C Thermodynamic Relationships Involving the Equilibrium Constant1
For the gas-phase reaction
1. The true (dimensionless) equilibrium constant
where ai is the activity of species i
where fi = fugacity of species i
= fugacity of species i at the standard state. For gases, the standard state is 1 bar, which is in the noise level of 1 atm, so we will use atm.
where γi is the activity coefficient
K = True equilibrium constant
Ky = Activity equilibrium constant
Kp = Pressure equilibrium constant
Kc = Concentration equilibrium constant

Kγ has units of
Kp has units of
For ideal gases Kγ = 1.0 atm-δ
2. For the generic reaction (2-2), the pressure equilibrium constant KP is
3. For the generic reaction (2-2), the concentration equilibrium constant
It is important to be able to relate K, Kγ, Kc, and Kp.
4. For ideal gases, Kc and Kp are related by
Where for the generic reaction (2-2),
5. KP is a function of temperature only, and the temperature dependence of KP is given by van’t Hoff’s equation:
Van’t Hoff’s equation
(http://www.umich.edu/~elements/5e/11chap/summary-biovan.html).
6. Integrating, we have
Kp and Kc are related by
then
7. Kp neglecting ΔCp. Given the equilibrium constant at one temperature, T1, KP (Ti), and the heat of reaction, , the partial pressure equilibrium constant at any temperature T is
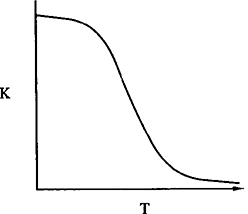
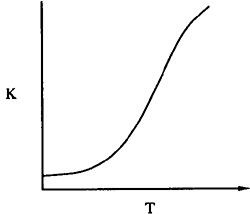
8. From Le Châtelier’s principle we know that for exothermic reactions, the equilibrium shifts to the left (i.e., K and Xe decrease) as the temperature increases. Figures C-1 and C-2 show how the equilibrium constant varies with temperature for an exothermic reaction and for an endothermic reaction, respectively.
Variation of equilibrium constant with temperature
9. The equilibrium constant for the reaction (2-2) at temperature T can be calculated from the change in the Gibbs free energy using
10. Tables that list the standard Gibbs free energy of formation of a given species are available in the literature (webbook.nist.gov).
11. The relationship between the change in Gibbs free energy and enthalpy, H, and entropy, S, is
See bilbo.chm.uri.edu/CHM112/lectures/lecture31.htm. An example on how to calculate the equivalent conversion for AG is given on the Web site.
Example C-1 Water-Gas Shift Reaction
The water-gas shift reaction to produce hydrogen
is to be carried out at 1000 K and 10 atm. For an equimolar mixture of water and carbon monoxide, calculate the equilibrium conversion and concentration of each species.
Data: At 1000 K and 10 atm, the Gibbs free energies of formation are .
Solution
We first calculate the equilibrium constant. The first step in calculating K is to calculate the change in Gibbs free energy for the reaction. Applying Equation (C-10) gives us
then
Expressing the equilibrium constant first in terms of activities and then finally in terms of concentration, we have
where ai is the activity, fi is the fugacity, γi is the activity coefficient (which we shall take to be 1.0 owing to high temperature and low pressure), and γi is the mole fraction of species i.2 Substituting for the mole fractions in terms of partial pressures gives
Calculate
Calculate K
In terms of conversion for an equimolar feed, we have

Figure EC-1.1 Modell, Michael, and Reid, Robert, Thermodynamics and Its Applications, 2nd ed., © 1983. Reprinted and electronically reproduced by permission of Pearson Education, Inc., Upper Saddle River, NJ.
Relate K and Xe
From Figure EC-1.1 we read at 1000 K that log KP = 0.15; therefore, KP = 1.41, which is close to the calculated value. We note that there is no net change in the number of moles for this reaction (i.e., δ = 0); therefore,
K = Kp = Kc (dimensionless)
Taking the square root of Equation (EC-1.8) yields
Solving for Xe , we obtain
Then

Figure EC-1.1 gives the equilibrium constant as a function of temperature for a number of reactions. Reactions in which the lines increase from left to right are exothermic.
The following links give thermochemical data. (Heats of Formation, CP, etc.)
1) www.uic.edu/~mansoori/Thermodynamic.Data.and.Propertyjhtml
Also see Chem. Tech., 28 (3) (March), 19 (1998).
Calculate Xe, the equilibrium conversion
Calculate CCO,e, the equilibrium conversion of CO

Links
1 For the limitations and for further explanation of these relationships, see, for example, K. Denbigh, The Principles of Chemical Equilibrium, 3rd ed. (Cambridge: Cambridge University Press, 1971), p. 138.
2 See Chapter 17 in J. R. Elliott and C. T. Lira, Introductory Chemical Engineering Thermodynamics, 2nd ed. (Upper Saddle River, NJ: Prentice Hall, 2012), and Chapter 16 in J. W. Tester and M. Modell, Thermodynamics and Its Applications, 3rd ed. (Upper Saddle River, NJ: Prentice Hall, 1997).