9. Reaction Mechanisms, Pathways, Bioreactions, and Bioreactors
The next best thing to knowing something is knowing where to find it.
—Samuel Johnson (1709–1784)
9.1 Active Intermediates and Nonelementary Rate Laws
In Chapter 3 a number of simple power-law models, e.g.,
were presented, where n was an integer of 0, 1, or 2 corresponding to a zero-, first-, or second-order reaction. However, for a large number of reactions, the orders are noninteger, such as the decomposition of acetaldehyde at 500˚C
where the rate law developed in problem P9-5B(b) is
The rate law could also have concentration terms in both the numerator and denominator such as the formation of HBr from hydrogen and bromine
where the rate law developed in problem P9-5B(c) is
Rate laws of this form usually involve a number of elementary reactions and at least one active intermediate. An active intermediate is a high-energy molecule that reacts virtually as fast as it is formed. As a result, it is present in very small concentrations. Active intermediates (e.g., A*) can be formed by collision or interaction with other molecules.
A + M → A* + M
Properties of an active intermediate A*
Here, the activation occurs when translational kinetic energy is transferred into internal energy, i.e., vibrational and rotational energy.1 An unstable molecule (i.e., active intermediate) is not formed solely as a consequence of the molecule moving at a high velocity (high translational kinetic energy). The energy must be absorbed into the chemical bonds, where high-amplitude oscillations will lead to bond ruptures, molecular rearrangement, and decomposition. In the absence of photochemical effects or similar phenomena, the transfer of translational energy to vibrational energy to produce an active intermediate can occur only as a consequence of molecular collision or interaction. Collision theory is discussed in the Professional Reference Shelf in Chapter 3 (http://www.umich.edu/~elements/5e/03chap/prof.html) on the CRE Web site. Other types of active intermediates that can be formed are free radicals (one or more unpaired electrons; e.g., CH3•), ionic intermediates (e.g., carbonium ion), and enzyme-substrate complexes, to mention a few (http://www.umich.edu/~elements/5e/09chap/prof.html).
1 W. J. Moore, Physical Chemistry (Reading, MA: Longman Publishing Group, 1998).
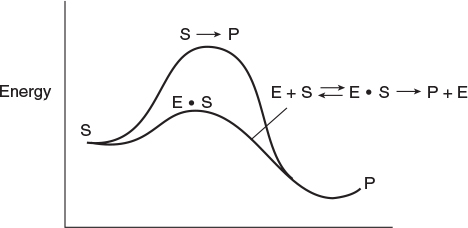
The idea of an active intermediate was first postulated in 1922 by F. A. Lindemann, who used it to explain changes in the reaction order with changes in reactant concentrations.2 Because the active intermediates were so short-lived and present in such low concentrations, their existence was not really definitively confirmed until the work of Ahmed Zewail, who received the Nobel Prize in Chemistry in 1999 for femtosecond spectroscopy.3 His work on cyclobutane showed that the reaction to form two ethylene molecules did not proceed directly, as shown in Figure 9-1(a), but formed the active intermediate shown in the small trough at the top of the energy barrier on the reaction-coordinate diagram in Figure 9-1(b). As discussed in Chapter 3, an estimation of the barrier height, E, can be obtained using computational software packages such as Spartan, Cerius2, or Gaussian as discussed in the Molecular Modeling Web Module in Chapter 3 on the CRE Web site, www.umich.edu/~elements/5e/index.html.

Figure 9-1 Reaction coordinate. Ivars Peterson, “Chemistry Nobel spotlights fast reactions,” Science News, 156, 247 (1999).
2 F. A. Lindemann, Trans. Faraday. Soc., 17, 598 (1922).
3 J. Peterson, Science News, 156, 247 (1999).

