2. Conversion and Reactor Sizing
Be more concerned with your character than with your reputation, because character is what you really are while reputation is merely what others think you are.
—John Wooden, former head coach, UCLA Bruins
2.1 Definition of Conversion
Conversion helps us measure how far a reaction has progressed. In defining conversion, we choose one of the reactants as the basis of calculation and then relate the other species involved in the reaction to this basis. In virtually all instances, we must choose the limiting reactant as the basis of calculation. For single reactions, we can use conversion to develop the stoichiometric relationships and design equations by considering the general reaction
The uppercase letters represent chemical species, and the lowercase letters represent stoichiometric coefficients. We shall choose species A as our limiting reactant and, thus, our basis of calculation. The limiting reactant is the reactant that will be completely consumed first after the reactants have been mixed. We next divide the reaction expression through by the stoichiometric coefficient of the limiting reactant, that is, of species A, in order to arrange the reaction stoichiometry in the form
“A” is our basis of calculation
to put every quantity on a “per mole of A” basis, our limiting reactant.
Now we ask such questions as “How can we quantify how far a reaction (e.g., Equation (2-2)) proceeds to the right?” or “How many moles of C are formed for every mole of A consumed?” A convenient way to answer these questions is to define a parameter called conversion. The conversion XA is the number of moles of A that have reacted per mole of A fed to the system:
Definition of X
Because we are defining conversion with respect to our basis of calculation (A in Equation (2-2)), we eliminate the subscript A for the sake of brevity and let X ≡ XA. For irreversible reactions, the maximum conversion is 1.0, that is, complete conversion. For reversible reactions, the maximum conversion is the equilibrium conversion Xe (i.e., Xmax = Xe). We will take a closer look at equilibrium conversion in Section 4.3.
2.2 Batch Reactor Design Equations
In most batch reactors (BRs), the longer a reactant stays in the reactor, the more the reactant is converted to product until either equilibrium is reached or the reactant is exhausted. Consequently, in batch systems, the conversion X is a function of the time the reactants spend in the reactor. If NA0 is the number of moles of A initially present in the reactor (i.e., t = 0), then the total number of moles of A that have reacted (i.e., have been consumed) after a time t is [NA0X].
[Moles of A reacted (consumed)] [Moles of A fed] ·
Now, the number of moles of A that remain in the reactor after a time t, NA, can be expressed in terms of NA0 and X:
The number of moles of A in the reactor after a conversion X has been achieved is
Moles of A in the reactor at a time t
When no spatial variations in reaction rate exist, the mole balance on species A for a batch system is given by the following equation (cf. Equation (1-5)):
This equation is valid whether or not the reactor volume is constant. In the general reaction, Equation (2-2), reactant A is disappearing; therefore, we multiply both sides of Equation (2-5) by –1 to obtain the mole balance for the batch reactor in the form
The rate of disappearance of A, –rA, in this reaction might be given by a rate law similar to Equation (1-2), such as –rA = kCACB.
For batch reactors, we are interested in determining how long to leave the reactants in the reactor to achieve a certain conversion X. To determine this length of time, we write the mole balance, Equation (2-5), in terms of conversion by differentiating Equation (2-4) with respect to time, remembering that NA0 is the number of moles of A initially present in the reactor and is therefore a constant with respect to time.
Combining the above with Equation (2-5) yields
For a batch reactor, the design equation in differential form is
Batch reactor (BR) design equation
We call Equation (2-6) the differential form of the BR mole balance and it is often referred to as the design equation for a batch reactor because we have written the mole balance in terms of conversion. The differential forms of the batch reactor mole balances, Equations (2-5) and (2-6), are often used in the interpretation of reaction rate data (Chapter 7) and for reactors with heat effects (Chapters 11–13), respectively. Batch reactors are frequently used in industry for both gas-phase and liquid-phase reactions. The laboratory bomb calorimeter reactor is widely used for obtaining reaction rate data. Liquid-phase reactions are frequently carried out in batch reactors when small-scale production is desired or operating difficulties rule out the use of continuous-flow systems.
Uses of a BR
To determine the time to achieve a specified conversion X, we first separate the variables in Equation (2-6) as follows:
Batch time t to achieve a conversion X
Next, this equation is integrated with the limits that the reaction begins at time equals zero where there is no conversion initially (i.e., when t = 0, X = 0) and ends at time t when a conversion X is achieved (i.e., when t = t, then X = X). Carrying out the integration, we obtain the time t necessary to achieve a conversion X in a batch reactor
The longer the reactants are left in the reactor, the greater the conversion will be. Equation (2-6) is the differential form of the design equation, and Equation (2-7) is the integral form of the design equation for a batch reactor.
2.3 Design Equations for Flow Reactors
For a batch reactor, we saw that conversion increases with time spent in the reactor. For continuous-flow systems, this time usually increases with increasing reactor volume; for example, the bigger/longer the reactor, the more time it will take the reactants to flow completely through the reactor and thus, the more time to react. Consequently, the conversion X is a function of reactor volume V. If FA0 is the molar flow rate of species A fed to a system operated at steady state, the molar rate at which species A is reacting within the entire system will be FA0X.
The molar flow rate of A leaving the system FA equals the molar feed rate of A into the system FA0 minus the rate of reaction of A within the system (FA0X). The preceding sentence can be expressed mathematically as
Rearranging gives
The entering molar flow rate of species A, FA0 (mol/s), is just the product of the entering concentration, CA0 (mol/dm3), and the entering volumetric flow rate, υ0(dm3/s).
For liquid systems, the volumetric flow rate, υ, is constant and equal to υ0, and CA0 is commonly given in terms of molarity, for example, CA0 = 2 mol/dm3.
Liquid phase
For gas systems, CA0 can be calculated from the entering mole fraction, yA0, the temperature, T0, and pressure, P0, using the ideal gas law or some other gas law. For an ideal gas (see Appendix B):
Gas phase
Now that we have a relationship [Equation (2-8)] between the molar flow rate and conversion, it is possible to express the design equations (i.e., mole balances) in terms of conversion for the flow reactors examined in Chapter 1.
2.3.1 CSTR (Also Known as a Backmix Reactor or a Vat)
Recall that the CSTR is modeled as being well mixed such that there are no spatial variations in the reactor. For the general reaction
the CSTR mole balance Equation (1-7) can be arranged to
We now substitute for FA in terms of FA0 and X
and then substitute Equation (2-12) into (2-11)
Simplifying, we see that the CSTR volume necessary to achieve a specified conversion X is

Because the reactor is perfectly mixed, the exit composition from the reactor is identical to the composition inside the reactor, and, therefore, the rate of reaction, –rA, is evaluated at the exit conditions.
Evaluate –rA at the CSTR exit conditions!!
2.3.2 Tubular Flow Reactor (PFR)
We model the tubular reactor as having the fluid flowing in plug flow—that is, no radial gradients in concentration, temperature, or reaction rate.1 As the reactants enter and flow axially down the reactor, they are consumed and the conversion increases along the length of the reactor. To develop the PFR design equation, we first multiply both sides of the tubular reactor design equation given in Chapter 1, Equation (1-12), by –1. We then express the mole balance equation for species A in the reaction as
1 This constraint will be removed when we extend our analysis to nonideal (industrial) reactors in Chapters 16–18.
For a flow system, FA has previously been given in terms of the entering molar flow rate FA0 and the conversion X
Differentiating
dFA = –FA0 dX
and substituting into Equation (2-14) gives the differential form of the design equation for a plug-flow reactor (PFR)
We now separate the variables and integrate with the limits V = 0 when X = 0 to obtain the plug-flow reactor volume necessary to achieve a specified conversion X
To carry out the integrations in the batch and plug-flow reactor design equations (2-7) and (2-16), as well as to evaluate the CSTR design equation (2-13), we need to know how the reaction rate –rA varies with the concentration (hence conversion) of the reacting species. This relationship between reaction rate and concentration is developed in Chapter 3.
2.3.3 Packed-Bed Reactor (PBR)
Packed-bed reactors are tubular reactors filled with catalyst particles. In PBRs, it is the weight of catalyst W that is important, rather than the reactor volume. The derivation of the differential and integral forms of the design equations for packed-bed reactors are analogous to those for a PFR (cf. Equations (2-15) and (2-16)). That is, substituting Equation (2-12) for FA in Equation (1-15) gives
PBR design equation
The differential form of the design equation (i.e., Equation (2-17)) must be used when analyzing reactors that have a pressure drop along the length of the reactor. We discuss pressure drop in packed-bed reactors in Chapter 5.†
In the absence of pressure drop, that is, ΔP = 0, we can integrate Equation (2-17) with limits X = 0 at W = 0, and when W = W then X = X to obtain

