CHAPTER 22
Teaching and Learning the Many Faces of Chemistry
The goal of this chapter is to review research on teaching and learning science that focuses on the specific domain of chemistry, with particular emphasis on the high school level. Many chemistry topics can be either viewed or taught from three potential perspectives that are mutually related (Fig. 22.1). First, the macroscopic perspective, focusing on substances and phenomena that can be observed with the naked eye. Second, the submicroscopic perspective, focusing on molecules, atoms, ions, and so on. Third, the symbolic perspective, focusing on formulas, equations, ionic drawings, and the like. The use of this three-cornered relationship (Johnstone, 1991) is not exclusive to the chemical domain, but it plays a more dominant role here than in the domains of the other natural sciences. Many students experience difficulties in understanding the macro/submicro/symbolic triangle and, in particular, in appreciating how and when to make the transitions between the three perspectives. Teachers do not always realize the importance of modeling the relationships here, by being explicit about the perspective being used, and the transitions being made, and helping students to overcome their difficulties.
The present chapter includes a review of studies that highlight two difficult chemical topics at the high school level. Part 1 of the chapter deals with a key topic in junior high schools: chemical reactions. Part 2 of the chapter concerns a core topic in senior high schools: atomic structure and chemical bonding. For each topic, students’ main conceptual difficulties are presented. They are explained concisely by the use of another triangle: the related perspectives of teaching, chemistry content, and learning. Studies of courses designed to help students respond to their difficulties are also presented and discussed. Special attention is given to courses based on modern perspectives on teaching and leaning, such as context-based teaching strategies and approaches incorporating a constructivist view of learning. Suggestions for priority areas for further research and curriculum development are given at several places. Finally, part 3 of the chapter presents a look to the near future of chemical education.
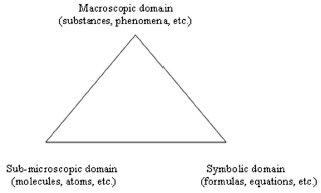
FIGURE 22–1. The triangle of meanings.
INTRODUCING MULTIPLE MEANINGS OF CHEMICAL REACTIONS
Multiple Meanings
In introductory chemical education, the central core content deals with chemical reactions. In elementary schools, if chemical reactions are introduced, students only have to learn the macroscopic meaning in terms of conversions of substances. High school students should also learn the submicroscopic meaning in terms of the rearrangement of particles (molecules, atoms, ions) and the symbolic meaning in terms of chemical equations (words, iconic drawings, formulas). These students also should become able to switch mentally between these meanings in an adequate and flexible way.
This section addresses studies of students’ conceptual difficulties, related to reactions that can be considered to proceed to completion, taking place in one direction. Difficulties in understanding more complex chemical reaction types can be found elsewhere, such as problems with understanding equilibrium reactions (Van Driel & Graeber, 2002) and redox reactions (De Jong & Treagust, 2002). The present section also offers some explanatory perspectives on students’ difficulties. Studies of efforts to prevent and to respond to their difficulties are discussed. Suggestions for further research and course development are also given.
Students’ Conceptual Difficulties
In the last two decades, numerous articles on students’ difficulties in understanding the multiple meanings of chemical reactions have been published. From studies and reviews, written by Ahtee and Varjola (1998), Andersson (1986), Fensham (1994), Gabel and Bunce, (1994), Johnson (2000), and Krnel, Watson, and Glazar (1998), a list of 12 of the most recurrent difficulties has been compiled and is presented below.
Vignette
In a junior high school class, a chemistry teacher puts a burning piece of wood in a glass with water. The burning stops.
| The teacher asks: | How is that possible? |
| Student #1 answers: | We do not understand, burning should go on because there is oxygen in the water, as we know because fish live in water. The teacher responds: But there is not enough oxygen in the water. |
| Student #2 argues: | We know that water is H2 O, so, one-third of water is oxygen, whereas it consists of oxygen for one-fifth only … so, teacher, how is that possible? |
In this vignette, student #2 compares water and air as providers of oxygen for a burning process. However, the student interprets the formula of water in an additive rather than from an interactive way: H2O is seen as H2 and O. This way of reasoning demonstrated a common difficulty in understanding symbolic representations.
Regarding the macroscopic meaning, recurrent difficulties are the following:
- Students may fail to recognize a process as a chemical change, through lack of sufficient knowledge of substance identity. For instance, students may interpret the product of a chemical change as a mixture where the original substances still persist.
- Students may believe that during chemical changes substances are displaced without any change of their properties. This is illustrated by students who think that parts of burning wood are driven off as smoke.
- Students tend to interpret chemical reactions as a process of modification, that is, chemical changes are seen as physical changes, and properties of substances are seen as changing, whereas the substances themselves remain the same. For instance, students may believe that the black coating formed on a piece of copper metal during heating represents black or burned copper.
- Students may interpret chemical changes as a transmutation of a given substance into another substance or into energy. This is demonstrated by students who believe that burned steel wool has been turned into carbon.
- Students sometimes seem to be unaware of the interactive role of “invisible” (gaseous) reactants or products. For instance, students may believe that the mass of a rusty nail is the same as that of the nail before rusting.
- Students tend to treat properties of substances as some kind of extra substance. This can be seen in students who believe that sugar disappears when it is dissolved in water, but the sweetness remains.
Regarding the submicroscopic meaning, recurrent difficulties are the following:
- Students often attribute macroscopic features to molecules or atoms and attribute submicroscopic features to substances. Typical examples are: students think that a molecule of water means a small drop of the liquid and may use expressions like “substances exchange outer electrons between them.”
- Students may fail to invoke atoms and molecules as explanatory constructs of chemical reactions, although they have knowledge of atoms and molecules.
- Students’ ability to give a submicroscopic explanation of chemical reactions in terms of a dynamic process is often limited. For instance, students may not refer to the rearrangement of atoms, that is, breaking of bonds and formation of new bonds.
Regarding the symbolic meaning, recurrent difficulties are as follows:
- Students tend to perceive a formula as representing one unit of a substance rather than a collection of particles, and they tend to interpret the formulas of compounds in an additive rather than from an interactive way. This is shown by students who interpret the formula H2O as H2 and O.
- Students may have difficulties in understanding the meaning of formula subscripts and equation coefficients. For instance, students tend to change the subscripts while balancing reaction equations.
- Students may consider balancing chemical reactions as mainly mathematical manipulation of symbols without much insight into the chemical meaning. A typical example: students may consider 3H2 as six linked atoms.
