10.8.2 Moving-Bed Reactors
Reaction systems with significant catalyst decay require the continual regeneration and/or replacement of the catalyst. Two types of reactors currently in commercial use that accommodate production with decaying catalysts are the moving-bed and straight-through transport reactor. A schematic diagram of a moving-bed reactor (used for catalytic cracking) is shown in Figure 10-32.
The freshly regenerated catalyst enters the top of the reactor and then moves through the reactor as a compact packed bed. The catalyst is coked continually as it moves through the reactor until it exits the reactor into the kiln, where air is used to burn off the carbon. The regenerated catalyst is lifted from the kiln by an airstream and then fed into a separator before it is returned to the reactor. The catalyst pellets are typically between one-quarter and one-eighth in. in diameter.
The reactant feed stream enters at the top of the reactor and flows rapidly through the reactor relative to the flow of the catalyst through the reactor (Figure 10-33). If the feed rates of the catalyst and the reactants do not vary with time, the reactor operates at steady state; that is, conditions at any point in the reactor do not change with time. The mole balance on reactant A over ΔW is
Figure 10-32 Thermofor catalytic cracking (TCC) unit. (Weekman, V. AIChE Monogr. Ser., 75(11), 4 (1979). With permission of the American Institute of Chemical Engineers. Copyright © 1979 AIChE. All rights reserved.)
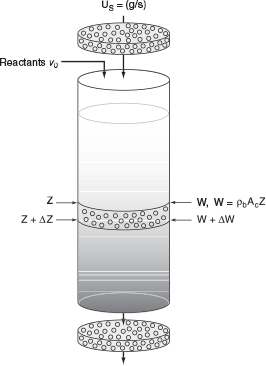
Figure 10-33 Moving-bed reactor schematic. The value of the catalyst contained in a reactor of this type is approximately $1 million.
Moving bed reactor, used for reactions with moderate rate of catalyst decay
Dividing by ΔW, letting ΔW approach zero, and expressing the flow rate in terms of conversion gives
Mole Balance
The rate of reaction at any time t is
The activity, as before, is a function of the time the catalyst has been in contact with the reacting gas stream. The decay rate law is
Decay Law
We now need to relate the contact time to the weight of the catalyst. Consider a point z in the reactor, where the reactant gas has passed co-currently through a catalyst weight W. Because the solid catalyst is moving through the bed at a rate U S (mass per unit time), the time t that the catalyst has been in contact with the gas when the catalyst reaches a point z is
If we now differentiate Equation (10-122)
and combine it with the decay rate law, we obtain
The activity equation is combined with the mole balance
The design equation for moving-bed reactors
these two coupled differential equations (i.e., Equations (10-124) and (10-125)) are numerically solved simultaneously with an ODE solver, e.g., Polymath.
Example 10-5 Catalytic Cracking in a Moving-Bed Reactor

The catalytic cracking of a gas-oil charge, A, to form C5 (B) and to form coke and dry gas (C) is to be carried out in a screw-type conveyor, moving-bed reactor at 900 F:
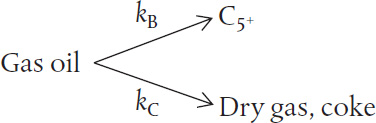
This cracking of gas oil can also be written as
While pure hydrocarbons are known to crack according to a first-order rate law, the fact that the gas-oil exhibits a wide spectrum of cracking rates gives rise to the fact that the lumped cracking rate is well represented by a second-order rate law (see Web Problem CDP5-HB) with the following specific reaction rate:32
The catalytic deactivation is independent of gas-phase concentration and follows a first-order decay rate law, with a decay constant, kd of 0.72 reciprocal minutes. The feed stream is diluted with nitrogen so that as a first approximation, volume changes can be neglected with reaction. The reactor contains 22 kg of catalyst that moves through the reactor at a rate of 10 kg/min. The gas-oil is fed at a rate of 30 mol/min at a concentration of 0.075 mol/dm3. Determine the conversion that can be achieved in this reactor.
Solution
1. Mole Balance:
2. Rate Law:
3. Decay Law. First-order decay
Moving beds: moderate rate of catalyst decay
Using Equation (10-124), we obtain
Integrating
4. Stoichiometry. If , then
5. Combining we have
The polymath program is shown below along with a conversion profile.
POLYMATH Report
Ordinary Differential Equations

For the simple rate law and activity law given here, we also could have solved this problem analytically.
6. Separating and integrating yields
7. Numerical evaluation:
X = 55%
If there were no catalyst decay, the conversion would be
Analysis: The purpose of this example was to show step-by-step how to apply the algorithm to a moving-bed reactor that has been used to reduce the effects of catalyst decay that would occur in a PBR.
We will now rearrange Equation (E10-5.8) to a form more commonly found in the literature. Let be a dimensionless decay time
and Da2 be the Damköhler number for a second-order reaction (a reaction rate divided by a transport rate) for a packed-bed reactor
Through a series of manipulations we arrive at the equation for the conversion in a moving bed where a second-order reaction is taking place33
Similar equations are given or can easily be obtained for other reaction orders or decay laws.
Second-order reaction in a moving-bed reactor
10.8.3 Straight-Through Transport Reactors (STTR)
This reactor is used for reaction systems in which the catalyst deactivates very rapidly. Commercially, the STTR is used in the production of gasoline from the cracking of heavier petroleum fractions where coking of the catalyst pellets occurs very rapidly. In the STTR, the catalyst pellets and the reactant feed enter together and are transported very rapidly through the reactor. The bulk density of the catalyst particle in the STTR is significantly smaller than in moving-bed reactors, and often the particles are carried through at the same velocity as the gas velocity. In some places the STTR is also referred to as a circulating fluid-ized bed (CFB). A schematic diagram is shown in Figure 10-34.