Equation (2-18) can be used to determine the catalyst weight W (i.e., mass) necessary to achieve a conversion X when the total pressure remains constant.
2.4 Sizing Continuous-Flow Reactors
In this section, we are going to show how we can size CSTRs and PFRs (i.e., determine their reactor volumes) using the appropriate mole balance in terms of conversion and knowledge of the rate of reaction, –rA, as a function of conversion, X (i.e., –rA = f(X)). The rate of disappearance of A, –rA, is almost always a function of the concentrations of the various species present (see Chapter 3). When only one reaction is occurring, each of the concentrations can be expressed as a function of the conversion X (see Chapter 4); consequently, –rA can be expressed as a function of X.
As an example, we shall choose a particularly simple functional dependence on concentration, that is, first-order.
–rA = kCA = kCA0 (1 – X)
Here, k is the specific reaction rate and is a function of temperature only, and CA0 is the entering concentration of A. We note in Equations (2-13) and (2-16) that the reactor volume is a function of the reciprocal of –rA. For this first-order dependence, a plot of the reciprocal rate of reaction (1/–rA) as a function of conversion yields a curve similar to the one shown in Figure 2-1, where
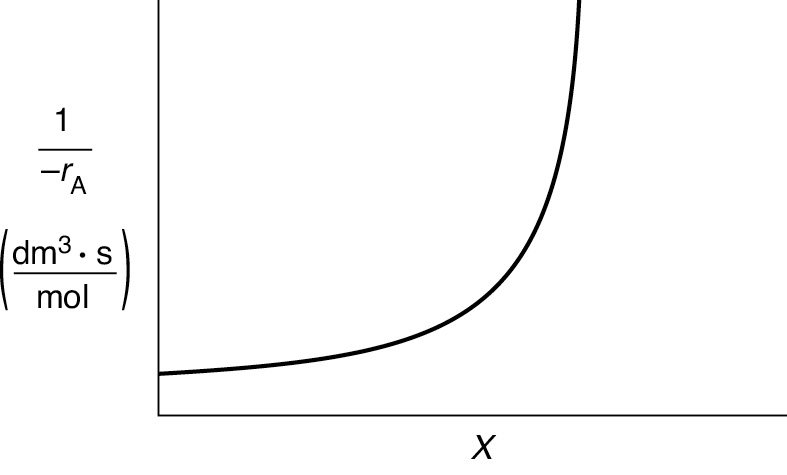
Figure 2-1 Typical curve of the reciprocal rate as a function of conversion.
† Be sure to watch the YouTube video (https://www.youtube.com/watch?v=S9mUAXmNqxs).
We can use Figure 2-1 to size CSTRs and PFRs for different entering flow rates. By sizing we mean either determine the reactor volume for a specified conversion or determine the conversion for a specified reactor volume. Before sizing flow reactors, let’s consider some insights. If a reaction is carried out isothermally, the rate is usually greatest at the start of the reaction when the concentration of reactant is greatest (i.e., when there is negligible conversion [X ≡ 0]). Hence, the reciprocal rate (1/–rA) will be small. Near the end of the reaction, when the reactant has been mostly used up and thus the concentration of A is small (i.e., the conversion is large), the reaction rate will be small. Consequently, the reciprocal rate (1/–rA) is large.
For all irreversible reactions of greater than zero order (see Chapter 3 for zero-order reactions), as we approach complete conversion where all the limiting reactant is used up, that is, X = 1, the reciprocal rate approaches infinity as does the reactor volume, that is,
A → B + C
Consequently, we see that an infinite reactor volume is necessary to reach complete conversion, X = 1.0.
“To infinity and beyond”—Buzz Lightyear
For reversible reactions (e.g., A ⇄ B), the maximum conversion is the equilibrium conversion Xe. At equilibrium, the reaction rate is zero (rA ≡ 0). Therefore,
A ⇄ B + C
and we see that an infinite reactor volume would also be necessary to obtain the exact equilibrium conversion, X = Xe. We will discuss Xe further in Chapter 4.
Examples of Reactor Design and Staging Given –rA = f(X)
To illustrate the design of continuous-flow reactors (i.e., CSTRs and PFRs), we consider the irreversible isothermal gas-phase isomerization
A → B
Researchers at the Central Lab performed experiments to determine the rate of chemical reaction as a function of the conversion of reactant A and the results are shown in Table 2-1. The temperature was constant at 500 K (440°F), the total pressure was constant at 830 kPa (8.2 atm), and the initial charge to the reactor was pure A. The entering molar flow of reactant A is FA0 = 0.4 mol/s.
TABLE 2-1 RAW DATA†

†Proprietary coded data courtesy of Jofostan Central Research Laboratory, Çölow, Jofostan, and published in Jofostan Journal of Chemical Engineering Research, Volume 21, page 73 (2023).
If we know –rA as a function of X, we can size any isothermal reaction system.
Recalling the CSTR and PFR design equations, (2-13) and (2-16), we see that the reactor volume varies directly with the molar flow rate FA0 and with the reciprocal of –rA, , for example, . Consequently, to size reactors, we first convert the raw data in Table 2-1, which gives –rA as a function of X first to as a function of X. Next, we multiply by the entering molar flow rate, FA0, to obtain as a function of X as shown in Table 2-2 of the processed data for FA0 = 0.4 mol/s.
TABLE 2-2 PROCESSED DATA
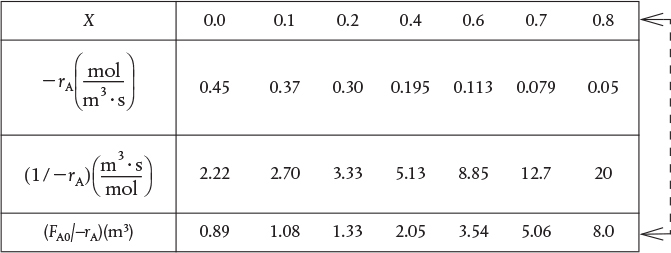
We shall use the data in this table for the next five Example Problems.
To size reactors for different entering molar flow rates, FA0, we would use rows 1 and 4, as shown by the arrows in Table 2-2 to construct the following figure:

Figure 2-2(a) Levenspiel plot of processed data 1 in Table 2-2.
However, for a given FA0, rather than using Figure 2-2(a) to size reactors, it is often more advantageous to plot as a function of X, which is called a Levenspiel plot.† We are now going to carry out a number of examples where we have specified the flow rate FA0 to be 0.4 mol A/s.
Plotting as a function of X using the data in Table 2-2 we obtain the plot shown in Figure 2-2(b).
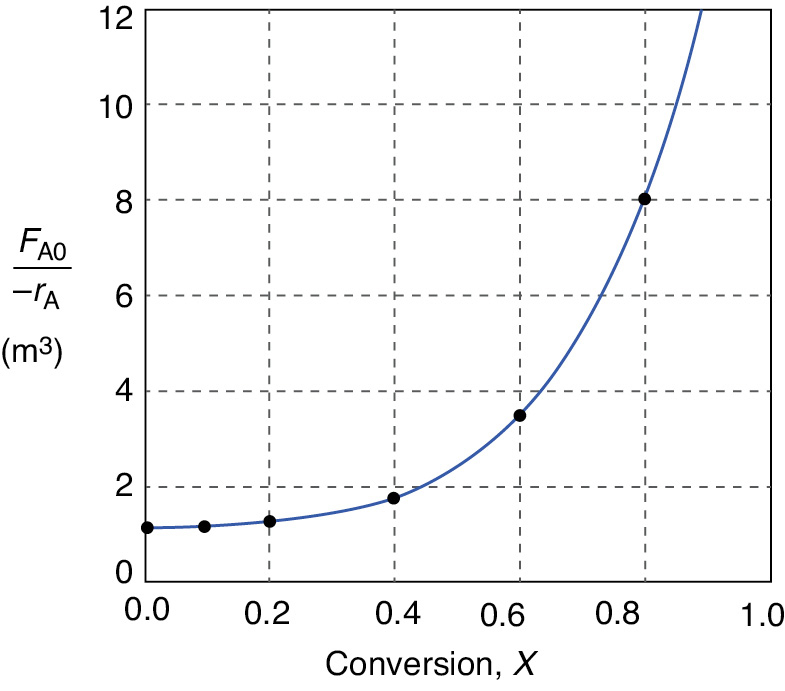
Figure 2-2(b) Levenspiel plot of processed data 2 in Table 2-2.
Levenspiel plot
We are now going to use the Levenspiel plot of the processed data (Figure 2-2(b)) to size a CSTR and a PFR.
†After Professor Octave Levenspiel (1/1/1926–3/5/2017), Oregon State University, Corvallis, OR.
The reaction described by the data in Table 2-2
A → B
is to be carried out in a CSTR. Species A enters the reactor at a molar flow rate of , which is the flow rate used to construct the Levenspiel plot in Figure 2-2(b).
Using the data in either Table 2-2 or Figure 2-2(b), calculate the volume necessary to achieve 80% conversion in a CSTR.
Shade the area in Figure 2-2(b) that would give the CSTR volume necessary to achieve 80% conversion.
Solutions
Equation (2-13) gives the volume of a CSTR as a function of FA0, X, and –rA
In a CSTR, the composition, temperature, and conversion of the effluent stream are identical to that of the fluid within the reactor, because perfect mixing is assumed. Therefore, we need to find the value of –rA (or reciprocal thereof) at X = 0.8. From either Table 2-2 or Figure 2-2(a), we see that when X = 0.8, then
Substitution into Equation (2-13) for an entering molar flow rate, FA0, of 0.4 mol A/s and X = 0.8 gives
V = 6.4 m3 = 6400 = dm3 = 6400 liters Answer
Shade the area in Figure 2-2(b) that yields the CSTR volume to achieve 80% conversion. Rearranging Equation (2-13) gives
In Figure E2-1.1, the volume is equal to the area of a rectangle with a height (FA0/–rA = 8 m3) and a base (X = 0.8). This rectangle is shaded in the figure.
V = Levenspiel rectangle area = height × width
V = [8 m3][0.8] = 6.4 m3 = 6400 dm3 = 6400 L Answer
Representative Industrial CSTR Dimensions