The reported difficulties can be explained from several perspectives. Three perspectives offering particular insight are:
Courses Developed from Modern Teaching and Learning Perspectives
The reported students’ conceptual difficulties have been found among students taught quite traditional chemistry courses. Efforts to prevent and to respond to their difficulties have led to a series of chemistry courses developed from modern teaching and learning perspectives. Studies of five exemplars are given below.
- A course that included three phases of the learning cycle, namely explication, concept introduction, and concept application, was investigated by Cavallo, McNeely, and Marek (2003). They reported on the development of understanding among 60 junior high-school students with respect to the three levels of meaning of chemical reactions. Findings indicate significant positive shifts in understanding. A minority (about 20%) of the students, however, showed persistent conceptual difficulties, especially regarding the difference between chemical change and physical change, and the relationship between atoms and substances.
- A course that introduced a teaching strategy based on the conceptual change perspective, that is, confronting students with “chemical events” that evoke cognitive conflicts because of existing everyday conceptions, was investigated by Nieswandt (2001). She reported on the development of understanding among 81 junior high school students with respect to macroscopic features of substances and chemical reactions (with particular emphasis on combustion). Results show a significant “erosion” of students’ everyday conceptions in favor of scientific concepts. A minority (about 25%) of the students, however, only developed “mixed” concepts, consisting of everyday concepts and chemistry explanations.
- A course that incorporated a context-based teaching approach by presenting chemistry concepts within the context of everyday events was investigated by Barker and Millar (1999). They reported on the development of understanding among 250 senior high school students with respect to the conservation of mass in closed- and open-system chemical reactions. Data indicate that students’ reasoning improved steadily as the course progressed. Nevertheless, a minority of the students retained misunderstandings about the conservation of mass in closed systems (23%) and open systems (29%), especially for reactions including gases.
- A course that included a constructivist view on learning by taking students’ own conceptions into account was investigated by Laverty and McGarvey (1991). They reported on the development of understanding among two classes of junior high school students with respect to macro, submicro, and symbolic meanings of chemical reactions. Results reveal good learning gains, although about 30% of the students were not able to identify particle diagrams correctly.
- A course designed from a mix of perspectives, namely conceptual change, context-led, and constructivist, was investigated by Solomonidou and Stavridou (2000). They reported on the development of understanding among 168 junior high school students with respect to macroscopic features of substances and various chemical reactions. Results show significant positive shifts in understanding. A minority (percentage not given) of the students, however, did not change their “concrete substance” idea toward the “unknown substance plus properties” scheme, and the “inert mixture” concept toward the “interaction between substances” concept.
Some of the reported studies cover only macroscopic features of chemical reactions (Barker & Millar, 1999; Nieswandt, 2001; Solomonidou & Stavidrou, 2000), whereas others also cover submicroscopic and symbolic features (Cavallo et al., 2003; Laverty & McGarvey, 1991). All studies report a positive development of students’ understanding, but all of them also indicate conceptual difficulties, despite the use of modern course designs and teaching strategies. This raises the question: what causes the persistency of the reported difficulties in these courses?
To answer this question, knowledge of the teaching-learning processes in the classroom could be helpful. Unfortunately, four of the studies only focused on learning outcomes, by using written questionnaires, sometimes combined with some interviews, in the context of pre-test/(repeated) post-test designs. As a consequence, they are not able to report on learning processes. Fortunately, in the fifth study, not only were a pre-test/post-test design and questionnaires used, but so were other instruments, such as audio records of lessons and classroom observations (Laverty & McGarvey, 1991). This study offers a better insight into students’ struggle for understanding. The researchers report how students design their own diagrammatic representations for the effect of heat on copper carbonate, why some of them mistake this decomposition for burning in air, and how they argue to find the best representation for the decomposition. In an older but still influential study of another constructivist course, De Vos and Verdonk (1985) also analyzed audiotaped classroom discussions. They found that junior high school students were able to develop primitive particle models of matter in the context of a chemical reaction, for example, for explaining the appearance of the brilliant yellow line, consisting of glittering tiny crystals in a continuous motion, when lead nitrate and potassium iodide are placed in opposite positions in a Petri dish filled with water.
In conclusion, more in-depth and longitudinal studies are needed to get a better “ecologically” valid insight into the factors and conditions that hinder or facilitate the development of students’ conceptions of the multiple meanings of chemical reactions.
The Dilemma of the Course Content Structure
The five reported studies deal with courses where the choice for a particular general teaching strategy is reported, but where the course content structure is hardly indicated, especially the issue of developing the idea of chemical reactions from a macroscopic level to a submicroscopic level by an early or late introduction of the particle theory. This issue is the subject of an old but ongoing debate in chemical education.
Several scholars have proposed a delayed introduction of molecules and atoms (e.g., Ahtee & Varjola, 1998; Fensham, 1994), but others have shown that students do not naturally have a concept of substance identity that allows them to recognize chemical change in a proper way (e.g., Johnson, 2000; Stavidrou & Solomonidou, 1998). For instance, although many courses introduce the burning of substances in an early stage, students experience a lot of difficulties in recognizing and understanding this event as a chemical reaction (Watson, Prieto, & Dillon, 1997). Johnson (2002) even found that students began to accept the idea of substances changing into other substances only after a teaching unit in which atoms had been introduced. The model of atoms and changes in bonding was not the explanation for the idea of chemical change, but the means by which chemical change was acknowledged. On the other hand, it is clear that premature introduction of the concepts of molecules and atoms is not indicated, because this approach will not enable students to consider particles as a fruitful concept for explaining chemical reactions, and may induce many difficulties at the submicroscopic level, as reported before. This raises the question: how should we escape from this content-related teaching approach dilemma?
A promising way out could be the development of context-led constructivist courses that use the students’ initial conceptions of substances and particles (most students have at least heard about atoms), although their conceptions are inevitably rather primitive and unscientific, to promote the development of knowledge of the multiple meanings of chemical reactions in a coherent and simultaneous way (see, e.g., Nakleh, Samarapungavan, & Saglam, 2005).
In conclusion, further research is required to get a deeper insight into the most effective course content structure for meaningful student learning of chemical reactions.