A mole balance on the reactant A over the differential reactor volume
is
Dividing by Δz and taking the limit as Δz → 0, letting ρb be the bulk density of the suspended catalyst and recalling that , we obtain
In terms of conversion and catalyst activity
For a catalyst particle traveling through the reactor with a particle velocity UP, the time the catalyst pellet has been in the reactor when it reaches a height z is just
Substituting for time t in terms of distance z [i.e., a(t) = a(z/UP)], the mole balance now becomes
The entering molar flow rate, FA0, can be expressed in terms of the gas velocity Ug, CA0, and AC
FA0 = UoACCA0
Substituting for FA0, we have
Example 10-6 Decay in a Straight-Through Transport Reactor
The vapor-phase cracking of a gas-oil is to be carried out in a straight-through transport reactor (STTR) that is 10-m high and 1.5 m in diameter. Gas-oil is a mixture of normal and branched paraffins (C12–C40), naphthenes, and aromatics, all of which will be lumped as a single species, A. We shall lump the primary hydrocarbon products according to distillate temperature into two respective groups, dry gas (C–C4) B and gasoline (C5–C14) C. The reaction

A typical cost of the catalyst in the reactor system is $1 million.
Gas-oil (g) → Products (g) + Coke
can be written symbolically as
A → B + C + Coke
Both B and C are adsorbed on the surface. The rate law for a gas-oil cracking reaction on fresh catalyst can be approximated by
with k’ = 0.0014 kmol/kg-cat·s·atm, KA = 0.05 atm-1, KB 0.15 atm-1, and KC 0.1 atm-1. The catalyst decays by the deposition of coke, which is produced in most cracking reactions along with the reaction products. The decay law is
Pure gas-oil enters at a pressure of 12 atm and a temperature of 400°C. The bulk density of catalyst in the STTR is 80 kg-cat/m3. Plot the activity and conversion of gas-oil up the reactor for entering gas velocity U0 = 2.5 m/s.
Solution
Mole Balance:
The height of the catalyst particle at time “t” after entering the STTR is
Differentiating, we can find a relation between the time the catalyst particle has been in the STTR and reached a height z, which we can use to find the activity a.
Rate Law:
On fresh catalyst
Combining Equations (E10-6.2) through (E10-6.4) gives
Decay Law. Assuming that the catalyst particle and gas travel up the reactor at the velocity UP = Ug = U, we obtain
where
Stoichiometry (gas-phase isothermal and no pressure drop):
Parameter Evaluation:
Equations (E10-6.1), (E10-6.5), (E10-6.7), and (E10-6.8) through (E10-6.10) are now combined and solved using an ODE solver. The Polymath program is shown in Table E10-6.1, and the computer output is shown in Figure E10-6.1.
TABLE E10-6.1 EQUATIONS FOR THE STTR: LANGMUIR–HINSHELWOOD KINETICS


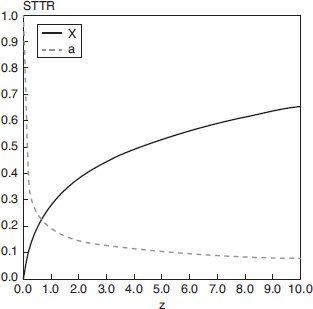
Use Wolfram to learn how the conversion and activity profiles shown above in Figure E10-6.1 change as you vary the parameters.
Analysis: In this example we applied the algorithm to a STTR in which the gas velocity and, hence, particle velocity increases as we move through the reactor. The decay is quite rapid and the activity is only 15% of its initial value at z = 3 m into the reactor and the conversion begins to plateau at z = 6 m at 60% conversion. If there were no catalyst decay (a = 0) the conversion would have been 97%.
Summary
1. Types of adsorption:
a. Chemisorption
b. Physical adsorption
2. The Langmuir isotherm relating the concentration of species A on the surface to the partial pressure of A in the gas phase is
3. The sequence of steps for the solid-catalyzed isomerization
is:
a. Mass transfer of A from the bulk fluid to the external surface of the pellet
b. Diffusion of A into the interior of the pellet
c. Adsorption of A onto the catalytic surface
d. Surface reaction of A to form B
e. Desorption of B from the surface
f. Diffusion of B from the pellet interior to the external surface
g. Mass transfer of B away from the solid surface to the bulk fluid
4. Assuming that mass transfer is not rate-limiting, the rate of adsorption is
The rate of desorption is
The rate of desorption is
At steady state
If there are no inhibitors present, the total concentration of sites is
5. If we assume that the surface reaction is rate-limiting, we set
and solve for CA S and CB S in terms of PA and PB. After substitution of these quantities in Equation (S10-4), the concentration of vacant sites is eliminated with the aid of Equation (S10-7)
Recall that the equilibrium constant for desorption of species B is the reciprocal of the equilibrium constant for the adsorption of species B
and the thermodynamic equilibrium constant, KP, is
6. Chemical vapor deposition
7. Catalyst deactivation. The catalyst activity is defined as
The rate of catalyst decay is
The rate of catalyst decay is
For first-order decay
For second-order decay
8. For slow catalyst decay, the idea of a temperature–time trajectory is to increase the temperature in such a way that the rate of reaction remains constant.