The CSTR volume necessary to achieve 80% conversion is 6.4 m3 when operated at 500 K, 830 kPa (8.2 atm), and with an entering molar flow rate of A of 0.4 mol/s. This volume corresponds to a reactor about 1.5 m in diameter and 3.6 m high. It’s a large CSTR, but this is a gas-phase reaction, and CSTRs are normally not used for gas-phase reactions. CSTRs are used primarily for liquid-phase reactions. Oops!

Figure E2-1.1 Levenspiel CSTR plot.
Plots of (FA0/=rA) versus X are often referred to as Levenspiel plots (after Prof. Octave Levenspiel).
Analysis: Given the conversion, the rate of reaction as a function of conversion along with the molar flow of the species A, we saw how to calculate the volume of a CSTR. From the data and information given, we calculated the volume to be 6.4 m3 for 80% conversion. We showed how to carry out this calculation using the design equation (2-13) and also using a Levenspiel plot.
The reaction described by the data in Tables 2-1 and 2-2 is to be carried out in a PFR. The entering molar flow rate of A is again, FA0 = 0.4 mol/s.
First, use one of the integration formulas given in Appendix A.4 to determine the PFR reactor volume necessary to achieve 80% conversion.
Next, shade the area in Figure 2-2(b) that would give the PFR volume necessary to achieve 80% conversion.
Finally, make a qualitative sketch of the conversion, X, and the rate of reaction, –rA, down the length (volume) of the reactor.
Solution
We start by repeating rows 1 and 4 of Table 2-2 to produce the results shown in Table 2-3
TABLE 2-3 PROCESSED DATA 2

(a) Numerically evaluate PFR volume. For the PFR, the differential form of the mole balance is
Rearranging and integrating gives
We shall use the five-point quadrature formula (Equation (A-23)) given in Appendix A.4 to numerically evaluate Equation (2-16). The five-point formula with a final conversion of 0.8 gives four equal segments between X = 0 and X = 0.8, with a segment length of . The function inside the integral is evaluated at X = 0, X = 0.2, X = 0.4, X = 0.6, and X = 0.8.
(E2-2.1)Using values of [FA0/(–rA)] corresponding to the different conversions in Table 2-3 yields

