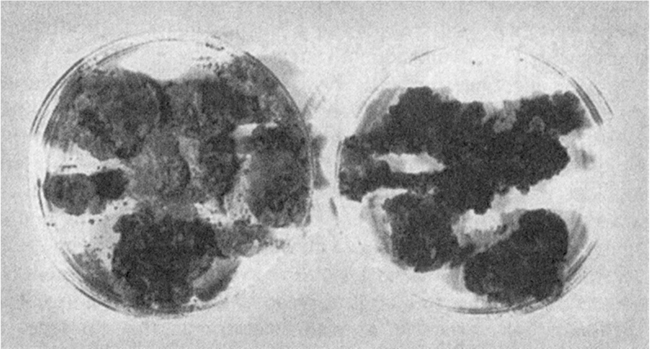
Figure 13.4. Calli of grape cultures. The plate on the left contains an unselected culture in which a few cells make high levels of reddish-purple pigments (anthocyanins). The plate on the right contains highly selected cultures in which most cells make pigment.
For both callus and suspension cultures, a chemically defined medium is used. Typically, cultures, especially suspension cultures, are maintained in the dark. While exposure to light may be used to regulate expression of specific pathways, light is rarely used solely to support growth as most cells are incapable of sustained photoautotrophic growth. Typical media use a carbon–energy source such as sucrose. Inorganic nutrients, vitamins, and hormones (or growth regulators) are included in media. Classes of plant hormones that are growth promoters are auxins, cytokinins, and gibberellins. Most media contain at least an auxin such as naphthalene acetic acid (NAA) or 2,4-dichlorophenoxyacetic acid (2,4-D) and a cytokinin such as kinetin or benzyladenine (BA). Ethylene is a plant hormone and is typically produced by the culture itself.
The establishment of suspension cultures (see Figure 13.5) from callus is generally straightforward if the callus is friable (easily breaks into small pieces). A piece of callus is placed in a liquid medium in a shake flask. With gentle to moderate agitation, cells or small aggregates of cells will slough off. Typical conditions are 27°C and a pH of 5.5 in the dark. These suspended cells then replicate. After 2 or 3 weeks, the suspended cells are transferred to fresh medium, and large aggregates or residual callus are discarded. Suspensions can grow to high cell densities (similar to applesauce). All suspension cultures contain aggregates, but it is possible to select for fine suspensions by discarding large aggregates at each subsequent transfer. However, due to cell-to-cell communication, cell aggregates are often more productive than a dispersed cell culture.
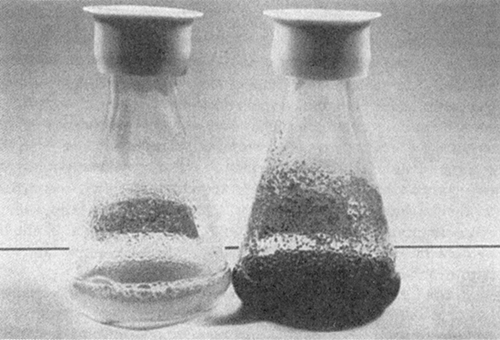
Figure 13.5. Suspension cultures of grape cells. The flask on the left is a culture with modest selection, while the culture on the right is highly selected for anthocyanin production.
The aggregates are due not so much to clumping as to the failure of progeny to separate. Cell-to-cell communication is very important in plants. Normally, all cells in a whole plant are connected to one another by small pores called the plasmodesmata. The plasmodesmata allow the interchange of lower-molecular-weight compounds (<800 da) from the cytoplasm of one cell to another. The plasmodesmata are formed principally during cell division. Cells in aggregates can communicate with one another through plasmodesmata and also through diffusable species. For example, plants generate ethylene, which acts as a hormone. In a large aggregate, there are concentration gradients of such metabolic products as well as nutrients (e.g., oxygen and hormones). These gradients lead to a wide variety of microenvironments, and as a consequence, cells in different positions in the aggregates may have greatly different biochemical and morphological structure. Although plant cell cultures are pure cultures in the sense that only one species is present (ideally, one genotype), they behave very much like a mixed culture. A mix of cell types may be necessary for the formation of some products.