9.1.1 Pseudo-Steady-State Hypothesis (PSSH)
In the theory of active intermediates, decomposition of the intermediate does not occur instantaneously after internal activation of the molecule; rather, there is a time lag, although infinitesimally small, during which the species remains activated. Zewail’s work was the first definitive proof of a gas-phase active intermediate that exists for an infinitesimally short time. Because a reactive intermediate reacts virtually as fast as it is formed, the net rate of formation of an active intermediate (e.g., A*) is zero, i.e.,
This condition is also referred to as the Pseudo-Steady-State Hypothesis (PSSH). If the active intermediate appears in n reactions, then
PSSH
To illustrate how rate laws of this type are formed, we shall first consider the gas-phase decomposition of azomethane, AZO, to give ethane and nitrogen
Experimental observations show that the rate of formation of ethane is first order with respect to AZO at pressures greater than 1 atm (relatively high concentrations)4
4 H. C. Ramsperger, J. Am. Chem. Soc., 49, 912.
and second order at pressures below 50 mmHg (low concentrations):
Why the reaction order changes
We could combine these two observations to postulate a rate law of the form
To find a mechanism that is consistent with the experimental observations, we use the steps shown in Table 9-1.
TABLE 9-1 STEPS TO DEDUCE A RATE LAW
|
1. Propose an active intermediate(s). 2. Propose a mechanism, utilizing the rate law obtained from experimental data, if possible. 3. Model each reaction in the mechanism sequence as an elementary reaction. 4. After writing rate laws for the rate of formation of desired product, write the rate laws for each of the active intermediates. 5. Write the net rate of formation for the active intermediate and use the PSSH. 6. Eliminate the concentrations of the intermediate species in the rate laws by solving the simultaneous equations developed in Steps 4 and 5. 7. If the derived rate law does not agree with experimental observation, assume a new mechanism and/or intermediates and go to Step 3. A strong background in organic and inorganic chemistry is helpful in predicting the activated intermediates for the reaction under consideration. |
We will now follow the steps in Table 9-1 to develop the rate law for azomethane (AZO) decomposition, –rAZO.
Step 1. Propose an active intermediate. We will choose as an active intermediate an azomethane molecule that has been excited through molecular collisions, to form AZO*, i.e., [(CH3)2N2]*.
Mechanism
In reaction 1, two AZO molecules collide and the kinetic energy of one AZO molecule is transferred to the internal rotational and vibrational energies of the other AZO molecule, and it becomes activated and highly reactive (i.e., AZO*). In reaction 2, the activated molecule (AZO*) is deactivated through collision with another AZO by transferring its internal energy to increase the kinetic energy of the molecules with which AZO* collides. In reaction 3, this highly activated AZO* molecule, which is wildly vibrating, spontaneously decomposes into ethane and nitrogen.
Step 3. Write rate laws.
Because each of the reaction steps is elementary, the corresponding rate laws for the active intermediate AZO* in reactions (1), (2), and (3) are
Note: The specific reaction rates, k, are all defined wrt the active intermediate AZO*.
(1)
(2)
(3)
The rate laws shown in Equations (9-3) through (9-5) are pretty much useless in the design of any reaction system because the concentration of the active intermediate AZO* is not readily measurable. Consequently, we will use the Pseudo-Steady-State-Hypothesis (PSSH) to obtain a rate law in terms of measurable concentrations.
Step 4. Write rate of formation of product.
We first write the rate of formation of product
Step 5. Write net rate of formation of the active intermediate and use the PSSH.
To find the concentration of the active intermediate AZO*, we set the net rate of formation of AZO* equal to zero,5 rAZO* ≡ 0.
5 For further elaboration on this section, see R. Aris, Am. Sci., 58, 419 (1970).
Solving for CAZO*
Step 6. Eliminate the concentration of the active intermediate species in the rate laws by solving the simultaneous equations developed in Steps 4 and 5. Substituting Equation (9-8) into Equation (9-6)
Step 7. Compare with experimental data.
At low AZO concentrations,
for which case we obtain the following second-order rate law:
At high concentrations
in which case the rate expression follows first-order kinetics
Apparent reaction orders
In describing reaction orders for this equation, one would say that the reaction is apparent first order at high azomethane concentrations and apparent second order at low azomethane concentrations.
9.1.2 If Two Molecules Must Collide, How Can the Rate Law Be First Order?
The PSSH can also explain why one observes so many first-order reactions such as
Symbolically, this reaction will be represented as A going to product P; that is,
A → P
with
–rA = kCA
The reaction is first order but the reaction is not elementary. The reaction proceeds by first forming an active intermediate, A*, from the collision of the reactant molecule and an inert molecule of M. Either this wildly oscillating active intermediate, A*, is deactivated by collision with inert M, or it decomposes to form product. See Figure 9-2.
The mechanism consists of the three elementary reactions:
1. Activation
2. Activation
3. Decomposition
Figure 9-2 Collision and activation of a vibrating A molecule.6
6 The reaction pathway for the reaction in Figure 9-2 is shown in the margin. We start at the top of the pathway with A and M, and move down to show the pathway of A and M touching k1 (collision) where the curved lines come together and then separate to form A* and M. The reverse of this reaction is shown starting with A* and M and moving upward along the pathway where arrows A* and M touch, k2, and then separate to form A and M. At the bottom, we see the arrow from A* to form P with k3.
Writing the rate of formation of product
and using the PSSH to find the concentrations of A* in a manner similar to the azomethane decomposition described earlier, the rate law can be shown to be
Because the concentration of the inert M is constant, we let
Reaction pathways6

to obtain the first-order rate law
–rA = kCA
Consequently, we see the reaction
A → P
follows an elementary rate law but is not an elementary reaction. Reactions similar to the AZO decomposition mechanism would also be apparent first order if the corresponding to the term (k2 CAZO) in the denominator of Equation (9-9) were larger than the other term k3 (i.e., (k2 CAZO) >> k3). The reaction pathway for this reaction is shown in the margin here. Reaction pathways for other reactions are given on the Web site (http://www.umich.edu/~elements/5e/09chap/prof-rxnpath.html).
First-order rate law for a nonelementary reaction
9.1.3 Searching for a Mechanism
In many instances the rate data are correlated before a mechanism is found. It is a normal procedure to reduce the additive constant in the denominator to 1.
We therefore divide the numerator and denominator of Equation (9-9) by k3 to obtain
General Considerations. Developing a mechanism is a difficult and time-consuming task. The rules of thumb listed in Table 9-2 are by no means inclusive but may be of some help in the development of a simple mechanism that is consistent with the experimental rate law.