INTRODUCING MULTIPLE MEANINGS OF ATOMIC STRUCTURE AND CHEMICAL BONDING
Introduction
In chemical education at the senior high school level, one of the key concepts is chemical bonding, because knowledge of this concept allows students to make predictions, and give explanations, about physical and chemical properties of substances. For a good understanding of the bonding concept, knowledge of the structure of the atom is a prerequisite. Students who progress to learn chemistry from the high school level to the university level will meet multiple models of both concepts. Regarding atomic structure, students usually have to learn initially the shell model in terms of a positive central nucleus surrounded by shells of negative electrons. Later on, they have to learn the orbital model in terms of subshells, orbitals, and electron density patterns. Regarding chemical bonding, students usually learn first about covalent bonding (initially described as electron sharing) and ionic bonding (often implied to be equivalent to electron transfer; see below). If metallic bonding is introduced it is often presented in terms of a sea-of-electrons model. Progression toward a more sophisticated understanding of these bonds types is expected later, as is knowledge of hydrogen bonding, van der Waals forces, and so forth.
Vignette
In a senior high school class, a chemistry teacher shows a sheet including the equation for the reaction starting H2(g) + F2(g) … (indicating that the reactants were in the form of molecules).
The teacher asks: In your own words, explain why you think hydrogen reacts with fluorine?
A student answers: Because both atoms need one extra electron in their outside shell to have a noble gas structure, so by sharing two electrons (one from each atom) in a covalent bond, hydrogen fluoride becomes a very stable molecule …
In this vignette, the student response demonstrates a confident reply, yet one that contradicts the information in the teacher's question. This is a common student response that exemplifies how students find the curriculum models of atomic structure and bonding problematic (Taber, 2002).
This section addresses studies of students’ difficulties in understanding these multiple models and offers some explanations of how these difficulties arise and ideas to prevent and to respond to them. Suggestions for further research and course development are also given.
Teaching and Learning about Atomic Structure
Students’ recurrent conceptual difficulties. In the last decade, the interest in students’ difficulties in understanding the concept of atomic structure has been growing. Typical examples of recurrent difficulties are reported below (see also Justi & Gilbert, 2000). Usually, at the senior high school level, teaching about atomic structure starts with the shell model of this concept. One of the main strengths of this model is that it can act as a major explanatory device linking atomic structure to chemical behavior in terms of the periodic table. Electronic configurations expressed in symbols, such as 2.7, 2.8.2, etc., may be readily related to the period and group of the element, which in turn link to patterns of chemical behavior. Indeed the periodic table as often currently presented in chemical education is described as a table of the elements (i.e., of substances), but often presents data about atoms, sometimes juxtaposed with macroscopic properties such as melting temperature (Schmidt, Baumgärtner, & Eybe, 2003).
The shell concept is often problematic for students. First of all, the idea that the atom has a certain structure may seem to be inconsistent with prior learning. Students at the junior high school level have learned that atoms are the fundamental components of matter. However, Harrison and Treagust (1996) found that students still often appreciate this concept in only a vague way, and tend to consider these constituent particles as solid spheres. A second area of difficulties relates to learning about the construction of the atom. For instance, Harrison and Treagust (1996) reported that some students believed that an electron shell is some form of protective coating on the atom. A third area of difficulty is in appreciating the nature of interactions within the atom. For instance, Taber (1998a) showed that many high school students feel that the positively charged nucleus gives rise to a certain amount of attraction that is shared out among the electrons present. Students also tend to use alternative notions when explaining the stability of the nucleus, for example suggesting that nuclear stability is due to a force from the electrons pushing the protons together. A common alternative explanation is the conception that neutrons neutralize the charge on the protons in some way so that the protons may be collected together in the nucleus (Schmidt, Baumgärtner, & Eybe, 2003).
In a second stage of teaching about atomic structure, the shell model is replaced (or at least supplemented) by the more subtle and complex orbital model, to provide more explanatory power. Many students experience this transition as difficult to understand. As Harrison and Treagust (2000) indicated, they often initially adopt the new terminology, but still largely think in terms of shells of orbiting electrons. Even chemistry undergraduates have been found to think largely in terms of simple Bohr-type models of the atom (Cros et al., 1986). Not surprisingly, concepts such as orbital hybridization have been found to be difficult for both high school students (Taber, 2004) and undergraduates (Nakiboglu, 2003) to master.
Students’ conceptual difficulties can be explained from the same three perspectives as mentioned in the previous section:
| (i) | The teaching perspective. Teachers may be hindered by their own familiarity with ideas about atomic structure from understanding why the model presented to students is unclear for them and is often inconsistent with students’ familiar notions. Furthermore, teachers often do not pay sufficient attention to the historical background and model characteristics of the shell and orbital versions of atomic structure met in the curriculum. |
| (ii) | The science content perspective. The nature of the atom is very abstract. A key idea is the electrostatic interactions between the charged particles present (the electrons and the nucleus), but although necessary, this is not sufficient to understand atomic structure. Not only is quantum theory needed to appreciate why electrons should occur in shells, but the stability of the nucleus requires a completely different type of force—something that is often ignored at this level. |
| (iii) | The learning perspective. Students have to learn counterintuitive ideas about the nature of atomic structure. Moreover, they have learned about electric forces and energy, but the application of these concepts to understanding the shell and orbital meaning of atoms leads to cognitive conflicts, as mentioned above. |
Courses developed from modern perspectives. Several studies have been reported about approaches to preventing and responding to students’ difficulties in this area. Many conventional courses introduce atomic structure by comparison with the solar system, which is assumed to be more familiar to students. This type of teaching by analogy is very powerful when done well, but needs to focus students on both similarities and differences between the two systems. However, in practice, as Taber (2002) found, students may not have a sound appreciation of the forces at work in either the solar or atomic systems.
In a study of teaching at the junior high school level, Moran and Vaughan (2000) reported on a course, developed from a conceptual change perspective, that included the use of model building to make atomic structure seem more concrete for students, and that set up discussion about potential cognitive conflicts. These features seem worthy; however, the cognitive conflicts involved spotting errors in fake students’ work, rather than engineering situations where the students’ own ideas could be challenged by evidence. Although the authors imply that the approach was successful, they offer no evaluation of the learning outcomes.
In a study related to teaching at the high school level, Petri and Niedderer (1998) reported on a computer-assisted instruction (CAI) approach to teaching about atomic structure based around the concepts of state and orbital, and the standing wave analogy for electrons in atoms. This approach, which included a constructivist view on learning, uses computers to allow students to undertake mathematical modeling— yet focuses on the models produced, not the mathematics. Their case study of student learning showed that although initial, relatively limited, models of the atom continued to be used by the student, these were augmented by more sophisticated models that were closer to the target knowledge. These findings are consistent with the study of Harrison and Treagust (2000).
