11. Nonisothermal Reactor Design: The Steady-State Energy Balance and Adiabatic PFR Applications
If you can’t stand the heat, get out of the kitchen.
—Harry S Truman
11.1 Rationale
To identify the additional information necessary to design nonisothermal reactors, we consider the following example, in which a highly exothermic reaction is carried out adiabatically in a plug-flow reactor.
Example 11–1 What Additional Information Is Required?
The first-order liquid-phase reaction
A → B
is carried out in a PFR. The reaction is exothermic and the reactor is operated adiabatically. As a result, the temperature will increase with conversion down the length of the reactor. Because T varies along the length of the reactor, k will also vary, which was not the case for isothermal plug-flow reactors.
Describe how to calculate the PFR reactor volume necessary for 70% conversion and plot the corresponding profiles for X and T.
Solution
The same CRE algorithm can be applied to nonisothermal reactions as to isothermal reactions by adding one more step, the energy balance.
Mole Balance (design equation):
Rate Law:
Recalling the Arrhenius equation,

we know that k is a function of temperature, T.
Stoichiometry (liquid phase): υ = υ0
Combining:
Combining Equations (E11-1.1), (E11-1.2), and (E11-1.4), and canceling the entering concentration, CA0, yields
Combining Equations (E11-1.3) and (E11-1.6) gives us
Why we need the energy balance
Oops! We see that we need another relationship relating X and T or T and V to solve this equation. The energy balance will provide us with this relationship.
So we add another step to our algorithm; this step is the energy balance.
Energy Balance:
In this step, we will find the appropriate energy balance to relate temperature and conversion or reaction rate. For example, if the reaction is adiabatic, we will show that for equal heat capacities, and , and a constant heat of reaction, , the temperature–conversion relationship for an adiabatic reaction (cf. Table 11-1) can be written in a form such as
T0
=
Entering CPB Temperature
=
Heat of Reaction
CPA
=
Heat Capacity of species A
We now have all the equations we need to solve for the conversion and temperature profiles. We simply type Equations (E11-1.7) and (E11-1.8) along with the parameters into Polymath, Wolfram, Python, or MATLAB; click the RUN button; and then carry out the calculation to a volume where 70% conversion is obtained.
Analysis: The purpose of this example was to demonstrate that for nonisothermal chemical reactions we need another step in our CRE algorithm, the energy balance. The energy balance allows us to solve for the reaction temperature, which is necessary in evaluating the specific reaction-rate constant k(T).
11.2 The Energy Balance
11.2.1 First Law of Thermodynamics
The goal of this section is to use the energy balance to develop user-friendly relationships with temperature that can be easily applied to chemical reactors. We begin with the application of the first law of thermodynamics, first to a closed system and then to an open system. A system is any bounded portion of the universe, moving or stationary, which is chosen for the application of the various thermodynamic equations. For a closed system, no mass crosses the system boundaries and the change in total energy of the system, dÊ, is equal to the heat flow to the system δQ, minus the work done by the system on the surroundings, δW. For a closed system, the energy balance is
Closed system
The δs signify that δQ and δW are not exact differentials of a state function.
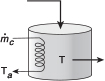
The continuous-flow reactors we have been discussing are open systems in which mass crosses the system boundary. We shall carry out an energy balance on the open system shown in Figure 11-1. For an open system in which some of the energy exchange is brought about by the flow of mass across the system boundaries, the energy balance for the case of only one species entering and leaving becomes
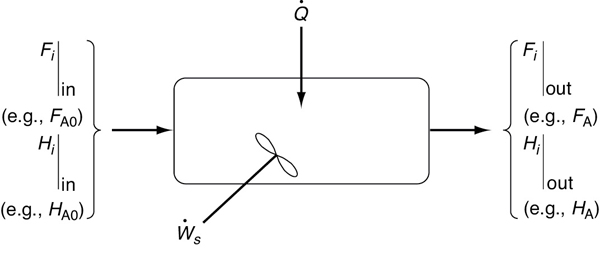
Figure 11-1 Energy balance on a well-mixed open system: schematic.
Energy balance on an open system
Typical units for each term in Equation (11-2) are (Joule/s). (Joule bio: http://www.corrosion-doctors.org/Biographies/JouleBio.htm.)
We will assume that the contents of the system volume are well mixed, an assumption that we could relax but that would require a couple of pages of text to develop, and the end result would be the same! The unsteady-state energy balance for an open well-mixed system that now has n species, each entering and leaving the system at their respective molar flow rate Fi (moles of i per time) and with their respective energy Ei (Joules per mole of i), is
The starting point
We will now discuss each of the terms in Equation (11-3).
11.2.2 Evaluating the Work Term
It is customary to separate the work term, , into flow work and other work, . The term , often referred to as the shaft work, could be produced from such things as a stirrer in a CSTR or a turbine in a PFR. Flow work is work that is necessary to get the mass into and out of the system. For example, when shear stresses are absent, we write
Flow work and shaft work
where P is the pressure (Pa) [1 Pa = 1 Newton/m2 = 1 kg·m/s2/m2] and is the specific molar volume of species i (m3/mol of i).
Let’s look at the units of the flow-work term, which is
where Fi is in mol/s, P is in Pa (1 Pa = 1 Newton/m2), and is in (m3/mol). Multiplying the units of each term in through, we obtain the units of flow work, that is,
We see that the units for flow work are consistent with the other terms in Equation (11-3), that is, (J/s) (http://www.corrosion-doctors.org/Biographies/WattBio.htm).
In most instances, the flow-work term is combined with those terms in the energy balance that represent the energy exchange by mass flow across the system boundaries. Substituting Equation (11-4) into (11-3) and grouping terms, we have
Convention
Heat Added
= + –10 J/s
Heat Removed
= + –10 J/s
Work Done by System
= + –10 J/s
Work Done on System
= + –10 J/s
The energy Ei is the sum of the internal energy (Ui), the kinetic energy , the potential energy (gzi), and any other energies, such as electric or magnetic energy or light
In almost all chemical reactor situations, the kinetic, potential, and “other” energy terms are negligible in comparison with the enthalpy, heat transfer, and work terms in the energy balance, and hence will be omitted, in which case
We recall that the enthalpy, Hi (J/mol), is defined in terms of the internal energy Ui (J/mol), and the product (1 Pa · m3/mol = 1 J/mol):
Enthalpy
Typical units of Hi are
Enthalpy carried into (or out of) the system can be expressed as the sum of the internal energy carried into (or out of) the system by mass flow plus the flow work:
Combining Equations (11-5), (11-7), and (11-8), we can now write the energy balance in the form
The energy of the system at any instant in time, Êsys, is the sum of the products of the number of moles of each species in the system multiplied by their respective energies. The derivative of the Esys term wrt time will be discussed in more detail when unsteady-state reactor operation is considered in Chapter 13.
We shall let the subscript “0” represent the inlet conditions. The conditions at the outlet of the chosen system volume will be represented by the unsubscripted variables.
In Section 11.1, we discussed that in order to solve reaction engineering problems with heat effects, we needed to relate temperature, conversion, and rate of reaction. The energy balance as given in Equation (11-9) is the most convenient starting point as we proceed to develop this relationship.
11.2.3 Overview of Energy Balances
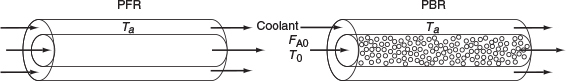
What is the plan? In the following pages, we manipulate Equation (11-9) in order to apply it to each of the reactor types we have been discussing: BR, PFR, PBR, and CSTR. The end result of the application of the energy balance to each type of reactor is shown in Table 11-1. These equations can be used in Step 5 of the algorithm discussed in Example 11-1. The equations in Table 11-1 relate temperature to conversion and to molar flow rates, and to the system parameters, such as the product of overall heat transfer coefficient, U, and heat exchange surface area, a, that is, Ua, with the corresponding ambient temperature, Ta, and the heat of reaction, ΔHRx.
Examples of How to Use Table 11-1. We now couple the energy balance equations in Table 11-1 with the appropriate reactor mole balance, rate law, and stoichiometry algorithm to solve reaction engineering problems with heat effects.
Table 11-1 gives the user-friendly reaction energy balances
TABLE 11-1 ENERGY BALANCES OF COMMON REACTORS
|
The equations in Table 11-1 are the ones we will use to solve reaction engineering problems with heat effects. Nomenclature: U = overall heat transfer coefficient, (J/m2 · s · K); A = CSTR heat-exchange area, (m2); a = PFR heat-exchange area per volume of reactor, (m2/m3); CPi = mean heat capacity of species i, (J/mol/K); CPc = the heat capacity of the coolant, (J/kg/K); = coolant flow rate, (kg/s); ΔHRx (T) = heat of reaction at temperature T, (J/mol A): A = heat of reaction at temperature TR; ΔHRxij = heat of reaction wrt species j in reaction i, (J/mol j): = heat added to the reactor, (J/s); and All other symbols are as defined in Chapters 1–10. |
For example, recall the rate law for a first-order reaction, Equation (E11-1.5) in Example 11-1
which can be combined with the mole balance to find the concentration, conversion and temperature profiles (i.e., BR, PBR, PFR), exit concentrations, and conversion and temperature in a CSTR. We will now consider four cases of heat exchange in a PFR and PBR: (1) adiabatic, (2) co-current, (3) countercurrent, and (4) constant exchanger fluid temperature. We focus on adiabatic operation in this chapter and the other three cases in Chapter 12.
Case 1: Adiabatic. If the reaction is carried out adiabatically, then we use Equation (T11-1.B) for the reaction A → B in Example 11-1 to obtain
Adiabatic
Consequently, we can now obtain –rA as a function of X alone by first choosing X, then calculating T from Equation (T11-1.B), then calculating k from Equation (E11-1.3), and then finally calculating (–rA) from Equation (E11-1.5)
The algorithm
We can use this sequence to prepare a table of (FA0/–rA) as a function of X. We can then proceed to size PFRs and CSTRs. In the absolute worst case scenario, we could use the techniques in Chapter 2 (e.g., Levenspiel plots or the quadrature formulas in Appendix A). However, instead of using a Levenspiel plot, we will most definitely use software packages such as MATLAB, Wolfram, Python, or Polymath to solve our coupled differential energy and mole balance equations.

