Chapter 19
Optical Sensors – Applications
As indicated in the previous chapter, there are a broad range of optical transduction methods that allow the development of a great diversity of optical sensor platforms. In order to develop a particular kind of optical sensor, one has to select a suitable recognition method and integrate the recognition element with an appropriate optical sensor platform.
This chapter presents typical strategies for the development of optical sensors for various classes of applications focusing on recognition methods for particular kinds of analytes. Included are optical sensors for inorganic species, such as the hydrogen ion, metal ions, and certain gaseous compounds. Optical sensors based on enzymes, affinity recognition receptors, and nucleic acids are also presented.
19.1 Optical Sensors Based on Acid–Base Indicators
19.1.1 Optical pH Sensors
Optical pH sensors make use of acid–base indicators (also called pH indicators) that are organic compounds capable of undergoing acid dissociation accompanied by a change in color:
Symbols in square parentheses represent activities of pertinent species. The equilibrium constant in Equation (19.1) is the indicator constant. An absorbance measurement at the specific wavelength of each indicator form (![]() or
or ![]() ) permits the solution pH to be determined.
) permits the solution pH to be determined.
An absorbance–pH relationship can be derived by combining the indicator constant expression (19.1) and the mass balance equation for the indicator (![]() , where ct is the total concentration of the indicator). Assuming that only the basic species
, where ct is the total concentration of the indicator). Assuming that only the basic species ![]() absorbs light and taking into account the pH definition (
absorbs light and taking into account the pH definition (![]() ) the concentration of the basic form of the indicator depends on pH as:
) the concentration of the basic form of the indicator depends on pH as:
(19.2) ![]()
A plot of ![]() absorbance vs. pH is shown in Figure 19.1A for the hypothetical case of an indicator with
absorbance vs. pH is shown in Figure 19.1A for the hypothetical case of an indicator with ![]() . The symmetrical, S-shaped curve shows a portion centered around
. The symmetrical, S-shaped curve shows a portion centered around ![]() where the absorbance variation is approximately linear. This is the indicator transition pH range, in brief the pH range. Therefore, pH determination based on a single indicator is limited to a narrow pH range of about 2–3 pH units, with strong deviation from linearity beyond this region. It is possible to broaden the pH range by incorporating in the sensing layer several pH indicators with pKi value selected so as to give, by absorbance superposition, an extended and pseudolinear response region.
where the absorbance variation is approximately linear. This is the indicator transition pH range, in brief the pH range. Therefore, pH determination based on a single indicator is limited to a narrow pH range of about 2–3 pH units, with strong deviation from linearity beyond this region. It is possible to broaden the pH range by incorporating in the sensing layer several pH indicators with pKi value selected so as to give, by absorbance superposition, an extended and pseudolinear response region.
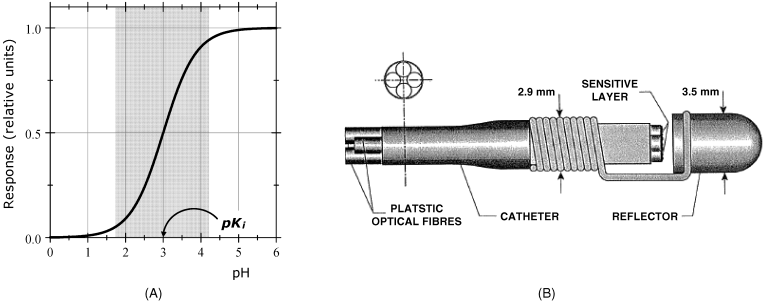
Figure 19.1 pH optical sensors. (A) pH response of a single-color indicator pH sensor. The gray area indicates the response range. (B) A miniature pH optical sensor for in vivo determination of pH in foregut. (B) Reprinted with permission from [1]. Copyright 1995 Elsevier.
As the indicator constant depends on the ionic strength of the solution and temperature, these parameters have to be kept under control.
Acid–base indicators can be immobilized by covalent binding or entrapment in cellulose membranes or polymeric materials, or by photochemical coupling to the fiber end. Sol-gel chemistry is also used for indicator immobilization by entrapment. Indicator immobilization can modify the absorption spectrum of the indicator, and, in favorable situations, expands the transition pH range.
An alternative to conventional acid–base indicators are conducting polymers (such as polyaniline or polypyrrole), that change their absorptivity in both the visible and near-infrared regions in response to pH change. The advantage of conducting polymer pH sensors is a wide response range, because these materials are polyelectrolytes with multiple acid dissociation constants. However, they are susceptible to interference by other ions and show a longer response time.
Diffuse reflectance provides essentially the same kind of response as light-absorption measurement but is more convenient from the standpoint of sensor fabrication. Figure 19.1B shows a miniature diffuse reflectance-based pH sensor for in vivo gastric pH monitoring. It is composed of two measuring channel, each of them fitted with a different pH indicator, namely thymol blue (pH range 1.2–2.8) and bromophenol blue (pH range 3–4.6). The pH indicators are covalently immobilized on controlled-pore glass beads attached to the end of a plastic optical fiber by heating. In each channel, one fiber carries the light from the source to the probe and the other fiber carries the reflected light from the probe to the light detector. A Teflon reflector placed in front of the fibers provides a better coupling of the modulated light. The reference signal is obtained at a wavelength far from the maximum absorption wavelength of each indicator. This sensor has a response range sufficiently wide for in vivo determination of pH in the digestive tract.
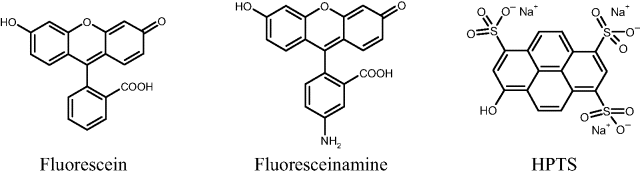
Fluorescent acid–base indicators display fluorescence intensity depending on pH similar to that shown in Figure 19.1A. As in the case of absorption- or reflectance-based pH sensors, the response range can be expanded by using combinations of indicators with different ![]() values. Common fluorescent pH indicators are fluorescein derivatives and 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS) (Figure 19.2). Fluoresceinamine can be immobilized at hydroxyl-containing membranes by covalent coupling with cyanuric chloride. HPTS is a strong polyacid and can be incorporated in an anion exchanger or in hydrophobic polymer as an ion pair with an organic hydrophobic cation. Its indicator constant (
values. Common fluorescent pH indicators are fluorescein derivatives and 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS) (Figure 19.2). Fluoresceinamine can be immobilized at hydroxyl-containing membranes by covalent coupling with cyanuric chloride. HPTS is a strong polyacid and can be incorporated in an anion exchanger or in hydrophobic polymer as an ion pair with an organic hydrophobic cation. Its indicator constant (![]() ) makes it suitable for pH determinations in the physiological range.
) makes it suitable for pH determinations in the physiological range.
Figure 19.2 Fluorescent indicators used in pH sensors.
The ratiometric measurement method can be implemented in pH sensors if both the acid and base form of the indicator absorb light or fluoresce at specific wavelengths. The ratiometric pH response is obtained by putting the indicator constant expression in (19.1) in the following form:
Compared with the pH glass electrode, which provides a very wide response range, the response range of optical pH sensors is limited to a narrow range around the ![]() value. However as already mentioned, the response range of optical pH sensors can be expanded by means of sensing layers including combinations of a series of properly selected indicators. Optical pH sensors have the advantages of easy miniaturization and reliable response in samples with a very low ion content, that are troublesome when using the pH glass electrode. An overview of pH optical sensor is presented in ref. [2].
value. However as already mentioned, the response range of optical pH sensors can be expanded by means of sensing layers including combinations of a series of properly selected indicators. Optical pH sensors have the advantages of easy miniaturization and reliable response in samples with a very low ion content, that are troublesome when using the pH glass electrode. An overview of pH optical sensor is presented in ref. [2].
19.1.2 Optical Sensors for Acidic and Basic Gases
Acidic gases (e.g., carbon dioxide) and basic gases (e.g., ammonia) impart on dissolution in water a particular pH that depends on the concentration in the gas sample. This property is exploited in the potentiometric sensor (Section 10.16) in which the pH change is monitored by a pH glass electrode. Similarly, optical gas sensors are obtained by means of optical pH probes. The indicator dye should be selected so as to match its indicator constant to the ionization constant of the dissolved gas. A pH buffer should be incorporated in the sensing layer in order to prevent complete protonation (or dissociation) of the indicator, which would significantly limit the response range of the sensor.
As in the case of potentiometric gas sensors, optical gas sensors are suitable for testing both liquid and gas samples with interferences arising only from other acidic or basic gases.
The response range of single indicator sensors is limited to about one order of magnitude, but it can be extended using an optimized mixture of indicators instead of a single one.
