Chapter 14
Amperometric Enzyme Sensors
Amperometry is a suitable transduction method when coupled with an enzymatic reaction involving oxidation/reduction steps, such as those catalyzed by oxidase and dehydrogenase enzymes. The prototype is the amperometric glucose sensor, which was first introduced by Clark and Lyons in 1962 [1], and that was the starting point for further impressive advances and improvements.
During the evolution of the technology of oxidase-based amperometric sensors three main transduction methods were developed. These are summarized below with reference to oxidase-type enzymes. The general scheme for an oxidase-catalyzed reaction is:
(14.1) ![]()
where S an P are the substrate and the product of the enzymatic reaction.
Hence, first-generation amperometric sensors relied on electrochemical monitoring of either oxygen depletion or hydrogen peroxide formation. In second-generation sensors oxygen is replaced by an artificial electron acceptor (that is, a redox mediator) that could be incorporated along with the enzyme in the biocatalytic layer [2]. In this way, the sensor becomes independent of any additional reagent in solution. Attempts at performing direct electron transfer from the enzyme to the electrode led to the third-generation, mediatorless sensors [2]. Current research is directed at improving sensor performance by rational use of new materials, particularly nanomaterials [3].
14.1 First-Generation Amperometric Enzyme Sensors
The most straightforward amperometric transduction relies on the detection of natural cosubstrates, such as oxygen in the case of oxidase catalyzed reactions. In its simplest form, such a sensor can be obtaining by entrapping an enzyme solution between the membrane of an oxygen probe and an additional semipermeable membrane (Figure 14.1A). Glucose, along with dissolved oxygen diffuses into the enzyme layer where the catalyzed substrate conversion results in a decrease in the oxygen concentration. Residual oxygen diffuses further to the electrode surface where its reduction generates the response current. The glucose concentration is indicated by the proportional decrease in the oxygen probe response relative to the response in the absence of the substrate. In more advanced versions, the enzyme was incorporated in structured materials using various immobilization methods [4, 5]. Although successful, this transduction scheme suffers shortcomings because the oxygen probe is sensitive to uncontrolled variations in the oxygen concentration within the sample, particularly in the case of in vivo applications. In addition, solution pH should be controlled by a pH buffer system.
Figure 14.1 Configurations of oxygen-linked (A) and hydrogen peroxide-linked (B) amperometric enzyme sensors. Adapted with permission from [6]. Copyright 2002, A. B![]() nic
nic![]() and F.G. B
and F.G. B![]() nic
nic![]() .
.
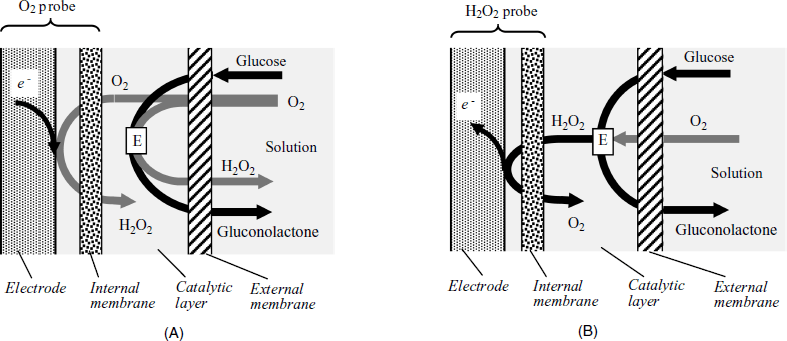
A second transduction alternative is based on hydrogen peroxide detection through its electrochemical anodic reaction (Figure 14.1B). Hydrogen peroxide, which is the product of the enzymatic conversion, travels to the anodically polarized electrode where it is oxidized to water. However, at the peroxide oxidation potential, endogenous interferents (such as urate, ascorbate and acetaminophen) also undergo anodic reactions resulting in a bias current. As some interferents are anions, their involvement can be avoided if the external membrane is made of a cation exchanger (such as Nafion). This membrane is permeable to small neutral molecules, such as glucose and oxygen, but rejects anionic species.
Another method for alleviating interference from electroactive concomitants relies on the lessening of the peroxide oxidation potential by means of a catalyst such as Prussian Blue [7]. This catalyst is amenable to integration into screen-print technology. However, Prussian Blue is not stable at pH > 7, which limits its application in conjunction with enzymes that display maximum activity in the alkaline region. Chemical, instead of electrochemical preparation of the Prussian Blue layer was reported to produce enhanced stability in this pH region.
An alternative catalyst is a peroxidase enzyme that can be integrated with an oxidase in the sensing layer. Hydrogen peroxide resulting from substrate conversion oxidizes the peroxidase and the resulting oxidized peroxidase then accepts electrons from the electrode either by direct transfer or via a mediator [8]. Thus, a glucose bienzyme sensor was obtained by integrating glucose oxidase and horseradish peroxidase immobilized on carbon nanotubes [9]. As usual, interference by anionic interferents was prevented by means of a Nafion external membrane. This sensor showed a high sensitivity for glucose detection at zero applied potential, which prevents interfences.
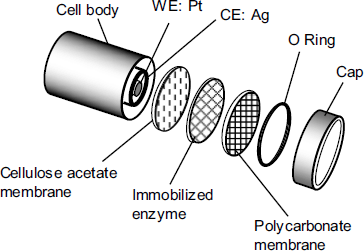
Similar transduction methods have been applied with a series of oxidase enzymes, enabling the determination of a large variety of compounds such as glucose, lactose, amino acids, cholesterol and phenols [4, 5]. The standard configuration of an amperometric sensor of this type is shown in Figure 14.2.
Figure 14.2 Typical arrangement of an amperometric sensor based on O2 or H2O2 detection. WE: working electrode; CE: counter (reference) electrode. Adapted with permission from [6]. Copyright 2002, A. B![]() nic
nic![]() and F.G. B
and F.G. B![]() nic
nic![]() .
.
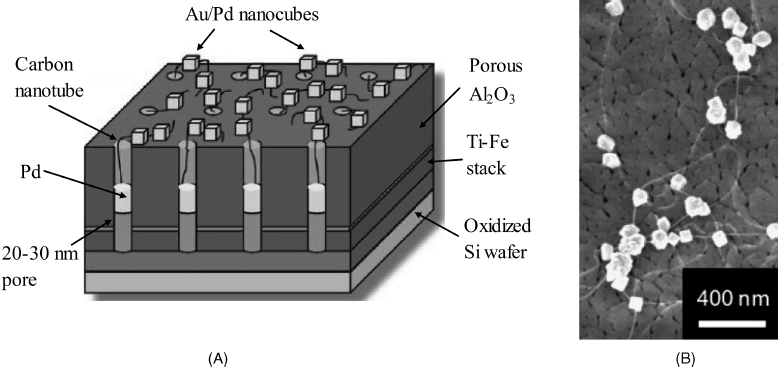
Despite the dependence on oxygen supply, the simplicity of this transduction method makes it still attractive for applications relying on new enzyme immobilization methods [10] or the use of nanomaterials [11, 12]. An example of nanomaterial application is shown in Figure 14.3, which represents a network of carbon nanotubes grown in a porous ![]() layer and connected to gold-covered palladium nanocubes [13]. Carbon nanotubes have been individually grown within the alumina pores by microwave plasma-enhanced chemical deposition. Formation of palladium nanocubes and subsequent gold decoration has been carried out by electrochemical deposition. In order to perform biofunctionalization, dithiobis(succinimidyl undecanoate) has been first attached as a linker via thiolate–gold bonding and then glucose oxidase has been covalently immobilized to this layer. Transduction has been performed by the anodic reaction of hydrogen peroxide at +0.5 V vs. Ag/AgCl. Excellent sensitivity (5.2 μA mM−1 cm−2), detection limit (1.3 μM) and a linear response range extending over 4 orders of magnitude have been reported. These satisfactory figures of merit can be ascribed to the high enzyme loading and to low electrical resistance pathways. At the same time, low site density prevents overlapping of individual diffusion layers and ensures radial diffusion taking place at individual sites with a positive effect on the rate of the mass transfer.
layer and connected to gold-covered palladium nanocubes [13]. Carbon nanotubes have been individually grown within the alumina pores by microwave plasma-enhanced chemical deposition. Formation of palladium nanocubes and subsequent gold decoration has been carried out by electrochemical deposition. In order to perform biofunctionalization, dithiobis(succinimidyl undecanoate) has been first attached as a linker via thiolate–gold bonding and then glucose oxidase has been covalently immobilized to this layer. Transduction has been performed by the anodic reaction of hydrogen peroxide at +0.5 V vs. Ag/AgCl. Excellent sensitivity (5.2 μA mM−1 cm−2), detection limit (1.3 μM) and a linear response range extending over 4 orders of magnitude have been reported. These satisfactory figures of merit can be ascribed to the high enzyme loading and to low electrical resistance pathways. At the same time, low site density prevents overlapping of individual diffusion layers and ensures radial diffusion taking place at individual sites with a positive effect on the rate of the mass transfer.
Figure 14.3 (A) Configuration of a nanocube-augmented carbon-nanotube network. The palladium deposit in each pore secures the electric contact of the carbon nanotube to the underlying titanium layer. (B) A field emission scanning electron microscopy picture showing carbon nanotubes protruding from the porous ![]() layer and attached Au/Pd nanocubes. Adapted with permission from [13]. Copyright 2009 American Chemical Society.
layer and attached Au/Pd nanocubes. Adapted with permission from [13]. Copyright 2009 American Chemical Society.