Levenspiel plot
Cases 2, 3, and 4: Correspond to Co-Current Heat Exchange, Countercurrent Heat Exchange, and Constant Coolant Temperature TC, respectively (Chapter 12). If there is cooling along the length of a PFR or PBR, we could then apply Equation (T11-1.D) to this reaction to arrive at two coupled differential equations.
In terms of conversion
Non-adiabatic PFR
In terms of molar flow rate
either of which needs to be coupled with the energy balance
which are easily solved using an ODE solver such as Polymath. If the temperature of the heat exchanger fluid (Ta) (e.g., coolant) is not constant, we add Equations (T11-1.K) or (T11-1.L) in Table 11-1 to the above equations and solve with Polymath, Wolfram, or MATLAB.
Heat Exchange in a CSTR. Similarly, for the case of the reaction A → B in Example 11-1 carried out in a CSTR, we could use Polymath, Python, Wolfram, or MATLAB to solve two nonlinear algebraic equations in X and T. These two equations are the combined mole balance
Non-adiabatic CSTR
and the application of Equation (T11-1.C), which is rearranged in the form
From these three cases, (1) adiabatic PFR and CSTR, (2) PFR and PBR with heat effects, and (3) CSTR with heat effects, one can see how to couple the energy balances and mole balances. In principle, one could simply use Table 11-1 to apply to different reactors and reaction systems without further discussion. However, understanding the derivation of these equations will greatly facilitate the proper application to and evaluation of various reactors and reaction systems. Consequently, we now derive the equations given in Table 11-1.
Why bother? Here is why!!
Why bother to derive the equations in Table 11-1? Because I have found that students can apply these equations much more accurately to solve reaction engineering problems with heat effects if they have gone through the derivation to understand the assumptions and manipulations used in arriving at the equations in Table 11.1. That is, understanding these derivations, students are more likely to put the correct number in the correct equation symbol.
11.3 The User-Friendly Energy Balance Equations
We will now dissect the molar flow rates and enthalpy terms in Equation (11-9) to arrive at a set of equations we can readily apply to a number of reactor situations.
11.3.1 Dissecting the Steady-State Molar Flow Rates to Obtain the Heat of Reaction
To begin our journey, we start with the energy balance Equation (11-9), and then proceed to finally arrive at the equations given in Table 11-1 by first dissecting two terms:
The molar flow rates, Fi and Fi0
The molar enthalpies, Hi, Hi0[Hi ≡ Hi(T), and Hi0 ≡ Hi(T0)]
An animated and “somewhat” humorous version of what follows for the derivation of the energy balance can be found in the reaction engineering games “Heat Effects 1” and “Heat Effects 2” on the CRE Web site, http://www.umich.edu/~elements/6e/icm/index.html. Here, equations talk to each other as they move around the screen, making substitutions and approximations to arrive at the equations shown in Table 11-1. Visual learners find these two ICGs a very useful resource (http://www.umich.edu/~elements/6e/icm/heatfx1.html).
We will now consider flow systems that are operated at steady state. The steady-state energy balance is obtained by setting (dÊsys/dt) equal to zero in Equation (11-9) in order to yield
Steady-state energy balance
To carry out the manipulations to write Equation (11-10) in terms of the heat of reaction, we shall use the generic reaction
The inlet and outlet summation terms in Equation (11-10) are expanded, respectively, to
In:
and
Out:
where the capital subscript I represents inert species.
We next express the molar flow rates in terms of conversion. In general, the molar flow rate of species i for the case of no accumulation and a stoichio-metric coefficient νi is
Fi = FA0 (Θi + νiX)
Specifically, for Reaction (2-2), , we have
FA = FA0 (1 — X)
Steady-state operation
We can substitute these symbols for the molar flow rates into Equations (11-11) and (11-12), then subtract Equation (11-12) from (11-11) to give (11-13)
The term in parentheses that is multiplied by FA0X is called the heat of reaction at temperature T and is designated ΔHRx(T).
Heat of reaction at temperature T
All enthalpies (e.g., HA, HB) are evaluated at a temperature T in the reactor and, consequently, [ΔHRx(T)] is the heat of reaction at that specific temperature T. The heat of reaction is always given per mole of the species that is the basis of calculation, that is, species A (Joules per mole of A reacted).
Substituting Equation (11-14) into (11-13) and reverting to summation notation for the species, Equation (11-13) becomes
Combining Equations (11-10) and (11-15), we can now write the steady-state, that is, (dÊsys/dt = 0), energy balance in a more usable form:
One can use this form of the steady-state energy balance if the enthalpies are available.
If a phase change takes place during the course of a reaction, this is the form of the energy balance (i.e., Equation (11-16)) that must be used.
11.3.2 Dissecting the Enthalpies
We are neglecting any enthalpy changes resulting from mixing so that the partial molal enthalpies are equal to the molal enthalpies of the pure components. The molal enthalpy of species i at a particular temperature and pressure, Hi, is usually expressed in terms of an enthalpy of formation of species i at some reference temperature TR, , plus the change in enthalpy, ΔHQi, that results when the temperature is raised from the reference temperature, TR, to some temperature T
The reference temperature at which is Hi°(TR) given is usually 25°C. For any substance i that is being heated from T1 to T2 in the absence of phase change
No phase change
Typical units of the heat capacity, CPi, are
A large number of chemical reactions carried out in industry do not involve phase change. Consequently, we shall further refine our energy balance to apply to single-phase chemical reactions. Under these conditions, the enthalpy of species i at temperature T is related to the enthalpy of formation at the reference temperature TR by
If phase changes do take place in going from the temperature for which the enthalpy of formation is given and the reaction temperature T, Equation (11-17) must be used instead of Equation (11-19).
The heat capacity at temperature T is frequently expressed as a quadratic function of temperature; that is
However, while this text will consider only constant heat capacities, the PRS R11.1 on the CRE Web site has examples with variable heat capacities (http://www.umich.edu/~elements/6e/11chap/prof.html).

To calculate the change in enthalpy (Hi — Hi0) when the reacting fluid is heated without phase change from its entrance temperature, Ti0, to a temperature T, we integrate Equation (11-19) for constant CPi to write
Substituting for Hi and Hi0 into Equation (11-16) yields
Result of dissecting the enthalpies
11.3.3 Relating ΔHRx(T), , and ΔCP
Recall that the heat of reaction at temperature T was given in terms of the enthalpy of each reacting species at temperature T in Equation (11-14); that is
where the enthalpy of each species is given by
If we now substitute for the enthalpy of each species, we have
For the generic reaction
The first term in brackets on the right-hand side of Equation (11-23) is the heat of reaction at the reference temperature TR
The enthalpies of formation of many compounds, , are usually tabulated at 25°C and can readily be found in the Handbook of Chemistry and Physics and similar handbooks.1 That is, we can look up the heats of formation at TR, then calculate the heat of reaction at this reference temperature. The heat of combustion (also available in these handbooks) can be used to determine the enthalpy of formation, , and this method of calculation is also described in these handbooks. From these values of the standard heat of formation, , we can calculate the heat of reaction at the reference temperature TR using Equation (11-24).
1 CRC Handbook of Chemistry and Physics, 95th ed. Boca Raton, FL: CRC Press, 2014.
The second term in brackets on the right-hand side of Equation (11-23) is the overall change in the heat capacity per mole of A reacted, ΔCP,
ΔCP
Combining Equations (11-25), (11-24), and (11-23) gives us
Heat of reaction at temperature T ΔHRx(T)
Equation (11-26) gives the heat of reaction at any temperature T in terms of the heat of reaction at a reference temperature (usually 298 K) and the ΔCP term. Techniques for determining the heat of reaction at pressures above atmospheric can be found in Chen.2 We note that for the reaction of hydrogen and nitrogen at 400°C, it was shown that the heat of reaction increased by only 6% as the pressure was raised from 1 to 200 atm! Consequently, we will neglect the effects of pressure on the heat of reaction.
2 N. H. Chen, Process Reactor Design, Needham Heights, MA: Allyn and Bacon, 1983, p. 26.
Calculate the heat of reaction for the synthesis of ammonia from hydrogen and nitrogen at 150°C in terms of (kcal/mol) of N2 reacted and also in terms of (kJ/mol) of H2 reacted.
Solution
N2 + 3H2 → 2NH3
To calculate the heat of reaction at the reference temperature TR, we will use the heats of formation of the reacting species obtained from Perry’s Chemical Engineers’ Handbook or the Handbook of Chemistry and Physics.3
3 D. W. Green and R. H. Perry, eds., Perry’s Chemical Engineers’ Handbook, 8th ed. New York: McGraw-Hill, 2008.
The enthalpies of formation at 25°C are
Note: The heats of formation of all elements (e.g., H2, N2) are by definition zero at 25°C.
We now can calculate , using Equation (11-24) and inserting the heats of formation of the products (e.g., NH3) multiplied by their appropriate stoichiometric coefficients (2 for NH3) minus the heats of formation of the reactants (e.g., N2, H2) multiplied by their stoichiometric coefficient (e.g., 3 for H2, 1 of N2)
or in terms of kJ/mol (recall: 1 kcal = 4.184 kJ)
Exothermic reaction
The minus sign indicates that the reaction is exothermic.
If the heat capacities are constant or if the mean heat capacities over the range 25°C–150°C are readily available, the determination of ΔHRx at 150°C is quite simple. (E11-2.2)
in terms of kJ/mol
The heat of reaction based on the moles of H2 reacted is
Analysis: This example showed (1) how to calculate the heat of reaction with respect to a given species, given the heats of formation of the reactants and the products, and (2) how to find the heat of reaction with respect to one species, given the heat of reaction with respect to another species in the reaction. Finally, (3) we also saw how the heat of reaction changed as we changed the temperature.
Now that we see that we can calculate the heat of reaction at any temperature, let’s substitute Equation (11-22) in terms of ΔHR(TR) and ΔCP, that is, Equation (11-26). The steady-state energy balance is now
Energy balance in terms of mean or constant heat capacities
From here on, for the sake of brevity we will let
unless otherwise specified.
In most systems, the work term, , can be neglected (note the exception in the California Professional Engineers’ Registration Exam Problem P12-6B at the end of Chapter 12). Neglecting , the energy balance becomes
We set Ti0 = T0
In almost all of the systems we will study, the reactants will be entering the system at the same temperature; therefore, we will set Ti0 = T0.
We can use Equation (11-28) to relate temperature and conversion and then proceed to evaluate the algorithm described in Example 11-1. However, unless the reaction is carried out adiabatically, Equation (11-28) is still difficult to evaluate because in non-adiabatic reactors, the heat added to or removed from the system can vary along the length of the reactor or can vary with time. This problem does not occur in reactors operated adiabatically, a situation often found in industry, therefore, the adiabatic tubular reactor will be analyzed first.
11.4 Adiabatic Operation ∴ = 0
Reactions in industry are frequently carried out adiabatically with heating or cooling provided either upstream or downstream. Consequently, analyzing and sizing adiabatic reactors is an important task.
11.4.1 Adiabatic Energy Balance
In the previous section, we derived Equation (11-28), which relates conversion to temperature and the heat added to the reactor, . Let’s stop a minute (actually it will probably be more like a couple of days) and consider a system with the special set of conditions of no work, , adiabatic operation , letting Ti0 = T0 and then rearranging Equation 11-28 into the form to express the conversion X as a function of temperature, that is,
For adiabatic operation, Example 11.1 can now be solved!
In many instances, the ΔCP(T — TR) term in the denominator of Equation (11-29) is negligible with respect to the term, so that a plot of X versus T will usually be linear, as shown in Figure 11-2. To remind us that the conversion in this plot was obtained from the energy balance rather than the mole balance, it is given the subscript EB (i.e., XEB) in Figure 11-2.
Relationship between X and T for adiabatic exothermic reactions
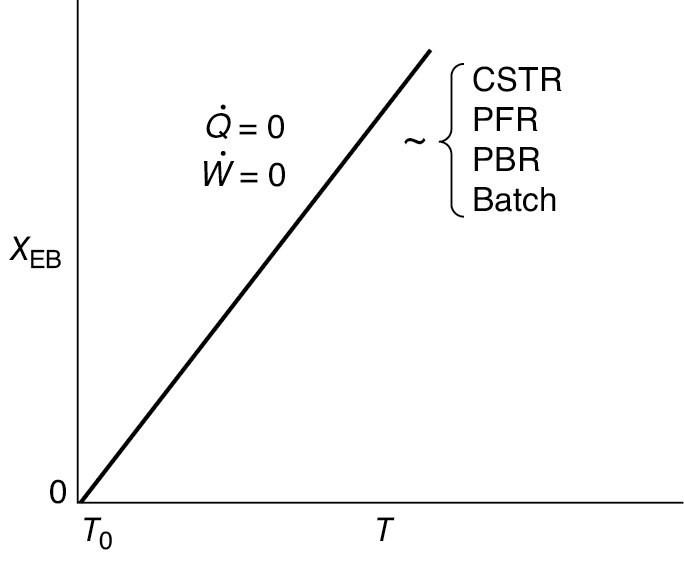
Figure 11-2 Adiabatic temperature–conversion relationship.
Equation (11-29) applies to a CSTR, PFR, or PBR, and also to a BR (as will be shown in Chapter 13). For Q̇ = 0 and Ẇs = 0, Equation (11-29) gives us the explicit relationship between X and T needed to be used in conjunction with the mole balance to solve a large variety of chemical reaction engineering problems as discussed in Section 11.1.
We can rearrange Equation (11-29) to solve for temperature as a function of conversion; that is
Energy balance for adiabatic operation of PFR
11.4.2 Adiabatic Tubular Reactor
Either Equation (11-29) or Equation (11-30) will be coupled with the differential mole balance
to obtain the temperature, conversion, and concentration profiles along the length of the reactor. The algorithm for solving PBRs and PFRs operated adiabatically will be illustrated by using a first-order reversible elementary reaction A ⇄ B as an example. Table 11-2 shows the algorithm using conversion, X, as the dependent variable while Table 11-3 shows the algorithm for solving the same reaction using molar flow rates instead of conversion.
The solution to reaction engineering problems today is to use software packages with ordinary differential equation (ODE) solvers, such as Polymath, MATLAB, Python, or Wolfram, to solve the coupled mole balance and energy balance differential equations.
We will now apply the algorithm in Table 11-2 and the solution procedure in Table 11-3 to a real reaction.