A common application is the optical carbon dioxide sensor, which is of interest to clinical chemistry, chemistry of aquatic systems and also to the food and beverage industry [3]. The sensing layer in an optical CO2 sensor is composed of an aqueous solution containing hydrogen carbonate as the pH buffer and a light-absorbing indicator dye. This solution is entrapped at the end of an optical fiber bundle by means of a ~ 10 μm thick Teflon membrane that is permeable to CO2 but not to ions. Sample CO2 passes through the membrane and dissolves in the internal solution, affecting its pH and hence the optical response produced by the pH indicator.
More rugged and reliable CO2 sensors are obtained by incorporating an anionic indicator dye in a plasticized polymer membrane as an ion pair with a hydrophobic cation. The ion pair binds also several water molecules that allow acid–base equilibria to occur as in the aqueous solution.
An ammonia sensor has been developed using two indicators: chlorophenol red (![]() ,
, ![]() nm) and bromothymol blue (
nm) and bromothymol blue (![]() ;
; ![]() nm) [4]. The sensing element in this sensor is a very thin layer of ammonium chloride buffer entrapped behind a microporous Teflon membrane. Light of a selected wavelength is conveyed to the indicator solution by two fibers. After crossing the solution, light is scattered back by the Teflon membrane, collected by a third fiber and transmitted to the light detector. The absorbance response is nonlinear and the response range stretches from 0.2 to 30 μM ammonia. Response and recovery times are ~ 10–20 min owing to the slow diffusion thorough the membrane.
nm) [4]. The sensing element in this sensor is a very thin layer of ammonium chloride buffer entrapped behind a microporous Teflon membrane. Light of a selected wavelength is conveyed to the indicator solution by two fibers. After crossing the solution, light is scattered back by the Teflon membrane, collected by a third fiber and transmitted to the light detector. The absorbance response is nonlinear and the response range stretches from 0.2 to 30 μM ammonia. Response and recovery times are ~ 10–20 min owing to the slow diffusion thorough the membrane.
Much shorter response times and a more rugged configuration are obtained by incorporation of the indicator dye in sol-gel materials or in a gas-permeable polymer matrix.
Fluorescence-based gas sensors have also been produced. For example, a carbon dioxide sensor has been developed using the HPTS fluorescent indicator incorporated in a silicone matrix as an emulsion of a room-temperature ionic liquid [5]. The response function of this sensor can be put in a linear form as follows:
(19.4) ![]()
where ![]() and F are fluorescence intensities in the absence and in the presence of
and F are fluorescence intensities in the absence and in the presence of ![]() , respectively and k is a constant depending upon the first dissociation constant of carbonic acid, the indicator constant and the total concentration of bicarbonate in the buffer solution incorporated in the sensing film. The dual lifetime referencing method has also been applied by incorporating in the sensing part a
, respectively and k is a constant depending upon the first dissociation constant of carbonic acid, the indicator constant and the total concentration of bicarbonate in the buffer solution incorporated in the sensing film. The dual lifetime referencing method has also been applied by incorporating in the sensing part a ![]() -insensitive, long-lifetime fluorophore in order to obtain a ratiometric response.
-insensitive, long-lifetime fluorophore in order to obtain a ratiometric response.
Phase-resolved fluorimetry combined with the dual lifetime referencing method (Section 18.3.6) has been applied to obtain a carbon dioxide sensor [6]. The HPTS fluorescent indicator was incorporated in a poly(dimethylsiloxane) matrix as an ion pair with a hydrophobic quaternary ammonium base. A ruthenium complex incorporated into silica microparticles and included in the immobilization matrix acts as the reference fluorophore. The measured phase shift is correlated with carbon dioxide partial pressure according to the following nonlinear function:
(19.5) ![]()
where ![]() is the phase angle at a particular
is the phase angle at a particular ![]() concentration,
concentration, ![]() the phase angle measured in the absence of
the phase angle measured in the absence of ![]() , and
, and ![]() the phase angle measured at saturation, when all indicator sites are in the protonation form. The
the phase angle measured at saturation, when all indicator sites are in the protonation form. The ![]() constant depends on the indicator constant and the basicity of the buffer used in the sensing membrane. The layout of such a sensor is presented in Figure 18.13.
constant depends on the indicator constant and the basicity of the buffer used in the sensing membrane. The layout of such a sensor is presented in Figure 18.13.
The main advantage of optical gas sensors over the potentiometric ones arises from the possibility of preparing the sensing layer as a solid membrane and not as an entrapped solution. This avoids serious drawbacks related to the difference between the osmotic pressure in the sample and that in the internal solution of the sensor. This difference produces water transfer to or from the internal solution, which affects the response of a potentiometric gas sensor.
19.2 Optical Ion Sensors
Optical ion sensors (also called ion optodes) can be developed as either direct or indirect sensors [7–9]. Selective ion recognition can be achieved by means of supramolecular chemical interactions.
19.2.1 Direct Optical Ion Sensors
Direct ion sensors are feasible when the receptor itself is a chromophore or a luminophore and experiences a change in optical properties upon binding to an analyte ion. Such a receptor should fulfill two critical conditions: (i) to display selectivity to the analyte ion and (ii) to change the absorption or fluorescence properties upon binding the analyte. Such double-function receptors can be obtained by tagging a selective ion receptor with a chromogenic or fluorogenic fragment. Ion binding brings an electric charge that shifts the absorption or fluorescence wavelength. Hence, at a fixed wavelength one can notice either an increase or a decrease of the optical signal. This principle has been applied to develop molecular ion sensors.
Sensing an alkaline metal ion by a macrocyclic receptor tagged with a fluorescent moiety is demonstrated in Figure 19.3. In the absence of the metal ion, fluorescence is quenched by electron transfer from the nitrogen atom at the macrocycle to the excited state of anthracene (photoinduced electron transfer). In order for quenching to occur the energy level of the donor orbital should be higher than that of the acceptor orbital. If the metal ion is bound to the receptor macrocycle, its charge lowers the energy level of the electron pair at the nitrogen atom and prevents the electron transfer. Consequently fluorescence intensity increases with increasing ion concentration. In the case of an alkali metal ion, the photoinduced electron transfer is shut off owing to the ion–dipole interaction between the ion and the electron donor site.
Figure 19.3 Ion sensing by photoinduced electron transfer. (A) Sensing of the potassium ion by a macrocyclic ligand tagged with a fluorescent moiety (anthracene); (B) sensing of a transition-metal ion (copper or nickel) by coordination to a polydentate ligand with an appended fluorescent moiety. Reprinted with permission from [10]. Copyright 1995 The Royal Society of Chemistry.
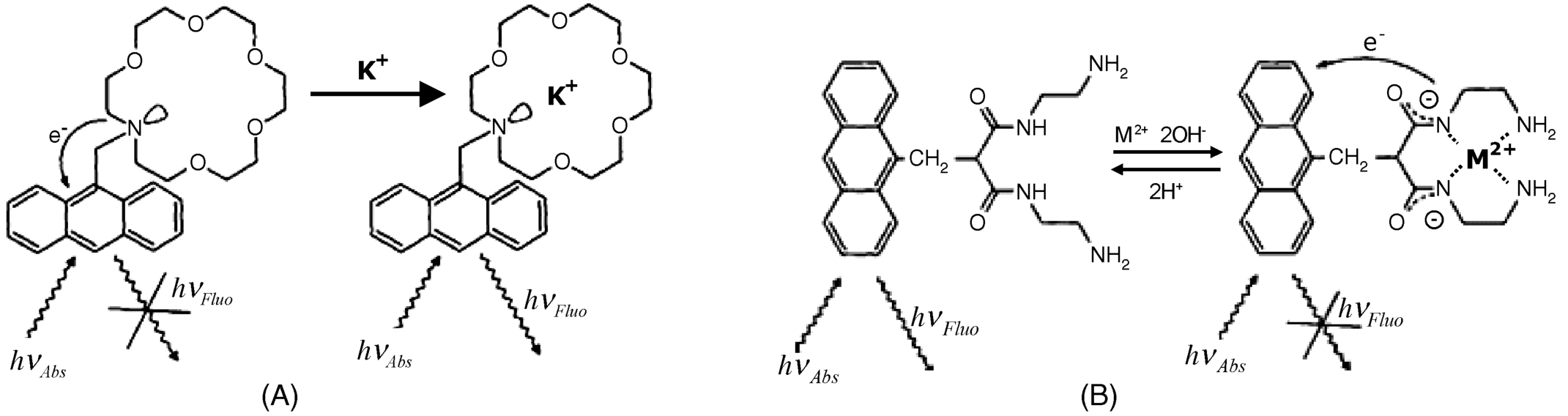
Transition-metal ions can be detected by a mechanism involving formation of donor sites in the receptor molecule after ion binding, as shown in Figure 19.3B. In this case, the fluorophore tag is bound to a carbon atom at the ion receptor and thereby photoinduced electron transfer cannot take place. Metal-ion binding by coordination leads to the ionization of amide nitrogens and creates negative sites that act as electron donors in photoinduced electron transfer. Therefore, ion binding results in fluorescence quenching.