14.2 Second-Generation Amperometric Enzyme Sensors
14.2.1 Principles
In order to alleviate the sensor's dependence on oxygen supply, an artificial electron acceptor (the mediator MO) was introduced as an oxygen substitute [14, 15]. The reaction scheme for such a sensor is:
(14.2) ![]()
So, in the first step, electrons are transferred from the enzyme active site (![]() ) to the substrate (S) that is converted into a product (P), whereas the active site of the enzyme is converted into the reduced form (
) to the substrate (S) that is converted into a product (P), whereas the active site of the enzyme is converted into the reduced form (![]() ). Next, the oxidized enzyme form is regenerated by electron transfer to the electron acceptor
). Next, the oxidized enzyme form is regenerated by electron transfer to the electron acceptor ![]() . The resulting reduced form
. The resulting reduced form ![]() undergoes further electrochemical reaction at the electrode, which regenerates the
undergoes further electrochemical reaction at the electrode, which regenerates the ![]() species. The overall process consists therefore of a stepwise electron transfer from the substrate to the electrode with the enzyme and the mediator as intermediate relays (see also Sections 13.9.2 and 13.9.3). Transduction is performed by the reaction (14.4) that yields the response current. The process is independent of any additional reactant except the hydrogen ion that may be involved in substrate conversion.
species. The overall process consists therefore of a stepwise electron transfer from the substrate to the electrode with the enzyme and the mediator as intermediate relays (see also Sections 13.9.2 and 13.9.3). Transduction is performed by the reaction (14.4) that yields the response current. The process is independent of any additional reactant except the hydrogen ion that may be involved in substrate conversion.
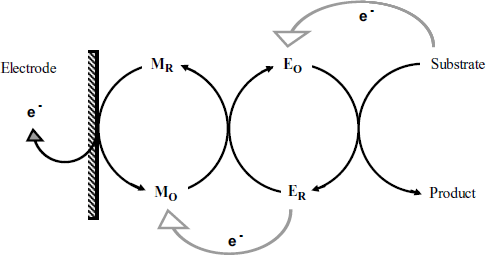
The process in a mediated amperometric sensor is schematically outlined in Figure 14.4. Charge transport to the electrode is a key process that can occur by various mechanisms depending on the architecture of the biocatalytic layer [2]. The mediator can, for example, be added to the sample solution and allowed to reach the biocatalytic layer by diffusion. Electrons are in this case conveyed to the electrode by ![]() diffusion. However, such a sensor is not a true reagentless one. It is best to get the mediator entrapped along with the enzyme within the biocatalytic layer. Often, mediated sensors are prepared by incorporating the enzyme and the mediator within a composite conductive material, such as carbon paste or screen-printing carbon ink. In such a case, electron transport will be achieved by sequential electron transfer between mediator molecules and conductive particles. If the sensing part consists of only one or a few molecular layers, direct electron transfer from mediator to the electrode can be achieved.
diffusion. However, such a sensor is not a true reagentless one. It is best to get the mediator entrapped along with the enzyme within the biocatalytic layer. Often, mediated sensors are prepared by incorporating the enzyme and the mediator within a composite conductive material, such as carbon paste or screen-printing carbon ink. In such a case, electron transport will be achieved by sequential electron transfer between mediator molecules and conductive particles. If the sensing part consists of only one or a few molecular layers, direct electron transfer from mediator to the electrode can be achieved.
Figure 14.4 Schematics of the reactions in a mediated amperometric enzyme sensor. Gray arrows indicate intermolecular electron transfer; dark arrows show actual chemical reactions.
The key problem in developing a mediated amperometric sensor is the selection of the mediator. The first condition a mediator should fulfill is the thermodynamic possibility of performing electron uptake from the enzyme. To this end, the standard electrode potential of the mediator must be positive relative to that of the enzyme. Investigation of redox processes in biological systems led to the discovery of a large variety of mediators characterized by various standard electrode potentials [16]. In addition, a suitable mediator should be very stable, compatible with the immobilization technique and, preferably, independent of hydrogen ions. Moreover, a mediator should exhibit as high a reaction rate as possible in both enzyme regeneration (reaction (14.3)) and mediator regeneration (reaction (14.4)) steps. Preferably, the electrochemical reaction (14.4) should be of reversibe (fast) type. In order to drive the process, the electrode should be polarized at a potential close to the standard potential of the mediator redox couple. A mediator with a lower standard potential performs best because the interference of electrochemical active concomitants can be avoided. If the sensor is designed for in vivo applications, care should be exercised to prevent a toxic mediator from leaking out into the tissue under investigation.
A common interference in mediated amperometric sensors arises from dissolved oxygen, which competes with the mediator in reaction (14.3) and gives rise to a bias in the response current [17]. This interference can be alleviated if the mediator concentration is very high and the enzyme reoxidation (14.3) as well as the electron transport is very fast. Oxygen interference can be prevented by immobilizing the enzyme in a permselective material that hampers oxygen access to the enzyme [18]. In some instances, an oxygen-independent enzyme can be used instead of an oxidase. Thus, PQQ-dependent glucose dehydrogenase can be a convenient alternative to glucose oxidase in amperometric enzyme sensors.
The next sections introduce the main classes of mediators for amperometric enzyme sensors.
14.2.2 Inorganic Mediators
Ferricyanide ion (![]() ) was among the first mediators that were tested. It undergoes a one-electron reduction to ferrocyanide with no hydrogen ion participation. Such an anion-mediator can be entrapped by electrostatic attraction into a positively charged matrix (e.g., oxidized polypyrrole). Ferricyanide has also been included in screen-printed structures along with carbon nanotubes that enhance the rate of the electron transport [19]. Ferricyanide is suitable for disposable sensors because mediator leakage is not a problem when a very short operation time is expected.
) was among the first mediators that were tested. It undergoes a one-electron reduction to ferrocyanide with no hydrogen ion participation. Such an anion-mediator can be entrapped by electrostatic attraction into a positively charged matrix (e.g., oxidized polypyrrole). Ferricyanide has also been included in screen-printed structures along with carbon nanotubes that enhance the rate of the electron transport [19]. Ferricyanide is suitable for disposable sensors because mediator leakage is not a problem when a very short operation time is expected.
Metal oxides, such as ![]() ,
, ![]()
![]() ,
, ![]() behave as efficient mediators when incorporated in carbon paste or screen-printing ink. Best results have been obtained, however, with platinum-group metal oxides, particularly
behave as efficient mediators when incorporated in carbon paste or screen-printing ink. Best results have been obtained, however, with platinum-group metal oxides, particularly ![]() , which allows the sensor to be operated at quite a low potential [20]. A tyrosinase-based catechol sensor was constructed by entrapping the enzyme and Fe3O4 nanoparticles in a chitosan matrix [21]. A high enzyme loading was achieved in this case as a result of the porous structure of chitosan. At the same time, the large specific area of the mediator nanoparticle secure a high reaction rate of the enzyme-mediator reaction.
, which allows the sensor to be operated at quite a low potential [20]. A tyrosinase-based catechol sensor was constructed by entrapping the enzyme and Fe3O4 nanoparticles in a chitosan matrix [21]. A high enzyme loading was achieved in this case as a result of the porous structure of chitosan. At the same time, the large specific area of the mediator nanoparticle secure a high reaction rate of the enzyme-mediator reaction.
14.2.3 Organic Mediators
Well-known organic redox couples can function as expedient mediators in redox enzyme-based biosensors. Thus, benzoquinone (Figure 14.5A) and its derivatives, as well as other diquinones (such as 1,2-naphthoquinone-4-sulfonate) have been successfully employed in mediated sensors. Many organic mediators have been selected from among the well-known class of redox indicators (redox dyes) that are used in redox titrimetry. To this class belong various derivatives of phenazine (Figure 14.5B) phenoxazine (Figure 14.5C) and phenothiazine (Figure 14.5D).
Figure 14.5 Examples of H+-dependent organic mediators used in amperometric enzyme sensors.
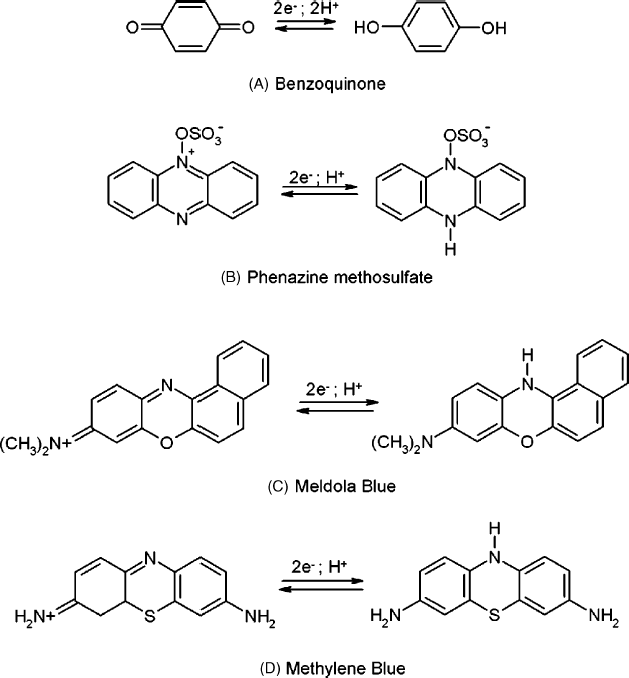
The redox reactions of such mediators involve hydrogen-ion transfer and, therefore, the standard electrode potential is pH dependent. Quinones undergo ![]() reactions, whereas the remaining compounds in Figure 14.5 experience
reactions, whereas the remaining compounds in Figure 14.5 experience ![]() reactions. Consequently, the sample pH should be controlled by means of a pH buffer. When selecting such a mediator, care should be exercised with regard to its possible instability under the effect of light or oxygen.
reactions. Consequently, the sample pH should be controlled by means of a pH buffer. When selecting such a mediator, care should be exercised with regard to its possible instability under the effect of light or oxygen.
