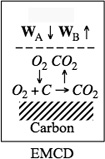
14. Mass Transfer Limitations in Reacting Systems
Giving up is the ultimate tragedy.
—Robert J. Donovan
or
It ain’t over ’til it’s over.
—Yogi Berra NY Yankees
The Algorithm
1. Mole balance
2. Rate law
3. Stoichiometry
4. Combine
5. Evaluate
14A Mass Transfer Fundamentals
14.1 Diffusion Fundamentals
The first step in our CRE algorithm is the mole balance, which we now need to extend to include the molar flux, WAz, and diffusional effects. The molar flow rate of A in a given direction, such as the z direction down the length of a tubular reactor, is just the product of the flux, WAz (mol/m2 • s), and the cross-sectional area, Ac (m2); that is,
FAz = Ac WAz
In the previous chapters, we have only considered plug flow with no diffusion superimposed, in which case
We now drop the plug-flow assumption and extend our discussion of mass transfer in catalytic and other mass transfer-limited reactions. In Chapter 10, we focused on the middle three steps (3, 4, and 5) in a catalytic reaction shown in Figure 14-1 and neglected steps (1), (2), (6), and (7) by assuming the reaction was surface-reaction limited. In this chapter, we describe the first and last steps (1) and (7), as well as showing other applications in which mass transfer plays a role.

Figure 14-1 Steps in a heterogeneous catalytic reaction.
Where are we going??†
† “If you don’t know where you are going, you’ll probably wind up some place else.” Yogi Berra, NY Yankees
We want to arrive at the mole balance that incorporates both diffusion and reaction effects, such as Equation (14-17) on page 746, that is,
We begin with Section 14.1.1 where we write the mole balance on Species A in three dimensions in terms of the molar flux, WA. In Section 14.1.2 we write WA in terms of the bulk flow of A in the fluid, BA and the diffusion flux JA of A that is superimposed on bulk flow. In Section 14.1.3 we use the previous two subsections as a basis to finally write the molar flux, WA, in terms of concentration using Fick’s first law, JA, and the bulk flow, BA. Next, in Section 14.2 we combine diffusion convective transport and reaction in our mole balance.
14.1.1 Definitions
Diffusion is the spontaneous intermingling or mixing of atoms or molecules by random thermal motion. It gives rise to motion of the species relative to motion of the mixture. In the absence of other gradients (such as temperature, electric potential, or gravitational potential), molecules of a given species within a single phase will always diffuse from regions of higher concentrations to regions of lower concentrations. This gradient results in a molar flux of the species (e.g., A), WA (moles/area=time), in the direction of the concentration gradient. The flux of A, WA, is relative to a fixed coordinate (e.g., the lab bench) and is a vector quantity with typical units of mol/m2 · s. In rectangular coordinates
WA = iWAx + jWAy + kWAz
We now apply the mole balance to species A, which flows and reacts in an element of volume ΔV = ΔxΔyΔz to obtain the variation of the molar fluxes in three dimensions.
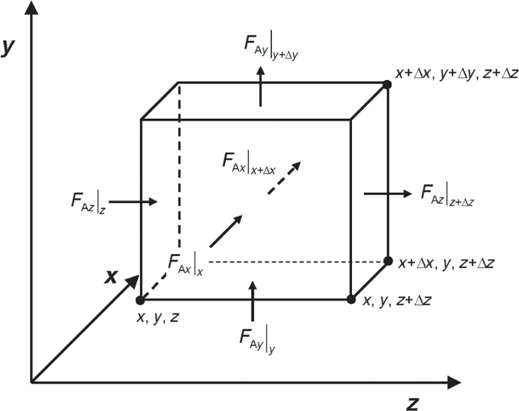
FAz = WAzΔxΔy
FAy = WAyΔxΔz
FAx = WAxΔzΔy
Mole balance
where rA is the rate of generation of A by reaction per unit volume (e.g., mol/m3/h).
Dividing by ΔxΔyΔz and taking the limit as they go to zero, we obtain the molar flux balance in rectangular coordinates
The corresponding balance in cylindrical coordinates with no variation in the rotation about the z-axis is
COMSOL
We will now evaluate the flux terms WA. We have taken the time to derive the molar flux equations in this form because they are now in a form that is consistent with the partial differential equation (PDE) solver COMSOL, which is accessible from the CRE Web site.
14.1.2 Molar Flux: WA
The molar flux of A, WA, is the result of two contributions: JA, the molecular diffusion flux relative to the bulk motion of the fluid produced by a concentration gradient, and BA, the flux resulting from the bulk motion of the fluid:
Total flux = diffusion + bulk motion
The bulk-flow term for species A is the total flux of all molecules relative to a fixed coordinate times the mole fraction of A, yA; that is, BA = yA Σ Wi.
For a two-component system of A diffusing in B, the flux of A is
The diffusional flux, JA, is the flux of A molecules that is superimposed on the bulk flow. It tells how fast A is moving ahead of the bulk flow velocity, that is, the molar average velocity.
The flux of species A, WA, is wrt a fixed coordinate system (e.g., the lab bench) and is just the concentration of A, CA, times the particle velocity of species A, UA, at that point
By particle velocities, we mean the vector average of millions of molecules of A at a given point. Similarly for species B: WB = UBCB; substituting into the bulk-flow term
BA = yA Σ Wi = yA (WA + WB) = yA (CA UA + CBUB
Writing the concentration of A and B in the generic form in terms of the mole fraction, yi, and the total concentration, c, that is, Ci = yic, and then factoring out the total concentration, c, the bulk flow, BA, is
BA = (c yA)(yA UA + yB UB) = CA U
Molar average velocity U
where U is the molar average velocity: U = Σyi Ui. The molar flux of A can now be written as
We now need to determine the equation for the molar flux of A, JA, that is superimposed on the molar average velocity.
14.1.3 Fick’s First Law
Experimentation with frog legs led to Fick’s first law.
Our discussion on diffusion will be restricted primarily to binary systems containing only species A and B. We now wish to determine how the molar diffusive flux of a species (i.e., JA) is related to its concentration gradient. As an aid in the discussion of the transport law that is ordinarily used to describe diffusion, recall similar laws from other transport processes. For example, in conductive heat transfer the constitutive equation relating the heat flux q and the temperature gradient is Fourier’s law, q = −kt ∇ T, where kt is the thermal conductivity.
Constitutive equations in heat, momentum, and mass transfer
In rectangular coordinates, the gradient is in the form
The mass transfer law for the diffusional flux of A resulting from a concentration gradient is analogous to Fourier’s law for heat transfer and is given by Fick’s first law†
† Adolf Fick was an interesting character as evidenced by his use of frogs to study diffusion.
DAB is the diffusivity of A in B . Combining Equations (14-5) and (14-6), we obtain an expression for the molar flux of A in terms of concentration for constant total concentration
Molar flux equation
In one dimension, that is, z, the molar flux term is
Where Uz is the axial velocity, in radial coordinates with no variations in the angular (θ) direction
Where Ur is the fluid’s radial velocity
14.2 Binary Diffusion
Although many systems involve more than two components, the diffusion of each species can be treated as if it were diffusing through another single species rather than through a mixture by defining an effective diffusivity.
14.2.1 Evaluating the Molar Flux
Now the task is to evaluate the bulk-flow term.
We now consider A diffusing in B. Substituting Equation (14-6) into Equation (14-4) we obtain
Before going to Section 14.2.2 it would be helpful to evaluate the bulk flow term (yA (WA + WB)) for five limiting situations. These situations are given by Equations 14-10 through 14-13 in Table 14-1 on page 745.
TABLE 14-1 EVALUATING WA FOR SPECIES A DIFFUSING IN SPECIES B
|
1 C. N. Satterfield, Mass Transfer in Heterogeneous Catalysis, Cambridge: MIT Press, 1970, pp. 41–42, discusses Knudsen flow in catalysis and gives the expression for calculating DK.
14.2.2 Diffusion and Convective Transport
When accounting for diffusional effects, the molar flow rate of species A, FA, in a specific direction z, is the product of molar flux in that direction, WAz, and the cross-sectional area normal to the direction of flow, Ac
FAz = AcWAz
In terms of concentration, the flux is
The molar flow rate is
Similar expressions follow for WAx and WAy. Substituting for the flux WAx, WAy, and WAz into Equation (14-1), we obtain
Flow, diffusion, and reaction
This form is used in COMSOL Multiphysics.
In terms of axial and radial coordinates with no angular variation and no radial velocity Ur we have
Equation (14-15) and (14-16) are in a user-friendly form to apply to the PDE solver, COMSOL. For one dimension at steady state, Equation (14-15) reduces to
In order to solve Equation (14-17) we need to specify the boundary conditions. In this chapter we will consider some of the simple boundary conditions, and in Chapter 18 we will consider the more complicated boundary conditions, such as the Danckwerts’ boundary conditions.
We will now use this form of the molar flow rate in our mole balance in the z direction of a tubular flow reactor
However, we first have to discuss the boundary conditions in solving this equation.
14.2.3 Boundary Conditions
The most common boundary conditions are presented in Table 14-2.
TABLE 14-2 TYPES OF BOUNDARY CONDITIONS
|
14.2.4 Temperature and Pressure Dependence of DAB
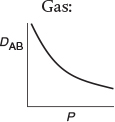
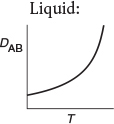
Before closing this brief discussion on mass-transfer fundamentals, further mention should be made of the diffusion coefficient. Equations for predicting gas diffusivities are given by Fuller and are also given in Perry’s Handbook.2,3 The orders of magnitude of the diffusivities for gases, liquids, and solids and the manner in which they vary with temperature and pressure are given in Table 14-3.4 We note that the Knudsen, liquid, and solid diffusivities are independent of total pressure.
2 E. N. Fuller, P. D. Schettler, and J. C. Giddings, Ind. Eng. Chem., 58(5), 19 (1966).
3 R. H. Perry and D. W. Green, Chemical Engineer’s Handbook, 7th ed. New York: McGraw-Hill, 1999.
4 To estimate liquid diffusivities for binary systems, see K. A. Reddy and L. K. Doraiswamy, Ind. Eng. Chem. Fund., 6, 77 (1967).
TABLE 14-3 DIFFUSIVITY RELATIONSHIPS FOR GASES, LIQUIDS, AND SOLIDS
|
Order of Magnitude |
|
|
|---|---|---|---|
| Phase | cm2/s | m2/s | Temperature and Pressure Dependencesa |
| Gas | |||
| Bulk | 10−1 | 10−5 | |
| Knudsen | 10−2 | 10−6 | |
| Liquid | 10−5 | 10−9 | |
| Solid | 10−9 | 10−13 | |
aμ1, μ2, liquid viscosities at temperatures T1 and T2, respectively; ED, diffusion activation energy.
It is important to know the magnitude and the T and P dependence of the diffusivity.
14.3 Modeling Diffusion with Chemical Reaction
We now consider the situation where species A does not react as it diffuses through a hypothetical stagnant film to a surface where it does react. In Chapter 15 we consider the case when species A does react as it diffuses through a stagnant film. Table 14-4 provides an algorithm for both of these situations.
TABLE 14-4 STEPS IN MODELING CHEMICAL SYSTEMS WITH DIFFUSION AND REACTION
|
aIn some instances it may be easier to integrate the resulting differential equation in Step 4 before substituting for WA.
The purpose of presenting algorithms (e.g., Table 14-4) to solve reaction engineering problems is to give the readers a starting point or framework with which to work if they were to get stuck. It is expected that once readers are familiar and comfortable using the algorithm/framework, they will be able to move in and out of the framework as they develop creative solutions to nonstandard chemical reaction engineering problems.
Use Table 14-4 to Move In ⇄ Out of the algorithm (Steps 1 → 10) to generate creative solutions.
14.3.1 Diffusion through a Stagnant Film to a Particle
We will first discuss the diffusion of reactants from the bulk fluid to the external surface of a particle that is either solid or liquid. Here our attention will focus on the flow past a single particle as shown in Figure 14-2(a) and its corresponding boundary layer shown in Figure 14-2(b). The particle can be either a liquid droplet, a catalyst pellet or a combustible solid grain. The reaction takes place only on the external surface and not in the fluid surrounding it. The fluid velocity in the vicinity of the spherical pellet will vary with position around the sphere. The hydrodynamic boundary layer is usually defined as the distance from a solid object to where the fluid velocity is 99% of the bulk velocity, U0. Similarly, the mass transfer boundary-layer thickness, =, is defined as the distance from a solid object to where the concentration of the diffusing species reaches 99% of the bulk concentration.
A reasonable representation of the concentration profile for a reactant A diffusing to the external surface is shown in Figure 14-2. As illustrated, the change in concentration of A from CAb to CAs takes place in a very narrow fluid layer next to the surface of the sphere. Nearly all of the resistance to mass transfer is found in this layer.