Understandably, there is a real debate about when and how quantum ideas ought to be taught in schools and universities. Some authors have argued that orbital concepts should be avoided completely for a longer time (e.g., Tsaparlis, 1997). Gillespie (1996) has argued that a conceptualization of atomic and molecular structure in terms of electron pair domains should be the preferred approach at the senior high school and introductory university levels.
In conclusion, the research highlights many of the difficulties students face in studying atomic structure. Unfortunately, the research does not provide clear advice to the teacher about how to proceed. In many cases, suggestions to delay the study of material may not be consistent with the prescribed curriculum. This underlines the need for further research, especially with respect to course designs that provide sufficient time for students to consolidate new ideas before being expected to develop them further.
Teaching and Learning about Chemical Bonding
Students’ recurrent conceptual difficulties. Research interest is growing in the area of students’ difficulties in understanding chemical bonding. Exemplars of recurrent difficulties are reported below. At the senior high school level, students commonly develop notions of two basic types of bonding that they take forward to more advanced levels of study: covalent and ionic. The covalent bond is often defined as electron-sharing; the ionic bond is often identified with the process of electron transfer between a metal atom and a nonmetal atom. These associations may be inappropriate as students often think that the notion of electron sharing is sufficient to explain the covalent bond, and that the ionic bond is an electron-transfer event, rather than the force holding ions together.
Regarding the understanding of the covalent bond, Barker and Millar (2000) showed that students may think that covalent bonds are weak, because many substances of which the constituent particles are considered to be covalently bound have low melting points. Other research indicated that students might think that covalent bonds break when a piece of material is reshaped, and may assume that high viscosity is due to covalent bonds (Peterson, Treagust, & Garnett, 1989). Tan and Treagust (1999) found that some students think that substances that actually have giant molecular structures contain discrete molecules with strong intermolecular forces.
Regarding the understanding of the ionic bond, research has shown that students tend to think that ionic substances consist of molecules, or ion-pair units that act like molecules, even if the actual term is not used (Taber, 1997; Barker & Millar, 2000). So, for the NaCl structure (which tends to be the archetypal teaching example, and is commonly quoted by learners) students would expect each ion to be involved in two types of interaction: with the one counter-ion within the same “molecule”/ion pair, and with the five other counter-ions that are not conceptualized as part of the same structural unit (Butts & Smith, 1987). A UK survey of over 300 students showed that a “molecular” model of ionic bonding was applied—to at least some extent—by most of the students, at both the junior high and senior high levels (Taber, 1997). Some students may believe that that the “molecules” assumed to be present in NaCl break up to give ions when the salt dissolves, but others consider the molecules/ion pairs to be the solvated species (Butts & Smith, 1987).
When students have to learn about polar bonds, they often tend to see these as a subclass of covalent bonds (Peterson et al., 1989). So, students may suggest that substances such as HCl exist as dissolved molecules in aqueous solutions (Barker & Millar, 2000).
Students often have a quite limited understanding of the metallic bond. Many expect molecules or ions to be present in metallic structures and, for that reason, think that the constituent particles are linked by covalent or ionic bonds (Taber, 2003).
Students’ conceptual difficulties can be examined from the same three perspectives applied earlier:
Developing research-based approaches. There are several studies that report courses informed by research into student learning difficulties in this area. In a study of teaching at the senior high school level, Barker and Millar (2000) reported on a context-based teaching approach that presents information about chemical bonding (and other theoretical notions) through a story line. The story line provides the context in which to introduce the concept as and when needed. Although the researchers found that the course seemed successful at teaching chemical bonding, they also found similar alternative conceptions about bonding among the students taking the course, as has been reported in the other studies discussed here.
Taber (1998b) reported a longitudinal study of teaching chemical bonding to senior high school students. He found that students often started their course with the idea that chemical bonding was always either covalent (= electron sharing) or ionic (= electron transfer). This finding can be related to research into the levels of intellectual development of students when they are introduced to these abstract concepts (e.g., Finster, 1991). They often had considerable difficulty adjusting their thinking to allow intermediate forms of bonding (i.e., polar) or accepting new categories of bond (e.g., hydrogen bonds). Unless a type of bond could be understood as a variation on the ionic or covalent case, it was often excluded from being considered a real bond and was seen as “just a force.” The study also reported on the progression through the course as involving a gradual process of coming to conceptualize chemical reactions and bonding in physical terms, rather than being about atoms trying to obtain octets of electrons. The results showed that this was a difficult succession that often required more time for learning than the course allowed, so that—at best—students were left with partly developed “multiple frameworks” for chemical bonding (Taber, 2001). This result is consistent with a study of Coll and Taylor (2002), who found that undergraduates and even post-graduate chemists may still often think in terms of the limited models acquired in school.
In conclusion, there has been a good deal of research on aspects of learning about chemical bonding, although much of this simply reports findings related to the alternative conceptions of rather disparate students. Although such research can inform teaching, it often fails to suggest how fundamental improvements in teaching strategies can occur. More detailed studies, exploring individual learners over time, can provide greater insights. The studies considered here led to recommendations for teaching strategy and course content structure that may be fruitful:
- Discuss bonding in physical terms (i.e., in terms of forces) rather than octets.
- Avoid anthropomorphic or animistic language when explaining why reactions occur or bonds form.
- Present discussions of bonding in an order of increasing complexity: metals, then ionic compounds (added complication, two types of ion present), then giant covalent structures (added complication—number of bonds determined by atomic structure), then simple covalent (added complication—two types of bond present).
However, more research is needed to find out if following such recommendations can actually improve student outcomes in practice.
As new learning is only meaningful and secure when it is constructed upon suitable foundations; then there needs to be a suitable period of time before any newly acquired concepts are suitable to be relied upon as prerequisite learning for new learning. This period of consolidation may be many months (e.g., Harrison & Treagust, 2000), and yet in chemistry teaching the curriculum often requires teachers to introduce such interdependent material in a much shorter time scale. As suggested above, this is another area where more research is indicated, as it is quite possibly a major factor in many of the difficulties that students face in learning about atomic structure and chemical bonding.