Plant cells can be very large, with cell diameters typically in the range of 10 to 100 μm. They grow slowly, with doubling times typically ranging from 20 to 100 h. Growth is usually nonphotosynthetic, with sucrose or glucose supplied exogenously as the carbon and energy source. Typical respiration rates are roughly 0.5 mmol O2/h-gDW, or about 5% to 15% of that in Escherichia coli. Plant cells can often be cultivated at very high densities, up to 70% of the total reactor volume as cells. Although the respiration rate is low, high cell density requires very good oxygen-transfer capabilities in a reactor.
Plant cells contain a higher percentage of water than do bacterial cells (90% to 95% versus 80%). This higher water content is due, in part, to the presence of the central vacuole, which can occupy as much as 95% of the intracellular volume in extreme cases. Plant cells tend to secrete relatively few compounds but sequester them inside the vacuole. Many of these compounds are secondary metabolites of commercial interest; many would also be cytotoxic if not removed from the cytoplasm. Their intracellular storage will have important implications in our discussions later in this chapter on bioreactor strategies.
One other biological aspect of critical importance is the role of elicitors. In whole plants, there are a number of defensive mechanisms that plants use to contain attacks from pathogenic fungi, bacteria, and viruses. Defense mechanisms can be activated if breakdown products of fungal or plant cell walls (oligosaccharides) are present. Other compounds, some even nonorganic, can also elicit a response. Many secondary metabolites are involved in the plant’s defensive mechanisms. The exposure of suspension cultures to elicitors has led in many cases to rapid increases in the accumulation of secondary products. The use of elicitors has been an important breakthrough in improving volumetric productivities in bioreactors. The most useful elicitor is methyl jasmonate. This compound is relatively inexpensive and readily introduced into large bioreactors. It regulates expression of a wide variety of plant genes.
Cells in suspension can be made to undergo differentiation and organization if the correct environmental conditions can be found. Nutrient and hormone levels must be adjusted. Embryos, shoots, and roots can then be made from either calli or aggregates in suspension. Root cultures can also be generated through the introduction of plant hormone genes by the plant pathogenic bacteria, Agrobacterium rhizogenes (see Figure 13.6). Hairy root cultures can grow in the absence of added hormone and are genetically stable, an advantage over cell suspension systems. However, the type of cell or tissue culture (i.e., cell suspensions, roots, or shoots) for producing the specialized metabolite is dependent on the desired product. For certain secondary metabolites, suspension cultures can be used as the production platform in traditional bioreactors using bioprocess strategies (such as cell line selection, elicitation, in situ product removal discussed in Section 13.3), while more differentiated cultures such as root or shoot cultures are required for the production of other metabolites.

Figure 13.6. Agrobacterium-mediated transformation method for the generation of Catharanthus roseus hairy roots. (From Noreen F. Rizvi, Miglia Carnejo, Kassi Stein, Jessica Weaver, Erin J. Cram, & Carolyn W. T. Lee-Parsons [2015], Plant Cell Tiss. Organ Cult. 120:475–487. With permission of Springer.)
Recent advances in genomics and our understanding of plant molecular biology make it possible to consider genetically engineering the plant cell’s biosynthetic pathways. It will soon be possible to redirect these toward desired chemicals.
13.3. Bioreactor Considerations
Our discussion of bioreactor design in Chapter 10 “Selection, Scale-Up, Operation, and Control of Bioreactors” laid out the principles of bioreactor design, but plant-cell cultures represent some unusual problems. Plant cells are large, they tend to form aggregates, and despite a strong cell wall they are sensitive to liquid shear. They grow slowly. In some cases, we need to cultivate fairly complex three-dimensional structures (e.g., root culture) that are sensitive to shear and create significant barriers to mass transfer, especially for oxygen and carbon dioxide. Control of plant cell differentiation into complex structures remains a challenge. Appropriate selection and design of bioreactors can address many of these factors.
13.3.1. Bioreactors for Suspension Cultures
Many of the differences between plant cell cultures and microbes have direct implications for the design and scale-up of suspension culture systems (see Table 13.2). In particular, the degree of physical mixing that is desirable is important. However, the basic reactor types we discussed in Chapter 10 can usually be adapted to plant cells.
Plant cells are large, and when they are exposed to turbulent shear fields where the eddy size approaches the cell size, the cells can be exposed to a twisting motion that can damage them. Lower levels of shear appear to affect cell surface receptors and nutrient transport. Reactors with high shear must be avoided. However, plant cells can withstand far more shear than animal cells, and shear-tolerant lines can sometimes be developed. Stirred tanks designed for the culture of bacteria are not good choices, but modified stirred tanks can be suitable. Reactors up to 75000 l have been used successfully.