Other mechanisms for direct sensing on metal ions are described in the literature [10–12]. Anion-sensing schemes based on photoinduced electron transfer have also been developed [13].
19.2.2 Indirect Optical Ion Sensors
The indirect approach to optical ion sensing relies on well-established selective ion receptors but avoiding their chemical modification that can impair the selectivity. As typical of indirect sensing, not the receptor or the ion-receptor complex is detectable and the transduction is performed by means of a signaling reagent involved in the sensing process.
A general indirect transduction scheme is based on the charge-balance restriction within the sensing membrane. Sensing membranes are similar to those used in potentiometric ion sensors, that is, they contains the receptor and additives entrapped in a plasticized polymer matrix [14–16]. For optical transduction purposes, a lipophilic pH indicator is included in the membrane as signaling reagent.
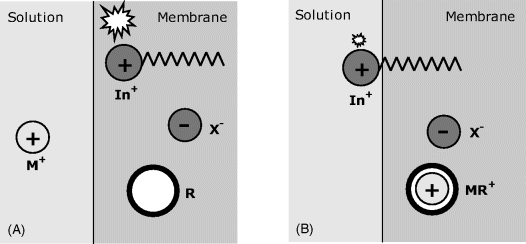
Figure 19.4 shows two possible indirect transduction schemes coupled with ion recognition by a neutral ion receptor (R). For cation sensing (Figure 19.4A), a hydrophobic indicator is incorporated as the protonated, neutral molecule (HIn). Owing to the very high stability constant of the MR metal complex, the cation M+ is extracted into the membrane but, for charge-balance reasons, a hydrogen ion is evicted leaving the indicator in the anionic form. Indicator ionization modifies its optical properties as a function of analyte concentration.
Figure 19.4 Indirect transduction in optical ion sensors based on neutral receptors (R) and a pH indicator. (A) Cation sensing by ion exchange involving the hydrogen ion. (B) Anion sensing based on coextraction of the hydrogen ions and the analyte. The receptor, the indicator, and the counterion (X+) are hydrophobic species that cannot be extracted into the solution.
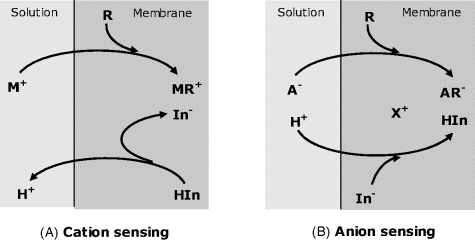
An anion-sensing scheme is shown in Figure 19.4B. In this case, the indicator is present in the membrane as an anion (In−) forming an ion pair with a hydrophobic counterion X+. The analyte anion A− is extracted into the membrane by strong coupling with the receptor and thus introduces an additional negative charge. For charge-balance reasons, the anionic indicator is protonated by extraction of a hydrogen ion from the solution. Protonation of the indicator alters its optical properties and thereby allows for the analyte concentration in the sample to be measured by means of an optical signal.
Depending on the optical properties of the indicator, either light absorption or fluorescence emission can be used to obtain the optical response.
Analogous transduction schemes have been developed for the case of ion recognition by charged receptors [15].
The merits of indirect optical ion sensors have been discussed in detail in ref. [15]. The response function has a sigmoid shape and the measured signal depends on the solution pH, which requires this parameter to be controlled by means of a pH buffer system.
A pH-independent transduction scheme is based on solvatochromism, which means solvent-dependent light absorption or emission. A sensing scheme based on this principle is shown in Figure 19.5. As shown in this Figure, a cationic fluorescent dye (In+), which is tagged with a long hydrocarbon tail, is incorporated in the sensing membrane along with the ion receptor (R) and a hydrophobic counterion (X−). Before ion extraction, the dye in the nonpolar membrane environment fluoresces strongly (Figure 19.5A). Ion extraction leads to the formation of a positively charged complex, which repels the charged head of the dye into the solution phase. In this polar environment, fluorescence is very weak. Therefore, fluorescence intensity decreases with increasing ion concentration in the solution. A potassium optical sensor based on this principle has been developed using valinomycin as receptor and alkyl-Acridine Orange as indicator [17]. The fluorescence response of this sensor decreases with analyte concentration as a linear function.
Figure 19.5 Indirect transduction in optical ion sensors based on the solvatochromic effect on the fluorescence of a signaling dye. (A) Before ion extraction (strong fluorescence); (B) after ion extraction (lessened fluorescence).
As potentiometric and indirect optical ion sensors are based on similar recognition receptors, it is useful to compare the performances of these to kinds of sensors [15]. Optode selectivity can be modeled by an equation similar to the Nikolskii-Eisenman equation used in potentiometry. The dynamic range of optical sensors stretches over 2–4 order of magnitudes. It is narrower than that of potentiometric sensors, which can be of 5–9 orders of magnitude. On the other hand, the detection limit of optical ion sensors can reach subnanomolar levels, whilst the limit of detection of potentiometric sensors is, in general, in the micromolar region. The better detection limit of optical sensors is due to the fact that the analyte ion is not present within the sensor system and cannot contaminate the sample, as occurs with potentiometric sensors. As far as the lifetime of the sensors is concerned, it is limited in both cases by slow leaching of membrane components into the sample. In contrast to potentiometric sensors, the ion optode response depends directly on the concentration of active components in the membrane and is therefore more sensitive to leaching effects.
An advantage of ion optodes comes for insensitivity of the response of mechanical damages of the membrane. In contrast, a physical hole in the potentiometric sensor membrane produces an electrical short between the internal and external solutions and, therefore, causes complete breakdown. The greater ruggedness of optodes allows for a wider variety of shapes and sizes of sensor to be used, as well as extreme miniaturization. Last, but not least, a potentiometric ion sensor depends on a reliable reference electrode, which complicates the sensor structure. Such a complication is absent in the case of ion optodes. However, it should not be forgotten that the readout instrumentation for potentiometric ion sensor is simpler.
19.3 Optical Oxygen Sensors
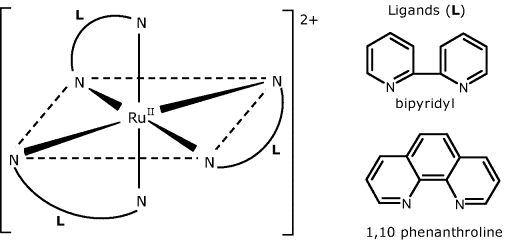
The property of oxygen to produce dynamic fluorescence quenching forms the basis of oxygen optical sensors [18–21] (see also Section 18.3.7). Common fluorophores used in oxygen sensors are ruthenium (II) complexes with 2,2′-bipyridine (bipyridyl), 1,10 phenanthroline and related compounds (Figure 19.6). Analogous complexes of other platinum group metals (Os2+, Ir3+) as well as certain organic dyes and polycyclic aromatic hydrocarbons have also been used.
Figure 19.6 Structure of Ru(II) complexes used in optical oxygen.
Luminophores can be immobilized within polymers such as cellulose acetate or polyvinyl chloride along with a plasticizer that enhances oxygen permeation [22]. Sol-gel entrapment is also a suitable immobilization method.
The response of oxygen sensors deviates from the Stern–Volmer equation (Equation (18.16)) owing to the effect of the immobilization matrix. Upon immobilization, the luminophores can be found in various types of site, each of them having a particular quenching constant. On the other hand, the response depends on the concentration of absorbed oxygen in the matrix, which is a nonlinear function of oxygen concentration in the sample. In many cases, Equation (19.6) (in which the a coefficients are empirical constants and ![]() is oxygen concentration in the sample) fits the following response function [18].
is oxygen concentration in the sample) fits the following response function [18].
As in Equation (18.16), F0 and F represent the fluorescence power in the absence and in the presence of the quencher, respectively. Good sensitivity is favored by long-lived excited states, high oxygen solubility and high rate of diffusion of oxygen in the immobilization matrix. In this respect, phosphorescent platinum and palladium porphyrin complexes are suited for very sensitive oxygen sensors because of their much longer lifetime of the excited state.
Nanomaterial application as luminophores opens up new prospects in the field of optical oxygen sensors. Thus, the fluorescent C70 fullerene has been incorporated in a sol-gel based on organically modified precursors [23]. It allows for oxygen sensing and imaging down to the ppb level.