9. The coupled differential equations to be solved for a moving-bed reactor are
For nth-order activity decay and m order in a gas-phase concentration of species i in a moving bed where the solids move up with the velocity US

10. The coupled equations to be solved in a straight-through transport reactor are
where Up is the particle velocity up the STTR. For coking
For the case when there is no slip between the catalyst particles and the gas velocity (S10-27)
Cre Web Site Materials
• Expanded Materials (http://umich.edu/~elements/5e/10chap/expanded.html)
1. Puzzle Problem “What’s Wrong with this Solution?” (http://umich.edu/~elements/5e/10chap/expanded_ch10_puzzle.pdf)
2. Turn Over Frequency (TOF) (http://umich.edu/~elements/5e/10chap/expanded_ch10_TOF.pdf)
3. Algorithm When More than One of the Three Steps in a Catalytic Reaction Mechanism (Adsorption, Surface Reaction, Desorption) Is Rate Limiting (http://umich.edu/~elements/5e/10chap/expanded_ch10_PSSH.pdf)
4. Additional Homework Problems (http://umich.edu/~elements/5e/10chap/add.html)
• Learning Resources (http://umich.edu/~elements/5e/10chap/learn.html)
1. Summary Notes (http://umich.edu/~elements/5e/10chap/summary.html)
2. SelfTests (http://umich.edu/~elements/5e/10chap/add.html)
A. Exercises (http://umich.edu/~elements/5e/10chap/add.html)
B. i>clicker Questions (http://umich.edu/~elements/5e/10chap/iclicker_ch10_q1.html)
3. Web Modules (http://umich.edu/~elements/5e/10chap/summary.html)
4. Interactive Computer Modules
A. Heterogeneous Catalysis (http://umich.edu/~elements/5e/icm/hetcat.html)

B. Analogy of the effects of cobra venom and adsorption on catalytic sites
(http://www.umich.edu/~elements/5e/web_mod/cobra/index.html)
C. Cobra Bites (http://umich.edu/~elements/5e/web_mod/cobra/index.html)

5. Solved Problems (http://umich.edu/~elements/5e/10chap/learn.html)
Example Chapter 10 CRE Web Site: 10-1 Analysis of a Heterogeneous Reaction [Class Problem University of Michigan]
Example Chapter 10 CRE Web Site: 10-2 Least Squares Analysis to Determine the Rate
Law Parameterss k, kT, and kB
Example Chapter 10 CRE Web Site: 10-3 Decay in a Straight-Through Reactor
Example Chapter 10 CRE Web Site: 10-4 Catalyst Poisoning in a Batch Reactor
• Living Example Problem (http://umich.edu/~elements/5e/10chap/live.html)
1. Example 10-1 Non-linear Regression Analysis and CV and CB S
2. Example 10-2 Catalytic Reactor Design (Fixed-Bed Reactor Design)
3. Example 10-3 Hydrogenation of Ethlyene to Ethane (Model Discrimination)
4. Example 10-4 Calculating Conversion with Catalyst Decay in Batch Reactors
5. Example 10-5 Catalytic Cracking in a Moving-Bed Reactor
6. Example 10-6 Decay in a Straight-Through Transport Reactor
• FAQs (Frequently Asked Questions)
(http://umich.edu/~elements/5e/10chap/faq.html)


• Professional Reference Shelf (http://umich.edu/~elements/5e/10chap/prof.html)
R10.1 Classification of Catalysts (http://umich.edu/~elements/5e/10chap/prof-classification.html)
R10.2 Hydrogen Adsorption (http://umich.edu/~elements/5e/10chap/prof-hydrogen.html)
A. Molecular Adsorption
B. Dissociative Adsorption
R10.3 Analysis of Catalyst Decay Laws
A. Differential Method (http://umich.edu/~elements/5e/10chap/pdf/CD-Ch10DecayAnalysis.pdf)
B. Integral Method (http://umich.edu/~elements/5e/10chap/prof-differential.html)
R10.4 Etching of Semiconductors (http://umich.edu/~elements/5e/10chap/prof-etching.html)
A. Dry Etching
B. Wet Etching
R10.5 Dissolution Catalysis (http://umich.edu/~elements/5e/10chap/prof-dissolution.html)
R10.6 Catalyst Deactivation (http://umich.edu/~elements/5e/10chap/prof-dissolution.html)
A. Type of Catalyst Deactivation
B. Temperature–Time Trajectories
C. Moving-Bed Reactors
D. Straight-Through Transport Reactors
After Reading Each Page in This Book, Ask Yourself a Question About What You Read
Questions and Problems
The subscript to each of the problem numbers indicates the level of difficulty: A, least difficult; D, most difficult.
Questions
Q10-1A i>clicker. Go to the Web site (http://www.umich.edu/~elements/5e/10chap/iclicker_ch10_q1.html) and view at least five i>clicker questions. Choose one that could be used as is, or a variation thereof, to be included on the next exam. You also could consider the opposite case: explaining why the question should not be on the next exam. In either case, explain your reasoning.
Q10-2A Read over the problems at the end of this chapter. Make up an original problem that uses the concepts presented in this chapter. See Problem P5-1A for guidelines. To obtain a solution:
(a) Create your data and reaction.
(b) Use a real reaction and real
data.
The journals listed at the end of Chapter 1 may be useful for part (b).
(c) Choose an FAQ from Chapter 10 and explain why it was most helpful.
(d) Listen to the audios ![]() on the Summary Notes, pick one, and explain why it was most helpful.
on the Summary Notes, pick one, and explain why it was most helpful.
Q10-3B For the decomposition of cumene discussed in this chapter, if an adsorbing inert is present, how would you compare the initial rate as a function of total pressure when desorption is the RLS as shown Figure 10-18?
Q10-4A Choose five Chapter 10 i>clicker questions, and then pick the one that was the most challenging and explain why.
Q10-5A What if you were asked to sketch the temperature–time trajectories and to find the catalyst lifetimes for first- and for second-order decay when EA = 35 kcal/mol, Ed = 10 kcal/mol, kd0 = 0.01 day–1, and T0 = 400 K? How would the trajectory of the catalyst lifetime change if EA = 10 kcal/mol and Ed = 35 kcal/mol? At what values of kd0 and ratios of Ed to EA would temperature–time trajectories not be effective? What would your temperature–time trajectory look like if n = 1 + Ed/EA?
Q10-6A Write a question for this problem that involves critical thinking and explain why it involves critical thinking.
Problems
Computer Simulations and Experiments
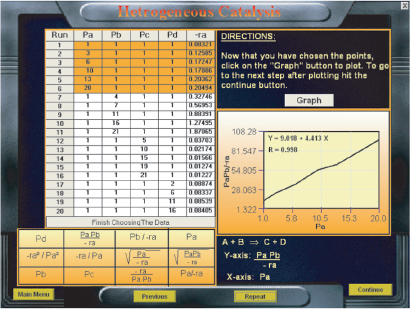
P10-1B (a) Example 10-1: Nonlinear Regression to Determine Model Parameters and Site Concentration Ratio = CT•S/CB•S
Wolfram
(i) Find the critical value of KT and KB at which fraction of vacant sites starts to increase with conversion? Vary only one parameter at a time.