Figure 14-2 Boundary layer around the surface of spherical particle.
The concept of a hypothetical stagnant film within which all the resistance to external mass transfer exists
A useful way of modeling diffusive transport is to treat the fluid layer next to a solid boundary as a hypothetical stagnant film of thickness δ, which we cannot measure. We say that all the resistance to mass transfer is found (i.e., lumped) within this hypothetical stagnant film of thickness δ, and the properties (i.e., concentration, temperature) of the fluid at the outer edge of the film are identical to those of the bulk fluid. This model can readily be used to solve the differential equation for diffusion through a stagnant film. The dashed line in Figure 14-2(b) represents the concentration profile predicted by the hypothetical stagnant film model, while the solid line gives the actual profile. If the film thickness is much smaller than the radius of the pellet (which is usually the case), curvature effects can be neglected. As a result, only the one-dimensional diffusion equation must be solved, as shown in Figure 14-3.
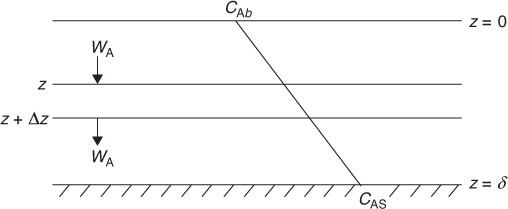
Figure 14-3 Concentration profile for dilute concentration in stagnant film mode.
We are going to carry out a mole balance on species A diffusing through the fluid between z = z and z = z + Δz at steady state for the unit cross-sectional area, Ac
dividing by Δz and taking the limit as Δz → 0
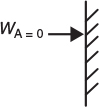
For diffusion through a stagnant film at dilute concentrations
or for EMCD, we have using Fick’s first law
Substituting for WAz and dividing by DAB we have
Integrating twice to get CA = K1z + K2, using the boundary conditions at
we obtain the concentration profile
To find the flux to the surface we substitute Equation (14-25) into Equation (14-24) to obtain
At steady state the flux of A to the surface will be equal to the rate of reaction of A on the surface. We also note that another example of diffusion through a stagnant film as applied to transdermal drug delivery is given in the Chapter 14 Expanded Material on the CRE Web site.
14.4 The Mass Transfer Coefficient
We now interpret the ratio (DAB/δ ) in Equation (14-26).
While the boundary-layer thickness will vary around the sphere, we will take it to have a mean film thickness δ. The ratio of the diffusivity DAB to the film thickness δ is the mass transfer coefficient, kc, that is,
Combining Equations (14-26) and (14-27), we obtain the average molar flux from the bulk fluid to the surface
The mass transfer coefficient
In this stagnant film model, we consider all the resistance to mass transfer to be lumped into the thickness δ. The reciprocal of the mass transfer coefficient can be thought of as this resistance
Molar flux of A to the surface
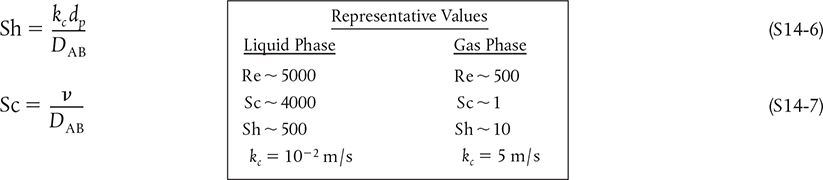
How Do I find the Mass Transfer Coefficient? The mass transfer coefficient is found either by experimentation or from correlations analogous to what one finds for a heat transfer coefficient. These correlations are usually in the form of the Sherwood number, Sh, as a function of the Reynolds number, Re, and the Schmidt number, Sc, that is,
where
Sherwood
Schmidt
Reynolds
where
″L″ is the characteristic length (m) (e.g., dP, diameter of the particle)
υ = Kinematic viscosity (m2/s) = μ/ρ
μ = viscosity (kg/m · s)
ρ = density (kg/m3)
U = free stream velocity (m/s)
DAB = diffusivity (m2/s)
As an example, the mass transfer coefficient for flow around a single spherical particle can be found from the Frössling correlation.†
†N. Frössling, Gerlands Beitr. Geophys., 52, 170 (1938).
For turbulent flow, the number 2 in this equation can be neglected with respect to the second term and the resulting correlation is shown in Table 14-5.‡
‡ Just out… An article in August 2019 issue of the AIChE Journal expands this correlation. Y. Wang and J G. Brasseur, “Enhancement of mass transfer from particles by local shear-rate and correlations with application to drug dissolution,” AIChE J., 65 (8), (August 2019). Equation (14-34) is derived for dissolution in an infinite fluid while Professor Wang’s article AIChE J. discusses dissolution in a confined domain. The corrections are particularly important at low Reynolds number and this article guides you through these corrections. However, the nomenclature is different. For example, the Sherwood number is given as Sh = R/δ, so it might help if you referred to Professor Wang’s earlier article 2012 Y. Yang, et.al., Mol. Pharm., 9, 1052 (2012).
The correlation given for low Re is reported as
Sh = Sh0 + 0.0177 Re0.46 Sc0.68
where Sh0 is a function of the Schmidt number Sc. For example, when
5 < Sc < 100
then
Sh0 = 1.2 + Sc0.82
Other values of Sh0 are given in Professor Wang’s article.
After calculating the numerical value of Sh, given the parameters to calculate Re and Sc, the mass transfer coefficient can be calculated
Correlation for geometries other than a single spherical particle are given in Table 14-5.
TABLE 14-5 MASS TRANSFER CORRELATIONS
Turbulent flow, mass transfer to pipe wall |
Sh = .332 (Re)1/2 (Sc)1/3 |
Mass transfer to a single sphere |
Sh = 2 = 0.6 Re1/2 Sc1/3 |
Mass transfer in fluidized beds |
|
Mass transfer to packed beds |
ϕJD = 0.453 Re0.453 |
What if I cannot find the mass transfer correlation for my situation or geometry? In this case, see whether a correlation for the heat transfer coefficient exists and then go to (http://www.umich.edu/~elements/6e/14chap/obj.html#/additional-materials/) to learn how to turn that heat transfer correlation into a mass transfer correlation.
The Sherwood, Reynolds, and Schmidt numbers are used in forced convection mass transfer correlations.
14B Applications
14.5 Mass Transfer to a Single Particle
Mass transfer to single particles is important in catalytic reactions and in dust explosions. In this section we consider two limiting cases of diffusion and reaction on a catalyst particle.5 In the first case, the reaction is so rapid that the rate of diffusion of the reactant to the surface limits the reaction rate. In the second case, the reaction is so slow that virtually no concentration gradient exists in the gas phase (i.e., rapid diffusion with respect to surface reaction).
5 A comprehensive list of correlations for mass transfer to particles is given by G. A. Hughmark, Ind. Eng. Chem. Fund., 19(2), 198 (1980).
14.5.1 First-Order Rate Laws
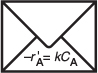
To easily show the limitations of mass transfer and reaction rate we will consider first order kinetics. For the case of either the burning of a combustible dust particle or for reaction on a catalyst surface at high temperatures, the rate law is taken as apparent first order.
Figure 14-4 shows the mass transfer flux of A to the surface, WA, the reaction on the surface , and the mass transfer flux of B away from the surface. In the examples discussed here species A can be thought of as oxygen and B as the combustion products, for example, CO2. The reaction on the surface is taken as apparent first order.
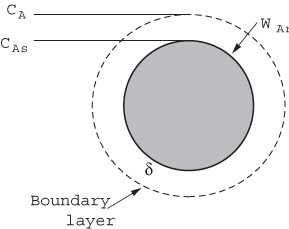
Figure 14-4 Diffusion to, and reaction on, external surface of pellet.
Using boundary conditions 2b. and 2c. in Table 14-2, we obtain
The concentration at the surface, CAs, is not as easily measured as is the bulk concentration, CA. Consequently, we need to eliminate CAs from the equation for the flux and rate of reaction. Solving Equation (14-38) for CAs yields
and the rate of reaction on the surface becomes
Molar flux of A to the surface is equal to the rate of consumption of A on the surface.
One will often find the flux to or from the surface written in terms of an effective transport coefficient keff
where
We will now consider the extremes of rapid and slow reaction at the particle surface.
14.5.2 Limiting Regimes
Rapid Reaction. We first consider how the overall rate of reaction may be increased when the rate of mass transfer to the surface limits the overall rate of reaction. Under these circumstances, the specific reaction-rate constant is much greater than the mass transfer coefficient
and
keff = kc
To increase the rate of reaction per unit surface area of a solid sphere, one must increase CA and/or kc. In this gas-phase catalytic reaction example, and for most liquids, the Schmidt number is sufficiently large that the number 2 in Equation (14-34) is negligible with respect to the second term when the Reynolds number is greater than 25. As a result, Equation (14-34) gives
It is important to know how the mass transfer coefficient varies with fluid velocity, particle size, and physical properties.
Mass Transfer Limited
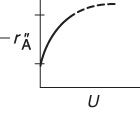
Term 1 is a function of the physical properties DAB and ν, which depend on temperature and pressure only. The diffusivity always increases with increasing temperature for both gas and liquid systems. However, the kinematic viscosity ν increases with temperature (ν ∝ T3/2) for gases and decreases exponentially with temperature for liquids. Term 2 is a function of flow conditions and particle size. Consequently, to increase kc and thus the overall rate of reaction per unit surface area, one may either decrease the particle size or increase the velocity of the fluid flowing past the particle. For this particular case of flow past a single sphere, we see that if the velocity is doubled, the mass transfer coefficient and consequently the rate of reaction is increased by a factor of
(U2/U1)0.5 = 20.5 = 1.41 or 41%
Reaction Rate Limited
Slow Reaction. Here, the specific reaction-rate constant is small with respect to the mass transfer coefficient
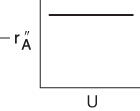
Mass transfer effects are not important when the reaction rate is limiting.
The specific reaction rate is independent of the velocity of fluid and for the solid sphere considered here, independent of particle size. However, for porous catalyst pellets, kr may depend on particle size for certain situations, as shown in Chapter 15.
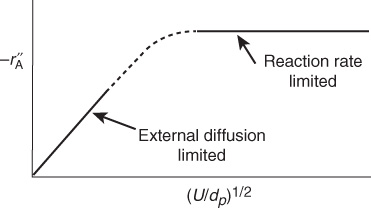
Figure 14-5 shows the variation in reaction rate with Term 2 in Equation (14-45), the ratio of velocity to particle size. At low velocities, the mass transfer boundary-layer thickness is large and diffusion limits the reaction. As the velocity past the sphere is increased, the boundary-layer thickness decreases, and the mass transfer across the boundary layer no longer limits the rate of reaction. One also notes that for a given (i.e., fixed) velocity, reaction-limiting conditions can be achieved by using very small particles. However, the smaller the particle size, the greater the pressure drop in a packed bed. When one is obtaining reaction-rate data in the laboratory, one must operate at sufficiently high velocities or sufficiently small particle sizes to ensure that the reaction is not mass transfer-limited when collecting data.
When collecting rate-law data, operate in the reaction-limited region.
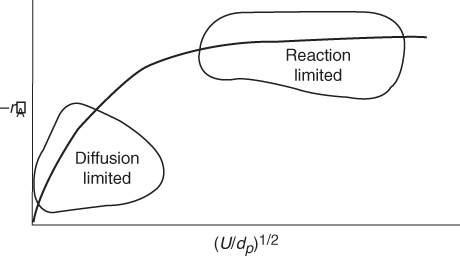
Figure 14-5 Regions of mass transfer-limited and reaction-limited reactions.
Before we analyze Equation (14-40) in more detail, we will describe examples involving a dust particle fire of granular carbon in air and a catalyst particle in a liquid slurry. In both cases we will use kr >> ks so that .
Example 14–1 Mass Transfer of Oxygen to a Burning Carbon Particle
A schematic of the transfer of oxygen to the particle is shown in Figure 14-4. The gas-phase diffusivity in the flame (1000 K) surrounding the particle is taken as 10–4 m2/s and the particle diameter is 50 μm (5 × 10–5 m). The concentration of oxygen in the bulk air (21% at 1 atm and 298 K) is 8.58 mol/m3. The reaction is virtually instantaneous so that the concentration of oxygen on the surface of the particle is zero, that is, CAs = 0. The heat of reaction is ΔHRx = –93.5 kJ/mol carbon.
Calculate molar flux, WA (mol/m2/s) and molar flow (mol/s) to the particle. For a dust cloud density of 200 g/m3, calculate the heat generated per particle , and the heat generated per volume of cloud.
Solution
The flux to the surface is
For particles that are sufficiently small such that they are in Stoke’s flow, (i.e., it follows the fluid) the fluid velocity relative to the particle is zero, (i.e., U ≌ 0). For a single particle in Stoke’s flow, the Sherwood number for a spherical particle
Sh = 2 + 0.61 (Re1/2 Sc1/3) ≅ 2
reduces to
The corresponding molar flux of oxygen to the surface where it reacts virtually instantaneously (CAs = 0) at the surface
The molar flux (mol/m2/s) of oxygen to one particle is
The molar flow (mol/s) of oxygen, , to one particle with surface area AP is
Heat Effects
Now let’s estimate the heat generated for this burning particle and then for the dust cloud with np, the number of particles per unit volume. Figure 8.40 of Ogle† gives the minimum dust explosion concentration, in air, Ccloud, as approximately 200 g/m3. The mass of one 50 μm carbon dust particle is
The corresponding particle concentration is
The heat generated by a single particle (kJ/particle) is just the molar flux of O2 (i.e., ) times the surface area of particle times the heat of reaction, that is,
The heat generated per unit volume of dust cloud, , with a concentration of dust particles, nP, is
The rate of heat generated per particle, , times the particle concentration, nP, gives the heat generated per unit volume of dust as
Analysis: We calculated the molar flux as well as the mass flow of oxygen to the surface of a burning particle. The molar flux of O2 to the surface is equal to the rate of reaction per unit surface area. Knowing the reaction rate, the dust density, and the heat of reaction we calculated the heat generated per particle and per unit volume of dust cloud.
We will continue our discussion on single dust particles when we discuss the shrinking core model. However, let’s first make a comparison with a larger particle, 0.1 cm, suspended in a body of flowing liquid.
Example 14–2 Rapid Liquid-Phase Reaction on the Surface of a Catalyst
Calculate the molar flux, WAr, of reactant A to a single catalyst pellet 1 cm in diameter suspended in a large body of liquid B. The reactant is present in dilute concentrations, and the reaction is considered to take place instantaneously at the external pellet surface (i.e., CAs ≃ 0). The bulk concentration of the reactant A is 1.0 M, and the free-stream liquid velocity past the sphere is 0.1 m/s. The kinematic viscosity (i.e., ) is 0.5 centistoke (cS; 1 centistoke = 10-6 m2/s), and the liquid diffusivity o A in B is DAB = 10–10 m2/s at 300 K.
If the surface reaction is rapid, then diffusion limits the overall rate.
Solution
For dilute concentrations of the solute, the radial flux is
Because reaction is assumed to occur instantaneously on the external surface of the pellet, CAs = 0. Also, CAb is given as 1 mol/dm3.
The mass transfer coefficient for single spheres is calculated from the Frössling correlation
Liquid Phase
Re = 2000
Sc = 5000
Sh = 460
kc = 4.6 × 10-6 m/s
Substituting these values into Equation (14-34) gives us
Substituting for kc and CAb in Equation (E14-2.2), the molar flux to the surface is
WAr = (4.61 × 10−6) m/s (103 − 0) mol/m3 = 4.61 × 10−3 mol/m2 · s
Because , this rate is also the rate of reaction per unit surface area of catalyst.
The mass transfer flow to the particle is
Analysis: In this example we calculated the rate of reaction on the external surface of a catalyst pellet in a liquid reactant when external mass transfer was limiting the reaction rate. To determine the rate of reaction, we used correlations to calculate the mass transfer coefficient and then used kc to calculate the flux to the surface, which in turn was equal to the rate of surface reaction.
14.6 The Shrinking Core Model
14.6.1 Dust Explosions, Particle Dissolution, and Catalyst Regeneration
Many situations arise in heterogeneous reactions where a gas-phase species reacts with a species contained in an inert solid matrix or solid combustible dust particles. The burning of a combustible dust is shown in Figure 14-6(a). The removal of carbon from catalyst particles that have been deactivated by fouling is shown in Figure 14-6(b) (see Section 10.7.1). The catalyst regeneration process to reactivate the catalyst by burning off the carbon is discussed in the expanded material on the CRE Web site.
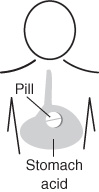
The shrinking core model is used to describe situations in which solid particles are being consumed either by dissolution or reaction and, as a result, the amount of the material being consumed is “shrinking.” For example, to design the time release of drugs into the body’s system, one must focus on the rate of dissolution of capsules and solid pills in the stomach. We now apply the shrinking core model to dust explosions where solid organic particles such as sugar are burned, to the formation of an ash layer around a burning coal particle, to catalyst regeneration. In this section we focus primarily on dust explosions and catalyst regeneration, and leave other applications such as drug delivery as exercises at the end of the chapter.
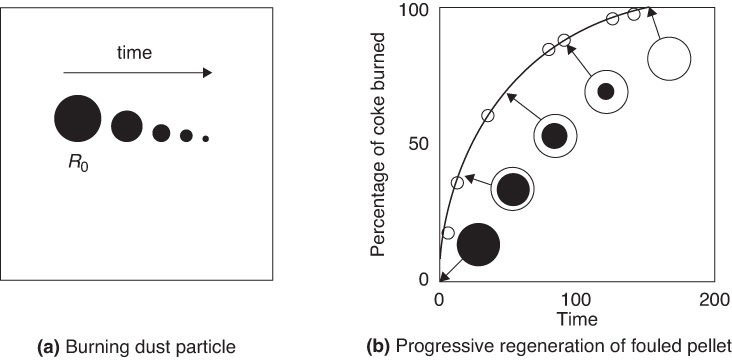
Figure 14-6 Shell progressive regeneration of fouled pellet.
To illustrate the principles of the shrinking core model, we shall consider the burning of carbon dust particle. As the carbon continues to burn the radius of dust particles shrink from R0 initially to R at time t as shown in Figure 14-6(a).
As shown in Figure 14-7, oxygen diffuses from the bulk gas at R∞ to the radius R, where it reacts with carbon to form carbon dioxide, which then diffuses out from the particle. The reaction
C + O2 → CO2
at the solid surface is very rapid, so the rate of oxygen diffusion to the surface controls the rate of carbon removal from the core. Although the core of
Oxygen must diffuse through the porous pellet matrix until it reaches the unreacted carbon core.
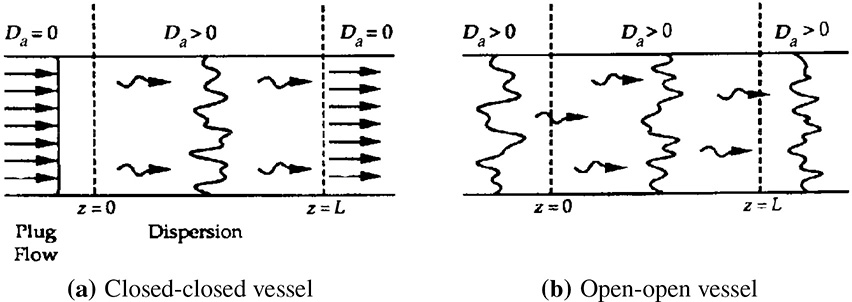
Figure 14-7 Sphere of radius R.
carbon is shrinking with time (an unsteady-state process), we assume the concentration profiles at any instant in time to be the steady-state profiles over the distance (R∞ – R) when R∞ is some larger distance from the surface of the particle, for example, R∞ ≈ ∞. This assumption is known as the quasi-steady state assumption (QSSA).
QSSA Use steady-state profiles
To study how the radius of unreacted carbon changes with time, we must first find the rate of diffusion of oxygen to the carbon surface. Next, we perform a mole balance on the elemental carbon and equate the rate of consumption of the carbon particle to the rate of diffusion of oxygen to the gas–carbon interface.
In applying a differential oxygen mole balance over the increment Δr located somewhere between R∞ and R, we recognize that O2 does not react in this region and reacts only when it reaches the solid carbon interface located at r = R. We shall let species A represent O2.
Step 1: The mole balance on O2 (i.e., A) between r and r + Δr is
Dividing through by –4πΔr and taking the limit gives
Mole balance on oxygen
Step 2: The constitutive equation for constant total molar concentration becomes