Fiber optic oxygen sensors can be designed in either the passive or the active (intrinsic) fiber configuration. An intrinsic sensor has been produced by forming a methylene blue layer on a decladded plastic optical fiber using the sol-gel method [24]. Dye excitation is performed by the evanescent wave, which brings about a more rapid response and very short recovery time. The response range stretches from 0.6 to 20.9%, which makes it applicable in the diagnosis of oxygen-deficiency diseases.
Oxygen microsensors can be obtained by photolithography. For example, the platinum octaethylporphyrin luminophore has been integrated in photoresist plastic material along with a plasticizer. The mixture has been deposited over a fused silica substrate and then patterned by ultraviolet irradiation in order to obtain arrays of 3 μm oxygen sensors for monitoring oxygen consumption rate in single cells [25].
Optical oxygen sensors function equally well in gas and aqueous solution analysis. They can be produced at a very small size for in vivo applications. Also, oxygen optical sensors can be integrated with optical pH and CO2 sensors to obtain multianalyte probes for in vivo determinations.
Moreover, an optical oxygen sensor can function as transduction element in oxygen-linked enzyme sensors.
Compared with the electrochemical oxygen sensor (Section 13.10.1), the optical one is more compact and rugged as it is an all-solid device. The optical oxygen sensor can be easily miniaturized and display shorter response and recovery times. On the other hand, the optical sensor is more expensive than the electrochemical one owing to the included optical and optoelectronic components.
19.4 Optical Enzymatic Sensors
19.4.1 Principles and Design
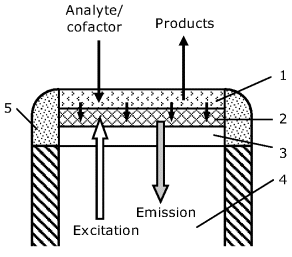
The functioning of an optical enzymatic sensor relies on the change in the optical properties of a fluorescent indicator in response of its interaction with a reactant or product involved in the enzymatic reaction. Common transduction procedures are based on optical monitoring of pH change or oxygen consumption. A typical configuration of a fiber optic enzymatic sensor is shown in Figure 19.7. For transduction purposes, a fluorescent indicator layer (2) is formed over a transparent support (3) at the end of the optical fiber. This layer is sensitive to the change in the concentration of a cosubstrate or a product involved in the enzymatic reaction. The recognition layer consisting of immobilized enzyme (1) interacts with the substrate, and the products or an unconsumed reactant diffuses further to the indicator layer and affect the fluorescence intensity. The indicator layer can include a pH indicator in order to monitor the production or consumption of hydrogen ions by the enzymatic reactions. In the case of an oxidase enzyme-base sensor, oxygen consumption can be monitored by fluorescence quenching. An alternative optical transduction scheme is based on the spectrochemical properties of the NAD+ coenzyme.
Figure 19.7 Schematics of a fiber optic enzymatic sensor. (1) Enzyme layer; (2) indicator layer; (3) transparent support; (4) optical fiber; (5) glue. Redrawn with permission from [26]. Copyright 2008 American Chemical Society.
19.4.2 Optical Monitoring of Reactants or Products
Common reactants or products involved in enzymatic reactions are hydrogen ions, oxygen, hydrogen peroxide, carbon dioxide, ammonia, and ammonium ions. In principle, an optical enzymatic sensor can be obtained by integrating an enzyme layer with a sensor for one of the above-mentioned species.
A common scheme relies on the pH-linked transduction method. Enzymes belonging to the oxidase or hydrolase classes are suitable for such applications. As is typical of pH-linked enzymatic sensors, proper functioning requires the presence of a low buffer capacity pH buffer. As sensor functioning is based on pH variation, care should be exercised to avoid drastic effects of pH change on enzyme activity which can affect unfavorably the response function.
Despite such problems, various applications of pH-linked optical transduction have been reported. For example, pH-based monitoring of acetylcholine esterase activity has proved useful in the development of inhibition-based sensors for pesticides and warfare agents.
Figure 19.8 shows a glucose optical sensor based on fluorescence quenching by residual oxygen in the glucose oxidase-catalyzed reaction. This sensor is designed in the catheter format and has been used for either in vitro or in vivo determination of glucose in fish blood. Glucose oxidase was immobilized within a polymer film over an ultrathin dialysis membrane (3). The rolled enzyme membrane was place in a metal needle (1) with lateral holes that allow the sample to get in contact with the enzyme membrane. For transduction purposes, a fiber optic oxygen probe (4) is installed inside the membrane roll. Before measurement, the sensor is filled with an oxygen-saturated neutral buffer solution and then the sensor is applied to the sample. Owing to the gradual oxygen consumption, the oxygen probe response decreases steadily. That is why the readout is measured after a predetermined time interval.
Figure 19.8 A catheter-type optical glucose sensor based on fluorescence quenching by oxygen. (1) Needle-type hollow container; (2) holes; (3) immobilized enzyme membrane; (4) optical fiber; (5) optical oxygen probe; (6) epoxy filling. Reprinted with permission from [27]. Copyright 2006 Elsevier.

Hydrogen peroxide is a product of reactions catalyzed by oxidase-type enzymes. Therefore, monitoring of this product by chemiluminescence or electrochemiluminescence provides alternative transduction methods in enzymatic optical sensors.
Electrochemiluminescence has been applied to the development of an array of six different enzymatic sensors [28]. This array is composed of six sensing spots formed at the surface of a carbon electrode. Each spot contains a particular oxidase enzyme for the determination of its substrate. Enzymes and luminol are loaded on anion-exchanger beads that are then entrapped in a polymer matrix. Chemiluminescence intensity produced at each spot is proportional to the amount of hydrogen peroxide resulting from the enzymatic oxidation of the substrate. Such a sensor array allows a series of analytes to be determined in parallel. Tests conducted with blood serum gave satisfactory results in the determination of glucose, lactate, and uric acid.
Ammonia monitoring by a pH indicator-based optical probe represents a viable transduction method in optical urea sensor based on the urease enzyme [29]. As ammonia is in equilibrium with the ammonium ion, an ammonium optode is suitable for performing transduction in urea sensors [30, 31].
19.4.3 Coenzyme-Based Optical Transduction
Nicotinamide adenine dinucleotide (NAD+) which functions as a coenzyme for certain dehydrogenases, displays characteristic absorption (350 nm) and fluorescence (450 nm) bands. This allows NAD+ to act as an optical marker in optical enzymatic sensors. In the initial stages of development, NAD+ was added to the sample solution. In a more advanced approach, a self-contained sensor has been produced by using NAD+ -tagged poly(ethylene glycol) in order to prevent NAD+ from crossing dialysis membranes [32]. This modified coenzyme along with two enzymes was entrapped within the sensing compartment between a dialysis membrane and the fiber optic tip. One of the enzymes (e.g., alcohol dehydrogenase) catalyzes substrate conversion using NAD+ as cofactor, whilst the second enzyme (lactate dehydrogenase) serves to regenerate NAD+ by reduction of NADH with pyruvate.
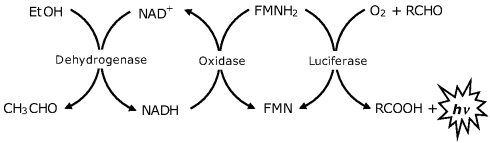
A two-enzymes system (an oxidase and a luciferase) extracted from the marine bacteria Vibrio harveyi or Vibrio fischeri has been employed in the determination of NADH or NADPH coenzymes by chemiluminescence produced in a two-step reaction In the first step (reaction (19.7)), which is catalyzed by an oxidoreductase, NAD(P)H is oxidized by flavin mononucleotide (FMN) giving reduced flavin mononucleotide (FMNH2), In the next step (reaction (19.8)), FMNH2 acts as cosubstrate in the oxidation of a long-chain aldehyde by luciferase, a reaction that produces chemiluminescence.
The above sequence of reactions provide an optical transduction method in sensors based on NAD(P)+-dependent dehydrogenases according to the mechanism in Figure 19.9. This transduction mechanism involving coenzyme recycling provides a limit of detection close to 0.4 μM, which is 1 or 2 orders of magnitude better than that obtained by direct detection of NADH.
Figure 19.9 Chemiluminescence ethanol assay by means of reactions (19.7) and (19.8).
19.4.4 Outlook
Optical enzymatic sensors can be obtained by combining an enzyme layer with an optical system in order to monitor reactants or products involved in the enzymatic reaction. Depending on the mechanism of the enzymatic reaction, either hydrogen ions, oxygen, hydrogen peroxide, gases (ammonia) or the ammonium ion can serve for transduction by means of optical probes for such species.
Recent advances in enzymatic optical sensors are surveyed in refs. [26, 33]. The field of optical glucose sensors is reviewed in ref. [34].