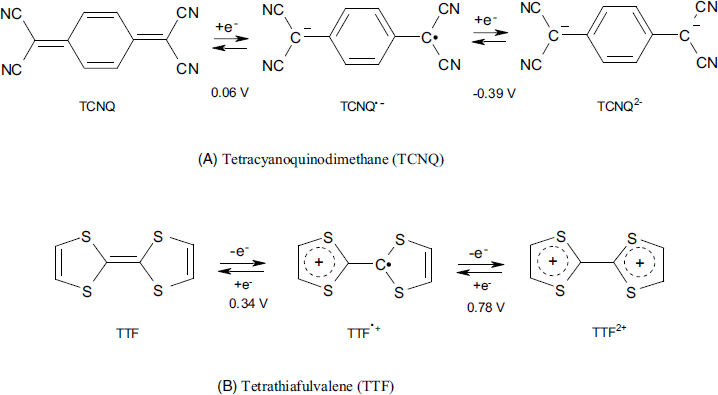
Another group of organic mediators are heteroatomic nonsaturated compounds whose ionic or radical forms are stabilized by charge delocalization over a conjugated ![]() orbitals system. As a result of this structure, such compounds undergo
orbitals system. As a result of this structure, such compounds undergo ![]() -independent redox reactions. Familiar examples are tetracyanoquinodimethane (TCNQ) and tetrathiafulvalene (TTF) shown in Figure 14.6. The first compound is an electron acceptor, whereas the second compound acts as an electron donor. Both of them undergo a sequence of two single-electron-transfer reactions leading to a radical-ion first and a di-ion finally. The standard potential of each redox step depends on the nature of the solvent and counterions in solution. As a rule, only the first electron transfer step is involved in biosensor applications. The properties of TTF and its derivatives have been extensively reviewed recently [22].
-independent redox reactions. Familiar examples are tetracyanoquinodimethane (TCNQ) and tetrathiafulvalene (TTF) shown in Figure 14.6. The first compound is an electron acceptor, whereas the second compound acts as an electron donor. Both of them undergo a sequence of two single-electron-transfer reactions leading to a radical-ion first and a di-ion finally. The standard potential of each redox step depends on the nature of the solvent and counterions in solution. As a rule, only the first electron transfer step is involved in biosensor applications. The properties of TTF and its derivatives have been extensively reviewed recently [22].
Figure 14.6 Structure and electrochemical reactions of tetrathiafulvalene (TTF) and tetracyanoquinodimethane (TCNQ). Specific potentials (vs. Ag/AgCl) have been determined in acetonitrile.
TTF-modified multiwalled carbon nanotubes are capable of facilitating electron transfers between the active sites of a redox enzyme and the electrode surface [23].
A TCNQ-modified graphite electrode was used for detecting the product of the acetylcholine esterase reaction in order to determine a series of insecticides by enzyme inhibition [24]. This method allows operation at an electrode potential lower than that required by the cobalt-phthalocyanine-mediated reaction [25].
14.2.4 Ferrocene Derivatives as Mediators
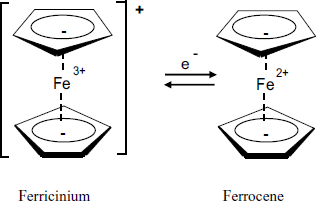
Among the large variety of investigated mediators, ferrocene derivatives, first introduced by Hill et al. [26], performs almost ideally. Ferrocene (Figure 14.7) is a charge-transfer complex of iron with the cyclopentadienyl aromatic anion. The chemical bonding in this compound results from the partial electron transfer from the ligand ![]() orbital to the empty orbitals at the iron ion. Electrochemical interconversion of the reduced and oxidized form proceeds reversibly with a half-wave potential of about +165 mV vs. SCE. Both solubility and the standard potential can be adjusted by adding a substituent to the ligand. Thus, electron-attracting groups, (such as carboxylate or aromatic groups) increase the standard potential, whereas electron-repelling groups (such as alkyls or amines) shift it to more negative values. Although ferrocene itself is soluble in water, solubility can be lessened by hydrophobic substituents. Ferrocene mediation was used in the first glucose sensor designed for home use [27].
orbital to the empty orbitals at the iron ion. Electrochemical interconversion of the reduced and oxidized form proceeds reversibly with a half-wave potential of about +165 mV vs. SCE. Both solubility and the standard potential can be adjusted by adding a substituent to the ligand. Thus, electron-attracting groups, (such as carboxylate or aromatic groups) increase the standard potential, whereas electron-repelling groups (such as alkyls or amines) shift it to more negative values. Although ferrocene itself is soluble in water, solubility can be lessened by hydrophobic substituents. Ferrocene mediation was used in the first glucose sensor designed for home use [27].
Figure 14.7 Structure and redox reaction of ferrocene. Note that each cyclopentadienyl ligand bears a negative charge (![]() electrons) delocalized over the whole ring.
electrons) delocalized over the whole ring.
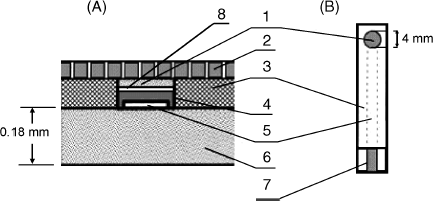
Construction of a ferrocene-mediated sensor is quite straightforward. A water-insoluble ferrocene (such as 1,1′-dimethyl ferrocene) is first deposited on the surface of a carbon electrode by evaporation of its solution in toluene. Next, the enzyme is immobilized by a suitable method, such as covalent binding or adsorption. Finally, the sensing layer can be coated with a perm-selective membrane. Alternatively, both the enzyme and mediator can be incorporated in a carbon paste or in a screen-printed structure [28]. Screen-printing technology is particularly appropriate for mass production of amperometric enzyme sensors. For example, the configuration in Figure 14.8 has been employed in glucose or ethanol sensors using pertinent PQQ-dependent dehydrogenases with 4-ferrocenylphenol as the electron-transfer mediator. The carbon ink layer is first covered with a perm-selective film formed by electrochemical polymerization of 2,4,7-trinitro-9-fluorenone (TNF). The mediator is adsorbed from acetone onto the carbon ink and, thereafter, the enzyme is immobilized by crosslinking with glutaraldehyde. The poly(TNF) coating prevents electroactive concomitants from reaching the electrode which improves the detection limit.
Figure 14.8 A screen-printed amperometric enzyme sensor. (A) cross section; (B) general view. 1) enzyme layer; 2) semipermeable terylene film; 3) insulating film; 4) carbon ink layer; 5) silver track; 6) polyethylene terephthalate film; 7) contact zone; 8) Perm-selective poly (TNF) film. Adapted with permission from [29]. Copyright 2001 Elsevier.
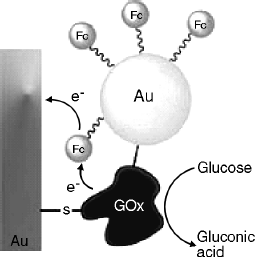
Recent research work in this field focuses on ferrocene integration with nanostructured materials [30] or nanoparticles such as gold [31] or carbon nanotubes [32]. As an example, Figure 14.9 shows a biocatalytic layer that includes glucose oxidase covalently linked to a gold electrode and to a gold nanoparticle. This device functions as a platform for appending ferrocene via a thiol bond in the close proximity of the enzyme molecule. In this configuration, the rate of electron transfer from the reduced enzyme to the electrode via the mediator is enhanced [33].
Figure 14.9 An electrically contacted Au nanoparticle–glucose oxidase composite electrode. 6-Ferrocenyl hexanethiol was attached to the nanoparticle in order to provide electron-transfer mediation. Reproduced with permission from [33]. Copyright 2008 John Wiley and Sons.
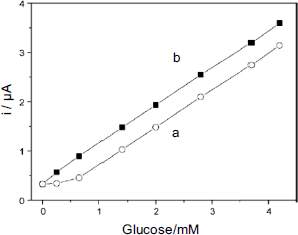
A carbon-nanotube-based glucose sensor was obtained by means of carbodiimide coupling of ferrocene monocarboxylic acid to carbon nanotubes previously derivatized by an amino-silane. The biocatalytic layer was then prepared by casting onto a graphite electrode a suspension of derivatized nanotubes in a chitosan and glucose oxidase solution [32]. The response graph of such a sensor is shown in Figure 14.10. Note the negative bias induced by dissolved oxygen that, as mentioned above, can occur with mediated sensors.