TABLE 11-2 ADIABATIC PFR/PBR ALGORITHM
The elementary reversible gas-phase reaction |
A ⇄ B |
is carried out in a PFR in which pressure drop is neglected and species A and inert I enter the reactor. |
|
TABLE 11-3 SOLUTION PROCEDURES FOR ELEMENTARY ADIABATIC GAS-PHASE PFR/PBR REACTOR
Moles as the Dependent Variable
|
Example 11–3 Adiabatic Liquid-Phase Isomerization of Normal Butane
Normal butane, C4H10, is to be isomerized to isobutane in a plug-flow reactor. Iso-butane is a valuable product that is used in the manufacture of gasoline additives. For example, isobutane can be further reacted to form iso-octane. The 2014 selling price of n-butane was $1.5/gal, while the trading price of isobutane was $1.75/gal.†
†Once again, you can buy a cheaper generic brand of n–C4H10 at the Sunday markets in downtown Riça, Jofostan, where there will be a special appearance and lecture by Jofostan’s own Prof. Dr. Sven Köttlov on February 29th, at the CRE booth.
This elementary reversible reaction is to be carried out adiabatically in the liquid phase under high pressure using essentially trace amounts of a liquid catalyst that gives a specific reaction rate of 31.1 h–1 at 360 K. We want to process 100000 gal/day (163 kmol/h) and achieve 70% conversion of n-butane from a mixture 90 mol % n-butane and 10 mol % i-pentane, which is considered an inert. The feed enters at 330 K.
Set up the CRE algorithm to calculate the PFR volume necessary to achieve 70% conversion.
Use an ODE software package (e.g., Polymath) to solve the CRE algorithm to plot and analyze X, Xe, T, and –rA down the length (volume) of a PFR to achieve 70% conversion.
Calculate the CSTR volume for 40% conversion.
The economic incentive $ = 1.75/gal versus 1.50/gal
Additional information:
Solution

It’s risky business to ask for 70% conversion in a reversible reaction.
Problem statement (a) said to set up the CRE algorithm to find the PFR volume necessary to achieve 70% conversion. This problem statement is risky. Why? Because the adiabatic equilibrium conversion may be less than 70%! Fortunately, it’s not so for the case discussed here, 0.7 < Xe. In general, it would be much safer and better to ask for the reactor volume to obtain 95% of the equilibrium conversion, Xf = 0.95 Xe.
Part (a) PFR algorithm

1. Mole Balance:
2. Rate Law:
The algorithm
with, for the case of δ = 0, KC = Ke,
3. Stoichiometry (liquid phase, υ = υ0):

4. Combine:
5. Energy Balance: Recalling Equation (11-27), we have
From the problem statement
Applying the preceding conditions to Equation (11-27) and rearranging gives
Nomenclature Note
6. Parameter Evaluation:
Because n-butane is 90% of the total molar feed, we calculate FA0 to be
where T is in degrees Kelvin.
Substituting for the activation energy, T1, k1 and in Equation (E11-3.3), we obtain
Substituting for , T2 and KC(T2 in Equation (E11-3.4) yields
Recalling the rate law gives us
7. Equilibrium Conversion:
At equilibrium
—rA ≡ 0
and therefore we can solve Equation (E11-3.7) for the equilibrium conversion
Because we know KC(T), we can find Xe as a function of temperature.
Note: It doesn’t matter what type of reactor we use, for example, BR, PFR, or CSTR, the equilibrium conversion, Xe, will be the same function of temperature.
Part (b) PFR computer solution to plot conversion and temperature profiles We now will use Polymath to solve the preceding set of equations to find the PFR reactor volume and to plot and analyze X, Xe, –rA, and T down the length (volume) of the reactor. The computer solution allows us to easily see how these reaction variables vary down the length of the reactor and also to study the reaction and the reactor by varying the system parameters such as CA0 and T0 (c.f. LEP P11-1A (b)).
Part B is the Polymath solution method we will use to solve most all CRE problems with “heat” effects.
The Polymath program using Equations (E11-3.1), (E11-3.7), (E11-3.9), (E11-3.10), (E11-3.11), and (E11-3.12) is shown in Table E11-3.1.
TABLE E11-3.1 POLYMATH PROGRAM ADIABATIC ISOMERIZATION

LEP Code: http://www.umich.edu/~elements/6e/live/chapter11/LEP-11-3.pol
From Table E11-3.1 and Figure E11-3.1(c) one observes that at the end of the 5 dm3 PFR both the conversion, X, and equilibrium conversion, Xe, reach 71.4%. One also notes in Figures E11-3.1(a) and (b) that the temperature reaches a plateau of 360.9 K where the net reaction rate approaches zero at this point in the reactor as the reactor conversion and equilibrium conversion become virtually the same as shown in Figure E11-3.1(c). Be sure to explore each of the three parts of Figure E11-3.1 using Wolfram.

Figure E11-3.1 (a) Adiabatic PFR temperature (K), (b) Reaction rate (kmol/m3/h), and (c) conversion profiles X and Xe.

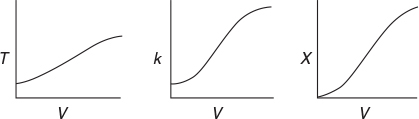
Look at the shape of the curves in Figure E11-3.1. Why do they look the way they do?
Analysis: Parts (a) and (b) for the PFR. The graphical output for the PFR is shown in Figure E11-3.1. We see from Figure E11-3.1(c) that a PFR volume of 1.15 m3 is required for 40% conversion. The temperature and reaction-rate profiles are also shown. Notice anything strange? One observes that the rate of reaction
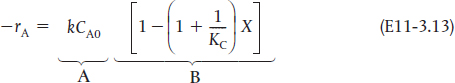
goes through a maximum. Near the entrance to the reactor, T increases as does k, causing term A to increase more rapidly than term B decreases, and thus the rate increases. Near the end of the reactor, term B is decreasing more rapidly than term A is increasing as we approach equilibrium. Consequently, because of these two competing effects, we have a maximum in the rate of reaction. Toward the end of the reactor, the temperature reaches a plateau as the reaction approaches equilibrium (i.e., X ≡ Xe at (V ≡ 3.5 m3)). As you know, and as do all chemical engineering students at Jofostan University in Riça, at equilibrium (–rA ≅ 0) no further changes in X, Xe, or T take place.
In the fifth edition, a solution by hand calculation was given to supposedly give more insight to the adiabatic algorithm. The students in Jofostan took a vote and the results showed it wasn’t that much help, so I decided to eliminate part (c) from fifth edition. However, it can still be found on the Web site under Additional Material.
Part (c) CSTR solution
First, take a guess, is VPFR > VCSTR or is VPFR < VCSTR? Let’s now calculate the adiabatic CSTR volume necessary to achieve 40% conversion, which is less than the equilibrium conversion of 71.5% shown in Figure E13-3.1 (c). Do you think the CSTR will be larger or smaller than the PFR?
Solution
The CSTR mole balance is
Using Equation (E11-3.7) in the mole balance, we obtain
From the energy balance, we have Equation (E11-3.9):
T = 330 + 43.4X
For 40% conversion
T = 330 + 43.4 (0.4) = 347.3K
Using Equations (E11-3.10) and (E11-3.11) or from Table E11-3.1, at T = 347.3 K, we find k and KC to be
k = 14.02 h-1
KC = 2.73
Then
We see that the CSTR volume (1 m3) to achieve 40% conversion in this adiabatic reaction is less than the PFR volume (1.15 m3) as shown in Figure E11-3.1(c). Who would have guessed VPFR > VCSTR?
By recalling the Levenspiel plots from Chapter 2, we can see that the reactor volume for 40% conversion is smaller for a CSTR than for a PFR. Plotting (FA0/–rA) as a function of X from the data in Table E11-3.1 is shown in Figure E11-3.2.
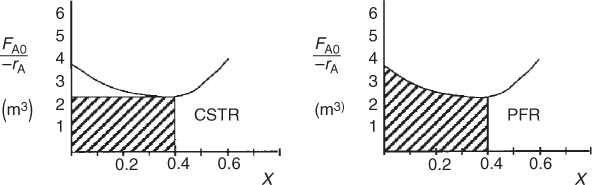
Figure E11-3.2 Levenspiel plots for a CSTR and a PFR.
The PFR area (volume) is greater than the CSTR area (volume).
In this example, the adiabatic CSTR volume is less than the PFR volume.
Analysis: In this example we applied the CRE algorithm to a reversible-first-order reaction carried out adiabatically in a PFR and in a CSTR. We note that at the CSTR volume necessary to achieve 40% conversion is smaller than the volume to achieve the same conversion in a PFR. In Figure E11-3.1(c) we also see that at a PFR volume of about 3.5 m3, equilibrium is essentially reached, and no further changes in temperature, reaction rate, equilibrium conversion, or conversion take place farther down the reactor.
AspenTech: Example 11-3 has also been formulated in AspenTech and this LEP can be loaded on your computer directly from the CRE Web site. Take a look and let me know what you think!