A LOOK TO THE NEAR FUTURE OF CHEMICAL EDUCATION
Chemical education reform is under way in many countries. An important reason for this reform is the growing dissatisfaction with the position of many chemistry curricula: quite isolated from students’ personal interest, society and technology issues, and modern chemistry. As a consequence, there is a growing interest in new issues, such as relevant and meaningful contexts (Bennett & Holman, 2002); multimedia tools, including computer software (Ardac & Akaygun, 2004); and multiple meanings of models (Justi & Gilbert, 2002). The new issues are not very specific to chemical education, but are also found in the curriculum reform of the other sciences. Most of them are elaborated in other parts of this book. Although the three exemplar issues—contexts, multimedia, and models—are already mentioned at several places in the previous sections, we will elaborate them more within the limited space available in the present chapter. First, we relate the issue of meaningful contexts and the issue of multimedia tools to the topic of chemical change. Second, we relate the issue of models to the topic of atomic structure and chemical bonding. At the end of the chapter, we briefly pay attention to the preparation of chemistry teachers for teaching multiple meanings, and, as a final note, we point out the need for a more coherent innovation of chemical education.
Teaching Multiple Meanings through Contexts
One of the most promising contributions to abolishing current curriculum isolation is the use of relevant and meaningful contexts for teaching chemistry topics (Bennett & Holman, 2002). Contexts are often considered as situations in which chemistry or other science concepts, rules, and so on, can help communicate meaning to students. They can come from several domains, such as students’ personal life, social life, and scientific life. Mahaffy (2004) even proposes to extend the triangle of meanings into a tetrahedron by adding the context issue (Fig. 22.2).
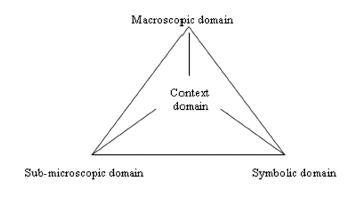
FIGURE 22–2. The tetrahedron of meanings.
Usually contexts are presented to students as illustrations of topics already presented as “theory,” but interest in another function of contexts is growing, namely contexts as a rationale or starting point for teaching topics. However, it would be naive to expect context-based chemistry courses to provide the solutions to all problems. These courses do not seem to help all students improve their reasoning about chemical reactions (see, e.g., Barker & Millar, 1999). Studies that compare the effects of context-based chemistry teaching with more traditional teaching are quite rare. Ramsden (1997) executed such a comparative study for introductory chemistry teaching at high schools. She found that there is little difference in understanding chemical change, but there appear to be some benefits associated with the context-based approach in terms of stimulating students’ interest in chemistry. Although this result may be somewhat disappointing from a cognitive development point of view, it is positive from an affective development point of view. The use of context-based approaches may not solve all of the reported problems with cognitive learning for several reasons. For instance, the contexts might have been too complex or quite unfamiliar to students, and the relation between a context and the intended accompanying concepts might not have been very meaningful to students. What is clear is that the results of existing studies suggest a need for further research in context-based chemistry teaching, especially in terms of the factors that contribute to cognitive learning outcomes.
Teaching Multiple Meanings Through Multimedia Tools
Within chemical education, there are a fast-growing number of articles on the use of multimedia tools, especially computer software with supplementary handouts. We will not review this literature, but only consider some experiences with teaching and learning the multiple meanings of chemical reactions.
Dynamic sequences of atomic and molecular interactions can be provided by computer-generated graphic representations. For instance, Garnett and Hackling (1999) reported on a short instructional intervention using a CD-ROM on balancing and interpreting chemical equations for high school students. The interactive program was designed to make extensive use of video illustrations of chemical reactions, followed by animations using dynamic graphics to show particle behavior and balancing chemical equations. Results indicate a growth of students’ understanding of chemical formulas and equations as well as in their skills in balancing these equations. Although these results are interesting, the value of the study is limited because it is unknown whether this intervention offers outcomes in excess of those that can be obtained by regular instruction. A comparison between both instructional conditions is made in a study of Ardac and Akaygun (2004) of a multimedia instructional unit that relates the multiple meanings of chemical reactions. They investigated the immediate and long-term (15 months) effects of high school students using this unit and found, for both time periods, that students from the experimental group showed a higher performance level than students who received regular instruction.
Despite success stories about the use of computerized learning environments, disappointing results are also reported. For instance, Wainwright (1989) evaluated a software package as a supplement to traditional instruction in balancing and interpreting chemical equations for high school students. The experimental group received reinforcement via the computer program, while the control group used parallel worksheets for concept reinforcement. After the intervention period, the control group performed significantly higher than the intervention group.
In conclusion, although the effectiveness of multimedia tools varies, they have the potential for enhancing students’ understanding of chemical reactions. They can improve students’ understanding of more complex reaction types, as studies have shown for acid-base reactions (Nakleh & Krajcik, 1994), redox reactions (Williamson & Abraham, 1995), and equilibrium reactions (Russell et al., 1997). In all cases, these tools require a very careful design and should be properly embedded in an overall teaching approach. More research is required to get a good insight into their use, especially concerning the factors that explain their effectiveness.
Teaching Multiple Meanings Through Models
In chemistry, it is very common to use models to represent molecular-level structures and processes. That these models are to some extent models of models—as any molecular level description of matter is, strictly, theoretical—complicates learning for students. Much has been written about the various types of models used in science and science education (Harrison & Treagust, 1996), and “model confusion” has been identified as a particular problem for learners in chemistry (Carr, 1984).
Indeed, it has been suggested that it is useful to distinguish between at least three different types of models that are important for chemistry education: scientific, curriculum, and teaching models (Justi & Gilbert, 2000). Scientific models are used extensively by chemists—for example to help visualization, theory development, and problem solving. Curriculum models are the (often simplified) versions that are set out in syllabuses as acceptable (or even desirable) target knowledge suitable for a particular age group. The level of simplification here may reflect considerations about the learners’ overall levels of cognitive development (e.g., the extent to which they are judged to be capable of formal abstract thinking), but also about their limited existing knowledge of a topic. Teaching models are constructed for or by teachers to help them communicate the curriculum models to students— such as a model to help students write Lewis structures when they are learning to understand acid-base reactions at a higher school level (Quilez Pardo, 1989).