Combining Equations (14-17) and (14-48) and dividing by (–DAB) gives
Step 3: Boundary conditions for burning of a dust particle At a large distance from the dust particle r ~ ∞ then CA = CA∞ At the dust particle’s surface, r = R then CA = 0
Step 4: Integrating twice yields
Using the boundary conditions for a spherical dust particle to eliminate K1 and K2, the concentration profiles is
A schematic representation of the profile of O2 is shown in Figure 14-8 at a time when the inner core has receded to a radius R. The zero on the r axis corresponds to the center of the sphere
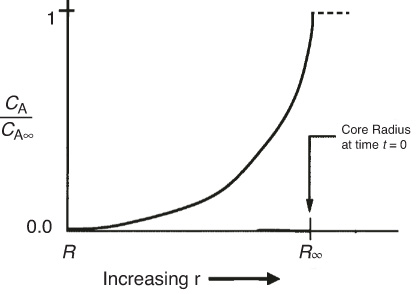
Figure 14-8 Oxygen concentration profile shown from the external radius of the pellet (R) to a large distance R∞ from the center of the burning particle. The gas–carbon interface is located at R.
Step 5: The molar flux of O2 to the gas–carbon interface for a dust particle is
Step 6: We now carry out an overall balance on elemental carbon. Elemental carbon does not enter or leave the particle.
Where ρc is the molar density of the solid carbon, simplifying gives
In terms of particle diameter, dP,
Step 7: The rate of disappearance of carbon is equal to the flux of O2 to the gas–carbon interface:
A. Surface reaction limiting
The initial conditions are