Plant cell cultures can achieve high cell densities and viscosities. Their reduced respiration rate, however, compensates in part for the need for vigorous agitation. Airlift reactors for low or moderate cell densities (<20 gDW/l) or paddle-type or helical-ribbon impellers for high-cell-density systems have been advocated as reactors that strike a good compromise between the need for good mixing and the shear sensitivity of plant cells.
More important than actual shear damage is the role that mixing plays in the biological response of some systems to scale-up. In at least some cases, the formation of aggregates appears to be necessary to achieving the mix of cell types essential for good secondary product formation. The degree of aggregate formation is influenced by the degree of mixing. Since the degree of mixing necessary to achieve equivalent oxygen transfer or equivalent shear changes upon scale-up, the degree of aggregation and productivity may change.
Mixing depends on a combination of sparging and mechanical agitation. Oversparging can be a problem for plant cell cultures. Even though photoautotrophic growth is not important in most cultures, elevated CO2 levels can enhance productivity. Plants make at least one volatile hormone, ethylene, and its rapid removal can affect productivity. With plant cell cultures, optimizing the gas composition, sparging rate, and degree of mechanical agitation is a difficult problem.
The low growth rates of plant cells present other problems for large-scale systems. The primary problem is maintenance of aseptic conditions for the 2 to 4 weeks often necessary to complete a batch cycle. Additionally, the low growth rates reduce volumetric productivities to levels that diminish the economic attractiveness of plant cell cultures.
A further problem is the apparent genetic instability of many cell lines. Often, suspension and callus cultures will produce a desired compound initially, but after 3 to 6 months, the capacity to make the product often decreases considerably. This loss of productivity may be due to inadequate nutrition or may have a genetic or epigenetic basis. Artificial cell culture tends to select for cells with higher ploidy levels (tetraploid instead of diploid†). The most frequent genetic changes include chromosome breakage and aneuploidy. With an atypical chromosome number, differentiation and regeneration become extremely difficult. Cryopreservation of cell lines is not possible in all cases, and many cell lines must be maintained through routine subculture, which may select for variants that grow better than the parental culture but are less productive.
† Recall that a diploid cell has two copies of each chromosome; a tetraploid cell has four copies.
This loss of activity can be counteracted in many cases by the active selection of productive subclones. Selection is facilitated when the product of interest is readily visible. In cases where extensive screening and selection have been done, stable, high-producing clones have been reported. A stable, high-producing cell line is essential to any commercial process. Since visible screens are not always possible, much screening is done currently with high-throughput metabolite assays.
One strategy that has proved to be useful in increasing productivity is the use of a two-phase culture. The first phase uses a medium optimized for growth, while a second phase uses a different medium optimized for product formation. The first commercial process with plant cell culture was shikonin production, which utilizes two batch reactors in series.
13.3.2. Reactors Using Cell Immobilization
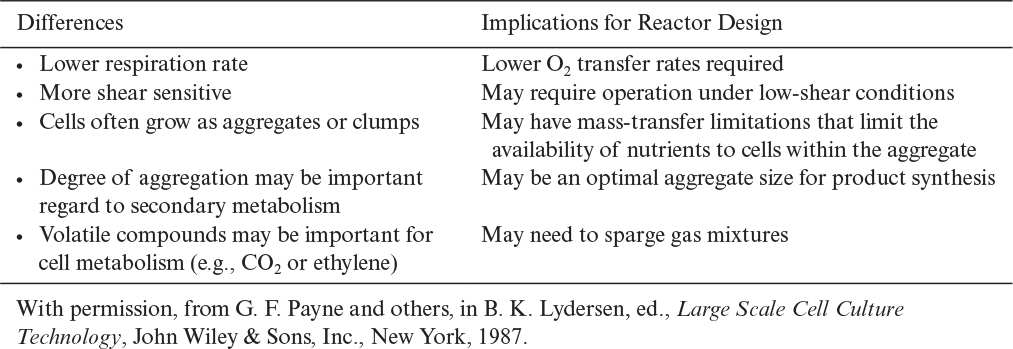
Further improvements in productivity may be possible due to cell immobilization. Immobilized-cell reactors inherently follow the two-phase approach. Cells are grown and then immobilized; once immobilized, conditions for product formation are optimized. Immobilized-cell systems are advantageous when continuous operation is possible and if the product is, or can be made, extracellular. Following are some possible advantages of immobilization.†† Probably the most important advantage is that the degree of cell-to-cell interaction can be manipulated.