TABLE 9-2 RULES OF THUMB FOR DEVELOPMENT OF A MECHANISM
1. Species having the concentration(s) appearing in the denominator of the rate law probably collide with the active intermediate; for example, 2. If a constant appears in the denominator, one of the reaction steps is probably the spontaneous decomposition of the active intermediate; for example, 3. Species having the concentration(s) appearing in the numerator of the rate law probably produce the active intermediate in one of the reaction steps; for example, |
Upon application of Table 9-2 to the azomethane example just discussed, we see the following from rate Equation (9-12):
1. The active intermediate, AZO*, collides with azomethane, AZO [Reaction 2], resulting in the concentration of AZO in the denominator.
2. AZO* decomposes spontaneously [Reaction 3], resulting in a constant in the denominator of the rate expression.
3. The appearance of AZO in the numerator suggests that the active intermediate AZO* is formed from AZO. Referring to [Reaction 1], we see that this case is indeed true.
Example 9-1 The Stern–Volmer Equation
Light is given off when a high-intensity ultrasonic wave is applied to water.7 You should be able to see the glow if you turn out the lights and irradiate water with a focused ultrasonic tip. This light results from microsize gas bubbles (0.1 mm) being formed by the ultrasonic wave and then being compressed by it. During the compression stage of the wave, the contents of the bubble (e.g., water and whatever else is dissolved in the water, e.g., CS2, O2, N2) are compressed adiabatically.
7 P. K. Chendke and H. S. Fogler, J. Phys. Chem., 87, 1362 (1983).
This compression gives rise to high temperatures and kinetic energies of the gas molecules, which through molecular collisions generate active intermediates and cause chemical reactions to occur in the bubble.
The intensity of the light given off, I, is proportional to the rate of deactivation of an activated water molecule that has been formed in the microbubble.
An order-of-magnitude increase in the intensity of sonoluminescence is observed when either carbon disulfide or carbon tetrachloride is added to the water. The intensity of luminescence, I, for the reaction is
is
A similar result exists for CCl4.
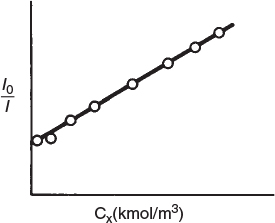
However, when an aliphatic alcohol, X, is added to the solution, the intensity decreases with increasing concentration of alcohol. The data are usually reported in terms of a Stern–Volmer plot in which relative intensity is given as a function of alcohol concentration, CX. (See Figure E9-1.1, where I0 is the sonoluminescence intensity in the absence of alcohol and I is the sonoluminescence intensity in the presence of alcohol.)
(a) Suggest a mechanism consistent with experimental observation.
(b) Derive a rate law consistent with Figure E9-1.1.
Stern–Volmer plot
Solution
(a) Mechanism
From the linear plot we know that
where CX ≡ (X) and A and B are numerical constants. Inverting Equation (E9-1.1) yields
From Rule 1 of Table 9-2, the denominator suggests that alcohol (X) collides with the active intermediate:
The alcohol acts as what is called a scavenger to deactivate the active intermediate. The fact that the addition of CCl4 or CS2 increases the intensity of the luminescence
Reaction pathways
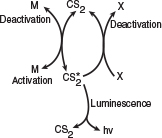
leads us to postulate (Rule 3 of Table 9-2) that the active intermediate was probably formed from the collision of either CS2 or another gas M or both in the collapsing bubble
where M is a third body (CS2, H2O, N2, etc.).
We also know that deactivation can occur by the reverse of reaction (E9-1.5). Combining this information, we have as our mechanism:
The mechanism
Activation:
Deactivation:
Deactivation:
Luminescence:
(b) Rate Law
Using the PSSH on in each of the above elementry reactions yields
Solving for () and substituting into Equation (E9-1.8) gives us
Wolfram Sliders
In the absence of alcohol
For constant concentrations of CS2 and the third body, M, we take a ratio of Equation (E9-1.10) to (E9-1.9)
which is of the same form as that suggested by Figure E9-1.1. Equation (E9-1.11) and similar equations involving scavengers are called Stern–Volmer equations.
Analysis: This example showed how to use the Rules of Thumb (Table 9-2) to develop a mechanism. Each step in the mechanism is assumed to follow an elementary rate law. The PSSH was applied to the net rate of reaction for the active intermediate in order to find the concentration of the active intermediate. This concentration was then substituted into the rate law for the rate of formation of product to give the rate law. The rate law from the mechanism was found to be consistent with experimental data.
A discussion of luminescence in the Web Module, Glow Sticks on the CRE Web site (www.umich.edu/~elements/5e/09chap/web.html). Here, the PSSH is applied to glow sticks. First, a mechanism for the reactions and luminescence is developed. Next, mole balance equations are written on each species and coupled with the rate law obtained using the PSSH; the resulting equations are solved and compared with experimental data.

Glow sticks Web Module
Steps in a chain reaction
9.1.4 Chain Reactions
A chain reaction consists of the following sequence:
1. Initiation: formation of an active intermediate
2. Propagation or chain transfer: interaction of an active intermediate with the reactant or product to produce another active intermediate
3. Termination: deactivation of the active intermediate to form products
Living Example Problem
An example comparing the application of the PSSH with the Polymath solution to the full set of equations is given in the Professional Reference Shelf R9.1, Chain Reactions, (http://www.umich.edu/~elements/5e/09chap/prof-chain.html). Also included in Professional Reference Shelf R9.2 is a discussion of Reaction Pathways and the chemistry of smog formation (http://www.umich.edu/~elements/5e/09chap/prof-rxnpath.html).
9.2 Enzymatic Reaction Fundamentals
An enzyme† is a high-molecular-weight protein or protein-like substance that acts on a substrate (reactant molecule) to transform it chemically at a greatly accelerated rate, usually 103 to 1017 times faster than the uncatalyzed rate. Without enzymes, essential biological reactions would not take place at a rate necessary to sustain life. Enzymes are usually present in small quantities and are not consumed during the course of the reaction, nor do they affect the chemical reaction equilibrium. Enzymes provide an alternate pathway for the reaction to occur, thereby requiring a lower activation energy. Figure 9-3 shows the reaction coordinate for the uncatalyzed reaction of a reactant molecule, called a substrate (S), to form a product (P)
† See http://www.rsc.org/Education/Teachers/Resources/cfb/enzymes.htm and https://en.wikipedia.org/wiki/Enzyme.
S → P
The figure also shows the catalyzed reaction pathway that proceeds through an active intermediate (E · S), called the enzyme–substrate complex, that is,
Because enzymatic pathways have lower activation energies, enhancements in reaction rates can be enormous, as in the degradation of urea by urease, where the degradation rate is on the order of 1014 higher than without the enzyme urease.
An important property of enzymes is that they are specific; that is, one enzyme can usually catalyze only one type of reaction. For example, a protease hydrolyzes only bonds between specific amino acids in proteins, an amylase works on bonds between glucose molecules in starch, and lipase attacks fats, degrading them to fatty acids and glycerol. Consequently, unwanted products are easily controlled in enzyme-catalyzed reactions. Enzymes are produced only by living organisms, and commercial enzymes are generally produced by bacteria. Enzymes usually work (i.e., catalyze reactions) under mild conditions: pH 4 to 9 and temperatures 75˚F to 160˚F. Most enzymes are named in terms of the reactions they catalyze. It is a customary practice to add the suffix -ase to a major part of the name of the substrate on which the enzyme acts. For example, the enzyme that catalyzes the decomposition of urea is urease and the enzyme that attacks tyrosine is tyrosinase. However, there are a few exceptions to the naming convention, such as a-amylase. The enzyme a-amylase catalyzes the transformation of starch in the first step in the production of the controversial soft drink (e.g., Red Pop) sweetener high-fructose corn syrup (HFCS) from corn starch, which is a $4 billion per year business.