Figure 14.10 Calibration graph for a glucose sensor mediated by carbon-nanotube-attached ferrocene in the presence (a) and in the absence of oxygen (b). Adapted with permission from [32]. Copyright 2008 Elsevier.
14.2.5 Electron-Transfer Mediation by Redox Polymers
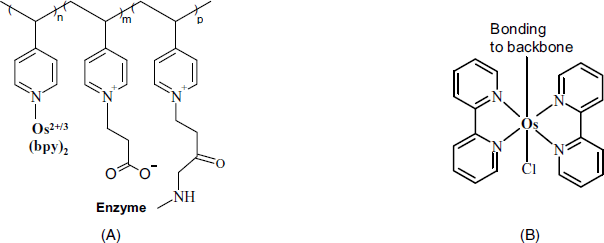
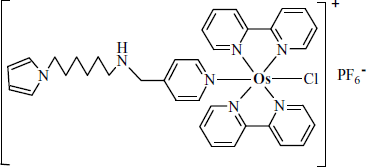
Initial applications of mediated electron transfer relayed on mediators as independent molecules randomly distributed within the biocatalytic layer. More efficient mediating was subsequently demonstrated by anchoring mediator moieties to a polymer backbone that allows, in addition, the enzyme to be immobilized by covalent bonding [34–36]. A typical structure of this type is shown in Figure 14.11A. It can be built up starting from poly(vinylpyridine). First, a fraction of the pyridine residues in the polymer is used for attaching by coordination an osmium complex (Figure 14.11B) to yield a redox polymer. Further, remaining pyridines are derivatized so as to allow enzyme immobilization by covalent bonding. The resulting macromolecular structure (Figure 14.11A) includes the enzyme and the osmium complex mediator firmly attached to the backbone. In addition, it contains charged moieties that impart hydrophilicity and allow hydration of the polymer network thus facilitating the access of substrate to the enzyme. The resulting material is termed a redox hydrogel.
Figure 14.11 General representation of enzyme wiring. (A) Polymer backbone and functional side groups. (B) A typical redox center/mediator, the [Os(bipirydine)Cl]2+/3+ complex.
In such a structure, electron transfer from enzyme to mediator is facilitated by the close proximity of the latter to the active site of the enzyme, although direct contact is prevented by steric factors. Further, electrons can be transferred directly from the mediator to the electrode within a few polymer monolayers. However, in a thicker system, some kind of electron diffusion must occur in order to achieve electrical communication between the redox centers and the electrode. This can be achieved by percolative electron hopping, long-range chain motion and short-range chain motion. Hence, electron diffusion is to a great extent dependent on the flexibility of the spacer chain between the mediator and the backbone. Electron diffusion can be limited by the diffusion velocity of counterions required for preserving electroneutrality in the system. The redox polymer accomplishes therefore a kind of electrical wiring between enzyme and electrode. Hence, it is said that an enzyme integrated with a redox polymer is a wired enzyme.
Other polymers, such as polysiloxanes and polyethylene oxide have been considered in order to construct more flexible structures with enhanced hydrophilicity. Also, various mediators such as ferrocene [35], tetrathiafulvalene, benzoquinone and viologen [36] have been tested in order to exploit their particular standard electrode potentials and their compatibility with particular enzymes.
Various applications of redox hydrogel enzyme sensors for determining oxidase and dehydrogenase substrates have been reported [36, 37]. It appears that redox hydrogels represent a versatile alternative for preparing biocatalytic layers for amperometric enzyme sensors. Their use guarantees a high mediator concentration and very fast electron transport, although this is still much slower than substrate diffusion.
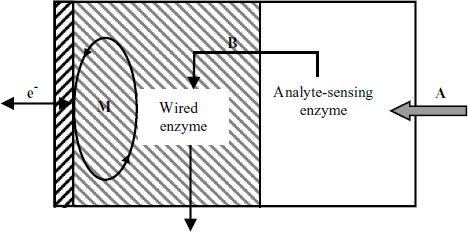
Redox hydrogels are also suitable for manufacturing multienzyme sensors, as demonstrated in Figure 14.12. In this design, the first enzyme layer converts the analyte A into a product B that acts as the substrate to a second enzyme that is wired to the electrode through a redox polymer. This approach allows the determination of an analyte that is not involved in an enzyme-catalyzed redox reaction.
Figure 14.12 A bienzyme sensor based on enzyme wiring. The enzyme in the first layer converts the analyte (A) into the intermediate product B that is further converted by the wired enzyme in order to generate the response current.
Interestingly, osmium redox polymers can also be applied to achieve efficient electrical communication between the gram-positive micro-organism Bacillus subtilis and an electrode in order to develop an amperometric succinate sensor [38].
14.2.6 Sensing by Organized Molecular Multilayer Structures
Preparation methods highlighted above lead as a rule to three-dimensional structures with the enzyme and mediator randomly distributed. Close control over the properties of the sensing layer can be accomplished by assembling successive molecular layers in order to integrate the component into a well-organized structure [10, 39–41] (see also Section 5.6.3). A sensing layer prepared by electrostatic, sequential self-assembly is depicted in Figure 14.13. In the first step, a negatively charged thiocarboxylate self-assembled monolayer was appended to a gold electrode surface. Next, the mediator, in the form of a cationic ferrocene polymer (poly(allylamine)) was attached to the first layer by electrostatic assembly. Finally, the negatively charged enzyme was allowed to form a monolayer over the second film. Such a bilayer film acts as the sensing element as it includes both the enzyme and a mediator. In order to increase the enzyme loading, successive, alternating mediator and enzyme layers can be assembled by repeating the previous procedure.
Figure 14.13 A wired enzyme biocatalytic layer built up by successive electrostatic assembly of various monolayers. Double pentagons represent ferrocene. Note the protrusion of ferrocene mediator moieties into the enzyme layer. PAA stands for poly(allylamine). Adapted with permission from [42]. Copyright 1997 Copyright American Chemical Society.
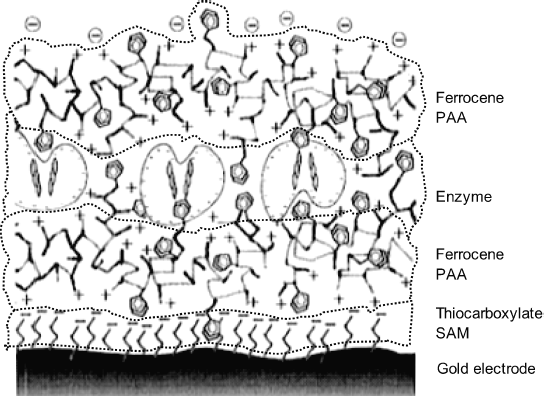
In the previous example, adhesion of successive layers was imparted by electrostatic interaction. The preparation method is very simple, but the resulting structure is somewhat sensitive to changes in pH and ionic strength that may causes electric charges to be altered. More robust catalytic films can be obtained by forming covalent bonds between consecutive layers. The usual methods and reagents for covalent immobilization of enzymes can be employed to this end. As an alternative, affinity reactions can be used for performing self-assembly of enzyme and redox polymer layers.
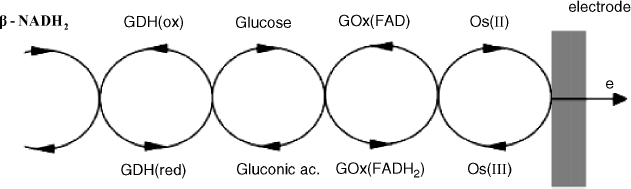
The layer-by-layer method outlined above allows a rigorous control of enzyme loading and also makes possible straightforward preparation of multienzyme layers. An example of a multienzyme sensor performing amplification by substrate recycling is presented in Figure 14.14. In this system, glucose oxidase converts the substrate (glucose) to gluconic acid that is recycled to glucose by a ![]() -assisted glucose dehydrogenase reaction. Wiring of glucose oxidase was achieved by an
-assisted glucose dehydrogenase reaction. Wiring of glucose oxidase was achieved by an ![]() functionalized polymer. As usually, substrate recycling results in greatly enhanced sensitivity.
functionalized polymer. As usually, substrate recycling results in greatly enhanced sensitivity.
Figure 14.14 Configuration of a glucose sensor with substrate amplification assembled by the layer-by-layer method. GDH-glucose dehydrogenase; GOx-glucose oxidase. Reproduced with permission from [40]. Copyright 2000 The Royal Society of Chemistry.
Incorporation of carbon nanotube–enzyme conjugates in redox hydrogels through a layer-by-layer self-assembly process leads to enhanced current density that gives better sensitivity relative to the film containing no nanotubes [43].
A redox polymer has been used in a flexible, implantable multienzyme sensor for the determination of glucose in blood. As shown in Figure 14.15, this sensor consists of several distinct layers at the surface of a recessed gold electrode. The first layer forms the actual sensing part and includes glucose oxidase entrapped in a redox polymer. The third layer contains a peroxidase coimmobilized with lactose oxidase and is intended to destroy common interferents in blood, such as urate, ascorbate and acetaminophen. In this layer, lactate (typically present in blood at about 1 mM) reacts with oxygen to form pyruvate and hydrogen peroxide that oxidizes the interferents (by peroxidase catalysis) and is itself reduced to water. The intermediate layer 2 consists of a polycationic polymer that secures insulation of the enzyme in the first layer. The outermost layer 4, which consists of tetraacrylated poly (ethylene oxide), provides biocompatibility.