†† G. F. Payne and others, in B. K. Lydersen, ed., Large Scale Cell Culture Technology, Wiley, New York, 1987.
• Continuous operation is facilitated.
• High cell concentrations are possible.
• Cell reuse may lead to increased efficiency.
• Cells can be protected from shear.
• Once immobilized, the slow growth and strain instability of plant cells are no longer problems.
• Media can be easily changed, which is important for processes that require a series of media for optimal production.
• Continuous removal of inhibitory metabolites may enhance the overall cellular metabolism or unmask biochemical pathways.
• The biological relationships between aggregation, morphological differentiation, and secondary metabolite production may be better exploited.
Following are potential problems with immobilization:†
† G. F. Payne and others, in B. K. Lydersen, ed., Large Scale Cell Culture Technology, Wiley, New York, 1987.
• Large-scale aseptic immobilization procedures must be developed.
• Mass-transfer limitations may significantly affect cell metabolism (positively and adversely).
• Products must be produced by nongrowing cells.
• Products must be released from the cell into the medium.
• Experience in the scale-up of immobilized-cell systems is limited.
Plant cells will often self-immobilize by preferentially attaching to or within a porous matrix. The resulting biofilm (if on a surface) has been shown to be very effective in a number of cases. Plant cells have also been entrapped in gels or between membranes. Immobilization generates concentration gradients that alter the biosynthetic capacity of the culture (sometimes negatively and sometimes positively). The cell-to-cell contact due to immobilization or the contact of the cell surface with the surrounding gel phase may also alter cell physiology. In some cases, immobilization has improved intrinsic production rates by more than an order of magnitude.
Plant cell cultures can be cultivated at three levels of cell-to-cell communication. In a fine suspension, aggregates are small and mass-transfer gradients are unimportant. Any diffusable species that might act as chemical messengers are diluted to a low concentration. Plasmodesmata interconnect only a small fraction of the cells. If cells are concentrated and entrapped, they form a pseudotissue; since the entrapping matrix is typically on the order of 1 to 10 mm, mass-transfer gradients become important. Diffusable chemical species can build to high local concentrations because of mass transfer resistances. However, plasmodesmata are relatively unimportant in pseudotissues. If a few cells are immobilized (say, between two membranes or in the pockets of a foam matrix) and allowed to grow in place, they will develop a tissuelike structure. Not only can diffusable species accumulate, but cells will be interconnected through the plasmodesmata. Then immobilization (and the method used for immobilization) can be used as an engineering design parameter to alter the degree and type of cell-to-cell communication. Immobilization can be coupled with other strategies to enhance product formation.
Consider the production of ajmalicine from periwinkle (Catharanthus roseus). Ajmalicine is stored primarily in the vacuole in cells in suspension culture, although a small amount (10%) is excreted. Ajmalicine has a pKa of 6.3, so that at the growth pH (5.6) much of the alkaloid is in the neutral form. A neutral resin can be used to remove ajmalicine in situ. The in situ removal of the product can enhance formation of a product by relief of feedback inhibition or protection from degradation or further conversion.
Figure 13.7 shows the results of experiments involving combinations of in situ adsorption, use of a fungal elicitor, immobilization in calcium alginate gels, and use of a production medium. Using all four approaches increases extracellular concentrations almost 100-fold. The purity is high. The closely related alkaloid serpentine has a much higher pKa (10.8) and will not adsorb into the resin. The adsorption of ajmalicine increases its synthesis preferentially. Under some circumstances, the intracellular levels of ajmalicine and serpentine are nearly equal. In this system, the ratio of extracellular ajmalicine to serpentine was 60-fold, with serpentine accounting for a vanishingly small amount of the adsorbed alkaloid. Perhaps the most important result of such an experiment is that at least some normally intracellular compounds can be excreted from viable cells when environmental conditions are correctly manipulated.