9.2.1 Enzyme–Substrate Complex
The key factor that sets enzymatic reactions apart from other catalyzed reactions is the formation of an enzyme–substrate complex, (E · S). Here, substrate binds with a specific active site of the enzyme to form this complex.8 Figure 9-4 shows a schematic of the enzyme chymotrypsin (MW = 25,000 Daltons), which catalyzes the hydrolytic cleavage of polypeptide bonds. In many cases, the enzyme’s active catalytic sites are found where the various folds or loops interact. For chymotrypsin, the catalytic sites are noted by the amino acid numbers 57, 102, and 195 in Figure 9-4. Much of the catalytic power is attributed to the binding energy of the substrate to the enzyme through multiple bonds with the specific functional groups on the enzyme (amino side chains, metal ions). The type of interactions that stabilize the enzyme–substrate complex include hydrogen bonding and hydrophobic, ionic, and London van der Waals forces. If the enzyme is exposed to extreme temperatures or pH environments (i.e., both high and low pH values), it may unfold, losing its active sites. When this occurs, the enzyme is said to be denatured (see Figure 9.8 and Problem P9-13B).
8 M. L. Shuler and F. Kargi, Bioprocess Engineering Basic Concepts, 2nd ed. (Upper Saddle River, NJ: Prentice Hall, 2002).

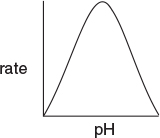
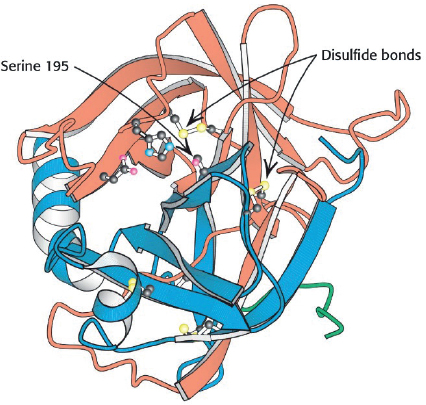
Figure 9-4 Enzyme chymotrypsin from Biochemistry, 7th ed., 2010 by Lubert Stryer, p. 258. Used with permission of W. H. Freeman and Company.
Two models for enzyme–substrate interactions are the lock-and-key model and the induced fit model, both of which are shown in Figure 9-5. For many years the lock-and-key model was preferred because of the sterospecific effects of one enzyme acting on one substrate. However, the induced fit model is the more useful model. In the induced fit model, both the enzyme molecule and the substrate molecules are distorted. These changes in conformation distort one or more of the substrate bonds, thereby stressing and weakening the bond to make the molecule more susceptible to rearrangement or attachment.
There are six classes of enzymes and only six:
1. Oxidoreductases |
AH2 + B + E → A + BH2 + E |
2. Transferases |
AB + C + E → AC + B + E |
3. Hydrolases |
AB + H2O + E → AH + BOH + E |
4. Isomerases |
A + E → isoA + E |
5. Lyases |
AB + E → A + B + E |
6. Ligases |
A + B + E → AB + E |
More information about enzymes can be found on the following two Web sites: http://us.expasy.org/enzyme/ and www.chem.qmw.ac.uk/iubmb/enzyme. These sites also give information about enzymatic reactions in general.