The students themselves may not even realize that the target knowledge is a model, as research shows that many students do not appreciate the meanings and roles of models and theories in science (Driver, Leach, Millar, & Scott, 1996). Indeed, studies even suggest that teachers themselves show widely varying sophistication in appreciating the importance and nature of the models they teach about and with (De Jong, Van Driel, & Verloop, 2005; Justi & Gilbert, 2002). In the present chapter, many difficulties are reviewed regarding junior high-school students’ understanding of the submicroscopic meaning of chemical reactions, especially how to relate this model to the macroscopic meaning of chemical reactions. At the senior high school level, students use submicroscopic models to explain substance properties and behavior, though these are often alternative models. Indeed, it is very common for the chemical explanations produced at this level to be animistic or even anthropomorphic. Not only does research suggest that junior high school students may think that atoms are alive and grow (Harrison & Treagust, 1996), but even senior high school students may tend to believe that atoms are alive (Griffiths & Preston, 1992). Students seem to use psychological and sociological metaphors to produce explanations of chemical properties and behavior. Atoms are said to need and want full shells and to be happy once they obtain them (Taber & Watts, 1996).
Given these considerations—the centrality of models and modeling in chemistry, yet the unsophisticated understanding of models shown by students—it seems especially important that curriculum models (which will be presented to students as target knowledge, and often accordingly imbued with a high status by students) should be carefully chosen. Now it is suggested here that there are three clear criteria for any suitable curriculum model:
| (i) | It must be presented at a level of complexity and abstraction that fits the developmental levels of the learners concerned. |
| (ii) | It must build upon conceptual foundations that are already familiar—that is, the prerequisite learning must already be in place. (This suggests that careful conceptual analysis of topics, diagnostic assessment of the range of current learning in a class, and individualized remedial instruction will be important in the effective teaching of these models.) |
| (iii) | It must form part of a suitable progression of models that facilitates learners’ subsequent learning of more advanced models. |
We believe that the research that is available suggests that the curriculum models used in high school and undergraduate chemistry education often fail to meet these criteria. For example, the research of Coll and Taylor (2002) suggests that in many teaching institutions the octet explanation for bonding (found by these authors to be commonly used by undergraduate and post-graduate chemists) has such currency in the curriculum that it should be considered as a curriculum model. So, the notion of ionic bond formation as electron transfer between isolated atoms has become a standard curriculum model (see, for example, the curriculum benchmarks recommended in the United States, AAAS, 1993). This model is not only inaccurate, but closely tied to common misconceptions of the ionic bond discussed above—for example, that each sodium atom can only form a bond to one chlorine atom.
We believe that the use of the octet rule as the basis of an explanatory scheme should not be part of any curriculum model. Chemical reactions and bond formation are not explained in chemistry in terms of atoms filling their shells, and this is not a suitable simplification of actual (current) scientific thinking. Moreover, the octet framework seems to act as an impediment to learning more chemically valid models, rather than a suitable intermediate. This suggests that it does not make an appropriate curriculum model.
It might seem odd that such dubious chemical models should be presented as target knowledge in the curriculum. However, a number of studies are now suggesting that many curriculum models reflect ideas that once had scientific currency, but which have fallen into disuse (Justi & Gilbert, 2000; Tsaparlis, 1997). Some of the problems could be overcome if it was ensured that scientific models (those that may no longer be current in chemistry, but still have currency as curriculum models) are presented in their historical perspective, for instance, models of atomic structure (Justi & Gilbert, 2000), or the historical significance of the octet rule in the development of ideas about affinity and valency. Research into other chemistry topics where less attention has so far been paid to curriculum models could be very valuable. In view of the difficulties so many students have understanding the models of chemistry, as revealed in the literature reviewed in this chapter, more research into the status of the prescribed curriculum models, and how learners can be encouraged to appreciate them as models, is needed to inform both curriculum change and the teaching of chemistry itself.
Preparation of Chemistry Teachers for Teaching Multiple Meanings
Teaching chemical topics through modern student-centred courses looks attractive, but it requires teachers to have a very good insight into the topic, because these courses, especially the courses that include a constructivist view on learning, often require students to address questions where the answers are not given in the textbook. This raises the question: are prospective teachers sufficiently prepared for answering authentic questions from students about chemical topics? This question will be considered in the context of teaching the topic of chemical reactions.
At the elementary school level, prospective teachers often show conceptual difficulties, especially when they have no high school background or incomplete high school background in chemistry, as many have. For instance, prospective elementary school teachers tend to believe that mass is not conserved when a piece of paper is burned in a closed system (Ryan, Jiminez, & De Torre, 1989). They may also ignore the conservation of particles when drawing diagrammatic representations of chemical change (Gabel, Samuel, & Hunn, 1987). In more recent studies, Kokkotas, Vlachos, and Kouladis (1998) indicated that prospective elementary school teachers may attribute macroscopic properties to particles, and Del Pozzo (2001) found that they may have difficulty in interrelating macro- and submicroscopic concepts describing the composition of matter in a proper way.
At the secondary school level, prospective chemistry teachers also show conceptual difficulties, although not so many as elementary school teachers. Nevertheless, prospective chemistry teachers may show good understanding of balancing chemical equations but lack the ability to apply the concepts of conservation of mass and the same number and kind of atoms (Haidar, 1997). They may be able to draw diagrams depicting chemical reactions in terms of particles, but tend to ignore the creation of intermediate products, and to draw loosely packed representations of particles in solid ionic substances (Lee, 1999). Finally, De Jong, Ahtee, Goodwin, Hatzinikita, and Kouladis (1999) found that prospective chemistry teachers are not very familiar with current students’ difficulties in understanding combustion at a macroscopic level.
Studies of courses focusing on helping prospective teachers to understand the multiple meanings of chemical reactions and to teach them are rather scarce. Kokkotas, Vlachos, and Kouladis (1998) examined a training course for prospective elementary teachers. The participants were confronted with students’ authentic ideas as they are expressed when the students answer questions about the macro- and submicroscopic meaning of the composition of matter and change. Results indicate that the participants show improvement in terms of scientific understanding and knowledge of students’ conceptual difficulties. In a study of a teacher training course for prospective chemistry teachers, De Jong and Van Driel (2004) reported that the participants became aware of the need to show students the relations among the multiple meanings in a much more explicit way than they initially tended to do and to ignore their own dominant orientation toward submicroscopic meanings. Moreover, they noticed the importance of the careful and consistent use of symbolic representations, for example, not using the formulas NaCl(s) and Na+Cl–(s) in the same context.