11.5 Adiabatic Equilibrium Conversion
The highest conversion that can be achieved in reversible reactions is the equilibrium conversion. For endothermic reactions, the equilibrium conversion increases with increasing temperature up to a maximum of 1.0. For exothermic reactions, the equilibrium conversion decreases with increasing temperature.
For reversible reactions, the equilibrium conversion, Xe, is usually calculated first.
11.5.1 Equilibrium Conversion
Exothermic Reactions. Figure 11-3(a) shows the variation of the concentration equilibrium constant, KC as a function of temperature for an exothermic reaction (see Appendix C), and Figure 11-3(b) shows the corresponding equilibrium conversion Xe as a function of temperature. In Example 11-3, we saw that for a first-order reaction, the equilibrium conversion could be calculated using Equation (E11-3.12)
First-order reversible reaction
The equilibrium conversion, Xe can be calculated as a function of temperature directly either by using Equation (E11-3.12) or from a plot of Equation (E11-3.12) such as that as shown in Figure 11-3(b).
For exothermic reactions, the equilibrium conversion decreases with increasing temperature.
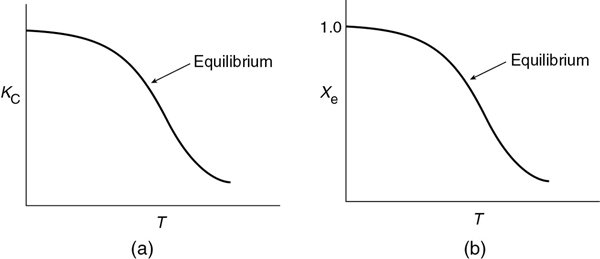
Figure 11-3 Variation of equilibrium constant and conversion with temperature for an exothermic reaction (http://www.umich.edu/~elements/6e/11chap/summary-biovan.html).
We note that the shape of the Xe versus T curve in Figure 11-3(b) will be similar for reactions that are other than first order.
To determine the maximum conversion that can be achieved in an exothermic reaction carried out adiabatically, we find the intersection of the equilibrium conversion as a function of temperature (Figure 11-3(b)) with temperature–conversion relationships from the energy balance (Figure 11-2 and Equation (T11-1.A)), as shown in Figure 11-4.
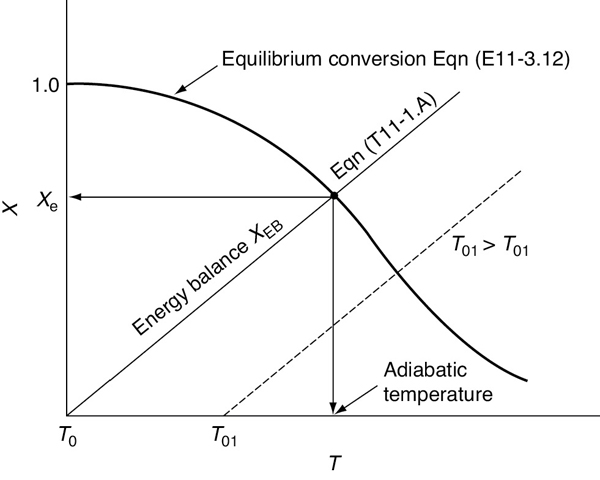
Figure 11-4 Graphical solution of equilibrium and energy balance equations to obtain the adiabatic temperature and the adiabatic equilibrium conversion Xe.
Adiabatic equilibrium conversion for exothermic reactions
This intersection of the energy balance line, XEB, with the thermodynamic equilibrium Xe curve gives the adiabatic-equilibrium conversion Xe and temperature for an entering temperature T0 as shown in Figure 11-4.
If the entering temperature is increased from T0 to T01, the energy balance line will be shifted to the right and be parallel to the original line, as shown by the dashed line. Note that as the inlet temperature increases, the adiabatic equilibrium conversion decreases.
Example 11–4 Calculating the Adiabatic Equilibrium Temperature and Conversion
For the elementary liquid-phase reaction
A ⇄ B
we want to make a plot of equilibrium conversion as a function of temperature. The reacting species in this industrially important reaction to Jofostan’s economy have been coded with the letters A and B for reasons of National Security and for the company’s proprietary reasons.
Combine the rate law and stoichiometry to write –rA as a function of k, CA0, X, and Xe.
Determine the adiabatic equilibrium temperature and conversion when species A and inert I are fed to the reactor at a temperature of 480 K.
What is the CSTR volume necessary to achieve 90% of the adiabatic equilibrium conversion for CA0 = 1.0 mol/dm3 and υ =0 = 5 dm3/min?
Additional information:†
†Jofostan Journal of Thermodynamic Data, Vol. 23, p. 74 (1999).
Solution
Combine rate law and stoichiometry to write -rA as a function of X
Mole Balance:
Rate Law:
Stoichiometry: Liquid (υ = υ0)
We follow the algorithm given in Table 11-2 except we apply it to a liquid-phase reaction in this case so that the concentrations are written as
At equilibrium: -rA = 0; so
Substituting for Ke in terms of Xe in Equation (E11-4.5) and simplifying
Solving Equation (E11-4.6) for Xe gives
(b) Find adiabatic equilibrium temperature and conversion
Equilibrium Constant: Calculate ΔCP, then Ke(T) as a function of temperature
ΔCP = CPB – CPA = 25 – 25 = 0 cal/mol · K
For ΔCP = 0, the equilibrium constant varies with temperature according to the van’t Hoff relation
Substituting Equation (E11-4.10) into (E11-4.8), we can calculate the equilibrium conversion as a function of temperature:
Equilibrium Conversion from Thermodynamics:
We will use the tutorial in the Chapter 11 LEP “Xe vs. T” on the Web site to generate a figure of the equilibrium conversion as a function of temperature. We do this by “fooling” Polymath by using a dummy independent variable “t” to generate Xe and XEB. Next we change variables in the plotting program to make T the independent variable and Xe and XEB the dependent variables to obtain Figure E11-4.1. See tutorial (http://www.umich.edu/~elements/6e/software/Polymath_fooling_tutorial.pdf).
The calculations are shown in Table E11-4.1.
TABLE E11-4.1 EQUILIBRIUM CONVERSION AS A FUNCTION OF TEMPERATURE
T(K)
Ke
Xe
k (min-1)
298
75,000.00
1.00
0.000035
350
2236.08
1.00
0.000430
400
180.57
0.99
0.002596
450
25.51
0.96
0.010507
500
5.33
0.84
0.032152
550
1.48
0.60
0.080279
620
0.35
0.26
0.225566
Note in this case the equilibrium constant Ke decreases by a factor of 105 and the reaction rate constant k increases by a factor of 104.
Energy Balance:
For a reaction carried out adiabatically, the energy balance, Equation (T11-1.A), reduces to
Conversion calculated from energy balance
Data from Table E11-4.1 and the following data are plotted in Figure E11-4.1.
T(K)
480
525
575
620
XEB
0
0.24
0.51
0.75


Figure E11-4.1 Finding the adiabatic equilibrium temperature (Te) and conversion (Xe). Note: Curve uses approximate interpolated points.
Adiabatic equilibrium conversion and temperature
The intersection of XEB(T) and Xe(T) gives Xe= 0.49 and Te = 572 K.
(C) Calculate the CSTR volume to achieve 90% of the adiabatic equilibrium conversion corresponding to an entering temperature of 480 K
From Figure E11-4.1 we see that for a feed temperature of 480 K, the adiabatic equilibrium temperature is 572 K and the corresponding adiabatic equilibrium conversion is only Xe = 0.49. For X = 0.9 Xe, the exit conversion is
X = 0.9 Xe = 0.9(0.49) = 0.44
From the adiabatic energy balance, the temperature corresponding to X = 0.44 is
T = 562 K
Now calculate V, for T = 562 K, Xe = 0.53, and k = 0.098 min-1
The molar flow rates will not affect the value of the equilibrium conversion!!
Analysis: The purpose of this example is to introduce the concept of the adiabatic equilibrium conversion and temperature. The adiabatic equilibrium conversion, Xe, is one of the first things to determine when carrying out an analysis involving reversible reactions. It is the maximum conversion one can achieve for a given entering temperature, T0, and feed composition. If Xe is too low to be economical, try lowering the feed temperature and/or adding inerts. From Equation (E11-4.6), we observe that changing the flow rate has no effect on the equilibrium conversion. For exothermic reactions, the adiabatic conversion decreases with increasing entering temperature T0, and for endothermic reactions the conversion increases with increasing entering T0. One can easily generate Figure E11-4.1 using Polymath with Equations (E11-4.5) and (E11-4.7).
If adding inerts or lowering the entering temperature is not feasible, then one should consider reactor staging.
11.6 Reactor Staging with Interstage Cooling or Heating
11.6.1 Exothermic Reactions
Conversions higher than those shown in Figure E11-4.1 can be achieved for adiabatic operations by connecting reactors in series with interstage cooling.