B. Diffusion Limiting
Again using the boundary conditions in Equation (14-57), we obtain
where KS is the burning rate constant, s-1.†
†R. A. Ogle Dust Explosion Dynamics, Amsterdam: Elsevier, 2017.
The time for complete combustion (dP = 0) of a particle of diameter dp0 is
Example 14–3 Combustion Time for a Single Particle
There is a 100 micron carbon dust particle in air at 1 atm. Calculate KS and the time for the particle to burn completely (dp = 0). The average value of the diffusivity near the burning particle is 10–4 m2/s and the density of the carbon particle is 2.26 × 106 g/m3.
Solution
The concentration of gas at 1 atm and 273 K is 0.046 mol/dm3. The corresponding concentration of O2 at the outside the boundary layer correcting for the temperature at 298 K, that is, CA∞ is
The molar density of the solid carbon particle is
The units used by R. A. Ogle in his book Dust Explosion Dynamic, Elsevier 2017, are μm and ms, in which case KS becomes
This value of KS is about an order of magnitude smaller than a burning liquid droplet. Calculate, tc, the time for the dust particle to completely burn
The time for a blink of an eye is approximately 400 ms.
Analysis: This analysis shows how to calculate time to consume a 100 micron carbon particle. The time is quite short, leading to a dust explosion. Be sure to view the Chemical Safety Board (CSB) video on the CRE Web site (http://www.csb.gov/imperial-sugar-company-dust-explosion-and-fire/).
14C Packed-Bed Applications
14.7 Mass Transfer-Limited Reactions in Packed Beds
A number of industrial reactions are potentially mass transfer-limited because they may be carried out at high temperatures without the occurrence of undesirable side reactions. In mass transfer-dominated reactions, the surface reaction is so rapid that the rate of transfer of reactant from the bulk gas or liquid phase to the surface limits the overall rate of reaction. Consequently, mass transfer-limited reactions respond quite differently to changes in temperature and flow conditions than do the rate-limited reactions discussed in previous chapters. In this section the basic equations describing the variation of conversion with the various PBR design parameters (catalyst weight, flow conditions) will be developed. To achieve this goal, we begin by carrying out a mole balance on the following generic mass transfer-limited reaction
carried out in a packed-bed reactor (Figure 14-9). A steady-state mole balance on reactant A in the reactor segment between z and z + Δz is

Figure 14-9 Packed-bed reactor.
where = rate of generation of A per unit of catalytic surface area, mol/s · m2
ac = external surface area of catalyst per volume of catalytic bed, m2/m3
= 6(1 = ϕ)/dp for packed beds, m2/m3
ϕ = porosity of the bed (i.e., void fraction)6
6 In the nomenclature for Chapter 4, for the Ergun equation for pressure drop.
dp = particle diameter, m
Ac = cross-sectional area of tube containing the catalyst, m2
Dividing Equation (14-62) by AcΔz and taking the limit as Δz → 0, we have
We now need to express FAz and in terms of concentration.
The molar flow rate of A in the axial direction is
Axial diffusion is neglected.
In almost all situations involving flow in packed-bed reactors, the amount of material transported by diffusion or dispersion in the axial direction is negligible compared with that transported by convection (i.e., bulk flow)
(In Chapter 18 we consider the case when dispersive effects (e.g., diffusion) must be taken into account.) Neglecting dispersion, Equation (14-14) becomes
where U is the superficial molar average velocity through the bed (m/s). Substituting for FAz in Equation (14-63) gives us
For the case of constant superficial velocity U
Differential equation describing flow and reaction in a packed bed
For reactions at steady state, the molar flux of A to the particle surface, WAr (mol/m2 · s) (see Figure 14-10), is equal to the rate of disappearance of A on the surface (mol/m2 · s); that is
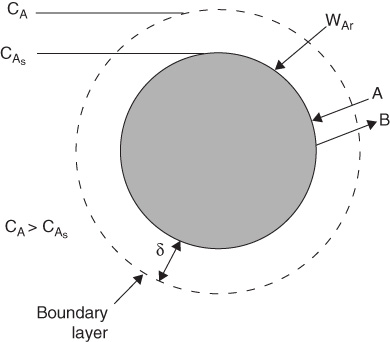
Figure 14-10 Diffusion across stagnant film surrounding catalyst pellet.