Figure 14.15 An implantable, multienzyme glucose electrode. Adapted with permission from [44]. Copyright 1994, American Chemical Society.
14.3 The Mediator as Analyte
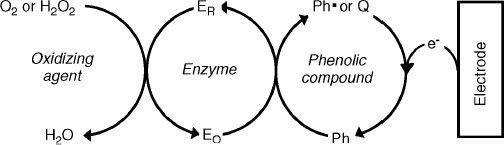
The key role played by the mediator in an amperometric enzyme sensor suggests a straightforward method for detecting a compound that can function as mediator in an enzymatic sensor. Typical examples are represented by phenolic compound sensors based on redox enzymes such as tyrosinase, laccase and peroxidase [45]. As shown in Figure 14.16, the analyte (a phenolic compound), along with an oxidizing agent, diffuses from the solution to the enzyme layer where the enzyme reaction, in conjunction with the electrode reaction recycles the analyte between two oxidation states. The oxidizing agent is ![]() in the case of peroxidase or
in the case of peroxidase or ![]() in the cases of laccase or tyrosinase enzyme.
in the cases of laccase or tyrosinase enzyme.
Figure 14.16 Mechanism of an amperometric phenol sensor. ![]() and
and ![]() are the reduced and oxidized forms, respectively, of the enzyme. Ph,
are the reduced and oxidized forms, respectively, of the enzyme. Ph, ![]() and Q are the phenolic molecule, its phenoxy radical and its quinone form, respectively. Adapted with permission from [45]. Copyright 1995, Elsevier.
and Q are the phenolic molecule, its phenoxy radical and its quinone form, respectively. Adapted with permission from [45]. Copyright 1995, Elsevier.
In contrast to this configuration, which is based on enzymatic oxidation reactions, dehydrogenases (such as cellobiose dehydrogenase or PQQ-dependent glucose dehydrogenase) allow the development of reduction–based sensors [46]. In this case, the phenolic analyte is first oxidized at the electrode and the product then accepts electrons via the enzyme from a suitable substrate. Whereas the oxidation-based sensor operates at a relatively low potential (−0.1 to 0 V vs. Ag/AgCl), the reduction-based version needs a relatively high anodic polarization (0.3–0.4 mV vs. Ag/AgCl) in order to perform electrochemical oxidation of the analyte. Consequently, the second version is subjected to the risks of interference by electroactive concomitants.
Substrate recycling can in some cases be assisted by a reducing agent, such as ascorbic agent. This task can also be performed by a second enzyme that converts the product of the electrochemical reaction into the substrate of the main enzyme. Thus, a phenol sensor was constructed by coimmobilization of poly-phenol oxidase and horse radish peroxidase by electrochemical polymerization [47]. The first enzyme converts phenol to o-quinone that can enter an amplification cycle involving electrochemical reduction to catechol, followed by peroxidase-catalyzed conversion of catechol to o-quinone. A theoretical model for such a bienzyme sensor has recently been worked out [48]. As some phenolic compounds are insoluble in water, they can be extracted into an organic solvent and then determined in the extract by means of a properly designed sensor [49, 50].
14.4 Conducting Polymers in Amperometric Enzyme Sensors
Conducting polymers can be used for various purposes in the design of enzyme amperometric sensors [51–54]. A simple application involves conducting polymers (such as polypyrrole or polyaniline) as entrapment material for enzyme immobilization. If amino groups are attached to the polymer backbone, they can act as a binding site in enzyme immobilization by covalent linking.
A more advanced application resorts to conducting polymers to form the backbone in the structure of a redox hydrogel. For example, PQQ-dependent glucose dehydrogenase was included in a three-dimensional polymeric structure along with osmium bipyridine by electrochemical copolymerization of pyrrole and the monomer in Figure 14.17 in the presence of the enzyme [55]. Enzyme immobilization was achieved by the reaction of the radical cations formed during the polymerization reaction with nucleophilic functions on the enzyme surface.
Figure 14.17 An osmium-bipyridine-derivatized pyrrole monomer used in the synthesis of a redox hydrogel by copolymerization with pyrrole.
Certain multifactor enzymes (e.g., PPQ and heme-c-based alcohol dehydrogenase) are able to perform direct electron transfer to the polypyrrole backbone. Therefore, enzyme sensors using a polypyrrole coated electrode can be designed with no need for a redox mediator. However, the number of enzymes that function in this way is limited.
14.5 Direct Electron Transfer: 3rd-Generation Amperometric Enzyme Sensors
Achieving direct electron transfer between the prosthetic group of an oxidoreductase and the electrode brings about a major simplification in the sensor configuration by precluding sensor dependence on a cosubstrate or a mediator. The next sections present several methods that proved successful in this respect
14.5.1 Conducting Organic Salt Electrodes
Direct electron transfer from redox enzymes can be achieved with electrodes consisting of conducting organic salts. Such compounds are formed by the combination of an electron donor and an electron acceptor such as tetrathiafulvalene (TTF) and tetracyanoquinodimethane (TCNQ), respectively (Figure 14.6). The ![]() salt can be prepared by direct reaction of the above compounds in acetonitrile. The product is a planar molecule with delocalized
salt can be prepared by direct reaction of the above compounds in acetonitrile. The product is a planar molecule with delocalized ![]() orbitals extending both above and below the molecular plane. Structurally, the solid
orbitals extending both above and below the molecular plane. Structurally, the solid ![]() salt consists of segregated stacks of cations and anions of the donor and the acceptor, respectively. Partial electron transfer ensures a relatively stable bonding between alternating donor–acceptor stacks. Compounds of this type belong therefore to the class of charge-transfer compounds and display semiconductor properties.
salt consists of segregated stacks of cations and anions of the donor and the acceptor, respectively. Partial electron transfer ensures a relatively stable bonding between alternating donor–acceptor stacks. Compounds of this type belong therefore to the class of charge-transfer compounds and display semiconductor properties.
The electrocatalytic properties of organic semiconductor salts towards various flavoenzymes was first reported in 1981 [56, 57]. An unequivocal interpretation of this effect is not yet available, but it may be associated with the exceptionally strong protein adsorption at the solid surface prompted by electrostatic attraction between the charged protein molecule and the intrinsic electric charge at the electrode surface.
The ![]() electrode can be prepared in several ways [58]. The pure compounds can be used either as a single crystal or as pressed pellets. Alternatively, the finely dispersed organic semiconductor mixed with an inert oil yields a conducting paste that can be manipulated in the same way as carbon paste electrodes. Slow evaporation of a salt solution applied to the surface of a graphite electrode (drop coating) is the most straightforward method for preparing conducting salt-modified electrodes.
electrode can be prepared in several ways [58]. The pure compounds can be used either as a single crystal or as pressed pellets. Alternatively, the finely dispersed organic semiconductor mixed with an inert oil yields a conducting paste that can be manipulated in the same way as carbon paste electrodes. Slow evaporation of a salt solution applied to the surface of a graphite electrode (drop coating) is the most straightforward method for preparing conducting salt-modified electrodes.
The potential window of a conducting salt electrode is determined by its electrochemical reactions. In neutral solutions, at a potential more positive than +0.4 V vs. SCE, the TCNQ− is oxidized to neutral TCNQ that is water insoluble and remains on the electrode surface. At potentials more negative than about −0.2 V, TTF and TCNQ− are released. Therefore, the working potential should be adjusted between these limits and not be allowed to drift outside it; otherwise the electrode surface may become completely covered with insoluble decomposition products.
The feasibility of conducting salt electrode-based enzyme sensors was demonstrated with various flavoenzymes such as glucose oxidase, D-amino acid oxidase and L-amino acid oxidase that enables the determination of glucose or amino acids, respectively. The enzyme was immobilized by adsorption onto the electrode surface. A wider linear response range is obtained by coating the sensing layer with a dialysis membrane that imposes a diffusion barrier. Screen printing is a suitable technology for mass fabrication of sensors of this type, which are characterized by very good long-term stability [59].
![]() integrated in a nonconducting poly(pyrrole) film has been used for fabricating a disposable glucose sensor (Figure 14.18) [60].
integrated in a nonconducting poly(pyrrole) film has been used for fabricating a disposable glucose sensor (Figure 14.18) [60]. ![]() crystals have been grown through the insulating polymer film yielding a highly branched structure with a very large effective area. Oxygen interference was detected only at glucose concentrations below 1 mM. Electroactive species (such as ascorbic acid, uric acid, cysteine, paracetamol, and acetaminophen) gave very low or undetectable signals. This favorable behavior was ascribed to the protecting effect of the negatively charged polymer that effectively rejects anionic interferents.
crystals have been grown through the insulating polymer film yielding a highly branched structure with a very large effective area. Oxygen interference was detected only at glucose concentrations below 1 mM. Electroactive species (such as ascorbic acid, uric acid, cysteine, paracetamol, and acetaminophen) gave very low or undetectable signals. This favorable behavior was ascribed to the protecting effect of the negatively charged polymer that effectively rejects anionic interferents.