9.2.2 Mechanisms
In developing some of the elementary principles of the kinetics of enzyme reactions, we shall discuss an enzymatic reaction that has been suggested by Levine and LaCourse as part of a system that would reduce the size of an artificial kidney.9 The desired result is a prototype of an artificial kidney that could be worn by the patient and would incorporate a replaceable unit for the elimination of the body’s nitrogenous waste products, such as uric acid and creatinine. A schematic of this device is shown in the CRE Web site (http://www.umich.edu/~elements/5e/09chap/summary.xhtml#sec2). In the microencapsulation scheme proposed by Levine and LaCourse, the enzyme urease would be used in the removal of urea from the bloodstream. Here, the catalytic action of urease would cause urea to decompose into ammonia and carbon dioxide. The mechanism of the reaction is believed to proceed by the following sequence of elementary reactions:
9 N. Levine and W. C. LaCourse, J. Biomed. Mater. Res., 1, 275.
The reaction mechanism
1. The unbound enzyme urease (E) reacts with the substrate urea (S) to form an enzyme–substrate complex (E · S):
2. This complex (E · S) can decompose back to urea (S) and urease (E):
3. Or, it can react with water (W) to give the products (P) ammonia and carbon dioxide, and recover the enzyme urease (E):
Symbolically, the overall reaction is written as
We see that some of the enzyme added to the solution binds to the urea, and some of the enzyme remains unbound. Although we can easily measure the total concentration of enzyme, (Et), it is difficult to measure either the concentration of free enzyme, (E), or the concentration of the bound enzyme (E · S).
Letting E, S, W, E · S, and P represent the enzyme, substrate, water, the enzyme–substrate complex, which is the active intermediate, and the reaction products, respectively, we can write Reactions (9-13), (9-14), and (9-15) symbolically in the forms
Here, P = 2NH3 + CO2.
The corresponding rate laws for Reactions (9-16), (9-17), and (9-18) are
where all the specific reaction rates are defined with respect to (E · S). The net rate of formation of product, rP, is
For the overall reaction
E + S → P + E
we know that the rate of consumption of the urea substrate equals the rate of formation of product CO2, i.e., –rS = rP.
This rate law, Equation (9-19), is of not much use to us in making reaction engineering calculations because we cannot measure the concentration of the active intermediate, which is the enzyme substrate complex (E · S). We will use the PSSH to express (E · S) in terms of measured variables.
The net rate of formation of the enzyme–substrate complex is
Substituting the rate laws, we obtain
Using the PSSH, rE·S = 0, we can now solve Equation (9-20) for (E · S)
and substitute for (E · S) into Equation (9-19)
We still cannot use this rate law because we cannot measure the unbound enzyme concentration (E); however, we can measure the total enzyme concentration, Et.
In the absence of enzyme denaturation, the total concentration of the enzyme in the system, (Et), is constant and equal to the sum of the concentrations of the free or unbounded enzyme, (E), and the enzyme–substrate complex, (E · S):
Total enzyme concentration = Bound + Free enzyme concentration.
Substituting for (E · S)
solving for (E)
and substituting for (E) in Equation (9-22), the rate law for substrate consumption is
Note: Throughout the following text, Et ≡ (Et) = total concentration of enzyme with typical units such as (kmol/m3) or (g/dm3).
9.2.3 Michaelis–Menten Equation
Because the reaction of urea and urease is carried out in an aqueous solution, water is, of course, in excess, and the concentration of water (W) is therefore considered constant, ca. 55 mol/dm3. Let
After dividing the numerator and denominator of Equation (9-24) by k1, we obtain a form of the Michaelis–Menten equation:
The final form of the rate law
Turnover number kcat
The parameter kcat is also referred to as the turnover number. It is the number of substrate molecules converted to product in a given time on a single-enzyme molecule when the enzyme is saturated with substrate (i.e., all the active sites on the single-enzyme molecule are occupied, (S)>>KM). For example, the turnover number for the decomposition of hydrogen-peroxide, H2O2, by the enzyme catalase is 40 × 106 s–1. That is, 40 million molecules of H2O2 are decomposed every second on a single-enzyme molecule saturated with H2O2. The constant KM (mol/dm3) is called the Michaelis constant and for simple systems is a measure of the attraction of the enzyme for its substrate, so it’s also called the affinity constant. The Michaelis constant, KM, for the decomposition of H2O2 discussed earlier is 1.1 M, while that for chymotrypsin is 0.1 M.10
10 D. L. Nelson and M. M. Cox, Lehninger Principles of Biochemistry, 3rd ed. (New York: Worth Publishers, 2000).
Michaelis constant KM
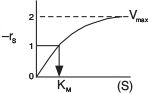
If, in addition, we let Vmax represent the maximum rate of reaction for a given total enzyme concentration
the Michaelis–Menten equation takes the familiar form
Michaelis–Menten equation
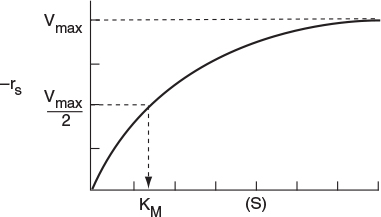
A sketch of the rate of disappearance of the substrate, –rs, at a given concentration of total enzyme, ET, is shown as a function of the substrate concentration, S, in Figure 9-6.
Michaelis–Menten plot
A plot of this type is sometimes called a Michaelis–Menten plot. At low substrate concentration, KM ≫ (S), Equation (9-26) reduces to
and the reaction is apparent first order in the substrate concentration. At high substrate concentrations,
Equation (9-26) reduces to
Interpretation of Michaelis constant
and we see the reaction is apparent zero order.
What does KM represent? Consider the case when the substrate concentration is such that the reaction rate is equal to one-half the maximum rate
then
Solving Equation (9-27) for the Michaelis constant yields
KM = (S1/2)
The Michaelis constant is equal to the substrate concentration at which the rate of reaction is equal to one-half the maximum rate, i.e., −rA = Vmax/2. The larger the value of KM, the higher the substrate concentration necessary for the reaction rate to reach half of its maximum value.
The parameters Vmax and KM characterize the enzymatic reactions that are described by Michaelis–Menten kinetics. Vmax is dependent on total enzyme concentration, whereas KM is not.
Two enzymes may have the same values for kcat but have different reaction rates because of different values of KM. One way to compare the catalytic efficiencies of different enzymes is to compare their ratios kcat/KM. When this ratio approaches 108 to 109 (dm3/mol/s), the reaction rate approaches becoming diffusion limited. That is, it takes a long time for the enzyme and substrate to diffuse around in the fluid and find each other, but once they do they react immediately. We will discuss diffusion-limited reactions in Chapters 14 and 15.
Example 9-2 Evaluation of Michaelis–Menten Parameters Vmax and KM
In this example we illustrate how to determine the enzymatic reaction rate parameters Vmax and KM for the reaction rate.
The rate of reaction is given as a function of urea concentration in Table E9-2.1, where (S) ≡ Curea.
Curea (kmol/m3) |
0.2 |
0.02 |
0.01 |
0.005 |
0.002 |
−rurea (kmol/m3 · s) |
1.08 |
0.55 |
0.38 |
0.2 |
0.09 |
(a) Determine the Michaelis–Menten parameters Vmax and KM for the reaction
(b) In addition to forming a Lineweaver–Burk Plot, form Hanes–Woolf and Eadie–Hofstee plots, and discuss the attributes of each type of plot.
(c) Use non-linear regression to find Vmax and KM.
Solution
Part (a)
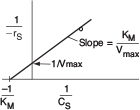
Inverting Equation (9-26) gives us the Lineweaver–Burk equation
or in terms of urea
A plot of the reciprocal reaction rate versus the reciprocal urea concentration should be a straight line with an intercept (1/Vmax) and slope (KM/Vmax). This type of plot is called a Lineweaver–Burk plot. We shall use the data in Table E9-2.2 to make two plots: a plot of –rurea as a function of Curea using Equation (9-26), which is called a Michaelis–Menten plot and is shown in Figure E9-2.1(a); and a plot of (1/–rurea) as a function (1/Curea), which is called a Lineweaver–Burk plot and is shown in Figure 9-2.1 (b).