In conclusion, the reported studies show the importance of courses for teachers that pay attention to improving prospective teachers’ knowledge of multiple meanings of chemical topics, and how to teach them. However, how prospective teachers link their “course” knowledge with their classroom practice is still not very clear. This is a general problem and requires further research.
LOOKING FORWARD
Innovations in chemical education should be carried out in a more coherent way than is currently the case. This requires the fine-tuning of at least the following components of innovations:
The integration of these three innovative steps implies an important challenge for the near future of chemical education.
ACKNOWLEDGMENTS
Thanks to George Bodner and Paul Hobden, who reviewed this chapter.
REFERENCES
A.A.A.S. (1993). Project 2061: Benchmarks, American Association for the Advancement of Science. (available at www.project2061.org/tools/benchol/bolframe.htm).
Ahtee, M., & Varjola, I. (1998). Students’ understanding of chemical reaction. International Journal of Science Education, 20, 305–316.
Andersson, B. (1986). Pupils’ explanations of some aspects of chemical reactions. Science Education, 70, 549–563.
Ardac, D., & Akaygun, S. (2004). Effectiveness on multimedia-based instruction that emphasizes molecular representations on students’ understanding of chemical change. Journal of Research in Science Teaching, 41, 317–337.
Barker, V., & Millar, R. (1999). Students’ reasoning about chemical reactions: what changes occur during a context-based post-16 chemistry course? International Journal of Science Education, 21, 645–665.
Barker, V., & Millar, R. (2000). Students’ reasoning about basic chemical thermodynamics and chemical bonding: what changes occur during a context-based post-16 chemistry course? International Journal of Science Education, 22, 1171–1200.
Bennett, J., & Holman, J. (2002). Context-based approaches to the teaching of chemistry: what are they and what are their effects? In J. K. Gilbert, O. De Jong, R. Justi, D. F. Treagust, & J. H. Van Driel (Eds.), Chemical education: Towards research-based practice (pp. 165–184). Dordrecht, the Netherlands: Kluwer Academic.
Butts, B., & Smith, R. (1987). HSC chemistry students’ understanding of the structure and properties of molecular and ionic compounds. Research in Science Education, 17, 192–201.
Carr, M. (1984). Model confusion in chemistry. Research in Science Education, 14, 97–103.
Cavallo, A. M. L., McNeely, J. C., & Marek, E. A. (2003). Eliciting students’ understanding of chemical reactions using two forms of essay questions during a learning cycle. International Journal of Science Education, 25, 583–603.
Coll, R. K., & Taylor, N. (2002). Mental models in chemistry: senior chemistry students’ mental models of chemical bonding. Chemistry Education: Research and Practice in Europe, 3, 175–184 (available at www.uoi.gr/cerp/2002_May/08.html).
Cros, D., Amouroux, R., Chastrette, M., Fayol, M., Leber, J., & Maurin, M. (1986). Conceptions of first year university students of the constitution of matter and the notions of acids and bases. European Journal of Science Education, 8, 305–313.
De Jong, O., Acampo, J., & Verdonk, A. H. (1995). Problems in teaching the topic of redox reactions: Actions and conceptions of chemistry teachers. Journal of Research in Science Teaching, 32, 1097–1110.
De Jong, O., Ahtee, M., Goodwin, A., Hatzinikita, V., & Kouladis, V. (1999). An international study of prospective teachers’ initial teaching conceptions and concerns: The case of teaching “combustion.” European Journal of Teacher Education, 22, 45–59.
De Jong, O., & Treagust, D. F. (2002). The teaching and learning of electrochemistry. In J. K. Gilbert, O. De Jong, R. Justi, D. F. Treagust, & J. H. Van Driel (Eds.), Chemical education: Towards research-based practice (pp. 317–337).
Dordrecht, the Netherlands: Kluwer Academic. De Jong, O., & Van Driel, J. (2004). Exploring the development of student teachers’ PCK of multiple meanings of chemistry topics. International Journal of Science and Mathematics Education, 2, 477–491.
De Jong, O., Van Driel, J., & Verloop, N. (2005). Preservice teachers’ pedagogical content knowledge of using particles models in teaching chemistry. Journal of Research in Science Teaching, 42(8), 947–964.
De Vos, W., & Verdonk, A. H. (1985). A new road to reactions, part 1. Journal of Chemical Education, 62, 238–240.
Del Pozo, R. M. (2001). Prospective teachers’ ideas about the relationships between concepts describing the composition of matter. International Journal of Science Education, 23, 353–371.
Driver, R., Leach, J., Millar, R., & Scott, P. (1996). Young people's images of science. Buckingham, UK: Open University Press.
Fensham, P. J. (1994). Beginning to teach chemistry. In P. J. Fensham, R. Gunstone, & R. White (Eds.), The content of science: A constructivist approach to its teaching and learning (pp. 14–28). London: Falmer Press.
Finster, D. C. (1991) Developmental instruction: Part 2. Application of Perry's model to general chemistry. Journal of Chemical Education, 68, 752–756.
Gabel, D. L., & Bunce, D. M. (1994). Research on problem solving: Chemistry. In D. L. Gabel (Ed.), Handbook of research on science teaching and learning (pp. 301–326). New York: Macmillan.
Gabel, D. L., Samuel, K. V., & Hunn, D. (1987). Understanding the particulate nature of matter. Journal in Chemical Education, 64, 695–697.
Garnett, P. J., & Hackling, M. W. (1999). Improving introductory chemistry students’ ability to visualise the particulate basis of chemical reactions. Chemeda: Australian Journal of Chemical Education, 51, 45–56.
Gillespie, R. J. (1996). Bonding without orbitals. Education in Chemistry, 33, 103–106.
Griffiths, A. K., & Preston, K. R (1992). Grade-12 students’ misconceptions relating to fundamental characteristics of atoms and molecules. Journal of Research in Science Teaching, 29, 611–628.
Haidar, A. H. (1997). Prospective chemistry teachers’ conceptions of the conservation of matter and related concepts. Journal of Research in Science Teaching, 34, 181–197.
Harrison, A., & Treagust, D. F. (1996). Secondary students’ mental models of atoms and molecules: implications for teaching chemistry. Science Education, 80, 509–534.
Harrison, A., & Treagust, D. F. (2000). Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in grade 11 chemistry. Science Education, 84, 352–381.
Johnson, P. (2000). Children's understanding of substances, part 1: Recognizing chemical change. International Journal of Science Education, 22, 719–737.