The PFR reactor volume necessary to achieve 80% conversion is 2165 dm3. This volume could be achieved by using a bank of 100 PFRs that are each 0.1 m in diameter with a length of 2.8 m (e.g., see margin figure or Figures 1-8(a) and (b)).
Note: As we proceed through later chapters and have analytical formulas for –rA, we will use integration tables or software packages (e.g., Polymath, rather than quadrate formulas, such as Equation (E2-2.1)).
The PFR volume, that is, the integral in Equation (2-16), can also be evaluated from the area under the curve of a plot of (FA0 /–rA) versus X.
= Area under the curve between X = 0 and X = 0.8
(see shaded area in Figure E2-2.1)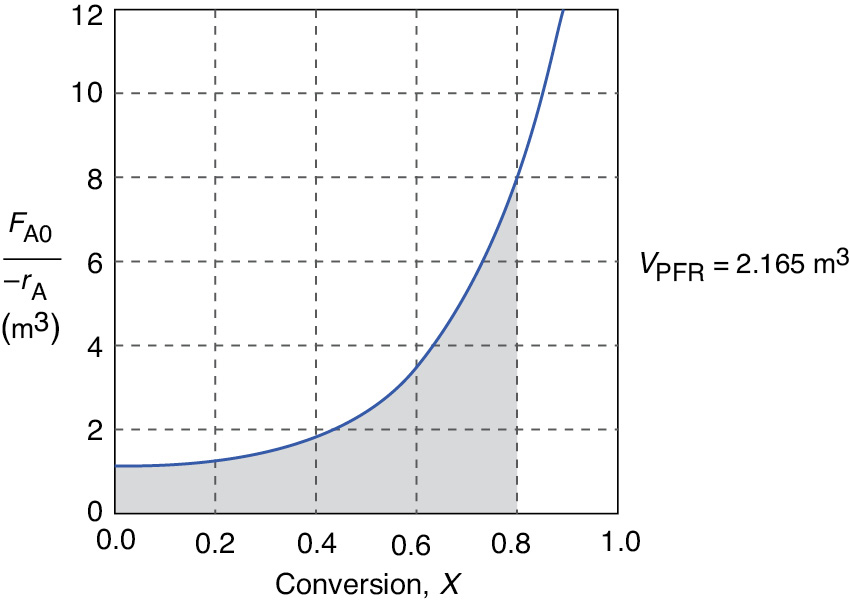
Figure E2-2.1 Levenspiel PFR plot.
PFR
The area under the curve will give the tubular reactor volume necessary to achieve the specified conversion of A. For 80% conversion, the shaded area is roughly equal to 2165 dm3 (2.165 m3).
Sketch the profiles of =rA and X down the length of the reactor.
We know that as we proceed down the reactor, the conversion increases as more and more reactant is converted to product. Consequently, as the reactant is consumed, the concentration of reactant decreases, as does the rate of disappearance of A for isothermal reactions.
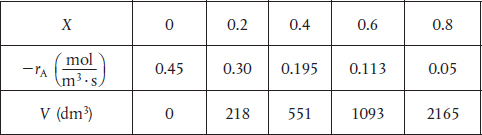
For X = 0.2, we calculate the corresponding reactor volume using Simpson’s rule (given in Appendix A.4 as Equation (A-21)) with increment =X = 0.1 and the data in rows 1 and 4 in Table 2-2.
This volume (218 m3) is the volume at which X = 0.2. From Table 2-2, we see the corresponding rate of reaction at X = 0.2 is .
Therefore at X = 0.2, then – rA = 0.3 mol/m3 · s and V = 218 dm3.
For X = 0.4, we can again use Table 2-3 and Simpson’s rule with ΔX = 0.2 to find the reactor volume necessary for a conversion of 40%.
From Table 2-2 we see that at X = 0.4, –rA = 0.195 mol m3 · s and V = 551 dm3.
We can continue in this manner to arrive at Table E2-2.1.
TABLE E2-2.1 CONVERSION AND REACTION RATE PROFILES
The data in Table E2-2.1 are plotted in Figures E2-2.2(a) and (b).
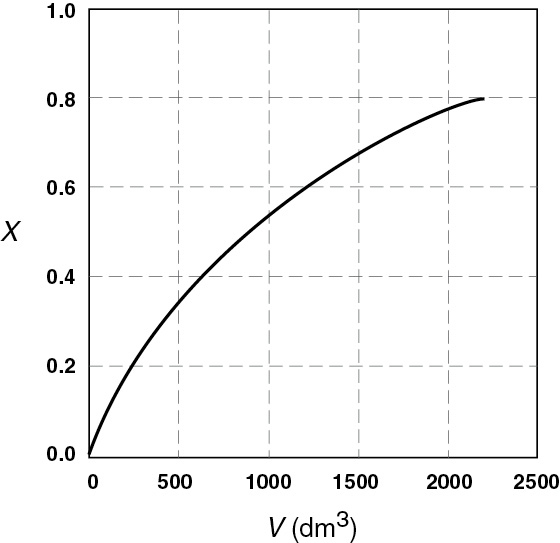
Figure E2-2.2(a) Conversion profile.
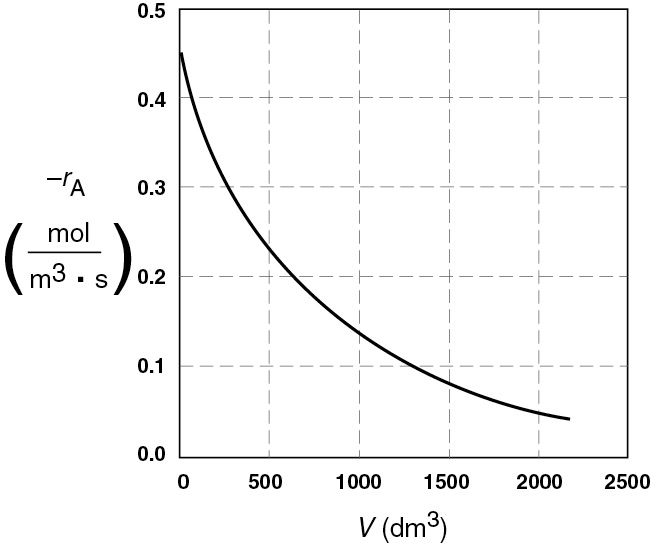
Figure E2-2.2(b) Reaction rate profile.
For isothermal reactions, the conversion increases and the rate decreases as we move down the PFR.
Analysis: One observes that for most all isothermal reactions the reaction rate, –rA, decreases as we move down the reactor while the conversion increases. These plots are typical for reactors operated isothermally.
Example 2–3 Comparing CSTR and PFR Sizes
Compare the volumes of a CSTR and a PFR required for the same conversion using the data in Figure 2-2(b). Which reactor would require the smaller volume to achieve a conversion of 80%: a CSTR or a PFR? The entering molar flow rate and the feed conditions are the same in both cases.
Solution
We will again use the data in Table 2-3.
TABLE 2-3 PROCESSED DATA 2
X |
0.0 |
0.1 |
0.2 |
0.4 |
0.6 |
0.7 |
0.8 |
(FA0/–rA)(m3) |
0.89 |
1.08 |
1.33 |
2.05 |
3.54 |
5.06 |
8.0 |
The CSTR volume was 6.4 m3 and the PFR volume was 2.165 m3. When we combine Figures E2-1.1 and E2-2.1 on the same graph, Figure 2-3.1(a), we see that the crosshatched area above the curve is the difference in the CSTR and PFR reactor volumes. Figure E2-3.1(b) shows the rate of reaction, –rA, as a function of conversion. We note that at 80% conversion the rate is approximately one-tenth the initial rate.
For isothermal reactions greater than zero order (see Chapter 3), the CSTR volume will always be greater than the PFR volume for the same conversion and reaction conditions (temperature, flow rate, etc.).
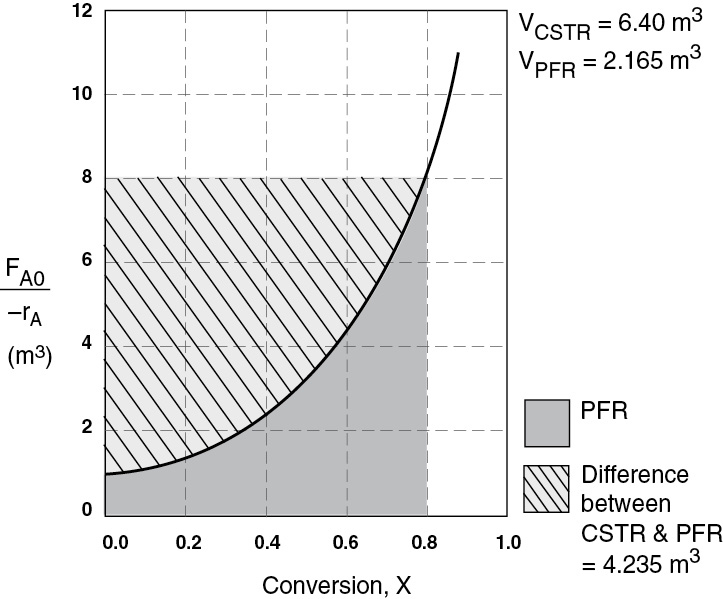
Figure E2-3.1(a) Comparison of CSTR and PFR reactor sizes for X = 0.8.
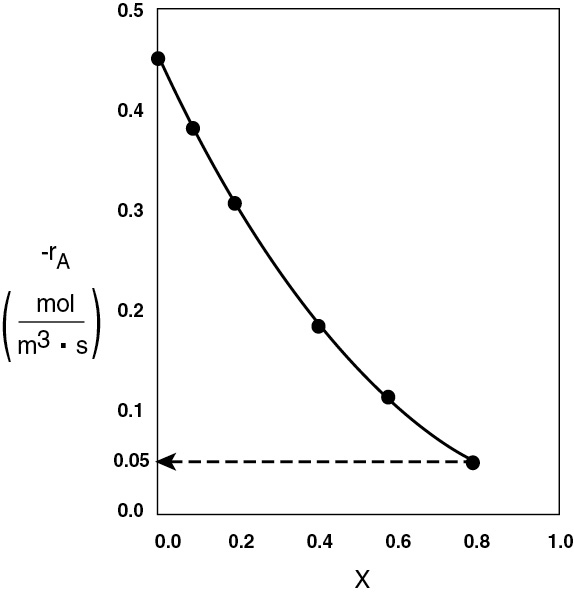
Figure E2-3.1(b) –rA as a function of X obtained from Table 2-2.1.
Analysis: We see that the reason the isothermal CSTR volume is usually greater than the PFR volume is that the CSTR is always operating at the lowest reaction rate (e.g., –rA = 0.05 mol m3 · s in Figure E2-3.1(b)). The PFR, on the other hand, starts at a high rate at the entrance and gradually decreases to the exit rate, thereby requiring less volume because the volume is inversely proportional to the rate. However, there are exceptions such as autocatalytic reactions, product-inhibited reactions, and nonisothermal exothermic reactions; these trends will not always be the case, as we will see in Chapters 9 and 11.
2.5 Reactors in Series
Many times, reactors are connected in series so that the exit stream of one reactor is the feed stream for another reactor. When this arrangement is used, it is often possible to speed calculations by defining conversion in terms of location at a point downstream rather than with respect to any single reactor. That is, the conversion X is the total number of moles of A that have reacted up to that point per mole of A fed to the first reactor.
For reactors in series
Only valid for NO side streams!!
However, this definition can only be used when the feed stream only enters the first reactor in the series and there are no side streams either fed or withdrawn. The molar flow rate of A at point i is equal to the moles of A fed to the first reactor, minus all the moles of A reacted up to point i.

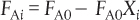
FAi = FA0 – FA0Xi
For the reactors shown in Figure 2-3, X1 at point i = 1 is the conversion achieved in the PFR, X2 at point i = 2 is the total conversion achieved at this point in the PFR and the CSTR, and X3 is the total conversion achieved by all three reactors.

Figure 2-3 Reactors in series.
To demonstrate these ideas, let us consider three different schemes of reactors in series: two CSTRs, two PFRs, and then a combination of PFRs and CSTRs in series. To size these reactors, we shall use the impeccable data in Table 2-1 from Jofostan Central Research laboratories that gives the reaction rate at different conversions.
2.5.1 CSTRs in Series
The first scheme to be considered is the two CSTRs in series shown in Figure 2-4.

Figure 2-4 Two CSTRs in series.
For the first reactor, the rate of disappearance of A is –rA1 at conversion X1.
A mole balance on reactor 1 gives
In – Out + Generation = 0
The molar flow rate of A at point 1 is
Combining Equations (2-19) and (2-20), and rearranging
Reactor 1
In the second reactor, the rate of disappearance of A, –rA2, is evaluated at the conversion of the exit stream of reactor 2, X2. A steady-state mole balance on the second reactor is
In – Out + Generation = 0
The molar flow rate of A at point 2 is
combining Equations (2-22) and (2-23) and rearranging
Reactor 2
For the second CSTR, recall that –rA2 is evaluated at X2 and then use (X2–X1) to calculate V2.
In the examples that follow, we shall again use the molar flow rate of A used in Example 2-1 (i.e., FA0 = 0.4 mol A/s) and the reaction conditions given in Table 2-3 in Example 2-3 processed data 2.
Example 2–4 Comparing Volumes for CSTRs in Series
For the two CSTRs in series, 40% conversion is achieved in the first reactor. What is the volume of each of the two reactors necessary to achieve 80% overall conversion of the entering species A? (See Table 2-3.)
TABLE 2-3 PROCESSED DATA 2