(ii) Find the value of KB such that fraction of sites occupied by benzene is doubled at 40% conversion of Toluene.
(iii) Find the value KB where the curve for versus conversion X changes from convex to concave and explain why this shape change occurs.
(b) Example 10-2: Catalytic Reactor Design Wolfram
(i) How would the conversion change if the entering pressure were (1) increased to 80 atm or (2) it were reduced 1 atm?
(ii) What if the molar flow rate were reduced by 50%; how would X and p change?
(iii) After reviewing Generating Ideas and Solutions on the Web site (http://www.umich.edu/~elements/5e/toc/SCPS,3rdEdBook(Ch07).pdf), choose one of the brainstorming techniques (e.g., lateral thinking) to suggest two questions that should be included in this problem.
(iv) Write two conclusions from your experiments with the sliders in this example.
Polymath
(v) What catalyst weight would be required for 60% conversion?
(vi) Which parameter will you vary so that PB = PH2 at the middle of the reactor (i.e., W = 5000Kg).
(c) Example 10-3: Hydrogenation Ethylene to Ethane
(1) Use Polymath to learn how your answers would change if the following data for run 10 were incorporated in your regression table.
(2) How do the rate laws (e) and (f)
compare with the nonlinear analysis with the other rate laws used to model the data?
(d) Example 10-4: Calculating Conversion with Catalyst Decay Wolfram
(i) What is the maximum conversion that can be achieved if there is no catalyst decay?
(ii) Vary k and kd and describe what you find. Can you explain why there is no effect of catalyst decay on conversion at a high value of k?
(iii) Vary E, Ed, k1, and k1d and then write a few sentences describing the results of your experiments.
(iv) Explain why conversion with catalyst decay increases with increasing Ed and decreases with increasing E.
Polymath
(v) Vary the ratio of (k/kd) and describe what you find.
(vi) Repeat this example (i.e., the plotting of X vs. t) for a second-order reaction with (CA0 = 1 mol/dm3) and first-order decay and describe the differences from the base case.
(vii) Repeat this example for a first-order reaction and first-order decay and describe the differences from the base case.
(viii) Repeat this example for a second-order reaction (CA0 = 1 mol/dm3) and a second-order decay and describe the differences from the base case.
(e) Example 10-5: Catalytic Cracking in a Moving Bed Polymath
(i) Use Polymath to learn what the conversion would be if there is no catalyst decay.
(ii) What if the solids and reactants entered from opposite ends of the reactor? How would your answers change?
(iii) What if the decay in the moving bed were second order? By how much must the catalyst charge, Us, be increased to obtain the same conversion?
(iv) What if ε = 2 (e.g., A → 3B) instead of zero. How would the results be affected?
(f) Example 10-6: Decay in a Straight-Through Transport Reactor Polymath
(i) What if you varied the parameters PA0, Ug, A, and k′ in the STTR? What parameter has the greatest effect on either increasing or decreasing the conversion?
(ii) Ask questions such as: What is the effect of varying the ratio of k to Ug or of k to A on the conversion? Make a plot of conversion versus distance as Ug is varied between 0.5 and 50 m/s.
(iii) Sketch the activity and conversion profiles for Ug = 0.025, 0.25, 2.5, and 25 m/s.
(iv) What generalizations can you make? Plot the exit conversion and activity as a function of gas velocity between velocities of 0.02 and 50 m/s.
(v) What gas velocity do you suggest operating at?
(vi) What is the corresponding entering volumetric flow rate?
(vii) What concerns do you have operating at the velocity you selected? Would you like to choose another velocity? If so, what is it? Which parameter will you vary so that conversion increases but activity decreases? Explain, if you can, this unusual behavior.
Interactive Computer Game
P10-2A Download the Interactive Computer Games (ICG) from the CRE Web site (http://www.umich.edu/~elements/5e/icm/install.html). Play the game and then record your performance number for the game, which indicates your mastery of the material. Your professor has the key to decode your performance number. (This ICG is a little longer than the other ICGs.)
ICG Heterogeneous Catalysis Performance # _____________
Problems
P10-3A t-Butyl alcohol (TBA) is an important octane enhancer that is used to replace lead additives in gasoline [Ind. Eng. Chem. Res., 27, 2224 (1988)]. TBA was produced by the liquid-phase hydration (W) of isobutene (I) over an Amberlyst-15 catalyst. The system is normally a multiphase mixture of hydrocarbon, water, and solid catalysts. However, the use of cosolvents or excess TBA can achieve reasonable miscibility.
The reaction mechanism is believed to be
Derive a rate law assuming:
(a) The surface reaction is rate-limiting.
(b) The adsorption of isobutene is limiting.
(c) The reaction follows Eley–Rideal kinetics
and the surface reaction is limiting.
(d) Isobutene (I) and water (W) are adsorbed on different sites.
TBA is not on the surface, and the surface reaction is rate-limiting.
(e) What generalization can you make by comparing the rate laws derived in parts (a) through (d)?
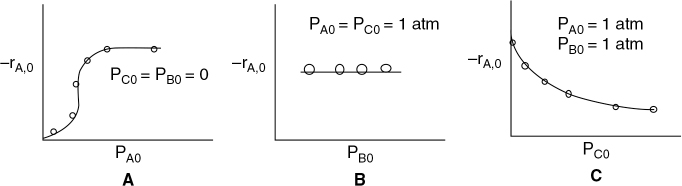
P10-4B Consider the catalytic reaction as a function of the initial partial pressures
The rate of disappearance of species A was obtained in a differential reactor and is shown below.
(a) What species are on the surface?
(b) What does Figure B tell you about the reversibility and what’s adsorbed on the surface?
(c) Derive the rate law and suggest a rate-liming step consistent with the above figures.