Figure 14.18 Schematics of a disposable glucose electrode based on ![]() integrated in an insulating poly(pyrrole) film. Glucose oxidase (GOx) was immobilized by crosslinking with glutaraldehyde and bovine serum albumin (BSA). Reproduced with permission from [60]. Copyright 2002, American Chemical Society.
integrated in an insulating poly(pyrrole) film. Glucose oxidase (GOx) was immobilized by crosslinking with glutaraldehyde and bovine serum albumin (BSA). Reproduced with permission from [60]. Copyright 2002, American Chemical Society.
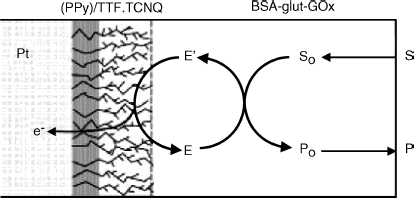
Conducting organic salt electrodes allowed the development of amperometric enzyme sensors for various analytes of biological interest [61].
14.5.2 Direct Electron Transfer with FAD-Heme Enzymes
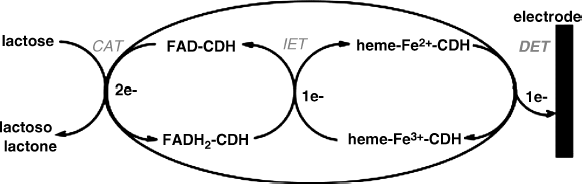
An interesting direct electron-transfer scheme was recently reported for the case of a cellobiose dehydrogenase-based lactose sensor [62]. Structurally, cellobiose dehydrogenase includes two prosthetic groups: a FAD and a heme cytochrome. The first catalytic step involves lactose conversion catalyzed by the FAD moiety (Figure 14.19). FAD is recycled by an internal electron transfer to the heme group that further conveys electrons to the electrode and thus achieves the detection/transduction step. Remarkably, this scheme allows the direct electron transfer to the usual electrode materials such as gold.
Figure 14.19 Schematics of reactions taking place at cellobiose dehydrogenase/electrode interface in a lactose biosensor. CAT: enzyme-catalyzed reaction; IET: intramolecular electron transfer; DET: electrochemical reaction. Reproduced with permission from. [62]. Copyright 2006, American Chemical Society.
The particular structure of cellobiose dehydrogenase has been cleverly exploited in the design of a fuel cell-type glucose biosensor [63]. A fuel cell is a kind of galvanic cell that generates a current by spontaneous electrochemical oxidation of a conventional fuel (such as glucose, in this case). In this glucose biosensor the anode reaction consists of glucose dehydrogenation by a scheme similar to that in Figure 14.19, using cellobiose dehydrogenase as catalyst. The cathode reaction is oxygen reduction catalyzed by bilirubin oxidase. This sensor can be operated either in a synthetic solution (phosphate buffer) or in human serum and is promising for in vivo applications.
14.5.3 Achieving Direct Electron Transfer by Means of Nanomaterials
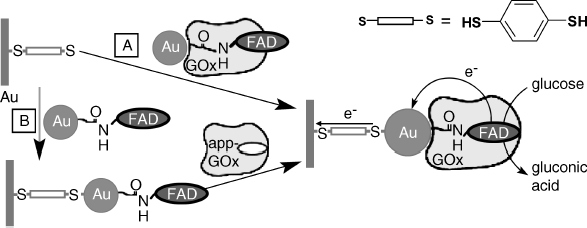
Although the mediated electron transfer method proved convenient in performing enzyme communication with the electrode, the geometry of the enzyme–mediator–electrode system at the molecular level is not optimal, which renders the electron transfer relatively inefficient. Close control of the architecture of the biocatalytic layer can be achieved by integrating the enzyme with nanoparticles in order to carry out direct electron transfer between the electrode and the enzyme's active site [64, 65]. An application of gold nanoparticles is demonstrated in Figure 14.20. According to path A in his scheme, gold nanoparticles are functionalized with a FAD derivative and this is allowed next to interact with apo-glucose oxidase in order to reconstitute the whole enzyme. Thus, a nanoparticle-enzyme conjugate is obtained. This adduct is further bridged with a dithiol-modified gold electrode via the gold nanoparticle. In an alternative approach (B), nanoparticle–FAD conjugates have first been assembled on the dithiol-modified gold electrode and next the enzyme was reconstituted on the resulting layer. Both methods led to a wired glucose oxidase monolayer that is characterized by a very fast electron transfer and responds linearly to glucose concentration over a broad range in the mM region, with no interference from dissolved oxygen.
Figure 14.20 Self-assembly of a glucose oxidase–gold nanoparticle conjugate in order to achieve direct electron transfer to the underlying gold electrode. Inset upper right: the dithiol used as linker. Adapted with permission from [66]. Copyright 2003, The American Association for the Advancement of Science.
Carbon nanotubes have also proved suitable for achieving direct electron transfer between an enzyme and the electrode. For example, Figure 14.21 displays a method for preparing a glucose sensor including carbon naotubes for enzyme wiring [67]. In this approach a gold electrode has been modified by coating with a self-assembled monolayer of cysteamine (A) Next, 120-nm single-walled carbon nanotubes have been covalently attached by amide bonding involving carboxyl terminal groups (B). Further, two different immobilization procedures have been employed for appending glucose oxidase to the upper end of the nanotubes. In alternative I, glucose oxidase itself was attached to the carbon nanotube layer (C). Procedure II involves attaching the FAD prosthetic group to carbon nanotubes (D), followed by reconstitution of the full enzyme by allowing the apoenzyme to interact with the FAD-modified electrode (E).
Figure 14.21 Main steps in the fabrication of a modified gold electrode for achieving glucose oxidase wiring via carbon nanotubes. Adapted with permission from [67]. Copyright 2005 Wiley-VCH Verlag GmBH & Co. KGaA.
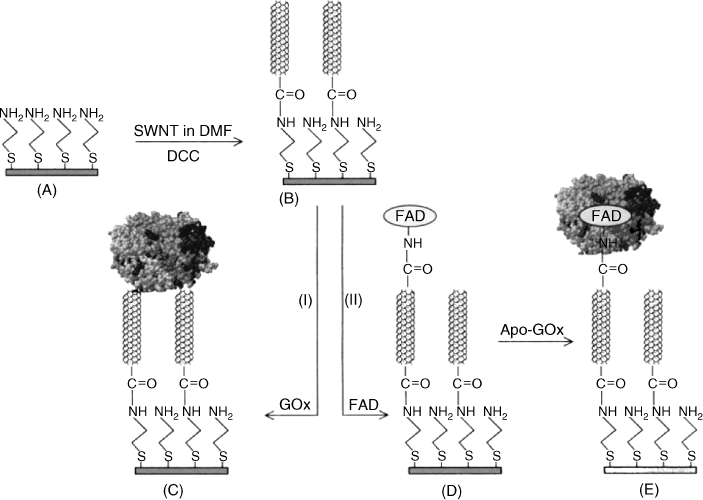
Both the above examples illustrate a general principle in enzyme immobilization and conjugation with nanomaterials, namely reconstitution of the enzyme by an affinity reaction between the apoenzyme and the catalytic molecular unit [68].
In conclusion, direct electron exchange between enzyme and electrodes can be achieved using different technologies and materials. This approach avoids certain complications that arise with other transduction methods. It is worth mentioning that copper-containing proteins [69] and ligninolytic redox enzymes [70] have been extensively studied from this standpoint and show a remarkable potential for application in amperometric enzyme sensors based on direct electron transfer.
14.6 NAD/NADH++ as Mediator in Biosensors
Many dehydrogenases use NAD(P)+/NAD(P)H as a cosubstrate. Hence, general transduction methods in conjunction with such enzymes are based on the detection of these cofactors [71, 72]. Electrochemical oxidation of NADH to NAD+ is, at first sight the obvious choice, but in fact at metal or carbon electrodes this reaction occurs with a large overvoltage. However, NADH oxidation occurs with a low overvoltage at organic conductor salt electrodes, which enables the development of convenient amperometric sensors such as, for example, an ethanol sensor [73]. In such a sensor, the NADH/NAD+ couple functions as a mediator that conveys the electron from the enzyme to the electrode by free diffusion.
At the same time, much effort has been devoted to produce sensors based on mediated electron transfer from NADH to metal or carbon electrodes in what can be called a double-mediation system (Figure 14.22). In this approach, the electron exchange between NADH and the electrode is effected by a redox mediator. A large number of mediators have been tested to this end looking for long-term stability and low operating potential. Among the most convenient, Meldola Blue, N-methylphenazinium, TTF and TCNQ can be mentioned.
Figure 14.22 Scheme of a mediated NADH/NAD+ sensor.

Enzymatic reactions involving NAD+/NADH have also proved useful for detecting an inorganic cosubstrate such as the ammonium ion. An ammonium sensor has been obtained using the glutamate dehydrogenase enzyme (GLDH), 2-oxoglutaric acid as substrate and Meldola Blue (MB) as mediator [74]. The reaction sequence in this sensor is:
(14.5) ![]()
(14.6) ![]()
(14.7) ![]()
This sensor has been fabricated by screen printing for the purpose of field analysis applications.