From Section 14.4, the boundary condition at the external surface is
where kc = mass transfer coefficient = (DAB/δ), (m/s)
CA = bulk concentration of A (mol/m3)
CAs = concentration of A at the catalytic surface (mol/m3)
Substituting for in Equation (14-67), we have
In reactions that are completely mass transfer-limited, it is not necessary to know the rate law.
In most mass transfer-limited reactions, the surface concentration is negligible with respect to the bulk concentration (i.e., )
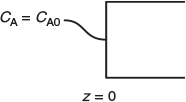
Integrating with the limit, at z = 0, CA = CA0
The corresponding variation of reaction rate along the length of the reactor is
The concentration and conversion profiles down a reactor of length L are shown in Figure 14-11.
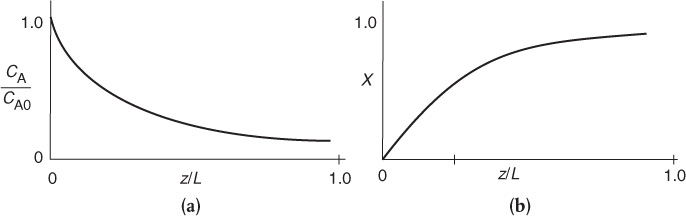
Figure 14-11 Axial concentration (a) and conversion (b) profiles in a packed bed.
To determine the reactor length L necessary to achieve a conversion X, we combine the definition of conversion
Reactor concentration profile for a mass transfer-limited reaction
with the evaluation of Equation (14-72) at z = L to obtain
14.8 Robert the Worrier
Robert is an engineer who is always worried (which is a Jofostanian trait). He always thinks something bad will happen if we change an operating condition such as flow rate or temperature, or an equipment parameter such as particle size. Robert’s motto is “If it ain’t broke, don’t fix it.” We can help Robert be a little more adventuresome by analyzing how the important parameters vary as we change operating conditions in order to predict the outcome of such a change. We first look at Equation (14-75) and see that conversion depends on the parameters kc, ac, U, and L. We now examine how each of these parameters will change as we change operating conditions. We first consider the effects of temperature and flow rate on conversion.
To learn the effect of flow rate on conversion, we need to know how flow rate affects the mass transfer coefficient. That is, we must determine the correlation for the mass transfer coefficient for the particular geometry and flow field. For flow through a packed bed, the correlation given by Thoenes and Kramers for 0.25 < ϕ < 0.5, 40 < Re′ < 4000, and 1 < Sc < 4000 is7
7 D. Thoenes, Jr. and H. Kramers, Chem. Eng. Sci., 8, 271 (1958).

Thoenes–Kramers correlation for flow through packed beds
where
dp = particle diameter (equivalent diameter of a sphere of the same volume), m
= [(6/π) (volume of pellet)]1/3, m
ϕ = void fraction (porosity) of packed bed
γ = shape factor (external surface area divided by )
and
U, ρ, μ, v, and DAB are as previously defined.
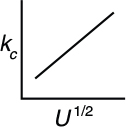
For constant fluid properties and particle diameter
We see that the mass transfer coefficient increases with the square root of the superficial velocity through the bed. Therefore, for a fixed concentration, CA, such as that found in a differential reactor, the rate of reaction should vary with U1/2
For diffusion-limited reactions, reaction rate depends on particle size and fluid velocity.
However, if the gas velocity is continually increased, a point is reached where the reaction becomes reaction rate–limited and, consequently, is independent of the superficial gas velocity, as shown in Figure 14-5.
Many mass transfer correlations in the literature are often reported in terms of the Colburn J factor (i.e., JD), which is a function of the Reynolds number. The relationship between JD and the numbers we have been discussing is
Figure 14-12 shows data from a number of investigations for the J factor as a function of the Reynolds number for a wide range of particle shapes and gas-flow conditions. Note: There are serious deviations from the Colburn analogy when the concentration gradient and temperature gradient are coupled, as shown by Venkatesan and Fogler.8
8 R. Venkatesan and H. S. Fogler, AIChE J., 50, 1623 (July 2004).
Colburn J factor
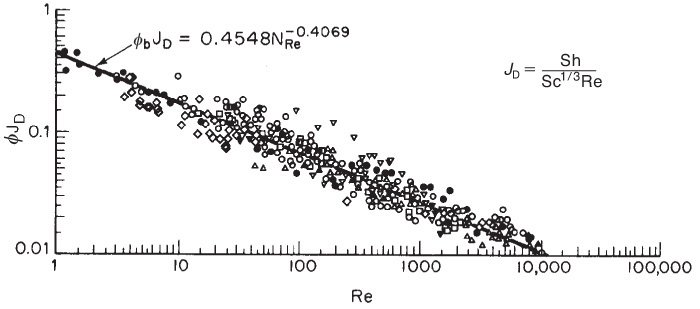
Figure 14-12 Mass transfer correlation for packed beds. ϕb≡ϕ [Reprinted by permission. Copyright © 1977, American Chemical Society. Dwivedi, P. N. and S. N. Upadhyay, “Particle-Fluid Mass Transfer in Fixed and Fluidized Beds.” Industrial & Engineering Chemistry Process Design and Development, 1977, 16 (2), 157–165.]
A correlation for flow through packed beds in terms of the Colburn J factor
Dwidevi and Upadhyay review a number of mass transfer correlations for both fixed and fluidized beds and arrive at the following correlation, which is valid for both gases (Re > 10) and liquids (Re > 0.01) in either fixed or fluid-ized beds:9
9 P. N. Dwidevi and S. N. Upadhyay, Ind. Eng. Chem. Process Des. Dev., 16, 157 (1977).
For nonspherical particles, the equivalent diameter used in the Reynolds and Sherwood numbers is , where Ap is the external surface area of the pellet.
To obtain correlations for mass transfer coefficients for a variety of systems and geometries, see either D. Kunii and O. Levenspiel, Fluidization Engineering, 2nd ed. Butterworth-Heinemann, 1991, Chap. 7, or W. L. McCabe, J. C. Smith, and P. Harriott, Unit Operations in Chemical Engineering, 6th ed. New York: McGraw-Hill, 2000. For other correlations for packed beds with different packing arrangements, see I. Colquhoun-Lee and J. Stepanek, Chemical Engineer, 108 (Feb. 1974).
Example 14–4 Mass Transfer Effects in Maneuvering a Space Satellite
Hydrazine has been studied extensively for use in monopropellant thrusters for space flights of long duration. Thrusters are used for altitude control of communication satellites. Here, the decomposition of hydrazine over a packed bed of alumina-supported iridium catalyst is of interest.10 In a proposed study, a 2% hydrazine in 98% helium mixture is to be passed over a packed bed of cylindrical particles 0.25 cm in diameter and 0.5 cm in length at a gas-phase velocity of 150 m/s and a temperature of 450 K. The kinematic viscosity of helium at this temperature is 4.94 × 10–5 m2/s. The hydrazine decomposition reaction is believed to be externally mass transfer-limited under these conditions. If the packed bed is 0.05 m in length, what conversion can be expected? Assume isothermal operation.
10 O. I. Smith and W. C. Solomon, Ind. Eng. Chem. Fund., 21, 374.
Actual case history and current application
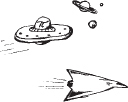
Figure E14-4.1 PBR.
Additional information:
DAB = 0.69 × 10–4 m2/s at 298 K
Bed porosity: 40%
Bed fluidicity: 95.7%
Solution
The following solution is detailed and a bit tedious, but it is important to know the details of how a mass transfer coefficient is calculated.
Rearranging Equation (14-64) gives us
(a) Using the Thoenes–Kramers correlation to calculate the mass transfer coefficient, kc
First we find the volume-average particle diameter
Surface area per volume of bed

Tedious reading and calculations, but we gotta know how to do the nitty–gritty.
Mass transfer coefficient
For cylindrical pellets
Correcting the diffusivity to 450 K using Table 14-3 gives us
Representative values
Gas Phase
Re′ = 15173
Sc = 0.35
Sh′ = 86.66
kc = 6.15 m/s
Substituting Re′ and Sc into Equation (14-65) yields
The conversion is
We find 87% conversion.
(b) Colburn JD factor to calculate kc. To find kc, we first calculate the surface-area-average particle diameter.
For cylindrical pellets, the external surface area is

Typical values
Gas Phase
Re = 11996
JD = 0.025
Sc = 0.35
Sh = 212
kc = 7.63 m/s
Fluidicity?? Red herring!
If there were such a thing as the bed fluidicity, given in the problem statement, it would be a useless piece of information. Make sure that you know what information you need to solve problems, and go after it. Do not let additional data confuse you or lead you astray with useless information or facts that represent someone else’s bias, and which are probably not well founded.
14.9 What If . . . ? (Parameter Sensitivity)
As we have stressed many times, one of the most important skills of an engineer is to be able to predict the effects of changes of system variables on the operation of a process. The engineer needs to determine these effects quickly through approximate but reasonably close calculations, which are sometimes referred to as “back-of-the-envelope calculations.”11 This type of calculation is used to answer such questions as “What will happen if I decrease the particle size?” “What if I triple the flow rate through the reactor?”
11 Prof. J. D. Goddard, University of Michigan, 1963–1976. Professor Emeritus at the University of California, San Diego.
J. D. Goddard’s
Back of the Envelope
To help answer these questions, we recall Equation (14-45) and our discussion on page 754. There, we showed the mass transfer coefficient for a packed bed was related to the product of two terms: Term 1 was dependent on the physical properties and Term 2 was dependent on the system properties. Re-writing Equation (14-45) as
one observes from this equation that the mass transfer coefficient increases as the particle size decreases. The use of sufficiently small particles offers another technique to escape from the mass transfer-limited regime into the reaction-rate-limited regime.
Find out how the mass transfer coefficient varies with changes in physical properties and system properties.
Example 14–5 The Case of Divide and Be Conquered
A mass transfer-limited reaction is being carried out in two reactors of equal volume and packing, connected in series as shown in Figure E14-5.1. Currently, 86.5% conversion is being achieved with this arrangement. It is suggested that the reactors be separated and the flow rate be divided equally among each of the two reactors (Figure E14-5.2) to decrease the pressure drop and hence the pumping requirements. In terms of achieving a higher conversion, Robert is wondering if this is a good idea.


Figure E14-5.1 Series arrangement.
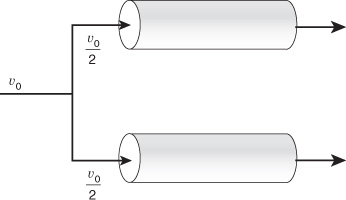
Figure E14-5.2 Parallel arrangement.
Reactors in series versus reactors in parallel
Solution
For the series arrangement we were given, X1 = 0.865, and for the parallel arrangement, the conversion is unknown, that is, X2 = ? As a first approximation, we neglect the effects of small changes in temperature and pressure on mass transfer.
We recall Equation (14-75), which gives conversion as a function of reactor length. For a mass transfer-limited reaction
For case 1, the undivided system
For case 2, the divided system
We now take the ratio of case 2 (divided system) to case 1 (undivided system)
The surface area per unit volume ac is the same for both systems.
From the conditions of the problem statement we know that
However, we must also consider the effect of the division on the mass transfer coefficient. From Equation (14-81) we know that
kc ∝ U1/2
Multiplying by the ratio of superficial velocities yields
Solving for X2 gives us
X2 = 0.76 Answer 2
Analysis: Consequently, we see that although the divided arrangement will have the advantage of a smaller pressure drop across the bed, it is a bad idea in terms of conversion. Recall that the series arrangement gave X1 = 0.865; therefore (X2 < X1). Bad idea!! But every chemical engineering student in Jofostan knew that! Recall that if the reaction were reaction rate–limited, both arrangements would give the same conversion.
Bad idea!! Robert was right to worry.
Example 14–6 The Case of the Overenthusiastic Engineers
The same reaction as that in Example 14-5 is being carried out in the same two reactors in series. A new engineer suggests that the rate of reaction could be increased by a factor of 210 by increasing the reaction temperature from 400°C to 500°C, reasoning that the reaction rate doubles for every 10 = C increase in temperature. Another engineer arrives on the scene and berates the new engineer with quotations from Chapter 3 concerning this rule of thumb. She points out that it is valid only for a specific activation energy within a specific temperature range. She then suggests that he go ahead with the proposed temperature increase but should only expect an increase on the order of 23 or 24. What do you think? Who is correct?