Figure 11-5 Reactor in series with interstage cooling.
In Figure 11-5, we show the case of an exothermic adiabatic reaction taking place in a PBR reactor train. The exit temperature from Reactor 1 is very high, 800 K, and the equilibrium conversion, Xe, is low as is the reactor conversion X1, which approaches Xe. Next, we pass the exit stream from adiabatic reactor 1 through a heat exchanger to lower the temperature back to 500 K where Xe is high but X is still low. To increase the conversion, the stream then enters adiabatic reactor 2 where the conversion increases to X2, which is followed by a heat exchanger and the process is repeated.
The conversion–temperature plot for this scheme is shown in Figure 11-6. We see that with three interstage coolers, 88% conversion can be achieved, compared to an equilibrium conversion of 35% for no interstage cooling.
11.6.2 Endothermic Reactions
Typical values for gasoline composition
Another example of the need for interstage heat transfer in a series of reactors can be found when upgrading the octane number of gasoline. The more compact the hydrocarbon molecule for a given number of carbon atoms is, the higher the octane rating (see Section 10.3.5). Consequently, it is desirable to convert straight-chain hydrocarbons to branched isomers, naphthenes, and aromatics. The reaction sequence is
Interstage cooling used for exothermic reversible reactions
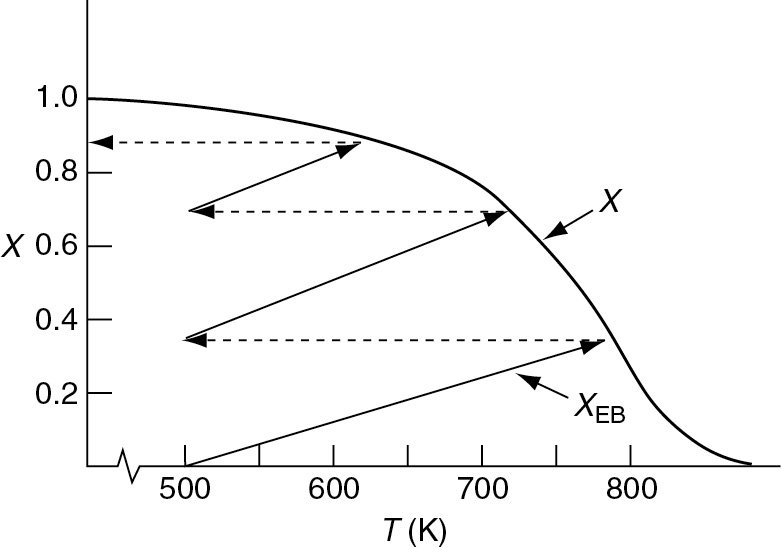
Figure 11-6 Increasing conversion by interstage cooling for an exothermic reaction. Note: Lines and curves are approximate.
Gasoline |
|
C5 |
10% |
C6 |
10% |
C7 |
20% |
C8 |
25% |
C9 |
20% |
C10 |
10% |
C11-C12 |
5% |
The first reaction step (k1) is slow compared to the second step, and each step is highly endothermic. The allowable temperature range for which this reaction can be carried out is quite narrow: Above 530°C undesirable side reactions occur, and below 430°C the reaction virtually does not take place to any reasonable extent due to the slow reaction kinetics. A typical feed stock might consist of 75% straight chains, 15% naphthas, and 10% aromatics.
One arrangement currently used to carry out these reactions is shown in Figure 11-7. Note that the reactors are not all the same size. Typical sizes are on the order of 10–20 m high and 2–5 m in diameter. A typical feed rate of gasoline is approximately 200 m3/h at 2 atm. Hydrogen is usually separated from the product stream and recycled.
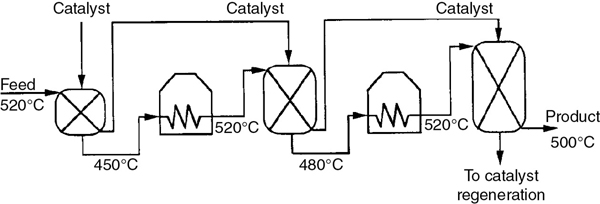
Figure 11-7 Interstage heating for gasoline production in moving-bed reactors.
Summer 2019 $2.85/gal for octane number (ON) ON = 89
Because the reaction is endothermic, the equilibrium conversion increases with increasing temperature. A typical equilibrium curve and temperature conversion trajectory for the reactor sequence are shown in Figure 11-8.
Interstage heating
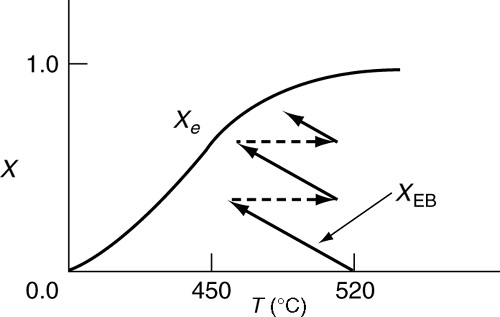
Figure 11-8 Temperature–conversion trajectory for interstage heating of an endothermic reaction analogous to Figure 11-6.
Example 11–5 Interstage Cooling for Highly Exothermic Reactions
What conversion could be achieved in Example 11-4 if two interstage coolers that had the capacity to cool the exit stream to 480 K were available? Next, determine the heat duty of each exchanger for a molar feed rate of A of 40 mol/s. Assume that 95% of the equilibrium conversion is achieved in each reactor. The feed temperature to the first reactor is 480 K.
Solution
Calculate Exit Temperature
For the reaction in Example 11-4, that is,
A ⇄ B
we saw that for an entering temperature of 480 K, the adiabatic equilibrium conversion was 0.49. For 95% of the equilibrium conversion (Xe = 0.49), the conversion exiting the first reactor is 0.47.
The exit temperature is found from a rearrangement of Equation (E11-4.12)
T = 568 K Answer
We will now cool the gas stream exiting the reactor at T1 = 568 K back down to T2 = T0 = 480 K in a heat exchanger (Figure E11-5.1).
Calculate the Heat Load on the Heat Exchanger
The following calculations may be familiar from your course in Heat Transfer. There is no work done on the reaction gas mixture in the exchanger, and the reaction does not take place in the exchanger. Under these conditions , the energy balance given by Equation (11-10)
for s = 0 becomes
Energy balance on the reaction gas mixture in the heat exchanger
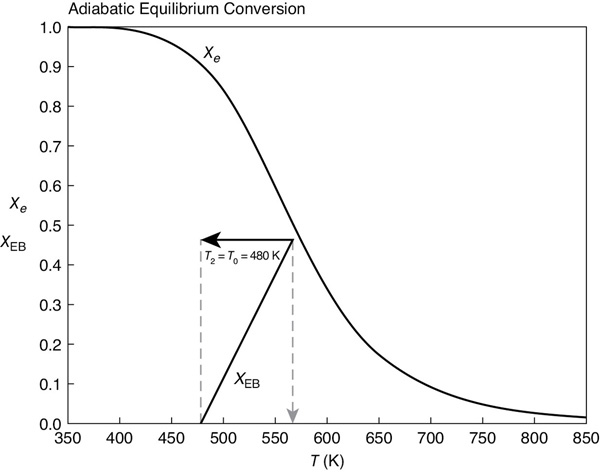
Figure E11-5.1 Reaching 95% of adiabatic equilibrium conversion, that is, X = 0.47, and then cooling back down to 480 K.
But CPA = CPB
Also, for this example, FA0 = FA = FB
Answer
That is, 264 kcal/s must be removed to cool the reacting mixture from 568 K to 480 K for a feed rate of 40 mol/s.
Second Reactor
Now let’s return to determine the conversion in the second reactor. Rearranging Equation (E11-4.12) for the second reactor
The conditions entering the second reactor are T20 = 480 K and X = 0.47. The energy balance starting from this point is shown in Figure E11-5.2. The corresponding adiabatic equilibrium conversion is 0.72. Ninety-five percent of the equilibrium conversion is 68.1% and the corresponding exit temperature is T = 480 = (0.68 = 0.47)186.7 = 519 K.
X = 0.9 Xe = 0.9.0.72
∴X = 0.681
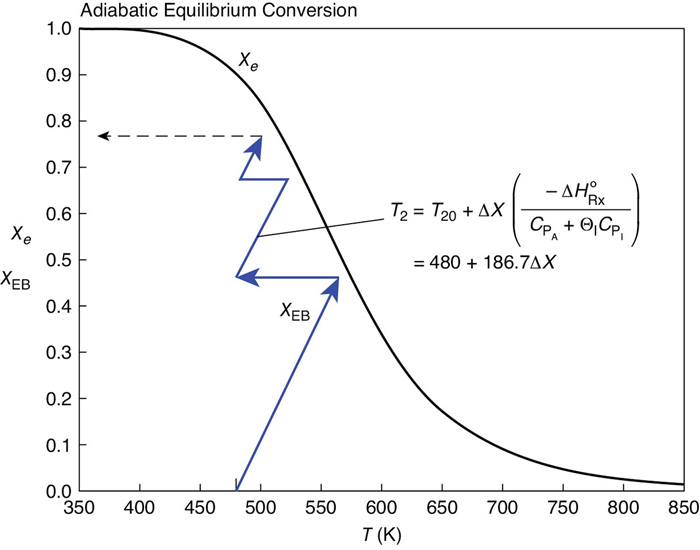
Figure E11-5.2 Three reactors in series with interstage cooling. Note: Curve uses approximate interpolated points.
Heat Load
The heat-exchange duty to cool the reacting mixture from 519 K back to 480 K can again be calculated from Equation (E11-5.5)