19.5 Optical Affinity Sensors
19.5.1 Optical Immunosensors
Optical immunosensors can be designed in either the competitive or sandwich format. Owing to its outstanding sensitivity, fluorescence is the preferred optical transduction method. Common fluorescent labels in immunoassay are cyanine dyes (Figure 19.10). Depending on structure details, cyanine dyes can fluoresce over a wide wavelength region, from the ultraviolet to the near-infrared. For labeling purposes, reactive groups are attached to the dye molecule in order to allow covalent bonding to proteins. Luminescent semiconductor nanocrystals (quantum dots) represent a promising alternative to organic dyes, as shown in detail in Chapter 20.
Figure 19.10 Cyanine fluorescent labels.
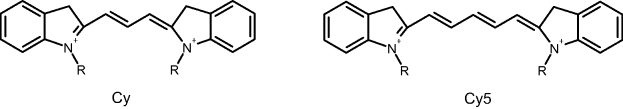
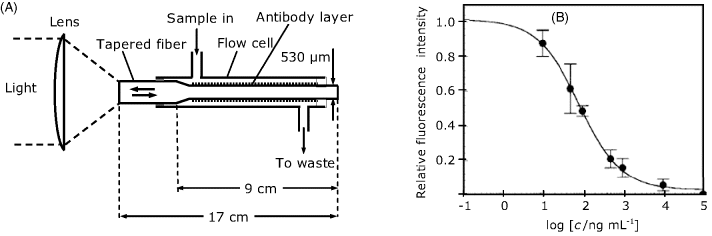
Figure 19.11A shows an intrinsic optical immunosensor in which the sensing layer is formed at the end of a tapered optical fiber. In order to perform flow analysis, the sensor is installed in a flow-through cell. Antibodies can be immobilized at the silica fiber surface by bifunctional silane coupling. The fluorescence light is separated from the excitation light by a dichroic mirror.
Figure 19.11 (A) An intrinsic optical immunosensor installed in a flow cell; (B) response of a sensor like that in (A) to the fumonisin B1 mycotoxin in a competitive assay based on a monoclonal antibody receptor. Adapted with permission from [35]. Copyright 1996 American Chemical Society.
In order to perform a competitive binding assay, the antibody layer is first saturated with the fluorescence-labeled analyte. Next, the sensor is incubated with the sample solution that also contains the labeled analyte at a predetermined concentration. Competition between the analyte and the labeled analyte leads to a partial replacement of the antibody-bound labeled analyte and thus reduces the fluorescence intensity. Sensor regeneration is performed by means of a solution of the labeled analyte that restores the saturation of the sensing layer.
As fluorescence excitation occurs through the evanescent wave, labels in the solution cannot be excited, which makes any washing-out step unnecessary.
A sensor of this type has been designed for the determination of the fumonisin B1 mycotoxin in corn extracts [35]. Figure 19.11B shows the response of this sensor and demonstrates that the working range extends from 10 to 1000 ng/mL. This sensor is highly selective towards fumonisin B1 and does not crossreact with structurally related compounds.
Besides fluorescence, resonance energy transfer is a very convenient transduction method in immunosensors (see Section 18.3.8).
Optical immunosensors have been developed for the determination of haptene-type analytes such as toxins, drugs, and pesticides. Antigens, such as bacterial cells can be sensed by means of immobilized antibodies. Alternative recognition elements for bacteria are antibacterial peptides which are produced by living organisms for protection against harmful microbes.
Immobilized aptamers, which can act as antibody substitutes, have also been exploited in the development of affinity optical sensors [36].
Label-free transduction in immunosensors and protein arrays represents a practical alternative to the schemes based on fluorescent labels. Surface plasmon resonance spectrometry, interferometry, as well as other methods presented in Sections 18.6 and 18.7 allow the labeling process to be precluded at the expense of a more intricate design of the sensor platform. Application of such methods is favored by the significant variation of the refractive index in response to the recognition process.
19.5.2 Optical Sensors Based on Biological Receptors
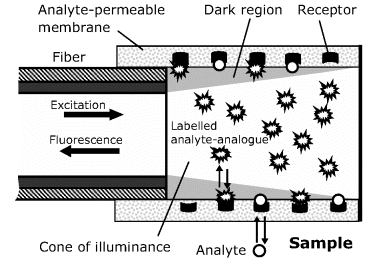
Optical glucose sensing can be achieved by means of the natural receptor Concanavalin A (Con A), which displays affinity to various carbohydrate compounds. An indirect, competitive binding sensor for glucose based on this receptor is shown in Figure 19.12. The receptor is immobilized at the surface of a glucose-permeable tubular membrane at the end of an optical fiber. The membrane-entrapped solution contains a labeled analyte-analog such as dextran. Dextran is a glucose-based polymer and can function as a glucose analog because glucose residues in its structure form affinity complexes with Con A. Being a bulky molecule, dextran cannot pass through the membrane. In contact with a glucose-containing sample, the following equilibrium is established:
Figure 19.12 A reversible affinity optical sensor based on competitive binding. The membrane is a hollow dialysis fiber that is permeable to glucose but not to the large dextran molecule. Redrawn with permission from [37]. Copyright 1987 John Wiley and Sons.
where ![]() is dextran tagged with a fluorescent label and Gluc is glucose.
is dextran tagged with a fluorescent label and Gluc is glucose.
Owing to the geometry of the cone of illuminance, labels bound to the receptors at the membrane surface are not excited and the fluorescence intensity depends only on the label concentration in the illumination region. In contact with the sample, labeled dextran is displaced by glucose molecules, which increases label concentration within the illumination zone and hence enhances the fluorescence intensity. As a result, fluorescence intensity increases nonlinearly with the analyte concentration.
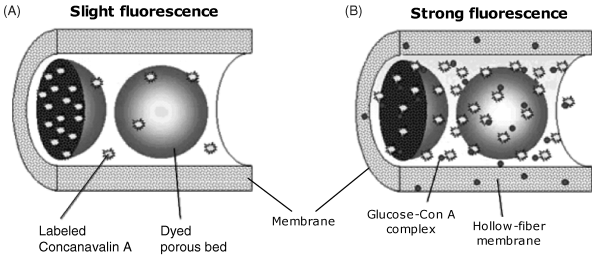
The above sensing scheme has been further improved by using labeled Con A immobilized at Sephadex microparticles as shown in Figure 19.13A. Sephadex is a crosslinked dextran gel that displays a large specific surface area which is available for affinity interaction with Con A. Hence, Con A tagged with a fluorescent label has been attached to Sephadex. In addition, Sephadex particles have been modified with two organic dyes that absorb both the excitation and the fluorescence light. Modified Sephadex particles have been entrapped in a hollow-fiber dialysis membrane attached at the end of an optical fiber in the same way as in Figure 19.12. Owing to the included dyes, fluorescence intensity in this configuration is very low. In contact with a glucose-containing sample, glucose molecules pass through the membrane and form glucose–Con A associations that are free to diffuse into the internal solution. This process can be represented by the following reaction scheme in which (Con A)∗ indicate labelled Con A (see also Figure 19.13B):
(19.10) ![]()
This competitive binding process releases the labeled receptor into the internal solution where excitation and fluorescence emission are possible. Consequently, fluorescence intensity increases as a function of the glucose concentration.
Figure 19.13 A competitive-binding glucose sensor based on dye modified Sephadex. Adapted with permission from [39]. Copyright 2000 American Chemical Society.
This sensing scheme is characterized by a very large density of receptor sites within the active region. Consequently, small variations in glucose concentration result in large variations of the response signal, which enhances the accuracy and expands the dynamic range of the sensor. This sensor can be produced in a very small size (0.5 × 0.2 mm) and is therefore suitable for transdermal glucose monitoring. By using a near-infrared fluorescent label, interference from the fluorescence background produced by the skin tissue is avoided [38].
A major drawback of Con A is its lack of selectivity. Better selectivity towards glucose has been obtained by the glucose oxidase enzyme in the inactive form (apoenzyme) that has been used in a competitive binding format sensor based on resonance energy transfer transduction [40]. Another selective glucose receptor is the Escherichia coli glucose-binding protein in [41].
19.5.3 Outlook
Luminescence is widely used to perform transduction in immunosensors. Although fluorescence is the most widely used transduction process, chemiluminescence and electrochemiluminescence are also of interest as these methods do no need an excitation light source. Fluorescence resonance energy transfer is another method of choice in optical immunosensors. Label-free transduction methods represent a promising alternative to label-based schemes owing to the simplification of the assay protocol.
Affinity reactions based on natural receptors allows the development of optical sensors for small molecules such as glucose. Such sensors have a relatively simple structure and can be easily fabricated at a small size that suits in vivo applications.