14.7 Summary
Amperometric enzyme sensors are based on redox enzymes that are catalysts in the redox reactions of analyte substrates. In order to perform electrochemical transduction, the electron-transfer process involved in the enzymatic reaction should be coupled in some way with an electrode in order to obtain a current. There are three main approaches to amperometric transduction in amperometric enzyme sensors. If a natural cofactor or a product is electrochemically active, amperometric monitoring of one of these components provides the response current and sensors based on this principle belong to the first generation of amperometric enzyme sensors. In this design, the sensor is dependent on the natural cofactor, whose concentration must be controlled. A reagentless sensor is obtained using an artificial mediator as cofactor. By incorporating the mediator within the sensing layer, the sensor becomes independent of any possible reagent, which makes the operation much more facile. As the mediator is recycled by the electrochemical reaction, it is not consumed during the run. Sensors of this type are called second-generation amperometric enzyme sensors. A great number of organic and inorganic mediators have been tested. Among them, metal complexes such as ferrocenes and osmium complexes have proved to be the most convenient. This is due to the fact that their electrochemical reactions do not involve hydrogen ions and, consequently, are not pH-dependent. The most advanced design in mediated sensors is based on redox polymer layers that incorporate both the enzyme and the mediator, the last being covalently attached to the polymer backbone. Well-structured sensing films can be obtained by self-assembly using the layer-by-layer preparation method.
In both first- and second-generation sensors, electron exchange between electrode and the enzyme active site is achieved indirectly, through a natural or artificial cofactor. A great simplification in the sensor design is achieved if the electron exchange between electrode and the enzyme occurs directly, with no intermediates. This design is a third-generation amperometric enzyme sensor. Direct electron transfer was first achieved using particular organic semiconductors as electrodes. In a more advanced approach, conducting nanoparticles are conjugated with the enzyme to promote direct electron exchange with the electrode. Certain enzymes are able to perform direct electron transfer to conducting polymers deposited at the electrode surface. Therefore, in third-generation sensors, there are no molecular compounds acting as electron conveyors between the enzyme and the electrode.
From the standpoint of mass production, the most convenient technology is that based on screen printing [75, 76]. This technology enables fabrication of cheap sensors suitable for single-use applications. As the running time is very short, simple immobilization by adsorption is preferred since the leaking out of the components of the sensing layer is not critical under such conditions. Immobilization by entrapment or electrochemical polymerization is the suitable alternative for fabricating more rugged sensors. Planar biosensors produced by screen printing are suitable for a wide range of applications, from “drop-on assay,” which can be useful in field analysis, to automated analysis methods such as flow-injection analysis.
The most impressive application of amperometric enzyme sensors is the glucose sensor that is widely used in diabetes monitoring and also in a series of industrial applications, [77, 78]. Amperometric enzyme sensors for other analytes have also been developed for various applications in medicine, biotechnology, food and drinks industry [79] and environment monitoring.
As enzymes are widely used as labels in electrochemical immunosensors and nucleic acid sensors, the basic principles of amperometric enzyme sensors form the basis for the development of various types of sensor based on affinity reactions or nucleic acid hybridization.
1. Clark, L.C. and Lyons, C. (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci., 102, 29–45.
2. Habermuller, L., Mosbach, M., and Schuhmann, W. (2000) Electron-transfer mechanisms in amperometric biosensors. Fresenius J. Anal. Chem., 366, 560–568.
3. Pingarron, J.M., Yanez-Sedeno, P., and Gonzalez-Cortes, A. (2008) Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta, 53, 5848–5866.
4. Scheller, F.W. and Schubert, F. (1992) Biosensors, Elsevier, Amsterdam.
5. Kauffmann, J.M. and Guilbault, G.G. (1992) Enzyme electrode biosensors – theory and applications. Methods Biochem. Anal., 36, 63–113.
6. Ion, A. and B![]() nic
nic![]() , F.G. (2002) Electrochemical Methods in Analytical Chemistry, Ars Docendi, Bucharest.
, F.G. (2002) Electrochemical Methods in Analytical Chemistry, Ars Docendi, Bucharest.
7. Ricci, F. and Palleschi, G. (2005) Sensor and biosensor preparation, optimisation and applications of Prussian Blue modified electrodes. Biosens. Bioelectron., 21, 389–407.
8. Lindgren, A., Ruzgas, T., Gorton, L. et al. (2000) Biosensors based on novel peroxidases with improved properties in direct and mediated electron transfer. Biosens. Bioelectron., 15, 491–497.
9. Yao, Y.L. and Shiu, K.K. (2008) A mediator-free bienzyme amperometric biosensor based on horseradish peroxidase and glucose oxidase immobilized on carbon nanotube modified electrode. Electroanalysis, 20, 2090–2095.
10. Willner, I. and Katz, E. (2000) Integration of layered redox proteins and conductive supports for bioelectronic applications. Angew. Chem. Int. Edit., 39, 1180–1218.
11. Katz, E. and Willner, I. (2004) Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. -Int. Edit., 43, 6042–6108.
12. Kerman, K., Saito, M., Yamamura, S. et al. (2008) Nanomaterial-based electrochemical biosensors for medical applications. TrAC-Trends Anal. Chem., 27, 585–592.
13. Claussen, J.C., Franklin, A.D., ul Haque, A. et al. (2009) Electrochemical biosensor of nanocube-augmented carbon nanotube networks. ACS Nano, 3, 37–44.
14. Chaubey, A. and Malhotra, B.D. (2002) Mediated biosensors. Biosens. Bioelectron., 17, 441–456.
15. Calvo, E.J. and Danilowicz, C. (1997) Amperometric enzyme electrodes. J. Braz. Chem. Soc., 8, 563–574.
16. Fultz, M.L. and Durst, R.A. (1982) Mediator compounds for the electrochemical study of biological redox systems – a compilation. Anal. Chim. Acta, 140, 1–18.
17. Martens, N., Hindle, A., and Hall, E.A.H. (1995) An assessment of mediators as oxidants for glucose-oxidase in the presence of oxygen. Biosens. Bioelectron., 10, 393–403.
18. McMahon, C.P., Rocchitta, G., Kirwan, S.M. et al. (2007) Oxygen tolerance of an implantable polymer/enzyme composite glutamate biosensor displaying polycation-enhanced substrate sensitivity. Biosens. Bioelectron., 22, 1466–1473.
19. Li, G., Xu, H., Huang, W.J. et al. (2008) A pyrrole quinoline quinone glucose dehydrogenase biosensor based on screen-printed carbon paste electrodes modified by carbon nanotubes. Meas. Sci. Technol., 19, 7.
20. Kotzian, P., Brazdilova, P., Kalcher, K. et al. (2007) Oxides of platinum metal group as potential catalysts in carbonaceous amperometric biosensors based on oxidases. Sens. Actuators B-Chem., 124, 297–302.
21. Wang, S.F., Tan, Y.M., Zhao, D.M. et al. (2008) Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles-chitosan nanocomposite. Biosens. Bioelectron., 23, 1781–1787.
22. Canevet, D., Salle, M., Zhang, G.X. et al. (2009) Tetrathiafulvalene (TTF) derivatives: key building-blocks for switchable processes. Chem. Commun., 2245–2269.
23. Kowalewska, B. and Kulesza, P.J. (2009) Application of tetrathiafulvalene-modified carbon nanotubes to preparation of integrated mediating system for bioelectrocatalytic oxidation of glucose. Electroanalysis, 21, 351–359.
24. Nunes, G.S., Montesinos, T., Marques, P.B.O. et al. (2001) Acetylcholine enzyme sensor for determining methamidophos insecticide - Evaluation of some genetically modified acetylcholinesterases from Drosophila melanogaster. Anal. Chim. Acta, 434, 1–8.
25. Skladal, P. (1992) Detection of organophosphate and carbamate pesticides using disposable biosensors based on chemically modified electrodes and immobilized cholinesterase. Anal. Chim. Acta, 269, 281–287.
26. Cass, A.E.G., Davis, G., Francis, G.D. et al. (1984) Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem., 56, 667–671.
27. Higgins, J.J., Hill, H.A.O., and Plotkin, E.V. (1985). US Pat. 4545382.
28. Gorton, L. (1995) Carbon-paste electrodes modified with enzymes, tissues, and cells. Electroanalysis, 7, 23–45.
29. Razumiene, J., Gureviciene, V., Laurinavicius, V. et al. (2001) Amperometric detection of glucose and ethanol in beverages using flow cell and immobilised on screen-printed carbon electrode PQQ-dependent glucose or alcohol dehydrogenases. Sens. Actuators B-Chem., 78, 243–248.
30. Frasconi, M., Deriu, D., D'Annibale, A. et al. (2009) Nanostructured materials based on the integration of ferrocenyl-tethered dendrimer and redox proteins on self-assembled monolayers: an efficient biosensor interface. Nanotechnology, 20, Article nr. 505501.
31. Chen, M. and Diao, G.W. (2009) Electrochemical study of mono-6-thio-beta-cyclodextrin/ferrocene capped on gold nanoparticles: Characterization and application to the design of glucose amperometric biosensor. Talanta, 80, 815–820.
32. Qiu, J.D., Deng, M.Q., Liang, R.P. et al. (2008) Ferrocene-modified multiwalled carbon nanotubes as building block for construction of reagentless enzyme-based biosensors. Sens. Actuators B-Chem., 135, 181–187.
33. Yan, Y.M., Tel-Vered, R., Yehezkeli, O. et al. (2008) Biocatalytic growth of Au nanoparticles immobilized on glucose oxidase enhances the ferrocene-mediated bioelectrocatalytic oxidation of glucose. Adv. Mater., 20, 2365–2370.