(d) How would you plot your data to linearize the initial rate data in Figure A?
(e) Assuming pure A is fed, and the adsorption constants for A and C are KA = 0.5 atm–1 and KC = 0.25 atm–1 respectively, at what conversion are the number of sites with A adsorbed on the surface and C adsorbed on the surface equal? (Ans.: X = 0.66)
P10-5A The rate law for the hydrogenation (H) of ethylene (E) to form ethane (A) over a cobalt-molybdenum catalyst [Collection Czech. Chem. Commun., 51, 2760 (1988)] is
(a) Suggest a mechanism and rate-limiting step consistent with the above rate law.
(b) What was the most difficult part in finding the mechanism?
P10-6B The formation of propanol on a catalytic surface is believed to proceed by the following mechanism
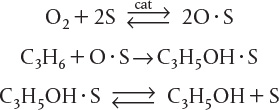
Suggest a rate-limiting step and derive a rate law.
P10-7B The dehydration of n-butyl alcohol (butanol) over an alumina-silica catalyst was investigated by J. F. Maurer (Ph.D. thesis, University of Michigan). The data in Figure P10-7B were obtained at 750°F in a modified differential reactor. The feed consisted of pure butanol.
(a) Suggest a mechanism and rate-limiting step that are consistent with the experimental data.
(b) Evaluate the rate-law parameters.
(c) At the point where the initial rate is a maximum, what is the fraction of vacant sites? What is the fraction of occupied sites by both A and B? % vacant = 0.41
(d) What generalizations can you make from studying this problem?
(e) Write a question that requires critical thinking and then explain why your question requires critical thinking. (Hint: See Preface Section G.)
(f) Apply one or more of the six ideas in Preface Table P-4, page xxvii, to this problem.
P10-8B The catalytic dehydration of methanol (ME) to form dimethyl ether (DME) and water was carried out over an ion exchange catalyst [K. Klusacek, Collection Czech. Chem. Commun., 49, 170 (1984)]. The packed bed was initially filled with nitrogen, and at t = 0. The N2 feed is switched to pure methanol vapor entering the reactor at 413 K, 100 kPa, and 0.2 cm3/s. The following partial pressures were recorded at the exit to the differential reactor containing 1.0 g of catalyst in 4.5 cm3 of reactor volume.
| t(s) | |||||||||
| 0 | 10 | 50 | 100 | 150 | 200 | 300 | |||
| PN2 (kPa) | 100 | 50 | 10 | 2 | 0 | 0 | 0 | ||
| PME (kPa) | 0 | 2 | 15 | 23 | 25 | 26 | 26 | ||
| PH2O (kPa) | 0 | 10 | 15 | 30 | 35 | 37 | 37 | ||
| PDME (kPa) | 0 | 38 | 60 | 45 | 40 | 37 | 37 | ||
Use parts (a) through (f) to lead you to suggest a mechanism, rate-limiting step, and rate law consistent with this data.
(a) Using the data above, sketch the exit concentrations as a function of time.
(b) Which species took longer than others to exit the reactor in the gas phase? What could have caused this difference in exit times?
(c) What species are adsorbed on the surface?
(d) Are any species not adsorbed on the surface? If so, which ones?
(e) Which set of figures, (1)-(4) below, correctly describes the func t ionality of the chemical reaction rate with the partial pressures PW, PDME, and PME?
(f) Derive a rate law for the catalytic dehydration of methanol. Dimethyl Either
P10-9B In 1981, the U.S. government put forth the following plan for automobile manufacturers to reduce emissions from automobiles over the next few years.
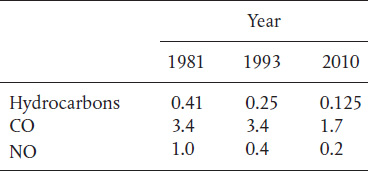

All values are in grams per mile. An automobile emitting 3.74 lbm of CO and 0.37 lbm of NO on a journey of 1000 miles would meet the current government requirements.
To remove oxides of nitrogen (assumed to be NO) from automobile exhaust, a scheme has been proposed that uses unburned carbon monoxide (CO) in the exhaust to reduce the NO over a solid catalyst, according to the reaction
Experimental data for a particular solid catalyst indicate that the reaction rate can be well represented over a large range of temperatures by
where
| PN = gas-phase partial pressure of NO |
| PC = gas-phase partial pressure of CO |
| k, K1, K2 = coefficients depending only on temperature |
(a) Propose an adsorption–surface reaction–desorption mechanism and rate-limiting step that are consistent with the experimentally observed rate law. Do you need to assume any species are weakly adsorbed to get agreement with Equation (P10-9.1)?
(b) A certain engineer thinks that it would be desirable to operate with a very large stoichiometric excess of CO to minimize catalytic reactor volume. Do you agree or disagree? Explain.
(c) What would be the relevance of the problem if everyone were driving a hybrid by 2018? A driverless car by 2020?
P10-10B Methyl ethyl ketone (MEK) is an important industrial solvent that can be produced from the dehydrogenation of butan-2-ol (Bu) over a zinc oxide catalyst [Ind. Eng. Chem. Res., 27, 2050 (1988)]:
The following data giving the reaction rate for MEK were obtained in a differential reactor at 490°C.
| PBu (atm) | 2 | 0.1 | 0.5 | 1 | 2 | 1 |
| PMEK (atm) | 5 | 0 | 2 | 1 | 0 | 0 |
| PH2 (atm) | 0 | 0 | 1 | 1 | 0 | 10 |
| 0.044 | 0.040 | 0.069 | 0.060 | 0.043 | 0.059 |
(a) Suggest a rate law consistent with the experimental data.
(b) Suggest a reaction mechanism and rate-limiting step consistent with the rate law. Hint: Some species might be weakly adsorbed.
(c) Apply one or more of the six ideas in Preface Table P-4, page xxvii, to this problem.
(d) Plot conversion (up to 90%) and reaction rate as a function of catalyst weight for an entering molar flow rate of pure butan-2-ol of 10 mol/min at an entering pressure P0 = 10 atm up to a catalyst weight Wmax 23 kg.