Robert worries if this temperature increase will be worth the trouble.
Solution
Because almost all surface reaction rates increase more rapidly with temperature than do diffusion rates, increasing the temperature will only increase the degree to which the reaction is mass transfer-limited.
We now consider the following two cases:
Taking the ratio of case 2 to case 1 and noting that the reactor length is the same for both cases (L1 = L2), we obtain
The molar feed rate FT0 remains unchanged
the pressure remains constant so
Because υ = AcU, the superficial velocity at temperature T2 is
J. D. G.
We now wish to learn the dependence of the mass transfer coefficient on temperature
Taking the ratio of case 2 to case 1 and realizing that the particle diameter is the same for both cases gives us
The temperature dependence of the gas-phase diffusivity is (from Table 14-3)
For most gases, viscosity increases with increasing temperature according to the relation
μ ∝ T1/2
From the ideal gas law
ρ ∝ T-1
Then
It’s really important to know how to do this type of analysis.
Rearranging Equation (E14-6.1) in the form
Analysis: Consequently, we see that increasing the temperature from 400°C to 500°C increases the conversion by only 1.7%, that is, X = 0.865 compared to X = 0.88. Bad idea! Bad, bad idea! Both engineers would have benefited from a more thorough study of this chapter.
Bad idea!! Robert was right to worry.
For a packed catalyst bed, the temperature-dependence part of the mass transfer coefficient for a gas-phase reaction can be written as
Taking the ratio of U to U0 and T to T0 and kc0 (T0, U0) the mass transfer coefficient at any other T and U can be found from the Equation
One can use Equation (14-81) when studying mass transfer-limited gas-phase reaction.
Depending on how one fixes or changes the molar feed rate, FT0, U may also depend on the feed temperature.
As an engineer, it is extremely important that you reason out the effects of changing conditions, as illustrated in the preceding two examples.

Important concept
Example 14–7 Flow, Diffusion, and Reaction in a Packed Bed
Let’s revisit a packed-bed reactor shown in Figure E14-4.1 in Example 14-4. We want to put the algorithm in a form where one can use Wolfram or Python to vary all the physical parameters and operating variables in order to study a first-order gas-phase reaction in a PBR. The reaction will be carried out isothermally and pressure drop is neglected.
Study the conversion and reaction rate profile for a reaction that is partially diffusion limited by first plotting the conversion as a function of catalyst weight up to a value W = 100 kg. Next use Wolfram to vary all the parameters and describe what you find.
Solution
Mole Balance
Rate Law
2A. First order,
At steady state the molar flux to the surface is equal to the rate of reaction on the surface

where
kr = reaction-rate constant on the surface (m/s)
kc = mass transfer coefficient (m/s)
(m/s) overall transfer coefficient
= external surface area of the catalyst per unit mass of catalyst (m2/kg-cat)
2B. Using the Thoenes and Kramer’s correlation, Equations (14-76) and (14-77)
Correlation for kc is
Stoichiometry with P = P0 and T = T0
Combine Polymath
We will now combine Equations (E14-7.1) through (E14-7.11) into Polymath, Wolfram, or Python to vary the parameters. The Polymath program is shown in Table E14-7.2 and the Wolfram solution in Figure E14-7.1.
Evaluate
The nominal values of the parameter to be varied are shown in Table E14-7.1.
TABLE E14-7.1 NOMINAL PARAMETER VALUES
Parameters
Liquids
Gases
υ
10–6 m2/s
10–5 m2/s
ρ
1000 kg/m3
1 kg/m3
DAB
10–8 m2/s
10–5 m2/s
μ
10–3 kg/m/s
10–5 kg/m/s
U
1 m/s
100 m/s
dp
10–3 m
10–2 m
kr
0.5 m/s
5 m/s
Ac
5*10–2 m2
5*10–2 m2
γ
1
1
ϕ
0.4
0.4
ρc
3000 kg/m3
3000 kg/m3
CA0
1 mol/m3
0.01 mol/m3 (Ca 1 atm)
Plot the conversion as a function of catalyst weight.
TABLE E14-7.2 POLYMATH PROGRAM FOR MASS TRANSFER-LIMITED LIQUID-PHASE REACTION IN A PBR

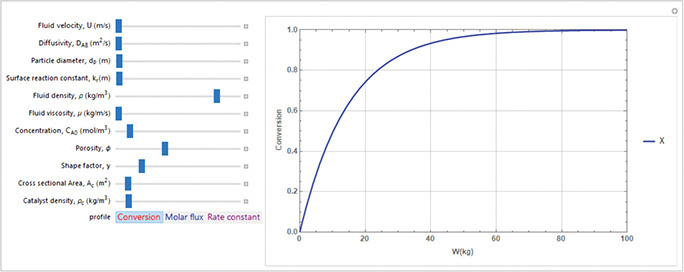
Figure E14-7.1 Wolfram solution.
Analysis: In this example we modeled a partially diffusion limited reaction in a PBR. The rest of the example is now up to you, the reader. Go to the LEP 14-7 and vary a number of physical parameters and operating variables, and write a set of observations and conclusions.
14.10 And Now… A Word from Our Sponsor—Safety 14 (AWFOS–S14 Sugar Dust Explosion)
Dust Explosion at Imperial Sugar Company
According to the Chemical Safety Board, the yearly average number of dust explosions has been 5 per year over the last 5 years. In Examples 14-1 and 14-3, we discussed and calculated the rate of mass transfer of oxygen to small carbon dust particles allowing the particle to burn and possibly causing a dust explosion. Dust explosions can produce deadly consequences. On February 7, 2008, combustible fine sugar was ignited at the Imperial Sugar Company causing an explosion. Because the fine granulated sugar has high surface area, it has the potential to rapidly burn and explode. Prior to the explosion the dust collection system was not working properly; as a result fine sugar that had spilled over on the floor and collected on the ceiling and piping became airborne. When the belt transport system was enclosed, the airborne sugar dust found an ignition source and ignited. The explosion was fueled by the large accumulation of airborne sugar dust throughout the packaging plant. The fire and explosion caused 14 deaths and 38 injuries, including life-threatening burns.


![]() (https://www.csb.gov/imperial-sugar-company-dust-explosion-and-fire/)
(https://www.csb.gov/imperial-sugar-company-dust-explosion-and-fire/)
As chemical reaction engineers, it is important that we understand this accident, why it happened, and how it could have been prevented to ensure similar accidents may be prevented. In order to become familiar with a strategy for accident awareness and prevention, view the Chemical Safety Board video on the explosion and determine whether all the important concepts to prevent and mitigate the incident were captured in the BowTie diagram below.
Two other excellent videos on dust explosions are (https://www.chemengonline.com/combustible-dust-fires-explosions-recent-data-lessons-learned/) and (https://www.aiche.org/academy/webinars/dust-explosion). The BowTie diagram for the sugar dust plan explosion is shown in Figure 14-13.
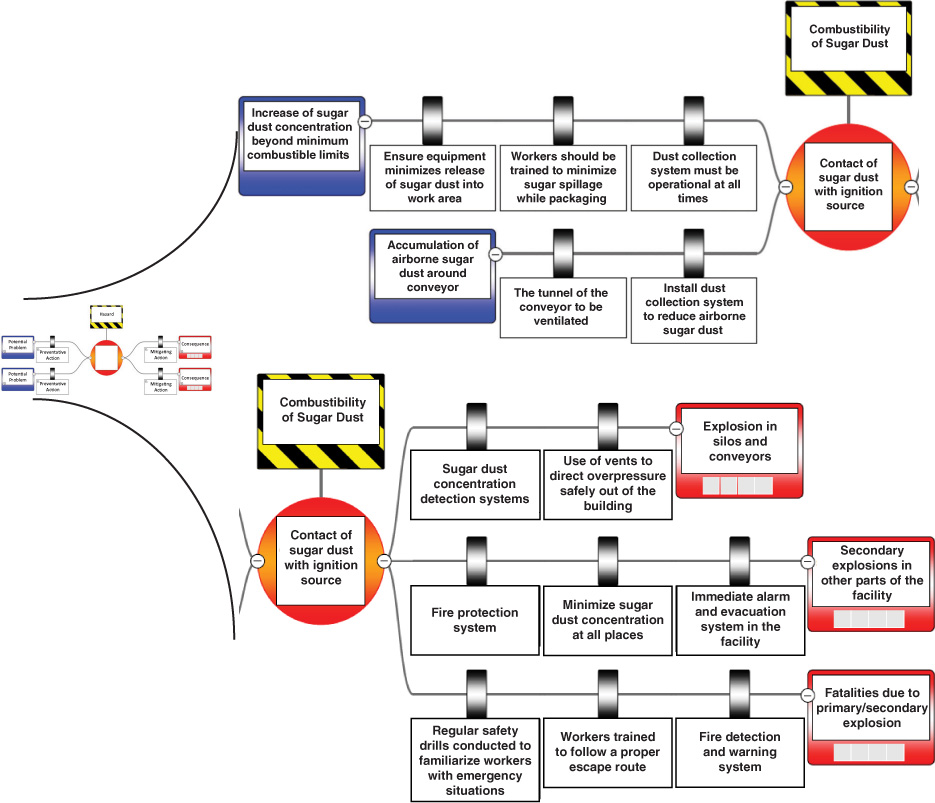
Figure 14-13 BowTie diagram for dust explosion.
Summary
The molar flux of A in a binary mixture of A and B is
For equimolar counterdiffusion (EMCD) or for dilute concentration of the solute
For diffusion through a stagnant gas
For negligible diffusion
The rate of mass transfer from the bulk fluid to a boundary at concentration CAs is
where kc is the mass transfer coefficient.
The Sherwood and Schmidt numbers are, respectively,
If a heat transfer correlation exists for a given system and geometry, the mass transfer correlation may be found by replacing the Nusselt number by the Sherwood number and the Prandtl number by the Schmidt number in the existing heat transfer correlation.
Increasing the gas-phase velocity and decreasing the particle size will increase the overall rate of reaction for reactions that are externally mass transfer-limited.
The conversion for externally mass transfer-limited reactions can be found from the equation
Back-of-the-envelope calculations should be carried out to determine the magnitude and direction that changes in process variables will have on conversion. What if . . .?
Cre Web Site Materials
(http://www.umich.edu/~elements/6e/14chap/obj.html#/)