Solution
For Reactor 1, we observe from either Table 2-3 or Figure 2-2(b) that when X = 0.4, then
Then, using Equation (2-13)
V1 = 820 dm3 (liters) Answer
For Reactor 2, when X2 = 0.8, then
Using Equation (2-24)
(2-24)
V2 = (8.0 m3)(0.8 – 0.4) = 3.2 m3 = 3200 dm3
V2 = 3200 dm3 (liters) Answer
To achieve the same overall conversion, the total volume for two CSTRs in series is less than that required for one CSTR.
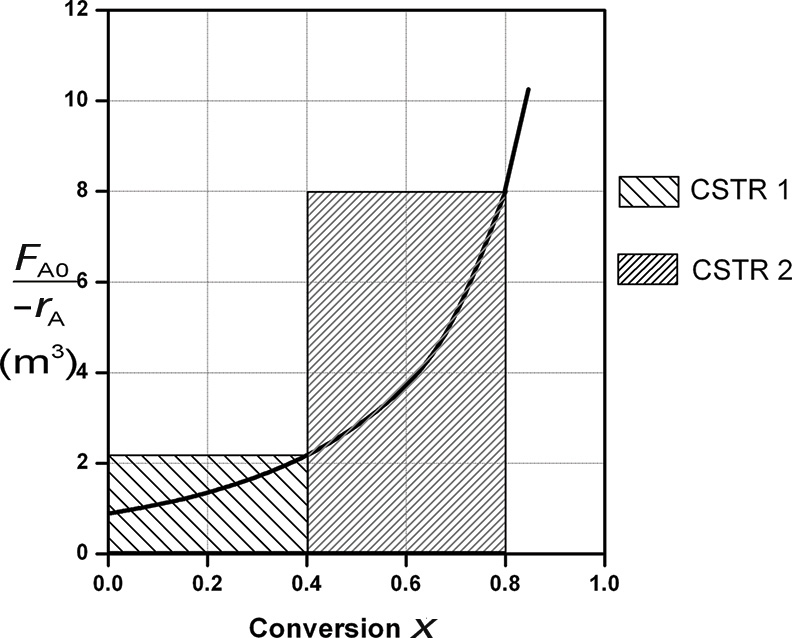
Figure E2-4.1 Two CSTRs in series.
The shaded areas in Figure E2-4.1 could also have been used to determine volumes of CSTR 1 and CSTR 2.
Note again that for CSTRs in series, the rate –rA1 is evaluated at a conversion of 0.4 and rate –rA2 is evaluated at a conversion of 0.8. The total volume for these two reactors in series is
V = V1 + V2 = 0.82 m3 + 3.2 m3 = 4.02 m3 = 4020 dm3 Answer
By comparison, the volume necessary to achieve 80% conversion in one CSTR is
Answer
We need only –rA = f(X) and FA0 to size reactors.
Notice in Example 2-4 that the sum of the two CSTR reactor volumes (4.02 m3) in series is less than the volume of one CSTR (6.4 m3) to achieve the same overall conversion.
Analysis: When we have reactors in series, we can speed our analysis and calculations by defining an overall conversion at a point in the series, rather than the conversion of each individual reactor. In this example, we saw that 40% was achieved at point 1, the exit to the first reactor, and that a total of 80% conversion was achieved by the time we exit the second reactor. Using two CSTRs in series will give a smaller total volume than one CSTR to achieve the same conversion.
Approximating a PFR by a Large Number of CSTRs in Series
Consider approximating a PFR with a number of small, equal-volume CSTRs of Vi in series (Figure 2-5). We want to compare the total volume of all these CSTRs in series with the volume of one plug-flow reactor for the same conversion, say 80%.

Figure 2-5 Modeling a PFR with CSTRs in series.
Useful Concept!
The fact that we can model a PFR with a large number of CSTRs is an important result.
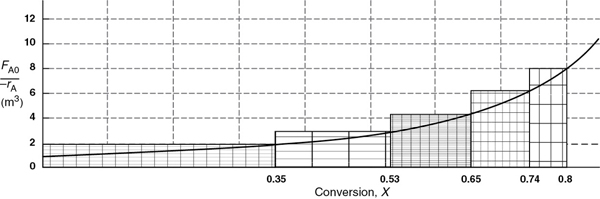
Figure 2-6 Levenspiel plot showing comparison of CSTRs in series with one PFR.
From Figure 2-6, we note a very important observation! The total volume to achieve 80% conversion for five CSTRs of equal volume in series is “roughly” the same as the volume of a PFR. As we make the volume of each CSTR smaller and increase the number of CSTRs, the total volume of the CSTRs in series and the volume of the PFR will become identical. That is, we can model a PFR with a large number of CSTRs in series. This concept of using many CSTRs in series to model a PFR will be used later in a number of situations, such as modeling catalyst decay in packed-bed reactors or transient heat effects in PFRs.
2.5.2 PFRs in Series
We saw that two CSTRs in series gave a smaller total volume than a single CSTR to achieve the same conversion. This case does not hold true for the two plug-flow reactors connected in series shown in Figure 2-7.

Figure 2-7 Two PFRs in series.
We can see from Figure 2-8 and from the following equation
PFRs in series
that it is immaterial whether you place two plug-flow reactors in series or have one continuous plug-flow reactor; the total reactor volume required to achieve the same conversion is identical!

Figure 2-8 Levenspiel plot for two PFRs in series.
The overall conversion of two PFRs in series is the same as one PFR with the same total volume.
2.5.3 Combinations of CSTRs and PFRs in Series
The final sequences we shall consider in this chapter are combinations of CSTRs and PFRs in series. An industrial example of reactors in series is shown in the photo in Figure 2-9 where two CSTRs in series are followed by a PFR. This sequence is used to dimerize propylene (A) into isohexanes (B), for example,
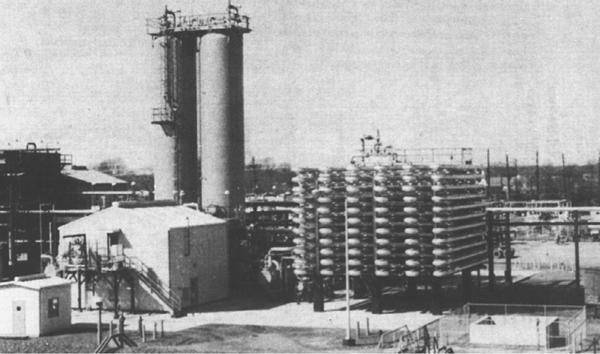
Figure 2-9 Dimersol G (an organometallic catalyst) unit (two CSTRs and one tubular reactor in series) to dimerize propylene into isohexanes. Institut Français du Pétrole process. Photo courtesy of Editions Technip (Institut Français du Pétrole).
Not sure whether the size of these CSTRs is in the Guiness Book of World Records.
A schematic of the industrial reactor system in Figure 2-9 is shown in Figure 2-10.
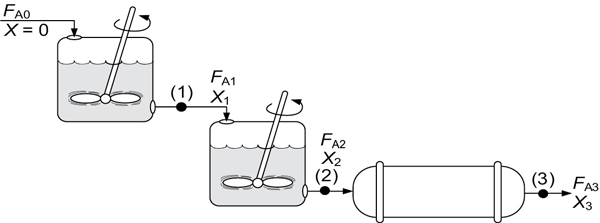
Figure 2-10 Schematic of a real system.
For the sake of illustration, let’s assume that the reaction carried out in the reactors in Figure 2-10 follows the same versus X curve given by Table 2-3.
The volumes of the first two CSTRs in series (see Example 2-4) are:
Reactor 1: (2-13)
Reactor 2: (2-14)
In this series arrangement, –rA2 is evaluated at X2 for the second CSTR.
Starting with the differential form of the PFR design equation
(2-15)
rearranging and integrating between limits, when V = 0, then X = X2, and when V = V3, then X = X3 we obtain
Reactor 3: (2-25)
The corresponding reactor volumes for each of the three reactors can be found from the shaded areas in Figure 2-11.

Figure 2-11 Levenspiel plot to determine the reactor volumes V1, V2, and V3.
The (FA0/–rA) versus X curves we have been using in the previous examples are typical of those found in isothermal reaction systems. We will now consider a real reaction system that is carried out adiabatically. Isothermal reaction systems are discussed in Chapter 5 and adiabatic systems in Chapter 11, where we will see that for adiabatic exothermic reactions the temperature increases with increasing conversion.
Example 2–5 An Adiabatic Liquid-Phase Isomerization
The isomerization of butane
n-C4H10 ⇄ i - C4H10
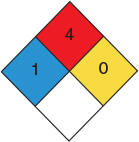
Butane NFPA Diamond
was carried out adiabatically in the liquid phase. The data for this reversible reaction are given in Table E2-5.1. (Example 11.3 shows how the data in Table E2-5.1 were generated.)
TABLE E2-5.1 RAW DATA
X |
0.0 |
0.2 |
0.4 |
0.6 |
0.65 |
–rA(kmol/m3 · h) |
39 |
53 |
59 |
38 |
25 |
Don’t worry how we got this data or why the (1/–rA) looks the way it does; we will see how to construct this table in Chapter 11, Example 11-3. It is real data for a real reaction carried out adiabatically, and the reactor scheme shown below in Figure E2-5.1 is used.