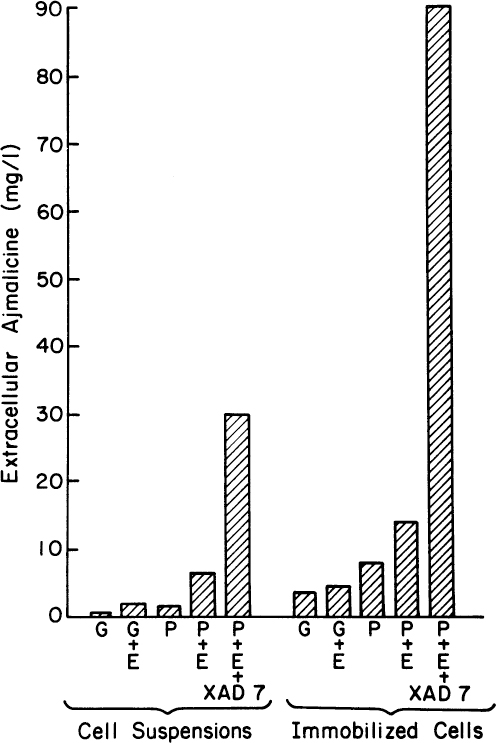
Figure 13.7. Summary of the effects of various treatments on the formation and release of ajmalicine from Catharanthus roseus. G stands for growth medium and P for production medium. E indicates elicitor addition (2%) of an autoclaved culture of the fungus, Phytophthora cactorum, for a 2-day exposure time; the neutral resin, Amberlite XAD-7, indicates the addition of resin. Samples were analyzed 23 days after inoculation. (With permission from M. Asada and M. L. Shuler, Stimulation of ajmalicine production and excretion from Catharanthus roseus: Effects of adsorption in situ, elicitors and alginate immobilization Appl. Microbiol. Biotechnol. 30(5):475–481, 1989. With permission of Springer.)
13.3.3. Bioreactors for Organized Tissues
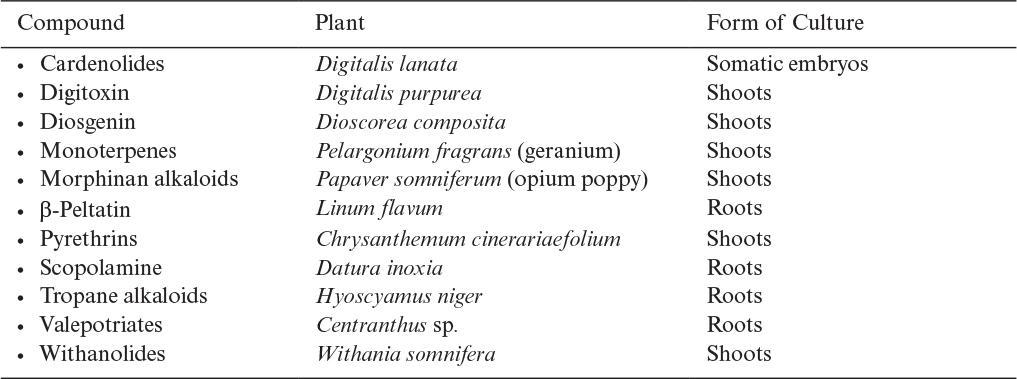
In many cases, neither suspension nor immobilized-cell cultures will produce satisfactory amounts of a desired metabolite. However, organ cultures from the same plant may give good yields (Table 13.3). In addition to high yields, organ cultures have a number of distinct advantages:
• Biosynthetic capacity often returns upon organogenesis.
• Product spectrum for flavors and fragrances returns to that for parent plant.
• Product secretion is enhanced in many cases (particularly root cultures).
• Genetic stability under growth conditions is greatly improved over callus or suspension culture.
• Organ cultures are often easier to genetically engineer than whole plants or cell cultures.
• Self-immobilization provides more optimal mix of cell types.
Following are disadvantages:
• Growth rates may be lower than suspension cultures in some, but not all, cases.
• Efficient, scalable reactors for organized tissues need to be developed.
• Control of microenvironmental conditions is often more difficult.
The primary disadvantages for organ cultures have been their apparently slow growth rates and difficulties in designing effective bioreactors to handle organized tissues. Recent advances suggest that both limitations may be circumvented, and in several cases, organ cultures that grow more rapidly than their corresponding suspension cultures have been found.