• Additional Material on the Web site
Transdermal Drug Delivery Example
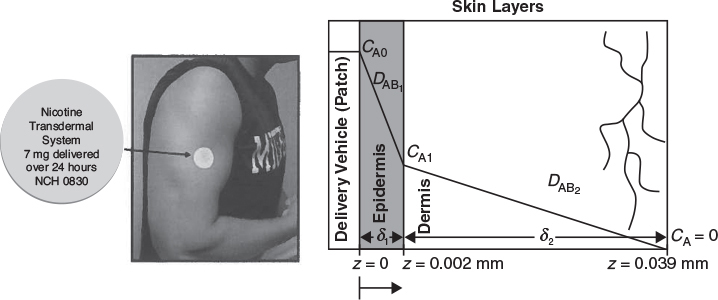
Transdermal drug delivery schematic.
Questions, Simulations, and Problems
The subscript to each of the problem numbers indicates the level of difficulty: A, least difficult; D, most difficult.
A = • B = ▪ C = ♦ D = ♦♦
Questions
Q14-1A QBR (Question Before Reading). Under what circumstances will the conversion predicted by mass transfer limitations be greater than that for surface reaction limitations?
Q14-2 Read over the problems at the end of this chapter. Make up an original problem that uses the concepts presented in this chapter. See Problem P5-1A for the guidelines. To obtain a solution:

Make up your data and reaction.
Use a real reaction and real data.
The journals listed at the end of Chapter 1 may be useful for part (b).
Q14-3 (Sergeant Ambercromby). Capt. Apollo is piloting a shuttlecraft on his way to space station Klingon. Just as he is about to maneuver to dock his craft using the hydrazine system discussed in Example 14-2, the shuttle craft’s thrusters do not respond properly and it crashes into the station, killing Capt. Apollo (Star Wars 7 (fall 2015)). An investigation reveals that Lt. Darkside prepared the packed beds used to maneuver the shuttle and Lt. Data prepared the hydrazine–helium gas mixture. Foul play is suspected and Sgt. Ambercromby arrives on the scene to investigate.
What are the first three questions he asks?
Make a list of possible explanations for the crash, supporting each one by an equation or reason.
Q14-4 How would your answers change if the temperature was increased by 50°C, the particle diameter was doubled, and fluid velocity was cut in half? Assume properties of water can be used for this system.
Q14-5 How would your answers change in Example 14-3 if you had a 50–50 mixture of hydrazine and helium? If you increase dp by a factor of 5?
Q14-6A After viewing the video, what was new to you with regard to dust explosions? Could you list three things one should do or have in place to prevent dust explosions?
Q14-7 Go to the LearnChemE screencast link for Chapter 14 (http://www.learncheme.com/screencasts/kinetics-reactor-design). View one or more of the screencast 5- to 6-minute videos and write a two-sentence evaluation of what you learned.
Q14-8 AWFOS–S14 View the CSB video to list two things in which dust explosions are different from those involving flammable liquids.
Computer Simulations and Experiments
P14-1B
Example 14-1: Mass Transfer of Oxygen to a Burning Carbon Particle
Wolfram and Python
Vary each slider to find the parameter to which the flux WAr is most sensitive.
What happens when the liquid diffusity and viscosity are both increased simultaneously?
Vary the velocity as shown in Figure 14-5 and describe its effect on the flux.
Write a set of conclusions based on the above experiments.
Example 14-2: Rapid Liquid-Phase Reaction on the Surface of a Catalyst Wolfram
and Python
Vary each of the parameters and tell which one the mass transfer coefficient (kc) is most sensitive.
Change DAB and U simultaneously both up and down and describe what you find.
Write a set of three conclusions from your experiments (i) and (ii).
Example 14-3: Combustion Time for a Single Particle
Wolfram and Python
Compare the burning time for a 100 μm porous carbon particle with a 10% solid with tc in Example 14-3.
Vary all the parameters you can think of that would give a burning time of 500 ms.
Write a set of conclusions from your Wolfram experiments (i) and (ii).
Example 14-4: Mass Transfer Effects in Maneuvering a Space Satellite. What if you were asked for representative values for Re, Sc, Sh, and kc for both liquid- and gas-phase systems for a velocity of 10 cm/s and a pipe diameter of 5 cm (or a packed-bed diameter of 0.2 cm)? What numbers would you give?
Example 14-5: The Case of Divide and Be Conquered. How would your answers change if the reaction were carried out in the liquid phase where kinematic viscosity, υ, varied as
Example 14-7: Flow, Diffusion, and Reaction in a Packed Bed
Wolfram and Python
Vary kr and U and view X, WA, and k as a function of W.
Vary DAB and dp and view k as a function of W.
Vary the ration (DAB/υ) and describe what you find.
Vary the parameters by a factor of 10 above and below their nominal values and describe what you find (e.g., what were the most sensitive and least sensitive parameters? Hint: See Equation (14-81).
Write a set of conclusions based on all your experiments.
Problems

P14-2B Assume the minimum respiration rate of a chipmunk is 1.5 micromoles of O2/min. The corresponding volumetric rate of gas intake is 0.05 dm3/min at STP.
What is the deepest a chipmunk can burrow a 3-cm diameter hole beneath the surface in Ann Arbor, Michigan? DAB = 1.8 × 10–5 m2/s
In Boulder, Colorado?
How would your answers to (a) and (b) change in the dead of winter when T = 0°F?
Critique and extend this problem (e.g., CO2 poisoning). Thanks to Professor Robert Kabel at Pennsylvania State University.
Hint: Review derivations and equations for WA and WB to see how they can be applied to this problem.
P14-3B Pure oxygen is being absorbed by xylene in a catalyzed reaction in the experimental apparatus sketched in Figure P14-3B. Under constant conditions of temperature and liquid composition, the following data were obtained:
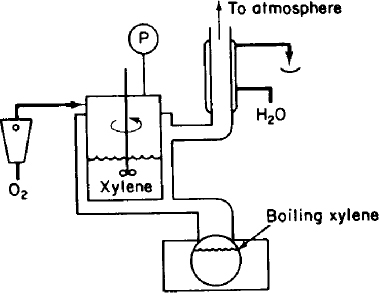
Figure P14-3B
Rate of Uptake of O2 (mL/h) for System Pressure (absolute) |
||||
|---|---|---|---|---|
Stirrer Speed (rpm) |
1.2 atm |
1.6 atm |
2.0 atm |
3.0 atm |
400 |
15 |
31 |
75 |
152 |
800 |
20 |
59 |
102 |
205 |
1200 |
21 |
62 |
105 |
208 |
1600 |
21 |
61 |
106 |
207 |
No gaseous products were formed by the chemical reaction. What would you conclude about the relative importance of liquid-phase diffusion and about the order of the kinetics of this reaction? (California Professional Engineers Exam)
P14-4B Derive an equation for the time necessary to completely burn a 100 μm carbon particle as a function of D0. Also calculate the burning rate constant. Use the KS values of the parameter values, for example, CA∞, given in Example 14-3.
diffusion controlled with DAB = 10–4 m2/s
reaction controlled with kr = 0.01 m/s
combined reaction and diffusion controlled
Additional Information:
ρc = 188 mol/m3
P14-5C In a diving-chamber experiment, a human subject breathed a mixture of O2 and He while small areas of his skin were exposed to nitrogen gas. After some time, the exposed areas became blotchy, with small blisters forming on the skin. Model the skin as consisting of two adjacent layers, one of thickness δ1 and the other of thickness δ2. If counterdiffusion of He out through the skin occurs at the same time as N2 diffuses into the skin, at what point in the skin layers is the sum of the partial pressures a maximum? If the saturation partial pressure for the sum of the gases is 101 kPa, can the blisters be a result of the sum of the gas partial pressures exceeding the saturation partial pressure and the gas coming out of the solution (i.e., the skin)?
Before answering any of these questions, derive the concentration profiles for N2 and He in the skin layers.
Diffusivity of He and N2 in the inner skin layer = 5 × 10−7 cm2/s and 1.5 × 10−7 cm2/s, respectively.
Diffusivity of He and N2 in the outer skin layer = 10−5 cm2/s and 3.3 × 10-4 cm2/s, respectively.
External Skin Boundary Partial Pressure |
Internal Skin Boundary Partial Pressure |
|
|---|---|---|
| N2 | 101 kPa |
0 |
| He | 0 |
81 kPa |
| δ1 | 20 μm |
Stratum corneum |
| δ2 | 80 μm |
Epidermis |
Hint: See Transdermal Drug Delivery in Expanded Material on the CRE Web site.
P14-6B The decomposition of cyclohexane to benzene and hydrogen is mass transfer-limited at high temperatures. The reaction is carried out in a 5-cm-ID pipe 20 m in length packed with cylindrical pellets 0.5 cm in diameter and 0.5 cm in length. The pellets are coated with the catalyst only on the outside. The bed porosity is 40%. The entering volumetric flow rate is 60 dm3/min.