19.6 Optical DNA Sensors and Arrays
Optical transduction is widely used in both nucleic acid sensors and in nucleic acid arrays [42–44]. Transduction based on luminescent labels is a simple method suited to both self-contained nucleic acid sensors and DNA microarrays [45, 46]. Label-free transduction can be achieved by a series of methods previously reviewed in Section 18.6 and 18.7. Application of label-free sensors simplifies the assay protocol but demands a more intricate fabrication technology.
19.6.1 Fluorescence Transduction in Nucleic Acid Sensors
The high sensitivity of fluorescence spectrometry is exploited on a large scale in the design of nucleic acids sensors and arrays. Fluorescence labels can be appended to nucleic acids in two ways: by covalent binding or by intercalation. Covalently bound labels can be included in a nucleic acid strand during the polymerase chain reaction by providing a fluorophore-tagged primer. Cyanine dyes are common fluorescent labels used in optical nucleic acid assays.
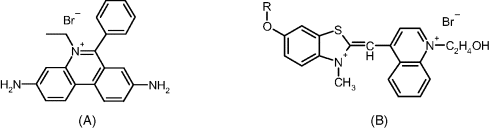
An alternative labeling method is based on fluorescent intercalating and groove binding of planar molecule dyes (see Section 7.3.2). When such a label is present in an aqueous solution, fluorescence is quenched by water molecules. After intercalation in a double-strand nucleic acid, fluorescence is strongly enhanced owing to the hydrophobic environment between the base pairs. Usual intercalating labels are shown in Figure 19.14.
Figure 19.14 Fluorescent dyes for DNA labeling by intercalation. (A) Ethidium bromide; (B) thiazole orange dyes. R is a methyl group in thiazole orange methoxyhydroxyethyl (TOMEHE).
Typical sensing strategies in nucleic acid optical sensors are summarized in Figure 19.15 (see also refs. [26], [43]). The panel (A) demonstrates the transduction by a fluorescent label attached to the target strand. Target labeling can be avoided by resorting to an intercalating fluorescent dye that binds to the double strand formed upon hybridization.
Figure 19.15 Typical sensing schemes in optical DNA sensors. (A) Conventional DNA sensor using a fluorescently labeled complementary strand. (B) molecular beacon sensor. Reprinted with permission from [26]. Copyright 2008 American Chemical Society.
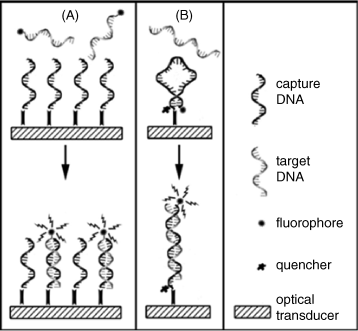
The molecular beacon approach is illustrated in Figure 19.15B. The molecular beacon capture probe is designed so as to include a fluorophore and a fluorescence quencher in close proximity. Probe–target hybridization shifts the fluorophore far from the quencher and results in fluorescence enhancement. This approach precludes the labeling of the target analyte.
Another method for avoiding the labeling of the target is based on the competitive hybridization approach. In this method, a labeled analyte analog is initially hybridized to the capture probe so as to achieve saturation. Next, the saturated receptor layer is allowed to interact with the sample. In this stage of the process, the sample analyte displaces a fraction of the labeled analog, leading to a decrease in the fluorescence.
Alternatively, transduction in optical nucleic acid sensors can be carried out in the sandwich format. In this approach, the probe is designed so that after hybridization with the target, an overhanging target fragment remains in the single-strand form. This fragment is then used to bind by hybridization a signaling probe tagged with a fluorescent label.
19.6.2 Fiber Optic Nucleic Acid Sensors
As shown in Figure 19.16, an intrinsic fiber optic DNA sensor has been obtained by immobilization of the capture probe onto a piece of decladded 400-μm diameter quartz fiber [47]. To this end, the fiber surface is first activated with a long-chain aliphatic spacer terminated in 5′-O-dimethoxytrityl-2′-deoxyribonucleoside. Starting with this molecular unit, solid-phase DNA synthesis has been performed at the fiber surface in order to obtain the capture probe.
Figure 19.16 Setup of an optical fiber DNA sensor. The sensing component is a piece of optical fiber onto which the capture probe is immobilized. The upper part of the figure shows a fluorescence microscope that includes the excitation source (an Ar laser), the photomultiplier light detector (PM) and a dichroic mirror that resolves excitation and fluorescence beams. Sample solutions, staining dye (ethidium bromide-EB) and hybridization buffer are delivered by syringes to the hybridization cell. Adapted with permission from [47]. Copyright 1994 Elsevier.
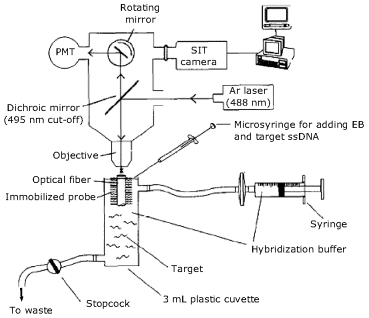
The transduction setup (Figure 19.16) includes the excitation source (an Ar laser) and a photomultiplier light detector. Discrimination of excitation and emission beams is achieved by a dichroic mirror.
Hybridization is conducted in a small plastic cell that contains the sample diluted with the hybridization buffer. After hybridization the cell is flushed with the buffer and the staining of the resulting duplex is achieved by incubation with the ethidium bromide intercalating dye. Finally, unreacted dye is flushed away by a stream of fresh buffer and the fluorescence signal is measured. In this step, the intercalated dye is excited by the evanescent wave. Emitted light is coupled back to the fiber and conveyed through the objective to the photomultiplier. The DNA-bound dye has an emission maximum at 595 nm that is well beyond the cut-off wavelength of the dichroic mirror (495 nm).
After each hybridization assay, the sensor should be regenerated by flushing with hot (85 °C) hybridization buffer, which promotes duplex denaturation.
The background-corrected fluorescence intensity serves as the analytical signal. The response is linear with respect to the target concentration up to 800 ng/mL and the reported limit of detection is 90 ng/mL.
The robustness of the quartz fiber and the nucleic acid probe, as well as the strength of the covalent probe binding, impart this sensor with excellent stability for long-term storage and for stringent cleaning conditions.
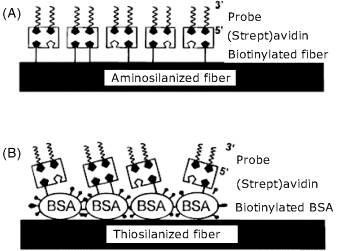
The next example refers to nucleic acid optical sensors produced by immobilization of preformed capture probes on the decladded surface of a quartz optical fiber in order to obtain intrinsic DNA sensors. Two immobilization methods are presented in Figure 19.17. In both cases, the first step consists of surface modification by silanization with reagents containing either amine (A) or thiol (B) terminal groups. In case (A), biotin was then covalently linked to the amine function and the biotinylated probe was attached finally to the previous layer by means of avidin bridges. In case (B), biotinylated bovine serum albumin (BSA) was adsorbed on the primary thiol layer and served as a linker for attaching the biotinylated probe by means of avidin. In order to detect the hybridization event, the target was labeled with fluorescein. This sensor has been included in a flow-through cell that allows the assay sequence to be run under automatic control.
Figure 19.17 Possible structures of the sensing part of an intrinsic fiber optic DNA sensor. (A) The biotinylated probe is immobilized through avidin bridges to a biotin layer covalently attached to an aminosilanized quartz fiber. (B) Biotinylated bovine serum albumin adsorbed on a thiosilanized fiber is used to attach the probe by biotin–avidin bridges. Reprinted with permission from [48]. Copyright 1996 American Chemical Society.
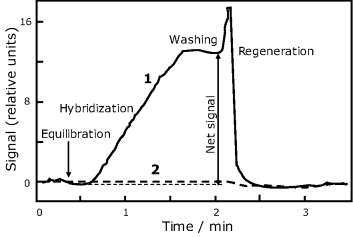
The fluorescent label is excited by the evanescent wave and emitted light couples back to the optical fiber and is detected in order to assess the response signal. The evolution of the sensor signal during an analysis cycle is shown in Figure 19.18 for both the target (curve 1) and a noncomplementary oligonucleotide (curve 2). Note that the last one gives rise to a negligible signal, which proves the absence of nonspecific interactions.