Wolfram Sliders
TABLE E9-2.2 RAW AND PROCESSED DATA
Curea (kmol/m3) |
−rurea (kmol/m3 · s) |
1/Curea (m3/kmol) |
1/−rurea (m3·s/kmol) |
0.20 |
1.08 |
5.0 |
0.93 |
0.02 |
0.55 |
50.0 |
1.82 |
0.01 |
0.38 |
100.0 |
2.63 |
0.005 |
0.20 |
200.0 |
5.00 |
0.002 |
0.09 |
500.0 |
11.11 |
Michaelis–Menten Plot
Lineweaver–Burk Plot

Wolfram Sliders
The intercept on Figure E9-2.1(b) is 0.75, so
Therefore, the maximum rate of reaction is
Vmax = 1.33 kmol/m3 · s = 1.33 mol/dm3 · s
From the slope, which is 0.02 s, we can calculate the Michaelis constant, KM
Solving for KM: KM = 0.0266 kmol/m3.
For enzymatic reactions, the two key rate-law parameters are Vmax and KM.
Substituting KM and Vmax into Equation (9-26) gives us
where Curea has units of (kmol/m3) and −rurea has units of (kmol/m3 · s). Levine and LaCourse suggest that the total concentration of urease, (Et), corresponding to the value of Vmax above is approximately 5 g/dm3.
Part (b)
In addition to the Lineweaver–Burk plot, one can also use a Hanes–Woolf plot or an Eadie–Hofstee plot. Here, S ≡ Curea, and –rS ≡ –rurea. Equation (9-26)

Eadie–Hofstee Plot
can be rearranged in the following forms. For the Eadie–Hofstee form
For the Hanes–Woolf form, we can rearrange Equation (9-26) to

Hanes–Woolf Plot
For the Eadie–Hofstee model, we plot –rS as a function of [–rS/(S)] and for the Hanes–Woolf model, we plot [(S)/–rS] as a function of (S).
When to use the different models? The Eadie–Hofstee plot does not bias the data points at low substrate concentrations, while the Hanes–Woolf plot gives a more accurate evaluation of Vmax. In Table E9-2.3, we add two columns to Table E9-2.1 to generate these plots (Curea ≡ S).

Wolfram Sliders
TABLE E9-2.3 RAW AND PROCESSED DATA
S (kmol/m3) |
–rS (kmol/m3 · s) |
1/S (m3/kmol) |
1/–rS (m3 · s/kmol) |
S/–rs (s) |
–rS/S (1/s) |
0.20 |
1.08 |
5.0 |
0.93 |
0.185 |
5.4 |
0.02 |
0.55 |
50.0 |
1.82 |
0.0364 |
27.5 |
0.01 |
0.38 |
100.0 |
2.63 |
0.0263 |
38 |
0.005 |
0.20 |
200.0 |
5.00 |
0.0250 |
40 |
0.002 |
0.09 |
500.0 |
11.11 |
0.0222 |
45 |
The slope of the Hanes Woolf plot in Figure E9-2.2 (i.e., (1/ Vmax) = 0.826 s · m3/kmol), gives Vmax = 1.2 kmol/m3 · s and from the intercept, KM/Vmax = 0.02 s, we obtain KM to be 0.024 kmol/m3.
Hanes–Woolf plot
We next generate an Eadie–Hofstee plot form the data in Table E9-2.3. From the slope at the Eadie-Hofstee plot in Figure E9-2.3 (–0.0244 kmol/m3), we find KM = 0.024 kmol/m3. Next, using the intercept at –rS = 0, i.e., Vmax/KM = 50 s–1, we calculate Vmax = 1.22 kmol/m3 · s.
Eadie–Hofstee plot
Part (c) Regression
Equation (9-26) and Table E9-2.2 were used in the regression program of Polymath with the following results for Vmax and KM, as shown in Table E9-2.4.
These values are within experimental error of those values of Vmax and Km deter-mined graphically.
Analysis: This example demonstrated how to evaluate the parameters Vmax and KM in the Michaelis–Menten rate law from enzymatic reaction data. Four techniques were used to evaluate Vmax and KM from experimental data: Lineweaver–Burk plot, Hanes–Woolf plot, Eadie–Hofstee plot, and non-linear regression. The advantage of each type of plot was discussed for evaluating the parameters Vmax and KM.
The Product-Enzyme Complex
In many reactions the enzyme and product complex (E · P) is formed directly from the enzyme substrate complex (E · S) according to the sequence
Briggs–Haldane Rate
Law
Applying the PSSH, after much effort and some approximations we obtain
which is often referred to as the Briggs–Haldane Equation [see P9-8B (a)] and the application of the PSSH to enzyme kinetics, often called the Briggs–Haldane approximation.
9.2.4 Batch-Reactor Calculations for Enzyme Reactions
A mole balance on urea in a batch reactor gives
Mole balance
Because this reaction is liquid phase, V = V0, the mole balance can be put in the following form:
The rate law for urea decomposition is
Rate law
Substituting Equation (9-31) into Equation (9-30) and then rearranging and integrating, we get
Combine
Integrate
We can write Equation (9-32) in terms of conversion as
Curea = Curea0 (1 – X)
Time to achieve a conversion X in a batch enzymatic reaction
The parameters KM and Vmax can readily be determined from batch-reactor data by using the integral method of analysis. Here, as shown in Figures 7-1, 7-2, and 7-3, we want to arrange our equation so that we can obtain a linear plot of our data. Letting S = Curea and S0 = Curea0, we arrange Equation (9-32) in the form
The corresponding plot in terms of substrate concentration is shown in Figure 9-7.
From Equation (9-33) we see that the slope of a plot of as a function of will be and the y intercept will be . In cases similar to Equation (9-33) where there is no possibility of confusion, we shall not bother to enclose the substrate or other species in parentheses to represent concentration [i.e., CS ≡ (S) ≡ S].
Example 9-3 Batch Enzymatic Reactors
Calculate the time needed to convert 99% of the urea to ammonia and carbon dioxide in a 0.5-dm3 batch reactor. The initial concentration of urea is 0.1 mol/dm3, and the urease concentration is 0.001 g/dm3. The reaction is to be carried out isothermally at the same temperature at which the data in Table E9-2.2 were obtained.