Subsequent Reactors
For the third and final reactor, we begin at T0 = 480 K and X = 0.68 and follow the line representing the equation for the energy balance along to the point of intersection with the equilibrium conversion, which is Xe = 0.82. Consequently, the final conversion achieved with three reactors and two interstage coolers is X = (0.95)(0.82) = 0.78.
Analysis: For highly exothermic, reversible reactions carried out adiabatically, reactor staging with interstage cooling can be used to obtain high conversions. One observes that the exit conversion and temperature from the first reactor are 47% and 568 K respectively, as shown by the energy balance line. The exit stream at this conversion is then cooled back down to 480 K where it enters the second reactor. In the second reactor, the overall conversion and temperature increase to 68% and 519 K. The slope of X versus T from the energy balance is the same as the first reactor. This example also showed how to calculate the heat load on each exchanger. We also note that the heat load on the third exchanger will be less than the first exchanger because the exit temperature from the second reactor (519 K) is lower than that of the first reactor (568 K). Consequently, less heat needs to be removed by the third exchanger.
A temperature conflict: High T rapid reaction but low Xe versus low T and slow reaction resulting in low X
11.7 Optimum Feed Temperature
We now consider an adiabatic reactor of fixed size or catalyst weight, and investigate what happens as the feed temperature is varied. The reaction is reversible and exothermic. At one extreme, using a very high feed temperature, the specific reaction rate will be large and the reaction will proceed rapidly, but the equilibrium conversion will be close to zero. Consequently, very little product will be formed. At the other extreme of low feed temperatures, the equilibrium conversion is high. So the question is, “Why not cool the feed to the lowest possible entering temperature, T0?” The answer is that we know at low temperatures the rate constant k is small and it’s possible the reaction would not proceed to any reasonable extent, resulting in virtually no or little conversion. So we have these two extremes, low conversion at high temperatures due to equilibrium limitations and low conversion at low temperatures because of a small rate of reaction. Example 11-6 explores how to find the optimum temperature.
Bonding with Unit Operations and Heat Transfer
Example 11–6 Finding the Optimum Entering Temperature for Adiabatic Operation
We are going to continue the reaction we have been discussing in Examples 11-4 and 11-5.
A ⇄ B
To illustrate the concept of an optimum inlet temperature, we are going to plot the conversion profiles for different inlet temperatures for this isomerization, cf. Figures E11-6.2 and E11-6.3. We will use all the same parameter values and conditions we used in Examples 11-4 and 11-5 and only vary the entering temperature. We start by applying Equations (T11-2.1)–(T11-2.8) to a liquid system so where there is no temperature dependence in the concentration term, that is, CA = CA0 (1 – X). Starting with the mole balance
we combine the mole balance, rate law, and stoichiometry equations to get
We next consider the energy balance for = 0
We will use the data and equations for Xe, k, and XEB as given in Example E11-4.
Changing the Adiabatic Equilibrium Temperature and Conversion
Again using the values of Xe as a function of T in Table E11-4.1 and the energy balance
Figure E11-6.1 shows a plot Xe and XEB on the same plot for different entering temperatures, that is, T0.
We see that for an entering temperature of T0 = 580 K, the adiabatic equilibrium conversion Xe is 0.24, and the corresponding adiabatic equilibrium temperature is 625 K. However, for an entering temperature of T0 = 380 K, Xe is 0.75 and the corresponding adiabatic equilibrium temperature is 520 K.
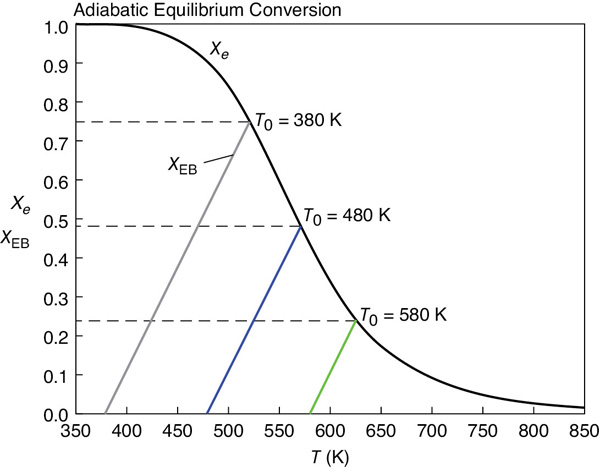
Figure E11-6.1 Adiabatic equilibrium temperature.
Conversion Profiles for X and Xe
To gain insight as to why there is an optimum feed temperature, let’s look at the conversion profiles for different entering temperatures, T0. First we enter the mole balance, rate law, stoichiometric, and energy balance equations (cf. Equations (E11-6.1)–(E11-6.5)) into Polymath, MATLAB, Python, and Wolfram. The Polymath program and output for T0 = 480 K is shown in Table E11-6.1.

TABLE E11-6.1 POLYMATH PROGRAM AND NUMERICAL OUTPUT
Differential equations 1 d(X)/d(V) = –ra/Fao Explicit equations 1 k1 = 0.000035 2 T2 = 298 3 dH = -14000 4 To = 480 5 Cao = 1 6 Fao = 5 7 R = 1.987 8 E = 10000 9 Ke2 = 75000 10 T = To-dH*X/(75) 11 Ke = Ke2*exp((dH/R)*((1/T2)-(1/T))) 12 T1 = 298 13 k = k1*exp((E/R)*((1/T1)-(1/T))) 14 ra = -k*Cao*((1-X)-(X/Ke)) 15 Xe = Ke/(1+Ke) |
|||
POLYMATH REPORT Ordinary Differential Equations Calculated values of DEQ variables |
|||
|
Variable |
Initial value |
Final value |
1 |
Cao |
1. |
1. |
2 |
dH |
-1.4E+04 |
-1.4E+04 |
3 |
E |
10000. |
10000. |
4 |
Fao |
5. |
5. |
5 |
k |
0.0211 0.021138 |
0.1092689 |
6 |
k1 |
3.6e-05 |
3.6e-05 |
7 |
Ke |
9.586656 |
0.9613053 |
8 |
Ke2 |
7.6e+04 |
7.6e+04 |
9 |
R |
1.987 |
1.987 |
10 |
ra |
-0.021138 |
-0.0027641 |
11 |
T |
480. |
569.1776 |
12 |
T1 |
298. |
298. |
13 |
T2 |
298. |
298. |
14 |
To |
480. |
480. |
15 |
V |
0 |
100. |
16 |
X |
0 |
0.477737 |
17 |
Xe |
0.9055415 |
0.4901355 |
The conversion profiles for X and Xe are shown in Figure E11-6.2 for T0 = 480 K where we see they approach each other up to the end of the 100 dm3 reactor.
Because the reactor temperature increases as we move along the reactor, the equilibrium conversion, Xe, also varies and decreases along the length of the reactor, as shown in Figure E11-6.2.
We next plot the corresponding conversion profiles down the length of the reactor for entering temperatures of 380 K, 480 K, and 580 K, as shown in Figure E11-6.3.
For an entering temperature of 580 K, we see that the conversion and temperature increase very rapidly over a short distance (i.e., a small amount of catalyst). This sharp increase is sometimes referred to as the “point” or temperature at which the

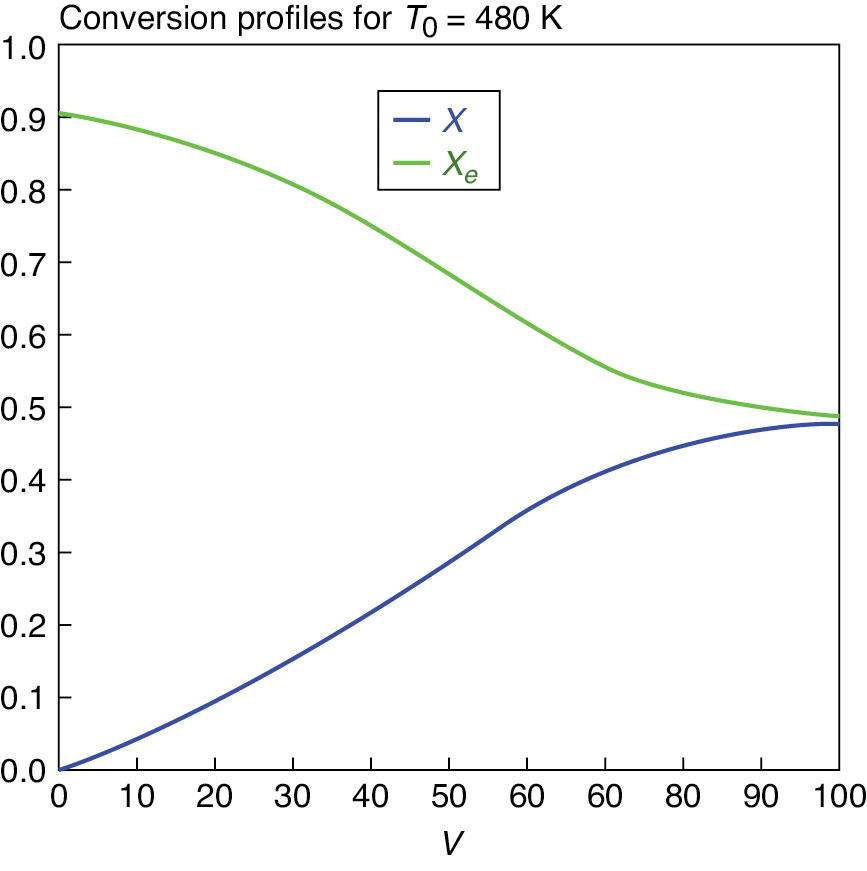
Figure E11-6.2 Conversion profiles.