(e) Write a question that requires critical thinking and then explain why your question requires critical thinking. (Hint: See Preface Section G.)
(f) Repeat part (d), accounting for pressure drop and 0.03 kg–1. Plot p and X as a function of catalyst weight down the reactor.
P10-11B Cyclohexanol was passed over a catalyst to form water and cyclohexene:
It is suspected that the reaction may involve a dual-site mechanism, but it is not known for certain. It is believed that the adsorption equilibrium constant for cyclohexanol is around 1.0 and is roughly one or two orders of magnitude greater than the adsorption equilibrium constants for the other compounds. Using these data:
TABLE P10-118 DATA FOR CATALYTIC FORMATION OF CYCLOHEXENE
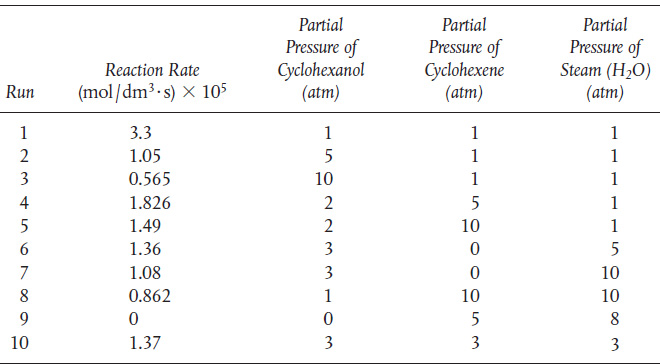
(a) Suggest a rate law and mechanism consistent with the data given here.
(b) Determine the values of the rate-law parameters. (Ind. Eng. Chem. Res., 32, 2626–2632.)
(c) Why do you think estimates of the rate-law parameters were given?
(d) For an entering molar flow rate of cychlohexanol of 10 mol/s at a partial pressure of 15 atm, what catalyst weight is necessary to achieve 85% conversion when the bulk density is 1500 gm/dm3?
P10-12B Experimental data for the gas-phase catalytic reaction
is shown below. The limiting step in the reaction is known to be irreversible, so that the overall reaction is irreversible. The reaction was carried out in a differential reactor to which A, B, and C were all fed.
Run Number |
PA (atm) |
PB (atm) |
PC (atm) |
Reaction rate (mol)/(g-cat • s) |
1 |
1 |
1 |
2 |
0.114 |
2 |
1 |
10 |
2 |
1.140 |
3 |
10 |
1 |
2 |
0.180 |
4 |
1 |
20 |
2 |
2.273 |
5 |
1 |
20 |
10 |
0.926 |
6 |
20 |
1 |
2 |
0.186 |
7 |
0.1 |
1 |
2 |
0.0243 |
(a) Suggest a rate law consistent with the experimental data. Hint: Sketch () as a function of PA, as a function of PB, and as a function of PC.
(b) From your rate expression, which species can you conclude are adsorbed on the surface?
(c) Suggest a rate law and then show that your mechanism is constant with the rate law in part (a)
(d) For an entering partial pressure of A of 2 atm in a PBR, what is the ratio of A to C sites at 80% conversion of A?
(e) At what conversion are the number of A and C sites equal? (Ans.: X = 0.235)
(f) What reactor volume is necessary to achieve 90% conversion of A for a stoichiometric feed and flow of A 2 mol/s? (Ans.: W = 8.9 g-cat)
If necessary, feel free to use none, any, or all of the following parameter values:
P10-13B Solar Energy Capture: Water Splitting. Hydrogen and O2 can be combined in fuel cells to generate electricity. Solar energy can be used to split water to generate the raw reactants H2 and O2 for fuel cells. One method of solar thermal reduction is with NiFe2O4 in the sequence

We note NiFe2O4 is regenerated in this process.34
(a) Derive a rate law for Step (2), assuming that water adsorbs on the solid solution as a single-site mechanism and that the reaction is irreversible.
(b) Repeat (a) when the reaction is reversible and the solid solution adsorption site for water is (S’) different than the NiFe2O4 site for the adsorption of H2, (S).
(c) How would your rate law change if we included Step 1?
P10-14A Vanadium oxides are of interest for various sensor applications, owing to the sharp metal–insulator transitions they undergo as a function of temperature, pressure, or stress. Vanadium triisopropoxide (VTIPO) was used to grow vanadium oxide films by chemical vapor deposition [J. Electrochem. Soc., 136, 897 (1989)]. The deposition rate as a function of VTIPO pressure for two different temperatures follows.
T = 120°C:
Growth Rate (μm/h) |
0.004 |
0.015 |
0.025 |
0.04 |
0.068 |
0.08 |
0.095 |
0.1 |
VTIPO Pressure (torr) |
0.1 |
0.2 |
0.3 |
0.5 |
0.8 |
1.0 |
1.5 |
2.0 |
T = 200°C:
Growth Rate (μm/h) |
0.025 |
0.045 |
1.8 |
2.8 |
7.2 |
VTIPO Pressure (torr) |
0.05 |
0.2 |
0.4 |
0.5 |
0.8 |
In light of the material presented in this chapter, analyze the data and describe your results. Specify where additional data should be taken.
P10-15A Titanium dioxide is a wide-bandgap semiconductor that is showing promise as an insulating dielectric in VLSI capacitors and for use in solar cells. Thin films of TiO2 are to be prepared by chemical vapor deposition from gaseous titanium tetraisopropoxide (TTIP). The overall reaction is
The reaction mechanism in a CVD reactor is believed to be [K. L. Siefering and G. L. Griffin, J. Electro-chem. Soc., 137, 814 (1990)]
where I is an active intermediate and P1 is one set of reaction products (e.g., H2O, C3H6), and P2 is another set. Assuming the homogeneous gas-phase reaction for TTIP is in equilibrium, derive a rate law for the deposition of TiO2. The experimental results show that at 200 C the reaction is second order at low partial pressures of TTIP and zero order at high partial pressures, while at 300 C the reaction is second order in TTIP over the entire pressure range. Discuss these results in light of the rate law you derived.