Some plants (typically dicots; monocots can be infected, but it is more difficult) will respond to infection by Agrobacterium rhizogenes with rapid root proliferation. This infection leads to hairy roots (see Figure 13.6). The best doubling times for hairy root cultures approach or exceed typical values for many suspension cultures. Even in species not susceptible to A. rhizogenes infection, recent results indicate that proper control of hormone content can accelerate growth rates to acceptable levels (3- to 5-day doubling times). Hairy root cultures are often easier to genetically engineer than cell cultures or whole plants. The biggest difficulty with the large-scale culture of roots is the formation of root mats. These mats restrict internal mass transfer, can entrap gas and float, and present significant problems in maintaining a scalable uniform environment. However, large-scale units for root culture have been built (20000 l for ginseng roots in Japan). Laboratory-scale reactors using a mist or forced convection of liquid nutrients appear promising and provide much better mass transfer in the center of root mats. Examples of commercial development of hairy root technology include Rootec (Switzerland) for key metabolites and Root Lines Technology (France) for production/secretion of complex recombinant enzymes.
One other advantage of using organ cultures over whole plants is the possibility of using precursor feeding and elicitors. For example, with species of onion and garlic, the use of precursors can greatly enhance the formation of flavor compounds. Different precursors give different levels of enhancement to particular components of the flavor spectrum. By using combinations of chemical precursors, it may be possible to custom-make flavors for specific applications.
Shoot cultures present additional problems. Light may be required for some shoot cultures, while roots can be grown easily in the dark. Shoot cultures may be mixotrophic, involving the exogenous supply of sugars as well as some photosynthesis. In some cases, control of the photoperiod (hours of exposure to light per day) is important. However, the most crucial need for light comes from the role that light often plays in cellular regulation. Exposure to light of certain wavelengths is essential to induce synthesis of some enzymes. In at least some cases, these enzymes play a crucial role in secondary metabolism. The maintenance of uniform light intensity in a large reactor is a challenging and partially unsolved problem.
One other perceived limitation on shoot cultures has been the belief that they could not be grown under totally submerged conditions. Recent experiments have shown that tobacco shoots will grow as well in submerged culture as in a standard shake flask (3-day doubling time in both cases), provided that the liquid phase is well mixed. In Israel, commercial production of plantlets using totally submerged culture has been reported.
Although organ cultures have not yet been extensively exploited, they are promising vehicles for the production of some important secondary metabolites.
13.4. Economics of Plant Cell Tissue Cultures
Far fewer commercial processes for plant cell culture have been established than for bacterial, fungal, and animal cell cultures. Currently, several large-scale systems (>1000 l) have been constructed throughout the world with the largest being 75,000 l. In North America, the possible commercial development of many products is underway (Table 13.1).
As a rule of thumb, most commercial fermentations yield revenue of more than 20¢/l-day. If the volumetric productivity of a process is known, the wholesale price necessary to achieve this level of revenue can be estimated. Table 13.4 lists the published values of the volumetric productivities reported for some of the more intensely studied cell lines. Bulk prices in excess of $220/kg would be necessary to yield 20¢/l-day for these products, and clearly prices in excess of $500/kg may be necessary for new technology.
13.5. Summary
Plants produce a wide range of commercially important compounds. For some of these chemicals, plant cell tissue culture is a potential technique for their production. In addition, the bioreactor techniques that are applied to plant cell culture will be useful in the micropropagation of plants.
Since most secondary products are not made in rapidly growing cultures, two-step cultivations are employed. The first phase is used to promote growth, and a second phase is optimized for product formation. Liquid suspensions can be cultured analogously to fermenters for microbes if the special characteristics of plant cells are taken into account. Alternatively, immobilized or entrapped cell systems can be used. In either case, elicitors can often be used to enhance product formation. A combination of immobilization and in situ separation techniques can often dramatically improve production.
Organized tissues (shoots, roots, and embryos) hold promise as techniques to improve yields. However, bioreactor designs that supply homogeneous environments for organ cultures are only now being developed.
Suggestions for Further Reading
DORAN, P. M., Design of Mixing Systems for Plant Cell Suspensions in Stirred Reactors, Biotechnol. Progr. 15: 319–355, 1999.
EIBL, R., AND D. EIBL, Design of Bioreactors Suitable for Plant Cell and Tissue Cell Cultures, Phytochem. Rev. 7: 593–598, 2008.
GEORGIEV, M. I., R. EIBL, AND J. J. ZHONG, Hosting the Plant Cells in Vitro: Recent Trends in Bioreactors, Appl. Microbiol. Biotechnol. 97: 3787–3800, 2013.