Figure E11-6.3 Equilibrium conversion for different feed temperatures.
reaction “ignites.” We also observe that the conversion, which is relatively small at a value of 0.24, remains constant from V = 15 dm3 to the reactor exit. If the inlet temperature were lowered to 480 K, the corresponding equilibrium conversion would increase to 0.49; however, the reaction rate is slower at this lower temperature so that this conversion is not achieved until close to the end of the reactor. If the entering temperature were lowered further to 380 K, the corresponding equilibrium conversion would be virtually 1.0, but the rate is so slow that a conversion of 0.03 is achieved for the specified reactor volume of 100 dm3. At very low feed temperatures, the specific reaction rate will be so small that virtually all of the reactant will pass through the reactor without reacting—that is, the reaction never “ignites” and little conversion is achieved.
It is apparent that with conversions close to zero for both high and low feed temperatures, there must be an optimum feed temperature that maximizes conversion. As the feed temperature is increased from a very low value, the specific reaction rate will increase, as will the conversion. The conversion will continue to increase with increasing feed temperature until the equilibrium conversion is approached in the reaction. Further increases in feed temperature for this exothermic reaction will only decrease the conversion due to the decreasing equilibrium conversion. This optimum inlet temperature is shown in Figure E11-6.4.
If we made similar plots for other entering temperatures, we would obtain the conversion at the exit of the reactor shown in Table E11-6.2.
TABLE E11-6.2 EXIT CONVERSION AS A FUNCTION OF ENTERING TEMPERATURE
T0 |
380 |
420 |
460 |
500 |
540 |
580 |
620 |
660 |
X |
0.029 |
0.12 |
0.43 |
0.43 |
0.33 |
0.24 |
0.17 |
0.11 |
These exit conversions are plotted as a function of the entering temperature in Figure E11-6.4.
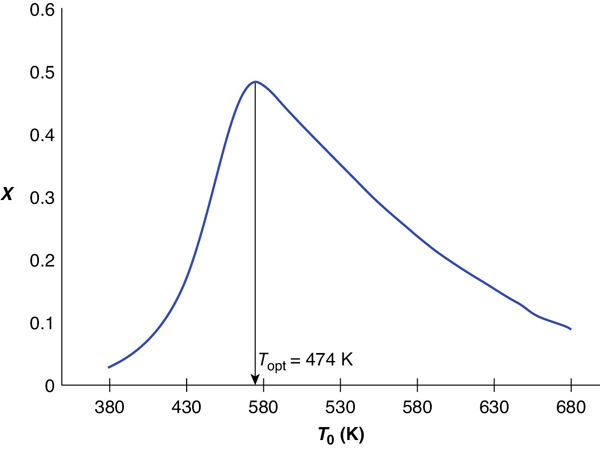
Figure E11-6.4 Conversion at exit of reactor.
Optimum inlet temperature
Analysis: We see that at high temperatures, the rate is rapid and equilibrium is reached near the entrance to the reactor and the conversion will be small. At the other extreme of low temperatures, the reaction never “ignites” and the reactants pass through the reactor virtually unreacted. At entering temperature between these two extremes we find there is an optimum entering feed temperature that maximizes conversion. The optimum feed temperature in this example is 474 K, and the corresponding optimum conversion is 0.48.
11.8 And Now… A Word from Our Sponsor–Safety 11 (AWFOS–S11 Acronyms)
The purpose of this tutorial is to get familiarized with commonly used acronyms in various Incident Investigation reports. Below is a list of acronyms which will be encountered frequently.
TABLE 11-4 ACRONYMS
Acronym |
Meaning |
Link |
AIChE |
American Institute of Chemical Engineers |
|
API |
American Petroleum Institute |
|
BLEVE |
Boiling Liquid Expanding Vapor Explosion |
|
CCPS |
Center for Chemical Process Safety |
|
CSB |
U.S. Chemical Safety and Hazard Investigation Board |
|
DCS |
Distributed Control System |
https://www.electricaltechnology.org/2016/08/distributed-control-system-dcs.html |
EPA |
Environmental Protection Agency |
|
HAZOP |
Hazard and Operability Study |
|
HSE |
Health, Safety and Environment |
https://www.workplacetesting.com/definition/16/health-safety-and-environment-hse |
LOPA |
Layer of Protection Analysis |
https://hseengineer.wordpress.com/lopa-layer-of-protection-analysis/ |
MOC |
Management of Change |
http://www.lni.wa.gov/safety/grantspartnerships/partnerships/vpp/pdfs/vppmocbestpractices.pdf |
MSDS |
Material Safety Data Sheet |
|
NFPA |
National Fire Protection Association |
|
OSHA |
Occupational Safety and Health Administration |
|
PPE |
Personal Protective Equipment |
|
P & IDs |
Piping and Instrumentation Diagrams |
|
PSSR |
Pre-Startup Safety Review |
https://www.chemicalprocessing.com/articles/2018/perform-a-proper-pre-startup-safety-review-5-steps/ |
PRVs |
Pressure Relief Valves |
|
PHA |
Process Hazard Analysis |
|
PSM |
Process Safety Management |
|
RMP |
Risk Management Program |
https://www.osha.gov/chemicalexecutiveorder/psm_terminology.html |
SIL |
Safety Integrity Levels |
https://www.crossco.com/blog/determining-safety-integrity-levels-sil-your-process-application |
SOPs |
Standard Operating Procedures |
Summary
For the reaction
The heat of reaction at temperature T, per mole of A, is
The mean heat capacity difference, ΔCP, per mole of A is
where CPi is the mean heat capacity of species i between temperatures and TR and T.
When there are no phase changes, the heat of reaction at temperature T is related to the heat of reaction at the standard reference temperature TR by
The steady-state energy balance on a system volume V is
We now couple the first four building blocks Mole Balance, Rate Law, Stoichiometry, and Combine with the fifth block, the Energy Balance, to solve nonisothermal reaction engineering problems as shown in the Closure box for this chapter.
For adiabatic operation ( ≡ 0) of a PFR, PBR, CSTR, or batch reactor (BR), and neglecting s, we solve Equation (S11-4) for the adiabatic conversion–temperature relationship, which is
Solving Equation (S11-5) for the adiabatic temperature–conversion relationship:
Using Equation (S11-4), one can solve nonisothermal adiabatic reactor problems to predict the exit conversion, concentrations, and temperature.
CRE Web Site Materials
(http://www.umich.edu/~elements/6e/11chap/obj.html#/)
Living Example Problem Formulated in AspenTech
A step-by-step AspenTech tutorial is given on the CRE Web site.
(http://www.umich.edu/~elements/6e/software/aspen.html)
Questions, Simulations, and Problems
The subscript to each of the problem numbers indicates the level of difficulty: A, least difficult; D, most difficult.
A = • B = ▪ C = ♦ D = ♦♦

In each of the questions and problems, rather than just drawing a box around your answer, write a sentence or two describing how you solved the problem, the assumptions you made, the reasonableness of your answer, what you learned, and any other facts that you want to include. See the Preface for additional generic parts (x), (y), (z) to the home problems.
Before solving the problems, state or sketch qualitatively the expected results or trends.
Questions
Q11-1A i>clicker. Go to the Web site (http://www.umich.edu/~elements/6e/11chap/iclicker_ch11_q1.html) and view at least five i>clicker questions. Choose one that could be used as is, or a variation thereof, to be included on the next exam. You also could consider the opposite case: explaining why the question should not be on the next exam. In either case, explain your reasoning.
Q11-2A Prepare a list of safety considerations for designing and operating chemical reactors. What would be the first four items on your list? For example, what safety concerns would you have for operating a reactor adiabatically? (See www.sache.org and www.siri.org/graphics.) The August 1985 issue of Chemical Engineering Progress may be useful.
Q11-3A Suppose the reaction in Table 11-2 were carried out in BR instead of PFR. What steps in Table 11-2 would be different?
Q11-4A Rework Problem P2-9D for the case of adiabatic operation.
Q11-5A What if you were asked to give an everyday example that demonstrates the principles discussed in this chapter? (Would sipping a teaspoon of Tabasco or other hot sauce be one?)
Q11-6A Example 11-1: What Additional Information Is Required? How would this example change if a CSTR were used instead of a PFR?
Q11-7A Example 11-2: Heat of Reaction. (1) What would the heat of reaction be if 50% inerts (e.g., helium) were added to the system? (2) What would be the % error if the ΔCP term were neglected?
Q11-8A Example 11-3: Adiabatic Liquid-Phase Isomerization of Normal Butane. Can you explain why the CSTR volume is smaller than the PFR volume? Hint: Equation (E11-3.13) may be helpful to your explanation.
Q11-9A Read over the problems at the end of this chapter. Make up an original problem that uses the concepts presented in this chapter. See Problem P5-1B for guidelines.
Q11-10A A new energy efficiency saving device, the Turbo Retro Thermo Encabulator has been previewed in the following YouTube video (https://www.youtube.com/watch?v=RXJKdh1KZ0w). Write a two-sentence evaluation of this device that is currently on sale at a reduced introductory price.
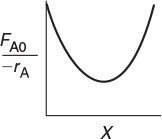
Q11-11A If you were to carry out a hand calculation using Simpson’s integral formula, write a step-by-step procedure to generate a table of X, T, –rA(X), and (FA0/–rb). Are there any circumstances in which you would get a plot similar to the following?
Q11-12A List three things that one should consider in the link to PPE.
Q11-13 Go to the LearnChemE screencasts link for Chapter 11 (http://www.umich.edu/~elements/6e/11chap/learn-cheme-videos.html). View one of the screencast 5- to 6-minute video tutorials and list two of the most important points.
Q11-14 AWFOS-S11 View two of the safety acronym links and write an evaluation as to whether or not the link was useful.
Computer Simulations and Experiments
P11-1A Download the following programs from the CRE Web site where appropriate:
Example Table 11.2: Algorithm for Gas-Phase Reaction
Wolfram and Python
What happens to X and Xe profiles as you vary T0? Can you explain this trend?
Use the base case for all of the variables. Which slider variable—Ke2, k1, or T0—has the greatest effect on the temperature profiles? Which slider variable—EA, FA0, or —has the greatest effect on X and Xe?
Describe how the reaction rate profile changes as you vary T0?
Write three conclusions on what you found in experiments (i)–(iii).
Example 11-3: Adiabatic Isomerization of Balance
Wolfram and Python
Describe how both X and Xe profiles change for each of the parameter values, T0, KC, and CA0.
What is the minimum feed temperature that would give that maximum possible conversion at the end of the reactor?
Vary and describe how the change in the temperature profile changes.
What parameter value—CA0, T0, or yA0—affects the reaction rate profile, -rA, the most, and in which ways does it change?
Write three conclusions on what you found in experiments (i)–(iv)
Polymath
What if the butane reaction were carried out in a 0.8-m3 PFR that can be pressurized to very high pressures? What would be the conversion?
What inlet temperature would you recommend?
Is there an optimum inlet temperature?
Plot the heat that must be removed along the reactor ( vs. V) to maintain isothermal operation.
AspenTech Example 11-3. Download the AspenTech program from the CRE Web site. (1) Repeat using AspenTech. (2) Vary the inlet flow rate and temperature, and describe what you find. (3) Write three conclusions on what you found by exploring this AspenTech example.
Example 11-4: Calculating the Adiabatic Equilibrium Temperature and Conversion
Wolfram and Python
Go to the extremes of the ranges of the sliders and describe how the adiabatic equilibrium conversion changes with each of the variables, T, (–), Ke, and ΘI.
What inlet temperatures, T0, will give the highest and lowest values of the adiabatic equation temperature?
Write a set of three conclusions from your experiments (i) and (ii).
Example 11-5: Interstage Cooling for Highly Exothermic Reactions. (1) Determine the molar flow rate of cooling water (CPw = 18 cal/mol·K) necessary to remove 220 kcal/s from the first exchanger as shown in Figure P11-1A (e). The cooling water enters at 270 K and leaves at 400 K. (2) Determine the necessary heat-transfer area A (m2) for an overall heat transfer coefficient, U, of 100 cal/s·m2·K. You must use the log-mean driving force in calculating .
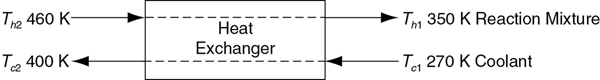
Figure P11-1A (e) Countercurrent heat exchanger.
Example 11-6: Optimum Feed Temperature
Wolfram and Python
Describe what happens to X and Xe as you increase ΘI from 0.3 to 4.
Vary ΔHRxand describe what you find.
Investigate the parameters Ke, k, and ΘI by setting each to their base case values and then vary T0 and describe what you find.
Set Ke2 at its maximum value, 150,000, and then vary T0. What differences do you observe from the base case?
Next set ΘI at 3, and again vary the other parameters. Write a sentence or two saying how each variable affects the optimum feed temperature.
Explain how inert, ΘI, affects the equilibrium conversion and actual conversion.
Write two conclusions about what you found in your experiments (i) through (vi).