Figure 19.18 Time evolution of the response signal during a DNA assay using the sensor in Figure 19.17(A). Curve 1: target; curve 2: noncomplementary oligonucleotide. Equilibration and washing was done using the hybridization buffer (pH 7). Chemical regeneration was performed by means of a 50% (w/w) urea solution at the hybridization temperature (26.7 °C). Target: (fluorescein-5′-GTTGTGTGGAATTGTG-3′); noncomplementary oligonucleotide: fluorescein-5′- CTGCAACACCTGACAAACCT -3′), each at 10 nM concentration. Adapted with permission from [48]. Copyright 1996 American Chemical Society.
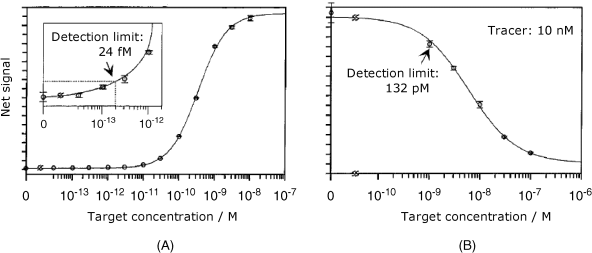
This sensor was used in two assay formats. The first format makes use of direct detection of the labeled target and produces the calibration curve shown in Figure 19.19A. In this case, the signal increases with the target concentration up to saturation at about 10 nM. The second version is based on a competitive assay. This consists of incubation with both the nonlabeled target and a tracer composed of a similar oligonucleotide tagged with a fluorophore. As is typical for competition assays, the target and the tracer compete for the same binding sites and, at a constant tracer concentration, the response decreases with increasing target concentration (Figure 19.19B). The detection limit is better in the first format, but the second format has the advantage of precluding the labeling of the target itself. Detection limits in Figure 19.19 have been achieved for 32.9 °C hybridization temperature and 60-min hybridization time.
Figure 19.19 Response curves for direct (A) and competitive hybridization assays (B) by the sensor in Figure 19.17B. The error bar corresponds to the standard deviation of three measurements. Reprinted with permission from [48]. Copyright 1996 American Chemical Society.
A critical issue is the sensor stability during a long sequence of assays. At a constant target concentration, the successive response values decay slowly according to an exponential law. In order to account for this drift, the corrected signal value (![]() ) should be calculated according to the following equation, where
) should be calculated according to the following equation, where ![]() is the measured signal, t is the utilization time and
is the measured signal, t is the utilization time and ![]() is the decay constant that should be determined prior the analysis:
is the decay constant that should be determined prior the analysis:
(19.11) ![]()
19.6.3 Fiber Optic Nucleic Acid Arrays
Nucleic acid arrays can be formed at the distal end of optical fiber bundles by the methods introduced in Section 19.6.2.
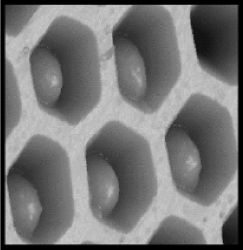
More control over the array design is achieved if the capture probe is immobilized on microspheres with diameters equal to the fiber core diameter [49, 50]. In order to couple microspheres to individual fibers, fiber ends are chemically etched to form microwells. Owing to the size complementarity, each microwell accommodates a single microsphere modified with a particular capture probe (Figure 19.20). As the microspheres are randomly distributed at the bundle surface, an optical decoding is required in order to identify the microsphere coupled to each individual fiber. Identification is achieved by encoding each sensing element with multiple fluorescent dyes prior to the array fabrication. The identity of each microsphere is ascertained from the wavelength and intensity of the fluorescence produced by the encoding dyes. Clearly, encoding dyes must have emission spectra distinct from those of the fluorophores used for sensing. This technique allows for coupling of about 100 different microspheres to a single bundle.
Figure 19.20 Atomic force micrograph of etched optical fiber bundle with 3-μm wells accommodating microspheres derivatized with recognition receptors. Reprinted with permission from [50]. Copyright 2003 The Royal Society of Chemistry.
Fiber optic DNA arrays allow for reliable identification of a pathogen microorganism by simultaneous detection of a series of characteristic DNA fragments. Moreover, this design is suitable for manufacturing of multipathogen detection arrays. The high number of individually addressable sensing elements makes this approach appropriate for genomics applications.
19.6.4 Optical DNA Microarrays
Optical detection by fluorescence is widely used in standard DNA microarrays that consists of a collections of microscopic DNA-probe spots attached to a solid surface [44, 51]. Each spot identifies by hybridization a particular target in a sample. Signal reading is carried out by means of an optical scanner that includes both laser excitation sources and very sensitive light detectors. The detection limit thus achieved is close to 1 fluorophore/μm2. However, owing to the small amount of available material, it is often necessary to perform prior DNA amplification by means of the polymerase chain reaction.
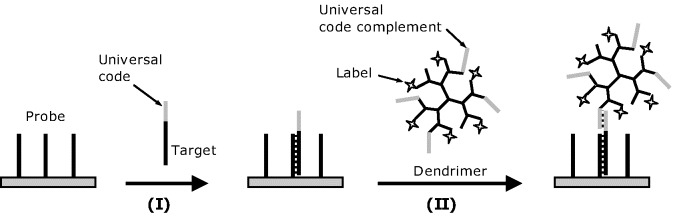
In order to avoid DNA amplification, various methods for enhancing the fluorescence signal have been advanced [52, 53]. For example, Figure 19.21 presents a sandwich format labeling method based on a dendrimer-tagged signaling oligonucleotide. Each dendrimer tag includes about 250 fluorescent molecules. In order to bind the dendrimer label to the hybrid, cDNA targets are coded with a single universal sequence tag that is complementary to the dendrimer-tagged oligonucleotide. After hybridization (step I), the hybrid is stained by coupling the overhanging universal code sequence with the complementary oligonucleotide attached to the dendrimer (step II). This procedure results in about a 16-fold improvement in the detection limit that allows the assay to be performed with much less starting RNA material in gene expression analysis.
Figure 19.21 DNA detection using fluorophore labeled dendrimers.
Signal amplification in fluorescence-based DNA microarrays can also be performed by means of nanomaterial tags such as semiconductor nanoparticles (quantum dots) or metal nanoparticles (see Chapter 20 for details).
Surface-enhanced Raman spectrometry is another optical method for ultrasensitive hybridization monitoring in DNA microarrays [53]. Application of Raman spectrometry in DNA microarrays can be approached in two ways. In one approach, the capture probe is immobilized at a nanoscale roughened silver layer. This layer induces an enhancement of the Raman signal produced by a label such as a Rhodamine dye [54]. A second approach is based on metal nanoparticle tags modified with a Raman label dye. The metal nanoparticle is attached to the hybrid in the sandwich format and induces a strong enhancement of the Raman signal produced by the dye label. Compared with the previous silver-layer-based method, the metal nanoparticle approach is characterized by a much lower scatter background because Raman scattering is produced only by nanoparticle tags. More details on metal nanoparticle applications in Raman spectrometry transduction are available in Chapter 20.
Label-free detection of the hybridization on microarrays can be achieved by surface plasmon resonance (SPR) [53, 55]. Detection limits achieved by standard SPR instruments are in the nanomolar range, which requires DNA amplification prior to the analysis. However, a limit of 54 nM has been attained with a high-resolution SPR instrument that discriminates an angle shift of ![]() .
.
19.6.5 Outlook
The design of optical nucleic acid sensors can be based on either label- or label-free transduction. Whilst label-based sensors are characterized by a simple design and facile multiplexing, the label-free approach involves a more intricate fabrication technology but presents a simpler assay protocol.
Luminescent labels can be introduced in the target molecule during the polymerase chain reaction. Alternatively, an intercalating luminescent label can be attached to the double helix formed upon target-probe hybridization. The sandwich format provides an additional method for labeling the probe-target hybrid. Another transduction procedure relies on the application of labeled capture probes in the molecular beacon configuration.
Label-based optical detection is widely used in standard DNA microarrays. In order to avoid the need to use DNA amplification using the polymerase chain reaction, one can resort to nanoparticles tagged with multiple signaling luminophore molecules. Label-free detection in DNA microarrays can be achieved by surface plasmon resonance spectrometry and other methods introduced in Section 18.6.
1. Baldini, F., Bechi, P., Bracci, S. et al. (1995) In-vivo optical-fiber pH sensor for gastroesophageal measurements. Sens. Actuators B-Chem., 29, 164–168.
2. Lin, J. (2000) Recent development and applications of optical and fiber-optic pH sensors. TrAC Trends Anal. Chem., 19, 541–552.
3. Mills, A. (2009) Optical sensors for carbon dioxide and their applications, in Sensors for Environment, Health and Security: Advanced Materials and Technologies (ed. M.I. Baraton), Springer, Dordrecht, pp. 347–370.
4. Rhines, T.D., and Arnold, M.A. (1988) Simplex optimization of a fiber-optic ammonia sensor based on multiple indicators. Anal. Chem., 60, 76–81.