Wolfram Sliders
Solution
We can use Equation (9-32)
From Table E9-2.3 we know KM = 0.0233 mol / dm3, Vmax = 1.2 mol / dm3 · s. The conditions given are X = 0.99 and Cureao = 0.1 mol / dm3 (i.e., 0.1 kmol/m3). However, for the conditions in the batch reactor, the enzyme concentration is only 0.001 g/dm3, compared with 5 g/dm3 in Example 9-2. Because Vmax = Et · k3, Vmax for the second enzyme concentration is
Substituting into Equation (9-32)
Analysis: This example shows a straightforward Chapter 5–type calculation of the batch reactor time to achieve a certain conversion X for an enzymatic reaction with a Michaelis–Menten rate law. This batch reaction time is very short; consequently, a continuous-flow reactor might be better suited for this reaction.
Effect of Temperature
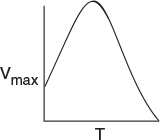
The effect of temperature on enzymatic reactions is very complex. If the enzyme structure would remain unchanged as the temperature is increased, the rate would probably follow the Arrhenius temperature dependence. However, as the temperature increases, the enzyme can unfold and/or become denatured and lose its catalytic activity. Consequently, as the temperature increases, the reaction rate, –rS, increases up to a maximum and then decreases as the temperature is increased further. The descending part of this curve is called temperature inactivation or thermal denaturizing.11 Figure 9-8 shows an example of this optimum in enzyme activity.12
11 M. L. Shuler and F. Kargi, Bioprocess Engineering Basic Concepts, 2nd ed. (Upper Saddle River, NJ: Prentice Hall, 2002), p. 77.
12 S. Aiba, A. E. Humphrey, and N. F. Mills, Biochemical Engineering (New York: Academic Press, 1973), p. 47.
9.3 Inhibition of Enzyme Reactions
In addition to temperature and solution pH, another factor that greatly influences the rates of enzyme-catalyzed reactions is the presence of an inhibitor. Inhibitors are species that interact with enzymes and render the enzyme either partially or totally ineffective to catalyze its specific reaction. The most dramatic consequences of enzyme inhibition are found in living organisms, where the inhibition of any particular enzyme involved in a primary metabolic pathway will render the entire pathway inoperative, resulting in either serious damage to or death of the organism. For example, the inhibition of a single enzyme, cytochrome oxidase, by cyanide will cause the aerobic oxidation process to stop; death occurs in a very few minutes. There are also beneficial inhibitors, such as the ones used in the treatment of leukemia and other neoplastic diseases. Aspirin inhibits the enzyme that catalyzes the synthesis of the module prostaglandin, which is involved in the pain-producing process. As shown in TV advertisements, the recent discovery of DDP-4 enzyme inhibitor Januvia has been approved for the treatment of Type 2 diabetes, a disease affecting 240 million people worldwide. Work Problem P9-15C and then as the TV ad said, “Ask your doctor if Januvia is right for you.”

Figure 9-8 Catalytic breakdown rate of H2O2 depending on temperature. Courtesy of S. Aiba, A. E. Humphrey, and N. F. Mills, Biochemical Engineering, Academic Press (1973).
The three most common types of reversible inhibition occurring in enzymatic reactions are competitive, uncompetitive, and noncompetitive. The enzyme molecule is analogous to a heterogeneous catalytic surface in that it contains active sites. When competitive inhibition occurs, the substrate and inhibitor are usually similar molecules that compete for the same site on the enzyme. Uncompetitive inhibition occurs when the inhibitor deactivates the enzyme–substrate complex, sometimes by attaching itself to both the substrate and enzyme molecules of the complex. Noncompetitive inhibition occurs with enzymes containing at least two different types of sites. The substrate attaches only to one type of site, and the inhibitor attaches only to the other to render the enzyme inactive.
9.3.1 Competitive Inhibition
Competitive inhibition is of particular importance in pharmacokinetics (drug therapy). If a patient were administered two or more drugs that react simultaneously within the body with a common enzyme, cofactor, or active species, this interaction could lead to competitive inhibition in the formation of the respective metabolites and produce serious consequences.
In competitive inhibition, another substance, i.e., I, competes with the substrate for the enzyme molecules to form an inhibitor–enzyme complex, as shown in Figure 9-9. That is, in addition to the three Michaelis–Menten reaction steps, there are two additional steps as the inhibitor (I) reversely ties up the enzyme, as shown in Reaction Steps 4 and 5.
The rate law for the formation of product in Reaction Step 3 is the same [cf. Equations (9-18A) and (9-19)] as it was before, i.e., in the absence of inhibitor
Competitive Inhibition Pathway

Figure 9-9 Competitive inhibition. Schematic drawing courtesy of Jofostan National Library, Lunčo, Jofostan, established 2019. New building completion expected in fall 2022.
Competitive inhibition pathway

Applying the PSSH, the net rate of reaction of the enzyme–substrate complex is
In a similar fashion, apply the PSSH to the net rate of formation of the inhibitor–substrate complex (E · I) is also zero
The total enzyme concentration is the sum of the bound and unbound enzyme concentrations
Combining Equations (9-35), (9-36), and (9-37), solving for (E · S) then substituting in Equation (9-34) and simplifying
Rate law for competitive inhibition
Vmax and KM are the same as before when no inhibitor is present; that is,
and the competitive inhibition constant KI (mol/dm3) is defined as
By letting , we can see that the effect of adding a competitive inhibitor is to increase the “apparent” Michaelis constant, . A consequence of the larger “apparent” Michaelis constant is that a larger substrate concentration is needed for the rate of substrate decomposition, –rS, to reach half its maximum rate.
Rearranging Equation (9-38) in order to generate a Lineweaver–Burk plot
From the Lineweaver–Burk plot (Figure 9-10), we see that as the inhibitor (I) concentration is increased, the slope increases (i.e., the rate decreases), while the intercept remains fixed.