GEORGIEV, M. I., J. WEBER, AND A. MACIUK, Bioprocessing of Plant Cell Cultures for Mass Production of Targeted Compounds, Appl. Microbiol. Biotechnol. 83:809–823, 2009.
HUANG, T. K., M. A. PLESHA, AND K. A. MCDONALD, Semicontinuous Bioreactor Production of a Recombinant Human Therapeutic Protein Using a Chemically Inducible Viral Amplicon Expression System in Transgenic Plant Cell Suspension Culture, Biotechnol. Bioeng. 106: 408–421, 2010.
KETCHUM, R. E. B., D. M. GIBSON, R. B. CROTEAU, AND M. L. SHULER, The Kinetics of Taxoid Accumulation in Cell Suspension Cultures of Taxus Following Elicitation with Methyl Jasmonate, Biotechnol. Bioeng. 62(1): 97–105, 1999.
MUSTAFA, N. R., W. DE WINTER, F. vAN IREN, AND R. VERPOORTE, Initiation, Growth and Cryopreservation of Plant Cell Suspension Cultures, Nat. Protoc. 6: 715–742, 2011.
PAYNE, G. F., V. BRINGI, C. L. PRINCE, AND M. L. SHULER, Plant Cell and Tissue Culture in Liquid Systems, Hanser Publ., New York (available through Wiley), 1992.
RIZVI, N., M. CORNEJO, K. STEIN, J. WEAVER, E. J. CRAM, AND C. W. T. LEE-PARSONS, An Efficient Transformation Method for Estrogen-Inducible Transgene Expression in Catharanthus roseus Hairy Roots, PCTOC 120(2): 475–487, 2015.
SRIVASTAVA, S., AND A. K. SRIVASTAVA, Hairy Root Cultures for Mass-Production of High-Value Secondary Metabolites, Crit. Rev. Biotechnol. 27: 29–43, 2007.
WEATHERS, P. J., M. J. TOWLER, AND J. XU, Bench to Batch: Advances in Plant Cell Culture for Producing Useful Products, Appl. Microbiol. Biotechnol. 85: 1339–1351, 2010.
WILSON, S. A., AND S. C. ROBERTS, Recent Advances Towards Development and Commercialization of Plant Cell Culture Processes for Synthesis of Biomolecules, Plant Biotechnol. J.10: 249–268, 2012.
ZHONG, J. J., Plant Cell Culture for Production of Paclitaxel and Other Taxanes, J. Biosci. Bioeng. 94: 591–599, 2002.
Problems
13.1. The uptake of the auxin indole acetic acid (IAA) by suspension cultures of Parthenocissus sp. is nearly zero order at 1 nmol/mg dry cell weight-min. The diffusivity of IAA in water is 5 × 10–6 cm2/s. Beads of calcium alginate are most conveniently made as spheres with a 4 mm diameter. Assume the beads are made 25% by volume of plant cells. Assume the plant cells are 90% water and that the diffusivity of IAA in the gel is the same as in water. If the external concentration is maintained at 2 μmol, will IAA penetrate to the center of the bead?
13.2. Gel-immobilized cells of Papaver somniferum (opium poppy) can make codeine from codeinone. The rate of codeinone uptake is first order, with a rate constant of 3.3 × 10–8 l/g cells dry weight-s. The diffusivity of codeinone in the gel is 0.2 × 10–9 m2/s. For a gel particle of 4 mm diameter with a 25% volume loading of cells (95% water), what will be the effectiveness factor?
13.3. The kLa of a small bubble column (2 l) has been measured as 20 h–1 at an airflow of 4 l/min. If the rate of oxygen uptake by a culture of Catharanthus roseus is 0.2 mmol O2/gDW-h and if the critical oxygen concentration must be above 10% of saturation (about 8 mg/l), what is the maximum concentration of cells that can be maintained in the reactor?
13.4. C. roseus cells immobilized in Ca-alginate beads of diameter 0.5 mm are used for production of indole alkaloids (IA) in a fluidized-bed bioreactor. The rate-limiting nutrient is glucose, and no intraparticle diffusion limitations exist. Growth is negligible and Monod kinetics is valid. Use the following data:

a. For 95% glucose conversion, determine required hydraulic residence time, volume, and the height of the column.
b. If Yp/s is 0.02 g IA/g glucose, determine IA concentration in the effluent and the productivity.