Johnson, P. (2002). Children's understanding of substances, part 2: Explaining chemical change. International Journal of Science Education, 24, 1037–1054.
Johnstone, A. H. (1991). Why is science difficult to learn? Things are seldom what they seem. Journal of Computer Assisted Instruction, 7, 75–83.
Justi, R., & Gilbert, J. K. (2000). History and philosophy of science through models: Some challenges in the case of “the atom.” International Journal of Science Education, 22, 993–1009.
Justi, R. S., & Gilbert, J. K. (2002). Models and modelling in chemical education. In J. K. Gilbert, O. De Jong, R. Justi, D. F. Treagust, & J. H. Van Driel (Eds.), Chemical education: Towards research-based practice (pp. 47–68). Dordrecht, the Netherlands: Kluwer Academic.
Kokkotas, P., Vlachos, L., & Kouladis, V. (1998). Teaching the topic of the particulate of matter in prospective teachers’ training courses. International Journal of Science Education, 20, 291–303.
Krnel, D., Watson, R., & Glazar, S. A. (1998). Survey of research related to the development of the concept of “matter.” International Journal of Science Education, 20, 257–289.
Laverty, D. T., & McGarvey, J. E. B. (1991). A “constructivist” approach to learning. Education in Chemistry, 28, 99–102.
Lee, K. L. (1999). A comparison of university lecturers’ and pre-service teachers’ understanding of a chemical reaction at the particulate level. Journal of Chemical Education, 76, 1008–1012.
Mahaffy, P. (2004). The future shape of chemistry education. Chemistry Education: Research and Practice, 5, 229–245 (available at www.uoi.gr/cerp/2004_October/05.html).
Moran, J., & Vaughan, S. (2000). Introducing CASE methodology at key stage 4: An example of bridging. School Science Review, 82, 47–55.
Nakiboglu, C. (2003). Instructional misconceptions of Turkish prospective chemistry teachers about atomic orbitals and hybridisation. Chemistry Education: Research and Practice, 4, 171–188 (available at www.uoi.gr/cerp/2003_May/06.html).
Nakleh, M. B., & Krajcik, J. S. (1994). Influence of levels of information as presented by different technologies on students’ understanding of acid, base, and pH concepts. Journal of Research in Science Teaching, 31, 1077–1096.
Nakleh, M. B., Samarapungavan, A., & Saglam, Y. (2005). Middle school students’ beliefs about matter. Journal of Research in Science Teaching, 42, 581–612.
Nieswandt, M. (2001). Problems and possibilities for learning in an introductory chemistry course from a conceptual change perspective. Science Education, 85, 158–179.
Peterson, R. F., Treagust, D. F., & Garnett, P. (1989). Development and application of a diagnostic instrument to evaluate grade-11 and -12 students’ concepts of covalent bonding and structure following a course of instruction. Journal of Research in Science Teaching, 26, 301–314.
Petri, J., & Niedderer, H. (1998). A learning pathway in high-school level quantum atomic physics. International Journal of Science Education, 20, 1075–1088.
Quilez Pardo, J. (1989). Teaching a model for writing Lewis structures. Journal of Chemical Education, 66, 456–458.
Ramsden, J. M. (1997). How does a context-based approach influence understanding of key chemical ideas at 16 +? International Journal of Science Education, 19, 697–710.
Russell, J. W., Kozma, R. B., Jones, T., Wykoff, J., Marx, N., & Davis, J. (1997). Use of simultaneous-synchronized macroscopic, sub-microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts. Journal of Chemical Education, 74, 330–334.
Ryan, C., Jiminez, J. M. S., & De Torre, A. M. O. (1989). Scientific ideas held by intending primary teachers in Britain and Spain. European Journal of Teacher Education, 12, 239–251.
Schmidt, H.-J., Baumgärtner, T., & Eybe, H. (2003). Changing ideas about the periodic table of elements and students’ alternative concepts of isotopes and allotropes. Journal of Research in Science Teaching, 40, 257–277.
Solomonidou, C., & Stavridou, H. (2000). From inert object to chemical substance: Students’ initial conceptions and conceptual development during an introductory experimental chemistry sequence. Science Education, 84, 382–400.
Stavidrou, H., & Solomonidou, C. (1998). Conceptual reorganization and the construction of the chemical reaction concept during secondary education. International Journal of Science Education, 20, 205–221.
Taber, K. S. (1997). Student understanding of ionic bonding: Molecular versus electrostatic thinking? School Science Review, 78, 85–95.
Taber, K. S. (1998a). The sharing-out of nuclear attraction: Or I can't think about physics in chemistry. International Journal of Science Education, 20, 1001–1014.
Taber, K. S. (1998b). An alternative conceptual framework from chemistry education. International Journal of Science Education, 20, 597–608.
Taber, K. S. (2001). Shifting sands: a case study of conceptual development as competition between alternative conceptions. International Journal of Science Education, 23, 731–753.
Taber, K. S. (2002). Chemical misconceptions—prevention, diagnosis and cure (2 Vols.). London: Royal Society of Chemistry.
Taber, K. S. (2003) Mediating mental models of metals: acknowledging the priority of the learner's prior learning. Science Education, 87, 732–758.
Taber, K. S., & Watts, M. (1996). The secret life of the chemical bond: students’ anthropomorphic and animistic references to bonding. International Journal of Science Education, 18, 557–568.
Tan, K.-C., & Treagust D. (1999). Evaluating students’ understanding of chemical bonding. School Science Review, 81, 75–83.
Tsaparlis, G. (1997). Atomic orbitals, molecular orbitals and related concepts: conceptual difficulties among chemistry students. Research in Science Education, 27, 271–287.
Van Driel, J. H., & Graeber, W. (2002). The teaching and learning of chemical equilibrium. In J. K. Gilbert, O. De Jong, R. Justi, D. F. Treagust, & J. H. Van Driel (Eds.), Chemical education: Towards research-based practice (pp. 271–292). Dordrecht, the Netherlands: Kluwer Academic.
Wainwright, C. L. (1989). The effectiveness of a computer-assisted instruction package in high school chemistry. Journal of Research in Science Teaching, 26, 275–290.
Watson, R., Prieto, T., & Dillon, J. S. (1997). Consistency of students’ explanations about combustion. Science Education, 81, 425–443.
Williamson, V. M., & Abraham, M. R. (1995). The effects of computer animation on the particulate mental models of college chemistry students. Journal of Research in Science Teaching, 32, 521–534.