P10-16B The dehydrogenation of methylcyclohexane (M) to produce toluene (T) was carried out over a 0.3% Pt/Al2O3 catalyst in a differential catalytic reactor. The reaction is carried out in the presence of hydrogen (H2) to avoid coking [J. Phys. Chem., 64, 1559 (1960)].
(a) Determine the model parameters for each of the following rate laws.
(1)
(2)
(3)
(4)
Use the data in Table P10-16B below.
(b) Which rate law best describes the data? Hint: Neither KH2 or KM can take on negative values.
(c) Where would you place additional data points?
(d) Suggest a mechanism and rate-limiting step consistent with the rate law you have chosen.
TABLE P10-16B DEHYDROGENATION OF METHYLCYCLOHEXANE
PH2 |
PM (atm) |
|
1 |
1 |
1.2 |
1.5 |
1 |
1.25 |
0.5 |
1 |
1.30 |
0.5 |
0.5 |
1.1 |
1 |
0.25 |
0.92 |
0.5 |
0.1 |
0.64 |
3 |
3 |
1.27 |
1 |
4 |
1.28 |
3 |
2 |
1.25 |
4 |
1 |
1.30 |
0.5 |
0.25 |
0.94 |
2 |
0.05 |
0.41 |
P10-17A Sketch qualitatively the reactant, product, and activity profiles as a function of length at various times for a packed-bed reactor for each of the following cases. In addition, sketch the effluent concentration of A as a function of time. The reaction is a simple isomerization:
(a) Rate law:
Decay law: rd = kdaCA
Case I: ≪ kd k, Case II: kd = k, Case III: kd ≫ k
(b)
(c)
(d) Sketch similar profiles for the rate laws in parts (a) and (c) in a moving-bed reactor with the solids entering at the same end of the reactor as the reactant.
(e) Repeat part (d) for the case where the solids and the reactant enter at opposite ends.
P10-18B The elementary irreversible gas-phase catalytic reaction
is to be carried out in a moving-bed reactor at constant temperature. The reactor contains 5 kg of catalyst. The feed is stoichiometric in A and B. The entering concentration of A is 0.2 mol/dm3. The catalyst decay law is zero order with kD = 0.2 s–1 and k = 1.0 dm6/(mol ⋅ kg-cat ⋅ s) and the volumetric flow rate is υ0 = 1 dm3/s.
(a) What conversion will be achieved for a catalyst feed rate of 0.5 kg/s? (Ans.: X = 0.2)
(b) Sketch the catalyst activity as a function of catalyst weight (i.e., distance) down the reactor length for a catalyst feed rate of 0.5 kg/s.
(c) What is the maximum conversion that could be achieved (i.e., at an infinite catalyst loading rate)?
(d) What catalyst loading rate is necessary to achieve 40% conversion? (Ans.: US = 1.5 kg/s)
(e) At what catalyst loading rate (kg/s) will the catalyst activity be exactly zero at the exit of the reactor?
(f) What does an activity of zero mean? Can catalyst activity be less than zero?
(g) How would your answer in part (a) change if the catalyst and reactant were fed at opposite ends? Compare with part (a).
(h) Now consider the reaction to be zero order with
k = 0.2 mol/kg-cat ⋅ min.
The economics:
• The product sells for $160 per gram mole.
• The cost of operating the bed is $10 per kilogram of catalyst exiting the bed.
What is the feed rate of solids (kg/min) that will give the maximum profit? (Ans.: Us = 4 kg/min.) (Note: For the purpose of this calculation, ignore all other costs, such as the cost of the reactant, the cost to the company of providing free lunches to workers, etc.)
P10-19B With the increasing demand for xylene in the petrochemical industry, the production of xylene from toluene disproportionation has gained attention in recent years [Ind. Eng. Chem. Res., 26, 1854 (1987)]. This reaction,
was studied over a hydrogen mordenite catalyst that decays with time. As a first approximation, assume that the catalyst follows second-order decay
and the rate law for low conversions is
with kT 20 g mol/h . kg-cat.atm and kd = 1.6 h-1 at 735 K.
(a) Compare the conversion-time curves in a batch reactor containing 5 kg-cat at different initial partial pressures (1 atm, 10 atm, etc.). The reaction volume containing pure toluene initially is 1 dm3 and the temperature is 735 K.
(b) What conversion can be achieved in a moving-bed reactor containing 50 kg of catalyst with a catalyst feed rate of 2 kg/h? Toluene is fed at a pressure of 2 atm and a rate of 10 mol/min.
(c) Explore the effect of catalyst feed rate on conversion.
(d) Suppose that ET = 25 kcal/mol and Ed = 10 kcal/mol. What would the temperature–time trajectory look like for a CSTR? What if ET 10 kcal/mol and Ed = 25 kcal/mol?
(e) The decay law more closely follows the equation
with kd = 0.2 atm-2 h-1. Redo parts (b) and (c) for these conditions.
P10-20A The vapor-phase cracking of gas-oil in Example 10-6 is carried out over a different catalyst, for which the rate law is
(a) Assuming that you can vary the entering pressure and gas velocity, what operating conditions would you recommend?
(b) What could go wrong with the conditions you chose?
Now assume the decay law is
where the concentration, Ccoke, in mol/dm3, can be determined from a stoichiometric table.
(c) For a temperature of 400°C and a reactor height of 15m, what gas velocity do you recommend? Explain. What is the corresponding conversion?
(d) The reaction is now to be carried in an STTR 15 m high and 1.5 m in diameter. The gas velocity is 2.5 m/s. You can operate in the temperature range between 100 and 500 C. What temperature do you choose, and what is the corresponding conversion?
(e) What would the temperature–time trajectory look like for a CSTR?