Problems
P11-2A For elementary reaction
A ⇄ B
the equilibrium conversion is 0.8 at 127°C and 0.5 at 227°C. What is the heat of reaction?
P11-3B OEQ (Old Exam Question). The equilibrium conversion is shown below as a function of catalyst weight down a PBR.
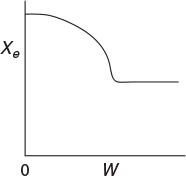
Please indicate which of the following statements are true and which are false. Explain each case.
The reaction could be first-order endothermic and carried out adiabatically.
The reaction is first-order endothermic and the reactor is heated along its length with Ta being constant.
The reaction is second-order exothermic and cooled along the length of the reactor with Ta being constant.
The reaction is second-order exothermic and carried out adiabatically.
P11-4A OEQ (Old Exam Question). The elementary, irreversible, organic liquid-phase reaction
A + B → C
is carried out adiabatically in a flow reactor. An equal molar feed in A and B enters at 27°C, and the volumetric flow rate is 2 dm3/s and CA0 = 0.1 kmol/m3.
Additional information:
PFR
Plot and then analyze the conversion and temperature as a function of PFR volume up to where X = 0.85. Describe the trends.
What is the maximum inlet temperature one could have so that the boiling point of the liquid (550 K) would not be exceeded even for complete conversion?
Plot the heat that must be removed along the reactor ( vs. V) to maintain isothermal operation.
Plot and then analyze the conversion and temperature profiles up to a PFR reactor volume of 10 dm3 for the case when the reaction is reversible with KC = 10 m3/kmol at 450 K. Plot the equilibrium conversion profile. How are the trends different than part (a)? (Ans: When V = 10 dm3 then X = 0.0051, Xeq= 0.517)
CSTR
What is the CSTR volume necessary to achieve 90% conversion?
BR
The reaction is next carried out in a 25 dm3 batch reactor charged with NA0 = 10 moles. Plot the number of moles of A, NA, the conversion, and the temperature as a function of time.
P11-5A The elementary, irreversible gas-phase reaction
A → B + C
is carried out adiabatically in a PFR packed with a catalyst. Pure A enters the reactor at a volumetric flow rate of 20 dm3/s, at a pressure of 10 atm, and a temperature of 450 K.
Additional information:
All heats of formation are referenced to 273 K.
Plot and then analyze the conversion and temperature down the plug-flow reactor until an 80% conversion (if possible) is reached. (The maximum catalyst weight that can be packed into the PFR is 50 kg.) Assume that ΔP = 0.0
Vary the inlet temperature and describe what you find.
Plot the heat that must be removed along the reactor ( vs. V) to maintain isothermal operation.
Now take the pressure drop into account in the PBR with ρb = 1 kg/dm3. The reactor can be packed with one of two particle sizes. Choose one.
α = 0.019/kg-cat for particle diameter D1
α = 0.0075/kg-cat for particle diameter D2
Plot and then analyze the temperature, conversion, and pressure along the length of the reactor. Vary the parameters α and P0 to learn the ranges of values in which they dramatically affect the conversion.
Apply to this problem one or more of the six ideas discussed in Table P-4 in the Complete preface-introduction on the Web site (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf).
P11-6B OEQ (Old Exam Question). The irreversible endothermic vapor-phase reaction follows an elementary rate law
CH3COCH3 → CH2CO + CH4
A → B + C
and is carried out adiabatically in a 500-dm3 PFR. Species A is fed to the reactor at a rate of 10 mol/min and a pressure of 2 atm. An inert stream is also fed to the reactor at 2 atm, as shown in Figure P11-6B. The entrance temperature of both streams is 1100 K.
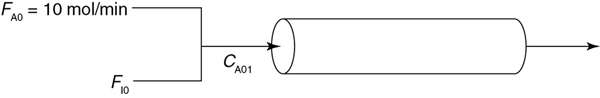
Figure P11-6B Adiabatic PFR with inerts.
Additional information:
k = exp (34.34) – 34222/T)1/s |
CPI 200 J/mol · K |
(T in degrees Kelvin, K) |
|
CPA = 700 J/mol · K |
CPB = 90 J/mol · K |
CPC = 80 J/mol · K |
= 80000 J/mol |
First derive an expression for CA01 as a function of CA0 and ΘI.
Sketch the conversion and temperature profiles for the case when no inerts are present. Using a dashed line, sketch the profiles when a moderate amount of inerts are added. Using a dotted line, sketch the profiles when a large amount of inerts are added. Qualitative sketches are fine. Describe the similarities and differences between the curves.
Sketch or plot and then analyze the exit conversion as a function of ΘI. Is there a ratio of the entering molar flow rates of inerts (I) to A (i.e., ΘI = FI0/FA0) at which the conversion is at a maximum? Explain why there “is” or “is not” a maximum.
What would change in parts (b) and (c) if reactions were exothermic and reversible with = –80 kJ/mol and KC = 2 dm3/mol at 1100 K?
Sketch or plot FB for parts (c) and (d), and describe what you find.
Plot the heat that must be removed along the reactor ( vs. V) to maintain isothermal operation for pure A fed and an exothermic reaction. Part (f) is “C” level of difficulty, that is, Problem P11-6C(f).
P11-7B OEQ (Old Exam Question). The gas-phase reversible reaction
A ⇄ B
is carried out under high pressure in a packed-bed reactor with pressure drop. The feed consists of both inerts I and Species A with the ratio of inerts to the species A being 2 to 1. The entering molar flow rate of A is 5 mol/min at a temperature of 300 K and a concentration of 2 mol/dm3. Work this problem in terms of volume. Hint:. V = W/ρB, rA = ρBr′A.
Additional information:
FA0 = 5.0 mol/min |
KC = 1000 at 300 K |
Ta0 = 300 K |
CA0 = 2 mol/dm3 |
CPB = 160 cal/mol/K |
V = 40 dm3 |
CI = 2CA0 |
ρB = 1.2 kg/dm3 |
αρb = 0.02 dm–3 |
CFI = 18 cal/mol/K |
T00 = 300 K |
Coolant |
CPA = 160 cal/mol/K |
Tt = 300 K |
C = 50 mol/min |
E = 10000 cal/mol |
k1 = 0.1 min–1 at 300 K |
CPCool = 20 cal/mol/K |
ΔHRx = –20000 cal/mol |
Ua = 150 cal/dm3/min/K |
Adiabatic Operation. Plot X, Xe, p, T, and the rate of disappearance as a function of V up to V = 40 dm3. Explain why the curves look the way they do.
Vary the ratio of inerts to A (0≤ΘI≤10) and the entering temperature, and describe what you find.
Plot the heat that must be removed along the reactor ( vs. V) to maintain isothermal operation. Part (c) is “C” level of difficulty.
We will continue this problem in Chapter 12.
P11-8B Algorithm for reaction in a PBR with heat effects
The elementary gas-phase reaction
A + B ⇄ 2C
is carried out in a packed-bed reactor. The entering molar flow rates are FA0 = 5 mol/s, FB0 = 2FA0, and FI = 2FA0 with CT0 = 0.3 mol/dm3. The entering temperature is 330 K.
Additional information:
KC = 1000 @298 K
Note: This problem is continued in Problem P12-1A with some of the entering conditions (e.g., FB0) modified.
Write the mole balance, the rate law, KC as a function of T, k as a function of T, and CA, CB, CC as a function of X, p, and T.
Write the rate law as a function of X, p, and T.
Show the equilibrium conversion is
and then plot Xe versus T.
What are ΣΘiCPi, ΔCP, T0, entering temperature T1 (rate law), and T2 (equilibrium constant)?
Write the energy balance for adiabatic operation.
Case 1 Adiabatic Operation. Plot and then analyze Xe, X, p, and T versus W when the reaction is carried out adiabatically. Describe why the profiles look the way they do. Identify those terms that will be affected by inerts. Sketch what you think the profiles Xe, X, p, and T will look like before you run the Polymath program to plot the profiles. (Ans: At W = 800 kg then X = 0.3583)
Plot the heat that must be removed along the reactor ( vs. V) to maintain isothermal operation. Part (g) is “C” level of difficulty, that is, Problem P11-8C(g).
P11-9A The reaction
A + B ⇄ C + D
is carried out adiabatically in a series of staged packed-bed reactors with interstage cooling (see Figure 11-5). The lowest temperature to which the reactant stream may be cooled is 27°C. The feed is equal molar in A and B, and the catalyst weight in each reactor is sufficient to achieve 99.9% of the equilibrium conversion. The feed enters at 27°C and the reaction is carried out adiabatically. If four reactors and three coolers are available, what conversion may be achieved?
Additional information:
30000 cal/mol A |
CPA = CPB = CPC = CPD = 25 cal/mol · K |
Ke (50°C) = 500000 |
FA0 = 10 mol A/min |
First prepare a plot of equilibrium conversion as a function of temperature. (Partial ans: T = 360 K, Xe = 0.984; T = 520 K, Xe = 0.09; T = 540 K, Xe = 0.057)
P11-10A Figure P11-10A shows the temperature–conversion trajectory for a train of reactors with interstage heating. Now consider replacing the interstage heating with injection of the feed stream in three equal portions, as shown in Figure P11-10A:
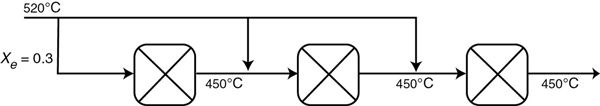
Figure P11-10A
Sketch the temperature–conversion trajectories for (a) an endothermic reaction with entering temperatures as shown, and (b) an exothermic reaction with the temperatures to and from the first reactor reversed, that is, T0 = 450°C.
Supplementary Reading
An excellent development of the energy balance is presented in
R. ARIS, Elementary Chemical Reactor Analysis. Upper Saddle River, NJ: Pearson, 1969, Chaps. 3 and 6.
A number of example problems dealing with nonisothermal reactors may or may not be found in
THORNTON W. BURGESS, The Adventures of Old Man Coyote. New York: Dover Publications, Inc., 1916.
JOHN B. BUTT, Reaction Kinetics and Reactor Design, Revised and Expanded, 2nd ed. New York: Marcel Dekker, Inc., 1999.
S. M. WALAS, Chemical Reaction Engineering Handbook of Solved Problems. Amsterdam: Gordon and Breach, 1995. See the following solved problems: 4.10.1, 4.10.08, 4.10.09, 4.10.13, 4.11.02, 4.11.09, 4.11.03, 4.10.11.
For a thorough discussion on the heat of reaction and equilibrium constant, one might also consult
K. G. DENBIGH, Principles of Chemical Equilibrium, 4th ed. Cambridge: Cambridge University Press, 1981.
The heats of formation, H(T), Gibbs free energies, Ge(TR), and the heat capacities of various compounds can be found in
D. W. GREEN, and R. H. PERRY, eds., Chemical Engineers’ Handbook, 8th ed. New York: McGraw-Hill, 2008.
R. C. REID, M. PRAUSNITZ, and T. K. SHERWOOD, The Properties of Gases and Liquids, 3rd ed. New York: McGraw-Hill, 1977.
R. C. WEAST, ed., CRC Handbook of Chemistry and Physics, 94th ed. Boca Raton, FL: CRC Press, 2013.