34. Heller, A. (1990) Electrical wiring of redox enzymes. Accounts Chem. Res., 23, 128–134.
35. Boguslavsky, L., Hale, P.D., Geng, L. et al. (1993) Applications of redox polymers in biosensors. Solid State Ion., 60, 189–197.
36. Karan, I.K. (2005) CT Enzyme biosensors containing polymeric electron transfer systems, in Biosensors and Modern Biospecific Analytical Techniques (ed. L. Gorton), Elsevier, Amsterdam, pp. 131–178.
37. Katakis, I. and Heller, A. (1997) CT Electron transfer via redox hydrogels between electrodes and enzymes, in Frontiers in Biosensorics, 1, Fundamental Aspects (eds F.W. Scheller, F. Schubert, and J. Fedrowitz), Birkhäuser, Basel, pp. 229–241.
38. Coman, V., Gustavsson, T., Finkelsteinas, A. et al. (2009) Electrical wiring of live, metabolically enhanced Bacillus subtilis cells with flexible osmium-redox polymers. J. Am. Chem. Soc., 131, 16171–16176.
39. Gooding, J.J. and Hibbert, D.B. (1999) The application of alkanethiol self-assembled monolayers to enzyme electrodes. TrAC-Trends Anal. Chem., 18, 525–533.
40. Calvo, E.J., Battaglini, F., Danilowicz, C. et al. (2000) Layer-by-layer electrostatic deposition of biomolecules on surfaces for molecular recognition, redox mediation and signal generation. Faraday Discuss., 116, 47–65.
41. Gooding, J.J., Mearns, F., Yang, W.R. et al. (2003) Self-assembled monolayers into the 21(st) century: Recent advances and applications. Electroanalysis, 15, 81–96.
42. Hodak, J., Etchenique, R., Calvo, E.J. et al. (1997) Layer-by-layer self-assembly of glucose oxidase with a poly(allylamine)ferrocene redox mediator. Langmuir, 13, 2708–2716.
43. Tsai, T.W., Heckert, G., Neves, L.F. et al. (2009) Adsorption of glucose oxidase onto single-walled carbon nanotubes and its application in layer-by-layer biosensors. Anal. Chem., 81, 7917–7925.
44. Csoregi, E., Quinn, C.P., Schmidtke, D.W. et al. (1994) Design, characterization, and one-point in-vivo calibration of a subcutaneously implanted glucose electrode. Anal. Chem., 66, 3131–3138.
45. Marko-Varga, G., Emneus, J., Gorton, L. et al. (1995) Development of enzyme-based amperometric sensors for the determination of phenolic-compounds. TrAC-Trends Anal. Chem., 14, 319–328.
46. Lindgren, A., Stoica, L., Ruzgas, T. et al. (1999) Development of a cellobiose dehydrogenase modified electrode for amperometric detection of diphenols. Analyst, 124, 527–532.
47. Cosnier, S. and Popescu, I.C. (1996) Poly(amphiphilic pyrrole)-tyrosinase-peroxidase electrode for amplified flow injection-amperometric detection of phenol. Anal. Chim. Acta, 319, 145–151.
48. Coche-Guerente, L., Labbe, P., and Mengeaud, V. (2001) Amplification of amperometric biosensor responses by electrochemical substrate recycling. 3. Theoretical and experimental study of the phenol-polyphenol oxidase system immobilized in Laponite hydrogels and layer-by-layer self-assembled structures. Anal. Chem., 73, 3206–3218.
49. Hall, G.F., Best, D.J., and Turner, A.P.F. (1988) The determination of p-cresol in chloroform with an enzyme electrode used in the organic-phase. Anal. Chim. Acta, 213, 113–119.
50. Saini, S. and Turner, A.P.F. (1995) Multiphase bioelectrochemical sensors. TrAC-Trends Anal. Chem., 14, 304–310.
51. Ramanavicius, A., Ramanaviciene, A., and Malinauskas, A. (2006) Electrochemical sensors based on conducting polymer- polypyrrole. Electrochim. Acta, 51, 6025–6037.
52. Schuhmann, W. (1995) Conducting polymer based amperometric enzyme electrodes. Mikrochim. Acta, 121, 1–29.
53. Gerard, M., Chaubey, A., and Malhotra, B.D. (2002) Application of conducting polymers to biosensors. Biosens. Bioelectron., 17, 345–359.
54. Bartlett, P.N. and Cooper, J.M. (1993) A review of the immobilization of enzymes in electropolymerized films. J. Electroanal. Chem., 362, 1–12.
55. Habermüller, K., Ramanavicius, A., Laurinavicius, V. et al. (2000) An oxygen-insensitive reagentless glucose biosensor based on osmium-complex modified polypyrrole. Electroanalysis, 12, 1383–1389.
56. Cenas, N.K. and Kulys, J.J. (1981) Biocatalytic oxidation of glucose on the conductive charge-transfer complexes. Bioelectrochem. Bioenerg., 8, 103–113.
57. Kulys, J.J. (1986) Enzyme electrodes based on organic metals. Biosensors, 2, 3–13.
58. Bartlett, P.N. (1990) CT Conducting organic salt electrodes, in Biosensors: A Practical Approach (ed. A.E.G. Cass), IRL Press, Oxford, pp. 47–95.
59. Khan, G.F. (1997) TTF-TCNQ complex based printed biosensor for long-term operation. Electroanalysis, 9, 325–329.
60. Palmisano, F., Zambonin, P.G., Centonze, D. et al. (2002) A disposable, reagentless, third-generation glucose biosensor based on overoxidized poly(pyrrole)/tetrathiafulvalene-tetracyanoquinodimethane composite. Anal. Chem., 74, 5913–5918.
61. Pauliukaite, R., Malinauskas, A., Zhylyak, G. et al. (2007) Conductive organic complex salt TTF-TCNQ as a mediator for biosensors. An overview. Electroanalysis, 19, 2491–2498.
62. Stoica, L., Ludwig, R., Haltrich, D. et al. (2006) Third-generation biosensor for lactose based on newly discovered cellobiose dehydrogenase. Anal. Chem., 78, 393–398.
63. Coman, V., Ludwig, R., Harreither, W. et al. (2010) A direct electron transfer-based glucose/oxygen biofuel cell operating in human serum. Fuel Cells, 10, 9–16.
64. Willner, B., Katz, E., and Willner, I. (2006) Electrical contacting of redox proteins by nanotechnological means. Curr. Opin. Biotechnol., 17, 589–596.
65. Willner, I., Willner, B., and Tel-Vered, R. (2011) Electroanalytical applications of metallic nanoparticles and supramolecular nanostructures. Electroanalysis, 23, 13–28.
66. Xiao, Y., Patolsky, F., Katz, E. et al. (2003) “ Plugging into enzymes”: Nanowiring of redox enzymes by a gold nanoparticle. Science, 299, 1877–1881.
67. Liu, J.Q., Chou, A., Rahmat, W. et al. (2005) Achieving direct electrical connection to glucose oxidase using aligned single walled carbon nanotube arrays. Electroanalysis, 17, 38–46.
68. Zayats, M., Willner, B., and Willner, I. (2008) Design of amperometric biosensors and biofuel cells by the reconstitution of electrically contacted enzyme electrodes. Electroanalysis, 20, 583–601.
69. Shleev, S., Tkac, J., Christenson, A. et al. (2005) Direct electron transfer between copper-containing proteins and electrodes. Biosens. Bioelectron., 20, 2517–2554.
70. Christenson, A., Dimcheva, N., Ferapontova, E.E. et al. (2004) Direct electron transfer between ligninolytic redox enzymes and electrodes. Electroanalysis, 16, 1074–1092.
71. Gorton, L. and Bartlett, P.N. (2008) NAD(P)-based biosensors, in Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications (ed. P.N. Bartlett), Wiley, Chichester, pp. 157–198.
72. Radoi, A. and Compagnone, D. (2009) Recent advances in NADH electrochemical sensing design. Bioelectrochemistry, 76, 126–134.
73. Albery, W.J., Bartlett, P.N., Cass, A.E.G. et al. (1987) Amperometric enzyme electrodes. 4. An enzyme electrode for ethanol. J. Electroanal. Chem., 218, 127–134.
74. Hart, J.P., Serban, S., Jones, L.J. et al. (2006) Selective and rapid biosensor integrated into a commercial hand-held instrument for the measurement of ammonium ion in sewage effluent. Anal. Lett., 39, 1657–1667.
75. Albareda-Sirvent, M., Merkoçi, A., and Alegret, S. (2000) Configurations used in the design of screen-printed enzymatic biosensors. A review. Sens. Actuators B-Chem., 69, 153–163.
76. Ricci, F., Moscone, D., and Palleschi, G. (2007) CT Mediated enzyme screen-printed electrode probes for clinical, environmental and food analysis, in Electrochemical Sensor Analysis (eds S. Alegret and A. Merkoçi), Elsevier, Amsterdam, pp. 559–584.
77. Wang, J. (2008) Electrochemical glucose biosensors. Chem. Rev., 108, 814–825.
78. Wang, J. (2001) Glucose biosensors: 40 years of advances and challenges. Electroanalysis, 13, 983–988.
79. Prodromidis, M.I. and Karayannis, M.I. (2002) Enzyme based amperometric biosensors for food analysis. Electroanalysis, 14, 241–261.