Additional information:
ER = 3000 cal/mol
ED = 15,000 cal/mol
P10-21C When the impurity cumene hydroperoxide is present in trace amounts in a cumene feed stream, it can deactivate the silica-alumina catalyst over which cumene is being cracked to form benzene and propylene. The following data were taken at 1 atm and 420°C in a differential reactor. The feed consists of cumene and a trace (0.08 mol %) of cumene hydroperoxide (CHP).
Benzene in Exit Stream (mol %) |
2 |
1.62 |
1.31 |
1.06 |
0.85 |
0.56 |
0.37 |
0.24 |
t (s) |
0 |
50 |
100 |
150 |
200 |
300 |
400 |
500 |
(a) Determine the order of decay and the decay constant. (Ans.: kd = 4.27 10-3 × S-1.)
(b) As a first approximation (actually a rather good one), we shall neglect the denominator of the catalytic rate law and consider the reaction to be first order in cumene. Given that the specific reaction rate with respect to cumene is k = 3.8 × 10-3 mol/kg fresh cat .s.atm, the molar flow rate of cumene (99.92% cumene, 0.08% CHP) is 200 mol/min, the entering concentration is 0.06 kmol/m3, the catalyst weight is 100 kg, and the velocity of solids is 1.0 kg/min, what conversion of cumene will be achieved in a moving-bed reactor?
P10-22C The decomposition of spartanol to wulfrene and CO2 is often carried out at high temperatures [J. Theor. Exp., 15, 15 (2014)]. Consequently, the denominator of the catalytic rate law is easily approximated as unity, and the reaction is first order with an activation energy of 150 kJ/mol. Fortunately, the reaction is irreversible. Unfortunately, the catalyst over which the reaction occurs decays with time on stream. The following conversion-time data were obtained in a differential reactor:
For T 500 K:
t (days) |
0 |
20 |
40 |
60 |
80 |
120 |
X (%) |
1 |
0.7 |
0.56 |
0.45 |
0.38 |
0.29 |
t (days) |
0 |
5 |
10 |
15 |
20 |
30 |
40 |
X (%) |
2 |
1.2 |
0.89 |
0.69 |
0.57 |
0.42 |
0.33 |
(a) If the initial temperature of the catalyst is 480 K, determine the temperature–time trajectory to maintain a constant conversion.
(b) What is the catalyst lifetime?
P10-23B The hydrogenation of ethylbenzene to ethylcyclohexane over a nickel-mordenite catalyst is zero order in both reactants up to an ethylbenzene conversion of 75% [Ind. Eng. Chem. Res., 28 (3), 260 (1989)]. At 553 K, k 5.8 mol ethylbenzene/(dm3 of catalyst.h). When a 100-ppm thiophene concentration entered the system, the ethylbenzene conversion began to drop.
Time (h) |
0 |
1 |
2 |
4 |
6 |
8 |
12 |
Conversion |
0.92 |
0.82 |
0.75 |
0.50 |
0.30 |
0.21 |
0.10 |
The reaction was carried out at 3 MPa and a molar ratio of H2/ETB = 10. Discuss the catalyst decay. Be quantitative where possible.
Supplementary Reading
1. A terrific discussion of heterogeneous catalytic mechanisms and rate-controlling steps may or may not be found in
BURGESS, THORNTON W., The Adventures of Grandfather Frog. New York: Dover Publications, Inc., 1915.
MASEL, R. I., Principles of Adsorption and Reaction on Solid Surfaces. New York: Wiley, 1996. A great reference.
SOMORJAI, G. A., Introduction to Surface Chemistry and Catalysis. New York: Wiley, 1994.
2. A truly excellent discussion of the types and rates of adsorption together with techniques used in measuring catalytic surface areas is presented in
MASEL, R. I., Principles of Adsorption and Reaction on Solid Surfaces. New York: Wiley, 1996.
3. Techniques for discriminating between mechanisms and models can be found in
BOX, G. E. P., J. S. HUNTER, and W. G. HUNTER, Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed. Hoboken, New Jersey: Wiley, 2005.
4. Examples of applications of catalytic principles to microelectronic manufacturing can be found in
BUTT, JOHN B., Reaction Kinetics and Reactor Design. Second Edition, Revised and Expanded. New York: Marcel Dekker, Inc., 1999.
DOBKIN, D. M., AND M. K. ZURAW. Principles of Chemical Vapor Deposition. The Netherlands: Kluwer Academic Publishers, 2003.
19 H. Ishii and Y. Takahashi, J. Electrochem. Soc., 135, p. 1539.
20 See G. F. Froment and K. B. Bishoff, Chemical Reaction Analysis and Design, 2nd ed. (New York: Wiley, 1990), p. 96.
21 J. B. Butt and E. E. Petersen, Activation, Deactivation and Poisoning of Catalysts (New York: Academic Press, 1988).
22 D. T. Lynch and G. Emig, Chem. Eng. Sci., 44(6), 1275–1280 (1989).
23 R. Hughes, Deactivation of Catalysts (San Diego: Academic Press, 1984).
24 See G. C. Kuczynski, ed., Sintering and Catalysis, vol. 10 of Materials Science Research
(New York: Plenum Press, 1975).
25 R. Hughes, Deactivation of Catalysts (San Diego: Academic Press, 1984).
26 A. Voorhies, Ind. Eng. Chem., 37, 318 (1945).
27 C. O. Prater and R. M. Lago, Adv. Catal., 8, 293 (1956).
28 M. A. Pacheco and E. E. Petersen, J. Catal., 86, 75 (1984).
29 R. J. Farrauto and C. H. Bartholomew, Fundamentals of Industrial Catalytic Processes, 2nd edition (New York: Blackie Academic and Professional, 2006). This book is one of the most definitive resources on catalyst decay.
30 Ibid.
31 Ibid.
32 Estimated from V. W. Weekman and D. M. Nace, AIChE J., 16, 397 (1970).
33 Ibid.
34 Scheffe, J. R., J. Li, and A. W. Weimer, “A Spinel Ferrite/Hercynite Water-Splitting Redox Cycle,” International Journal of Hydrogen Energy, 35, 3333–3340 (2010).