Figure E2-5.1 Reactors in series.
Real Data for a Real Reaction
Calculate the volume of each of the reactors for an entering molar flow rate of n-butane of 50 kmol/hr, that is, FA0 = 50 kmol/h.
Solution
Taking the reciprocal of –rA and multiplying by FA0, we obtain Table E2-5.2.
For example, at
TABLE E2-5.2 PROCESSED DATA†
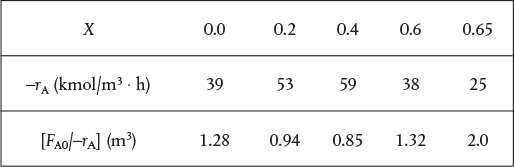
†Data from Table E11-3.1.
For the first CSTR,
when X = 0.2, then
For the PFR,
Using Simpson’s three-point formula with ΔX = (0.6 – 0.2)/2 = 0.2, and X1 = 0.2, X2 = 0.4, and X3 = 0.6
For the last reactor and the second CSTR, mole balance on A for the CSTR:
In – Out + Generation = 0
Rearranging

FA2 = FA0 – FA0X2
FA3 = FA0 – FA0X3
Simplifying
We find from Table E2-5.2 that at X3 = 0.65, then
V3 = 2 m3 (0.65 – 0.6) = 0.1 m3
A Levenspiel plot of (FA0/–rA) versus X is shown in Figure E2-5.2.

Figure E2-5.2 Levenspiel plot for adiabatic reactors in series.
For this adiabatic reaction, the three reactors in series resulted in an overall conversion of 65%. The maximum conversion we can achieve is the equilibrium conversion, which is 68%, and is shown by the dashed line in Figure E2-5.2. Recall that at equilibrium, the rate of reaction is zero and an infinite reactor volume is required to reach equilibrium .
Analysis: For exothermic reactions that are not carried out isothermally, the rate usually increases at the start of the reaction because reaction temperature increases. However, as the reaction proceeds the rate eventually decreases as the conversion increases because the reactants are consumed. These two competing effects give the bowed shape of the curve in Figure E2-5.2, which will be discussed in detail in Chapters 11 and 12. Under these circumstances, we saw that a CSTR will require a smaller volume than a PFR at low conversions.
2.5.4 Comparing the CSTR and PFR Volumes and Reactor Sequencing
If we look at Figure E2-5.2, the area under the curve (PFR volume) between X = 0 and X = 0.2, we see that the PFR area is greater than the rectangular area corresponding to the CSTR volume, that is, VPFR > VCSTR. However, if we compare the areas under the curve between X = 0.6 and X = 0.65, we see that the area under the curve (PFR volume) is smaller than the rectangular area corresponding to the CSTR volume, that is, VCSTR > VPFR. This result often occurs when the reaction is carried out adiabatically, which is discussed when we look at heat effects in Chapter 11.
Which arrangement is best?
In the sequencing of reactors, one is often asked, “Which reactor should go first to give the highest overall conversion? Should it be a PFR followed by a CSTR, or two CSTRs, then a PFR, or …?” The answer is “It depends.” It depends not only on the shape of the Levenspiel plot (FA0/–rA) versus X, but also on the relative reactor sizes. As an exercise, examine Figure E2-5.2 to learn if there is a better way to arrange the two CSTRs and one PFR. Suppose you were given a Levenspiel plot of (FA0/–rA) versus X for three reactors in series, along with their reactor volumes VCSTR1 = 3 m3, VCSTR2 = 2 m3, and VPFR = 1.2 m3, and were asked to find the highest possible conversion X. What would you do? The methods we used to calculate reactor volumes all apply, except the procedure is reversed and a trial-and-error solution is needed to find the exit overall conversion from each reactor (see Problem P2-5B).
The previous examples show that if we know the molar flow rate to the reactor and the reaction rate as a function of conversion, then we can calculate the reactor volume necessary to achieve a specified conversion. The reaction rate does not depend on conversion alone, however. It is also affected by the initial concentrations of the reactants, the temperature, and the pressure. Consequently, the experimental data obtained in the laboratory and presented in Table 2-1 as –rA as a function of X are useful only in the design of full-scale reactors that are to be operated at the identical conditions as the laboratory experiments (temperature, pressure, and initial reactant concentrations). However, such circumstances are seldom encountered and we must revert to the methods we describe in Chapters 3 and 4 to obtain –rA as a function of X.
It is important to remember that if the rate of reaction is available or can be obtained solely as a function of conversion, –rA = f(X), or if it can be generated by some intermediate calculations, one can design a variety of reactors and combinations of reactors.
Only need –rA = f(X) to size flow reactors
Ordinarily, laboratory data are used to formulate a rate law, and then the reaction rate–conversion functional dependence is determined using the rate law. The preceding sections show that with the reaction rate–conversion relationship, that is, –rA = f(X), different reactor schemes can readily be sized. In Chapters 3 and 4, we show how we obtain this relationship between reaction rate and conversion from rate law and reaction stoichiometry.

2.6 Some Further Definitions
Before proceeding to Chapter 3, some terms and equations commonly used in reaction engineering need to be defined. We also consider the special case of the plug-flow design equation when the volumetric flow rate is constant.
2.6.1 Space Time
The space time tau, τ, is obtained by dividing the reactor volume by the volu-metric flow rate entering the reactor
τ is an important quantity!
The space time is the time necessary to process one reactor volume of fluid based on entrance conditions. For example, consider the tubular reactor shown in Figure 2-12, which is 20 m long and 0.2 m3 in volume. The dashed line in Figure 2-12 represents 0.2 m3 of fluid directly upstream of the reactor. The time it takes for this upstream fluid volume to enter the reactor completely is called the space time tau. It is also called the holding time or mean residence time.
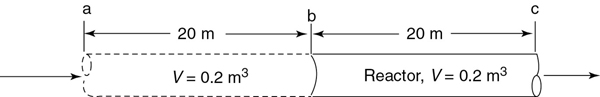
Figure 2-12 Tubular reactor showing identical volume upstream.
For example, if the reactor volume is 0.2 m3 and the inlet volumetric flow rate is 0.01 m3/s, it would take the upstream equivalent reactor volume (V = 0.2 m3), shown by the dashed lines, a time τ equal to
to enter the reactor (V = 0.2 m3). In other words, it would take 20 s for the fluid molecules at point a to move to point b, which corresponds to a space time of 20 s. We can substitute for FA0 = υ0CA0 in Equations (2-13) and (2-16) and then divide both sides by to write our mole balance in the following forms:
and
For plug flow, the space time is equal to the mean residence time in the reactor, tm (see Chapter 16). This time is the average time that the molecules spend in the reactor. A range of typical processing times in terms of the space time (residence time) for industrial reactors is shown in Table 2-4.
TABLE 2-4 TYPICAL SPACE TIME FOR INDUSTRIAL REACTORS2

2 P. Trambouze, H. Landeghem, and J. P. Wauquier, Chemical Reactors, Paris: Editions Technip, 1988; Houston: Gulf Publishing Company, 1988, p. 154.
Practical guidelines
Table 2-5 shows an order of magnitude of the space times for six industrial reactions and associated reactors.
TABLE 2-5 SAMPLE INDUSTRIAL SPACE TIMES3

Typical industrial reaction space times
Table 2-6 gives typical sizes for batch and CSTR reactors (along with the comparable size of a familiar object) and the costs associated with those sizes. All reactors are glass lined and the prices include heating/cooling jacket, motor, mixer, and baffles. The reactors can be operated at temperatures between 20°F and 450°F, and at pressures up to 100 psi.

TABLE 2-6 REPRESENTATIVE PFAUDLER CSTR/BATCH REACTOR SIZES AND PRICES†

†Doesn’t include instrumentation costs.
2.6.2 Space Velocity
The space velocity (SV) is another term used by reaction engineers and is defined as
3 Walas, S. M. Chemical Reactor Data, Chemical Engineering, 79 (October 14, 1985).
The space velocity might be regarded at first sight as the reciprocal of the space time. However, there can be a difference in the two quantities’ definitions. For the space time, the entering volumetric flow rate is measured at the entrance conditions, but for the space velocity, other conditions are often used. The two space velocities commonly used in industry are the liquid-hourly and gas-hourly space velocities, LHSV and GHSV, respectively. The entering volumetric flow rate, υ0, in the LHSV is frequently measured as that of a liquid feed rate at 60°F or 75°F, even though the feed to the reactor may be a vapor at some higher temperature. Strange but true.
(2-28)
Slight differences in space velocities
The gas volumetric flow rate, υ0, in the GHSV is normally reported at standard temperature and pressure (STP).
(2-29)
Example 2–6 Reactor Space Times and Space Velocities
Calculate the space time, τ, and space velocities for the reactor in Examples 2-1 and 2-3 for an entering volumetric flow rate of 2 dm3/s.
Solution
The entering volumetric flow rate is 2 dm3/s (0.002 m3/s).
From Example 2-1, the CSTR volume was 6.4 m3 and the corresponding space time, τ, and space velocity, SV are
It takes 0.89 h to put 6.4 m3 into the reactor and the corresponding space velocity, SV, is
From Example 2-3, the PFR volume was 2.165 m3, and the corresponding space time and space velocity are
Analysis: This example gives an important industrial concept. These space times are the times for each of the reactors to take the volume of fluid equivalent to one reactor volume and put it into the reactor.
Summary
In these last examples we have seen that in the design of reactors that are to be operated at conditions (e.g., temperature and initial concentration) identical to those at which the reaction rate–conversion data –rA = f(X) were obtained, we can size (determine the reactor volume) both CSTRs and PFRs alone or in various combinations. In principle, it may be possible to scale up a laboratory-bench or pilot-plant reaction system solely from knowledge of –rA as a function of X or CA. However, for most reactor systems in industry, a scale-up process cannot be achieved in this manner because knowledge of –rA solely as a function of X is seldom, if ever, available under identical conditions. By combining the information in Chapters 3 and 4, we shall see how we can obtain –rA = f(X) from information obtained either in the laboratory or from the literature. This relationship will be developed in a two-step process. In Step 1, we will find the rate law that gives the rate as a function of concentration (Table 3-1) and in Step 2, we will find the concentrations as a function of conversion (Figure 4-3). Combining Steps 1 and 2 in Chapters 3 and 4, we obtain –rA = f(X). We can then use the methods developed in this chapter, along with integral and numerical methods, to size reactors.
Coming attractions in Chapters 3 and 4
2.7 And Now… A Word from Our Sponsor–Safety 2 (AWFOS–S2 The NFPA Diamond)
In this AWFOS–S, we describe the National Fire Protection Agency (NFPA) Diamond which quickly identifies risks of hazardous materials. This identification is extremely important to first responders in an emergency. Each color in the diamond represents a different type of hazard, while the numbers in the hazard refer to a degree of concern one should have about the material. The different types of hazards and levels of concern of the NFPA diamond are shown in Figure 2-13.