Figure 9-10 Lineweaver–Burk plot for competitive inhibition. (http://www.umich.edu/~elements/5e/09chap/live.html)

Wolfram Sliders
9.3.2 Uncompetitive Inhibition
Here, the inhibitor has no affinity for the enzyme by itself and thus does not compete with the substrate for the enzyme; instead, it ties up the enzyme–substrate complex by forming an inhibitor–enzyme–substrate complex, (I · E · S), which is inactive. In uncompetitive inhibition, the inhibitor reversibly ties up enzyme–substrate complex after it has been formed.
As with competitive inhibition, two additional reaction steps are added to the Michaelis–Menten kinetics for uncompetitive inhibition, as shown in Reaction Steps 4 and 5 in Figure 9-11.
Reaction Steps
Uncompetitive Pathway
Uncompetitive inhibition pathway

Starting with the equation for the rate of formation of product, Equation (9-34), and then applying the pseudo-steady-state hypothesis to the intermediate (I · E · S), we arrive at the rate law for uncompetitive inhibition
Rate law for uncompetitive inhibition
The intermediate steps are shown in the Chapter 9 Summary Notes in Learning Resources on the CRE Web site (http://www.umich.edu/~elements/5e/09chap/summary-example4.html).
Rearranging Equation (9-40)
The Lineweaver–Burk plot for uncompetitive enzyme inhibition is shown in Figure 9-12 for different inhibitor concentrations. The slope (KM/Vmax) remains the same as the inhibitor (I) concentration is increased, while the intercept [(1/Vmax)(1 + (I)/KI)] increases.
9.3.3 Noncompetitive Inhibition (Mixed Inhibition)13
13 In some texts, mixed inhibition is a combination of competitive and uncompetitive inhibition.
In noncompetitive inhibition, also sometimes called mixed inhibition, the substrate and inhibitor molecules react with different types of sites on the enzyme molecule. Whenever the inhibitor is attached to the enzyme, it is inactive and cannot form products. Consequently, the deactivating complex (I · E · S) can be formed by two reversible reaction paths.
1. After a substrate molecule attaches to the enzyme molecule at the substrate site, then the inhibitor molecule attaches to the enzyme at the inhibitor site. (E • S + I ⇄ I • E • S)
2. After an inhibitor molecule attaches to the enzyme molecule at the inhibitor site, then the substrate molecule attaches to the enzyme at the substrate site. (E • I + S ⇄ I • E • S)
These paths, along with the formation of the product, P, are shown in Figure 9-13. In noncompetitive inhibition, the enzyme can be tied up in its inactive form either before or after forming the enzyme–substrate complex as shown in Steps 2, 3, and 4.
Again, starting with the rate law for the rate of formation of product and then applying the PSSH to the complexes (I · E) and (I · E · S), we arrive at the rate law for the noncompetitive inhibition

Rate law for noncompetitive inhibition
The derivation of the rate law is given in the Summary Notes on the CRE Web site (http://www.umich.edu/~elements/5e/09chap/summary-derive3.html). Equation (9-42) is in the form of the rate law that is given for an enzymatic reaction exhibiting noncompetitive inhibition. Heavy metal ions such as Pb2+, Ag+, and Hg2+, as well as inhibitors that react with the enzyme to form chemical derivatives, are typical examples of noncompetitive inhibitors.
Reaction Steps
Noncompetitive Pathway
Mixed inhibition

Rearranging Equation (9-42)
For noncompetitive inhibition, we see in Figure 9-14 that both the slope and intercept increase with increasing inhibitor concentration. In practice, uncompetitive inhibition and mixed inhibition are generally observed only for enzymes with two or more substrates, S1 and S2.
The three types of inhibition are compared with a reaction without inhibitors and are summarized on the Lineweaver–Burk plot shown in Figure 9-15.
In summary, we observe the following trends and relationships:
1. In competitive inhibition, the slope increases with increasing inhibitor concentration, while the intercept remains fixed.
2. In uncompetitive inhibition, the y-intercept increases with increasing inhibitor concentration, while the slope remains fixed.
3. In noncompetitive inhibition (mixed inhibition), both the y–intercept and slope will increase with increasing inhibitor concentration.
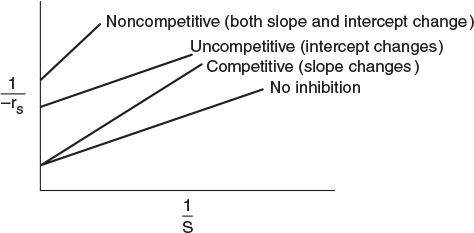
Figure 9-15 Summary: Lineweaver–Burk plots for three types of enzyme inhibition. (http://www.umich.edu/~elements/5e/09chap/live.html)
Summary plot of types of inhibition

Wolfram Sliders
Problem P9-12B asks you to find the type of inhibition for the enzyme catalyzed reaction of starch.
9.3.4 Substrate Inhibition
In a number of cases, the substrate itself can act as an inhibitor, especially at high substrate concentrations. In the case of uncompetitive inhibition, the inactive molecule (S · E · S) is formed by the reaction
S + E · S → S · E · S (inactive)
Consequently, we see that by replacing (I) by (S) in Equation (9-40), the rate law for –rS is
We see that at low substrate concentrations
and the rate increases linearly with increasing substrate concentration.
At high substrate concentrations ((S)2 / KI) >>(KM + (S)), then
Substrate inhibition
and we see that the rate decreases as the substrate concentration increases. Consequently, the rate of reaction goes through a maximum in the substrate concentration, as shown in Figure 9-16. We also see that there is an optimum substrate concentration at which to operate. This maximum is found by setting the derivative of –rS wrt S in Equation (9-44) equal to 0, to obtain
When substrate inhibition is possible, the substrate is fed to a semibatch reactor called a fed batch to maximize the reaction rate and conversion.

Figure 9-16 Substrate reaction rate as a function of substrate concentration for substrate inhibition.
Our discussion of enzymes is continued in the Professional Reference Shelf on the CRE Web site where we describe multiple enzyme and substrate systems, enzyme regeneration, and enzyme co-factors (see R9.6; http://www.umich.edu/~elements/5e/09chap/prof-07prof2.html