Calculate the number of pipes necessary to achieve 99.9% conversion of cyclohexane from an entering gas stream of 5% cyclohexane and 95% H2 at 2 atm and 500°C.
Plot conversion as a function of pipe length.
How much would your answer change if the pellet diameter and length were each cut in half?
How would your answer to part (a) change if the feed were pure cyclohexane?
What do you believe is the point of this problem? Is the focus really green CRE? How so?
P14-7C Lead titanate, PbTiO3, is a material having remarkable ferroelectric, pyroelectric, and piezoelectric properties [ J. Elec. Chem. Soc., 135, 3137 (1988)]. A thin film of PbTiO3 was deposited in a CVD reactor. The deposition rate is given below as a function of a temperature and flow rate over the film.
Flow Rate (SCCM) |
Temperature (°C) |
Deposition Rate (mg/cm2 · h) |
|---|---|---|
500 |
650 |
0.2 |
750 |
0.8 |
|
800 |
1.2 |
|
600 |
650 |
0.35 |
750 |
1.0 |
|
800 |
1.5 |
|
750 |
650 |
0.53 |
750 |
1.45 |
|
800 |
2.0 |
|
1000 |
650 |
0.55 |
750 |
1.5 |
|
800 |
2.0 |
What are all the things, qualitative and quantative, that you can learn from these data?
P14-8B OEQ (Old Exam Question). A plant is removing a trace of Cl2 from a waste-gas stream by passing it over a solid granulm absorbent in a tubular packed bed (Figure P14-8B). At present, 63.2% removal is being acomplished, but it is believed that greater removal could be achieved if the flow rate were increased by a factor of 4, the particle diameter were decreased by a factor of 3, and the packed tube length increased by 50%. What percentage of chlorine would be removed under the proposed scheme? (The chlorine transferring to the absorbent is removed completely by a virtually instantaneous chemical reaction.) (Ans: 98%)

Figure P14-8B
P14-9B OEQ (Old Exam Question). In a certain chemical plant, a reversible fluid-phase isomerization
is carried out over a solid catalyst in a tubular packed-bed reactor. If the reaction is so rapid that mass transfer between the catalyst surface and the bulk fluid is rate-limiting, show that the kinetics are described in terms of the bulk concentrations CA and CB by
where = moles of A reacting per unit area catalyst per
kA, kB = transfer coefficients for A and B
K = equilibrium constant
It is desired to double the capacity of the existing plant by processing twice the feed of reactant A while maintaining the same fractional conversion of A to B in the reactor. How much larger a reactor, in terms of catalyst weight, would be required if all other operating variables are held constant? You may use the Thoenes–Kramers correlation for mass transfer coefficients in a packed bed.
P14-10B The irreversible gas-phase reaction
is carried out adiabatically over a packed bed of solid catalyst particles. The reaction is first order in the concentration of A on the catalyst surface
The feed consists of 50% (mole) A and 50% inerts, and enters the bed at a temperature of 300 K. The entering volumetric flow rate is 10 dm3/s (i.e., 10000 cm3/s). The relationship between the Sherwood number and the Reynolds number is
Sh = 100 Re1/2
As a first approximation, one may neglect pressure drop. The entering concentration of A is 1.0 M. Calculate the catalyst weight necessary to achieve 60% conversion of A for
isothermal operation.
adiabatic operation.
Additional information:
Kinematic viscosity: μ/ρ = 0.02 cm2/s
Particle diameter: dp = 0.1 cm
Superficial velocity: U = 10 cm/s
Catalyst surface area/mass of catalyst bed: a = 60 cm2/g-cat
Diffusivity of A: De = 10−2 cm2/s
Heat of reaction: = −10000 cal/g mol A
Heat capacities: CpA = CpB = 25 cal/g mol · K, CpS (solvent) = 75 cal/g mol · K
k′ (300 K) = 0.01 cm3/s · g-cat with E = 4000 cal/mol
P14-11B Transdermal Drug Delivery. See photo on page 781. The principles of steady-state diffusion have been used in a number of drug-delivery systems. Specifically, medicated patches are commonly attached to the skin to deliver drugs for nicotine withdrawal, birth control, and motion sickness, to name a few. The U.S. transdermal drug-delivery is a multibillion dollar market. Equations similar to Equation (14-24) have been used to model the release, diffusion, and absorption of the drug from the patch into the body. The figure shown in the Additional Material and on page 781 shows a drug-delivery vehicle (patch) along with the concentration gradient in the epidermis and dermis skin layers.
Use a shell balance to show
Show the concentration profile in the epidermis layer
Show the concentration profile in the dermis layer
Equate the fluxes using and and to show
What are the concentration profiles in the dermis and epidermis layers?
Show the flux in the dermis layer is
What is the flux in the epidermis layer?
P14-12D(Estimating glacial ages) The following oxygen-18 data were obtained from soil samples taken at different depths in Ontario, Canada. Assuming that all the 18O was laid down during the last glacial age and that the transport of 18O to the surface takes place by molecular diffusion, estimate the number of years since the last glacial age from the following data. Independent measurements give the diffusivity of 18O in soil as 2.64 = 10−10 m2/s.
Figure P14-12D Glaciers.
(surface) |
||||||
|---|---|---|---|---|---|---|
| Depth (m) | 0 |
3 |
6 |
9 |
12 |
18 |
| 18O Conc. Ratio (C/C0) | 0 |
0.35 |
0.65 |
0.83 |
0.94 |
1.0 |
C0 is the concentration of 18O at 25 m. Hint: A knowledge of error function solutions may or may not be helpful. (Ans: t = 5,616 years)
P14-13B OEQ (Old Exam Question). A spherical particle is dissolving in a liquid. The rate of dissolution is first order in the solvent concentration, C. Assuming that the solvent is in excess, show that the following conversion-time relationships hold.
Rate-Limiting Regime |
Conversion–Time Relationship |
|---|---|
| Surface reaction | |
| Mass transfer | |
| Mixed |
P14-14C Derive the diffusion and reaction equation in spherical coordinates to describe the dissolution of a drug in the form of a spherical pellet. Plot the drug concentration as a function distance r and time t. Also plot the flux and particle diameter as a function of time.
P14-15B A powder is to be completely dissolved in an aqueous solution in a large, well-mixed tank. An acid must be added to the solution to render the spherical particle soluble. The particles are sufficiently small that they are unaffected by fluid velocity effects in the tank. For the case of excess acid, C0 = 2 M, derive an equation for the diameter of the particle as a function of time when
Mass transfer limits the dissolution: –WA = kcCA0
Reaction limits the dissolution:
What is the time for complete dissolution in each case?
Now assume that the acid is not in excess and that mass transfer is limiting the dissolution. One mole of acid is required to dissolve 1 mol of solid. The molar concentration of acid is 0.1 M, the tank is 100 L, and 9.8 mol of solid is added to the tank at time t = 0. Derive an expression for the radius of the particles as a function of time and calculate the time for the particles to dissolve completely.
How could you make the powder dissolve faster? Slower?
Additional information:
P14-16B (Pills) An antibiotic drug is contained in a solid inner core and is surrounded by an outer coating that makes it palatable. The outer coating and the drug are dissolved at different rates in the stomach, owing to their differences in equilibrium solubilities.
If D2 = 4 mm and D1 = 3 mm, calculate the time necessary for the pill to dissolve completely.
Assuming first-order kinetics (kA = 10 h–1) for the absorption of the dissolved drug (i.e., in solution in the stomach) into the bloodstream, plot the concentration in grams of the drug in the blood per gram of body weight as a function of time when the following three pills are taken simultaneously:

Discuss how you would maintain the drug level in the blood at a constant level using different-size pills?
How could you arrange a distribution of pill sizes so that the concentration in the blood was constant over a period (e.g., 3 hr) of time?
Additional information:
Amount of drug in inner core = 500 mg
Solubility of outer layer at stomach conditions = 1.0 mg/cm3
Solubility of inner layer at stomach conditions = 0.4 mg/cm3
Volume of fluid in stomach = 1.2 L
Typical body weight = 75 kg
Sh = 2, DAB = 6 × 10–4 cm2/min

P14-17B If disposal of industrial liquid wastes by incineration is to be a feasible process, it is important that the toxic chemicals be completely decomposed into harmless substances. One study carried out concerned the atomization and burning of a liquid stream of “principal” organic hazardous constituents (POHCs) [Environ. Prog., 8, 152 (1989)]. The following data give the burning droplet diameter as a function of time (both diameter and time are given in arbitrary units):
| Time | 20 |
40 |
50 |
70 |
90 |
110 |
| Diameter | 9.7 |
8.8 |
8.4 |
7.1 |
5.6 |
4.0 |
What can you learn from these data?
Supplementary Reading
The fundamentals of diffusional mass transfer may or may not be found in
R. B. BIRD, W. E. STEWART, and E. N. LIGHTFOOT, Transport Phenomena, 2nd ed. New York: Wiley, 2002, Chaps. 17 and 18.
S. Collins, Mockingjay (The Final Book of the Hunger Games). New York: Scholastic, 2014.
E. L. CUSSLER, Diffusion Mass Transfer in Fluid Systems, 3rd ed. New York: Cambridge University Press, 2009.
C. J. GEANKOPLIS, Transport Processes and Unit Operations. Upper Saddle River, NJ: Prentice Hall, 2003.
V. G. LEVICH, Physiochemical Hydrodynamics. Upper Saddle River, NJ: Prentice Hall, 1962, Chaps. 1 and 4.
Experimental values of the diffusivity can be found in a number of sources, two of which are
R. H. PERRY, D. W. GREEN, and J. O. MALONEY, Chemical Engineers’ Handbook, 8th ed. New York: McGraw-Hill, 2007.
T. K. SHERWOOD, R. L. PIGFORD, and C. R. WILKE, Mass Transfer. New York: McGraw-Hill, 1975.
A number of correlations for the mass transfer coefficient can be found in
A. L. LYDERSEN, Mass Transfer in Engineering Practice. New York: Wiley-Interscience, 1983, Chap. 1.
W. L. MCCABE, J. C. SMITH, and P. HARRIOTT, Unit Operations of Chemical Engineering, 6th ed. New York: McGraw-Hill, 2000, Chap. 17.