Figure 2-13 Components of each color of the NFPA Diamond. (Image: Dmitry Kovalchuk/Shutterstock)
A tutorial on how to fill in the numbers in the diamond for the insecticide dichlorodiphenyltrichloroethane (DDT) using the CAMEO Chemicals Website (https://cameochemicals.noaa.gov) is given in the NFPA Diamond tutorial on the safety Website (http://umich.edu/~safeche/nfpa.html, http://umich.edu/~safeche/assets/pdf/CAMEO.pdf). The NFPA Diamond for the chemical DDT discussed in Example 1-1, DDT, is shown in Figure 2-14.

Figure 2-14 NFPA Diamond Levels of Concern for DDT. (Image: Idea.s/Shutterstock)
We see that the number two in the red (top-most) diamond indicates DDT has a flash point below 200°F, the zero in the yellow (far-right) diamond indicates it is stable, the blank in the white (bottom-most) diamond indicates no specific hazard, and the two in the blue (far-left) diamond indicates DDT is hazardous to one’s health.
The following photographs show two examples of how the NFPA Diamond is used in industry.

Figure 2-15 NFPA Diamond in Action. (Photos: left, Gado Images/Getty; right, TFoxFoto/Shutterstock)
The NFPA Diamond along with its companion GHS Diamond (see AWFOS-S3) is shown on the Process Safety Across the Chemical Engineering Curriculum Web site. For tutorials and NFPA data sheets see
NFPA Diamond Tutorial: https://cse.wwu.edu/files/safety-tutorials/Gen_Chem_Tutorial/HTML/GCST_10.htm
CAMEO Chemicals Tutorial: http://umich.edu/~safeche/assets/pdf/CAMEO.pdf
Quick Card: https://www.nfpa.org/Assets/files/AboutTheCodes/704/NFPA704_HC2012_QCard.pdf
Data Sheets: https://cameochemicals.noaa.gov/chemical/694https://cameochemicals.noaa.gov/chemical/4145
FAQs: https://www.nfpa.org/Assets/files/AboutTheCodes/704/704_FAQs.pdf
Summary
The conversion X is the moles of A reacted per mole of A fed.
For batch systems:
(S2-1)For flow sytems:
(S2-2)For reactors in series with no side streams, the conversion at point i is
(S2-3)
The differential and integral forms of the reactor design equations (i.e., GMBEs) are shown in Table S2-1.
TABLE S2-1 MOLE BALANCE FOR BR, CSTR, PFR, AND PBR TERMS OF CONVERSION
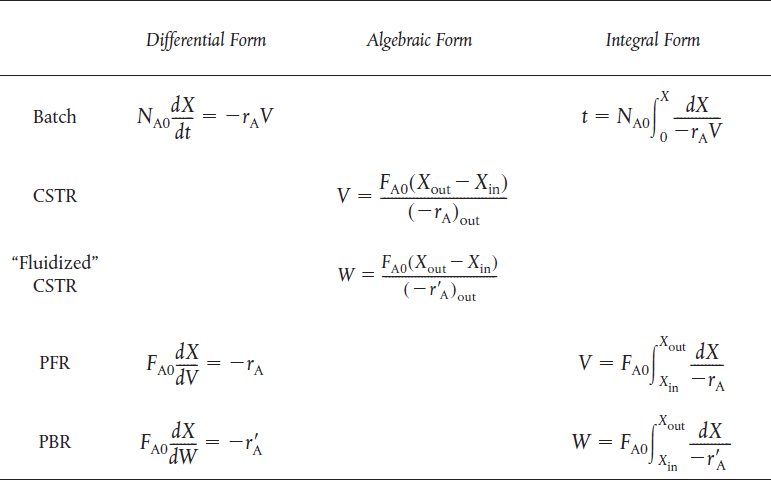
If the rate of disappearance of A is given as a function of conversion, the following graphical techniques can be used to size a CSTR and a plug-flow reactor.
Graphical Integration Using Levenspiel Plots
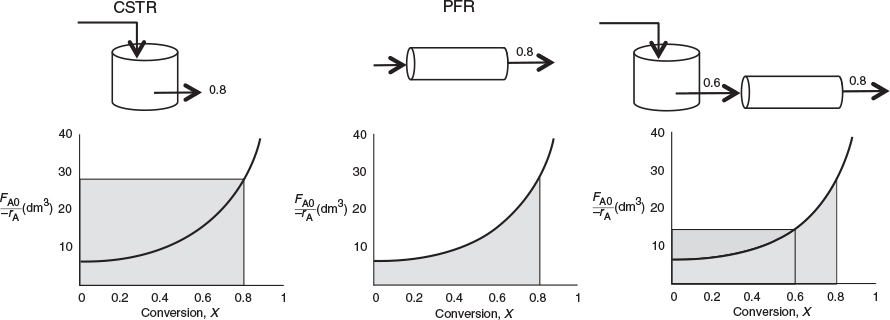
The PFR integral could also be evaluated by
Numerical Integration
See Appendix A.4 for quadrature formulas such as the five-point quadrature formula with ΔX = 0.8/4 of five equally spaced points, X1 = 0, X2 = 0.2, X3 = 0.4, X4 = 0.6, and X5 = 0.8.
Space time, τ, and space velocity, SV, are given by
(S2-4)
CRE Web Site Materials
(http://www.umich.edu/~elements/6e/02chap/obj.html)
Web Module Hippopotamus Digestive System
(http://umich.edu/~elements/6e/web_mod/hippo/index.htm)
Interactive Computer Games
Reactor Staging (http://umich.edu/~elements/6e/icm/staging.html)
Questions and Problems
The subscript to each of the problem numbers indicates the level of difficulty: A, least difficult; D, most difficult.
A = • B = ▪ C = ♦ D = ♦♦
Questions
Q2-1A QBR (Questions Before Reading). Will or could there ever be a case where the CSTR volume is represented by something other than a square or rectangle on a Levenspiel plot?
Q2-2A i>clicker. Go to the Web site http://www.umich.edu/~elements/6e/02chap/iclicker_ch2_q1.html and view at least five i>clicker questions. Choose one that could be used as is, or a variation thereof, to be included on the next exam. You also could consider the opposite case: explaining why the question should not be on the next exam. In either case, explain your reasoning.
Q2-3 (a) Without referring back, make a list of the most important items you learned in this chapter.
(b) Overall, what do you believe were the three major purposes of the chapter?
Q2-4 Go to the Web site www.engr.ncsu.edu/learningstyles/ilsweb.html. Take the Inventory of Learning Style test, and record your learning style according to the Soloman/Felder inventory, and then use Appendix I.2 to suggest two ways to facilitate your learning style in each of the four categories.
Global/Sequential_____
Active/Reflective_____
Visual/Verbal_____
Sensing/Intuitive_____
Q2-5A Go to the Chapter 2 Video Screencasts Web page (http://www.umich.edu/~elements/6e/02chap/learn-cheme-videos.html).
View one or more of the screencast 5- to 6-minute videos and write a two-sentence evaluation.
What did the speaker do well and what could be improved?
Q2-6A AWFOS-S2
What is the NFPA diamond and why is it important?
Go to Wikipedia and search ethylene oxide to find the NFPA diamond numbers.
Draw the NFPA diamond for the chemicals propylene, isohexane, and isobutane that we discussed in Figure 2-6, and Example 2-5.
Interactive Computer Games
P2-1A ICG Staging. Download the Interactive Computer Game (ICG) from the CRE Web site, http://www.umich.edu/~elements/6e/icm/staging.html. Play this game and then record your performance number, which indicates your mastery of the material. Your professor has the key to decode your performance number. Note: To play this game you must have Windows 2000 or a later version.
Write the ICG Reactor Staging Performance here # ______________________.
Problems
Before solving the problems, state or sketch qualitatively the expected results or trends.
P2-2A
Revisit the data in Table 2-1 Raw Data and calculate the batch reactor (BR) times to achieve 10%, 50%, and 80% conversion when 100 moles of A are charged to a 400 dm3 reactor.
Revisit Examples 2-1 through 2-3. How would your answers change if the flow rate, FA0, were cut in half? If it were doubled? What conversion can be achieved in a 4.5 m3 PFR and in a 4.5 m3 CSTR?
Revisit Example 2-2. Being a company about to go bankrupt, you can only afford a 2.5 m3 CSTR. What conversion can you achieve?
Revisit Example 2-3. What conversion could you achieve if you could convince your boss, Dr. Pennypincher, to spend more money to buy a 1.0 m3 PFR to attach to a 2.40 CSTR?
Revisit Example 2-4. How would your answers change if the two CSTRs (one 0.82 m3 and the other 3.2 m3) were placed in parallel with the flow, FA0, divided equally between the reactors?
Revisit Example 2-5. (1) What is the worst possible way to arrange the two CSTRs and one PFR? (2) What would be the reactor volumes if the two intermediate conversions were changed to 20% and 50%, respectively? (3) What would be the conversions, X1, X2, and X3, if all the reactors had the same volume of 100 dm3 and were placed in the same order?
Revisit Example 2-6. If the term is 2 seconds for 80% conversion, how much fluid (m3/min) can you process in a 3 m3 reactor?
P2-3B You have two CSTRs and two PFRs, each with a volume of 1.6 m3. Use Figure 2-2(b) on page 45 to calculate the conversion for each of the reactors in the following arrangements.
Two CSTRs in series. (Ans: X1 = 0.435, X2 = 0.66)
Two PFRs in series.
Two CSTRs in parallel with the feed, FA0, divided equally between the two reactors.
Two PFRs in parallel with the feed divided equally between the two reactors.
A PFR followed by a CSTR.
(f) A CSTR followed by a PFR. (Ans: XCSTR = 0.435, XPFR = 0.71)
A PFR followed by two CSTRs. Is this a good arrangement or is there a better one?
Caution: Part (h) is a C level problem. A CSTR and a PFR in parallel with the flow equally divided. Calculate the overall conversion, Xov
P2-4B The exothermic reaction of stillbene (A) to form the economically important trospophene (B) and methane (C), that is,
A → B+ C
was carried out adiabatically and the following data recorded:

The entering molar flow rate of A was 300 mol/min.
Calculate the batch reactor (BR) times to achieve 40% and 80% conversion when 400 moles of A are charged to a 400 dm3 reactor.
What are the PFR and CSTR volumes necessary to achieve 40% conversion? (VPFR = 72 dm3, VCSTR = 24 dm3)
Over what range of conversions would the CSTR and PFR reactor volumes be identical?
What is the maximum conversion that can be achieved in a 105-dm3 CSTR?
What conversion can be achieved if a 72-dm3 PFR is followed in series by a 24-dm3 CSTR?
What conversion can be achieved if a 24-dm3 CSTR is followed in a series by a 72-dm3 PFR?
Plot the conversion and rate of reaction as a function of PFR reactor volume up to a volume of 100 dm3.
P2-5B The financially important reaction to produce the valuable product B (not the real name) was carried out in Jesse Pinkman’s garage (see Breaking Bad Season 3, Episode 7). This Breaking Bad, fly-by-night company, is on a shoestring budget and has very little money to purchase equipment. Fortunately, cousin Bernie has a reactor surplus company and you can get reactors from Bernie. The reaction
A → B + C
takes place in the liquid phase. Below is the Levenspiel plot for this reaction. You have up to $10,000 to use to purchase reactors from those given below.

What reactors do you choose, how do you arrange them, and what is the highest conversion you can get for $10,000? Approximately what is the corresponding highest conversion with your arrangement of reactors?
Scheme and sketch your reactor volumes.
P2-6B Read the Web Module “Chemical Reaction Engineering of Hippopotamus Stomach” on the CRE Web site (http://www.umich.edu/~elements/6e/web_mod/hippo/index.htm).

Write five sentences summarizing what you learned from the Web Module.
Work problems (1) and (2) in the Hippo Web Module.
The hippo has picked up a river fungus, and now the effective volume of the CSTR stomach compartment is only 0.2 m3. The hippo needs 30% conversion to survive. Will the hippo survive?
The hippo had to have surgery to remove a blockage. Unfortunately, the surgeon, Dr. No, accidentally reversed the CSTR and the PFR during the operation. Oops!! What will be the conversion with the new digestive arrangement? Can the hippo survive?
P2-7B OEQ (Old Exam Question). The adiabatic exothermic irreversible gas-phase reaction
2A + B → 2C
is to be carried out in a flow reactor for an equimolar feed of A and B. A Levenspiel plot for this reaction is shown in Figure P2-7B.

Figure P2-7B Levenspiel plot.
What PFR volume is necessary to achieve 50% conversion?
What CSTR volume is necessary to achieve 50% conversion?
What is the volume of a second CSTR added in series to the first CSTR (Part b) necessary to achieve an overall conversion of 80%? (Ans: VCSTR = 1.5 × 105 m3)
What PFR volume must be added to the first CSTR (Part b) to raise the conversion to 80%?
What conversion can be achieved in a 6 × 104 m3 CSTR? In a 6 × 104 m3 PFR?
Think critically (cf. Introduction, Section H, page xxix) to critique the answers (numbers) to this problem.
P2-8A Estimate the reactor volumes of the two CSTRs and the PFR shown in the photo in Figure 2-9. Hint: Use the dimensions of the door as a scale.
P2-9D Don’t calculate anything. Just go home and relax.
P2-10C OEQ (Old Exam Question). The curve shown in Figure 2-1 is typical of a reaction carried out isothermally, and the curve shown in Figure P2-10C (see Example 11-3) is typical of a gas–solid catalytic exothermic reaction carried out adiabatically.
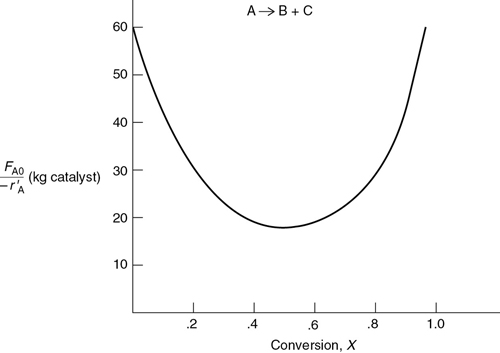
Figure P2-10C Levenspiel plot for an adiabatic exothermic heterogeneous reaction.
Assuming that you have a fluidized CSTR and a PBR containing equal weights of catalyst, how should they be arranged for this adiabatic reaction? Use the smallest amount of catalyst weight to achieve 80% conversion of A.
What is the catalyst weight necessary to achieve 80% conversion in a fluidized CSTR? (Ans: W = 23.2 kg of catalyst)
What fluidized CSTR weight is necessary to achieve 40% conversion?
What PBR weight is necessary to achieve 80% conversion?
What PBR weight is necessary to achieve 40% conversion?
Plot the rate of reaction and conversion as a function of PBR catalyst weight, W.
Additional information: FA0 = 2 mol/s.
P2-11 The experiments on reaction A → B were carried out and the conversion-rate data is given in Table P2-11.
TABLE P2-11 CONVERSION-RATE DATA
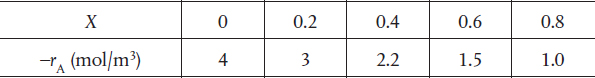
What are the batch reactor times to achieve 10%, 50%, and 80% for the reactor A → B when the initial concentration is 2 molar?
Plot the reaction rate, –rA, as a function of time up to a time necessary to achieve 80% conversion.
Additional Homework Problems can be found on the CRE Web site under Chapter 2 hot button.
Supplementary Reading
Further discussion of the proper staging of reactors in series for various rate laws, in which a plot of (=1/rA) versus X is given, may or may not be presented in
THORNTON W. BURGESS, The Adventures of Poor Mrs. Quack, New York: Dover Publications, Inc., 1917.
CHESTER L. KARRASS, Effective Negotiating: Workbook and Discussion Guide, Beverly Hills, CA: Karrass Ltd., 2004.
OCTAVE LEVENSPIEL, Chemical Reaction Engineering, 3rd ed. New York: Wiley, 1999, Chapter 6, pp. 139–156.