5. Borisov, S.M., Waldhier, M.C., Klimant, I. et al. (2007) Optical carbon dioxide sensors based on silicone-encapsulated room-temperature ionic liquids. Chem. Mater., 19, 6187–6194.
6. Burke, C.S., Markey, A., Nooney, R.I. et al. (2006) Development of an optical sensor probe for the detection of dissolved carbon dioxide. Sens. Actuators B-Chem., 119, 288–294.
7. Oehme, I., and Wolfbeis, O.S. (1997) Optical sensors for determination of heavy metal ions. Mikrochim. Acta, 126, 177–192.
8. Langry, K. and Rambabu, B. (1999) Ionic optodes: role in fiber optic chemical sensor technology. J. Chem. Technol. Biotechnol., 74, 717–732.
9. Bakker, E.B.E., Crespo, G., Grygolowicz-Pawlak, E. et al. (2011) Advancing membrane electrodes and optical ion sensors. Chimia, 65, 141–149.
10. Fabbrizzi, L. and Poggi, A. (1995) Sensors and Switches from Supramolecular Chemistry. Chem. Soc. Rev., 24, 197–202.
11. Valeur, B. and Leray, I. (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev., 205, 3–40.
12. Callan, J.F., de Silva, A.P., and Magri, D.C. (2005) Luminescent sensors and switches in the early 21st century. Tetrahedron, 61, 8551–8588.
13. Fabbrizzi, L., Licchelli, M., Rabaioli, G. et al. (2000) The design of luminescent sensors for anions and ionisable analytes. Coord. Chem. Rev., 205, 85–108.
14. Johnson, R.D. and Bachas, L.G. (2003) Ionophore-based ion-selective potentiometric and optical sensors. Anal. Bioanal. Chem., 376, 328–341.
15. Bakker, E., Buhlmann, P., and Pretsch, E. (1997) Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev., 97, 3083–3132.
16. Buhlmann, P., Bakker, E. and Pretsch, E. (1998) Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev., 98, 1593–1687.
17. Kawabata, Y., Tahara, R., Kamichika, T. et al. (1990) Fiber optic potassium ion sensor using alkyl-acridine orange in plasticized poly(vinyl chloride) membrane. Anal. Chem., 62, 1528–1531.
18. Mills, A. (1997) Optical oxygen sensors. Platinum Metals Rev., 41, 115–127.
19. Wang, X.-D., Chen, H.-X., Zhao, Y. et al. (2010) Optical oxygen sensors move towards colorimetric determination. Trac-Trends Anal. Chem., 29, 319–338.
20. Mills, A. (2009) Oxygen indicators in food packaging, in Sensors for Environment, Health and Security: Advanced Materials and Technologies (ed. M.I. Baraton), Springer, Dordrecht, pp. 371–388.
21. Grist, S.M., Chrostowski, L., and Cheung, K.C. (2010) Optical oxygen sensors for applications in microfluidic cell culture. Sensors, 10, 9286–9316.
22. Amao, Y. (2003) Probes and polymers for optical sensing of oxygen. Microchim. Acta, 143, 1–12.
23. Nagl, S., Baleizao, C., Borisov, S.M. et al. (2007) Optical sensing and imaging of trace oxygen with record response. Angew. Chem. Int. Ed., 46, 2317–2319.
24. Cao, W. and Duan, Y. (2006) Optical fiber evanescent wave sensor for oxygen deficiency detection. Sens. Actuators B-Chem., 119, 363–369.
25. Zhu, H., Tian, Y., Bhushan, S. et al. (2010) High throughput micropatterning of optical oxygen sensor. IEEE Sensors 2010 Conference, pp. 2053–2056.
26. Borisov, S.M. and Wolfbeis, O.S. (2008) Optical biosensors. Chem. Rev., 108, 423–461.
27. Endo, H., Yonemori, Y., Musiya, K. et al. (2006) A needle-type optical enzyme sensor system for determining glucose levels in fish blood. Anal. Chim. Acta, 573–574, 117–124.
28. Marquette, C.A., Degiuli, A., and Blum, L.J. (2003) Electrochemiluminescent biosensors array for the concomitant detection of choline, glucose, glutamate, lactate, lysine and urate. Biosens. Bioelectron., 19, 433–439.
29. Xie, X.F., Suleiman, A.A., and Guilbault, G.G. (1990) A urea fiber optic biosensor based on absorption measurement. Anal. Lett., 23, 2143–2153.
30. Wolfbeis, O.S. and Li, H. (1993) Fluorescence optical urea biosensor with an ammonium optrode as transducer. Biosens. Bioelectron., 8, 161–166.
31. Sansubrino, A. and Mascini, M. (1994) Development of an optical fiber sensor for ammonia, urea, urease and IgG. Biosens. Bioelectron., 9, 207–216.
32. Schelp, C., Scheper, T., Buckmann, F. et al. (1991) Two fiberoptic sensors with confined enzymes and coenzymes - Development and application. Anal. Chim. Acta, 255, 223–229.
33. Choi, M.M.F. (2004) Progress in enzyme-based biosensors using optical transducers. Microchim. Acta, 148, 107–132.
34. Pickup, J.C., Hussain, F., Evans, N.D. et al. (2005) Fluorescence-based glucose sensors. Biosens. Bioelectron., 20, 2555–2565.
35. Thompson, V.S. and Maragos, C.M. (1996) Fiber-optic immunosensor for the detection of fumonisin B1. J. Agric. Food Chem., 44, 1041–1046.
36. Sassolas, A., Blum, L.J., and Leca-Bouvier, B.D. (2011) Optical detection systems using immobilized aptamers. Biosens. Bioelectron., 26, 3725–3736.
37. Schultz, J.S. (1987) Sensitivity and dynamics of bioreceptor-based biosensors. Ann. N. Y. Acad. Sci., 506, 406–414.
38. Ballerstadt, R., Polak, A., Beuhler, A. et al. (2004) In vitro long-term performance study of a near-infrared fluorescence affinity sensor for glucose monitoring. Biosen. Bioelectron., 19, 905–914.
39. Ballerstadt, R. and Schultz, J.S. (2000) A fluorescence affinity hollow fiber sensor for continuous transdermal glucose monitoring. Anal. Chem., 72, 4185–4192.
40. Chinnayelka, S. and McShane, M.J. (2005) Microcapsule biosensors using competitive binding resonance energy transfer assays based on apoenzymes. Anal. Chem., 77, 5501–5511.
41. Ge, X.D., Tolosa, L., Simpson, J. et al. (2003) Genetically engineered binding proteins as biosensors for fermentation and cell culture. Biotechnol. Bioeng., 84, 723–731.
42. Kuswandi, B., Tombelli, S., Marazza, G. et al. (2005) Recent advances in optical DNA biosensors technology. Chimia, 59, 236–242.
43. Yang, M.S. McGovern, M.E., and Thompson, M. (1997) Genosensor technology and the detection of interfacial nucleic acid chemistry. Anal. Chim. Acta, 346, 259–275.
44. Sassolas, A., Leca-Bouvier, B.D., and Blum, L.J. (2008) DNA biosensors and microarrays. Chem. Rev., 108, 109–139.
45. Müller, H.J. and Röder, T. (2006) Microarrays, Elsevier, San Diego.
46. Baldi, P. and Hatfield, G.W. (2002) DNA Microarrays and gene Expression: From Experiments to Data Analysis and Modeling, Cambridge University Press, Cambridge.
47. Piunno, P.A.E., Krull, U.J., Hudson, R.H.E. et al. (1994) Fiber optic biosensor for fluorometric detection of DNA Hybridization. Anal. Chim. Acta, 288, 205–214.
48. Abel, A.P., Weller, M.G., Duveneck, G.L. et al. (1996) Fiber-optic evanescent wave biosensor for the detection of oligonucleotides. Anal. Chem., 68, 2905–2912.
49. Epstein, J.R., Leung, A.P.K., Lee, K.H. et al. (2003) High-density, microsphere-based fiber optic DNA microarrays. Biosens. Bioelectron., 18, 541–546.
50. Epstein, J.R. and Walt, D.R. (2003) Fluorescence-based fibre optic arrays: a universal platform for sensing. Chem. Soc. Rev., 32, 203–214.
51. Teles, F.R.R. and Fonseca, L.R. (2008) Trends in DNA biosensors. Talanta, 77, 606–623.
52. Willner, I., Shlyahovsky, B., Willner, B. et al. (2009) Amplified DNA biosensors, in Functional Nucleic Acids for Analytical Applications (eds Y. Li and Y. Lu), Springer New York, New York, pp. 199–252.
53. Storhoff, J.J., Marla, S.S., Garimella, V. et al. (2005) Labels and detection methods, in Microarray Technology and Its Applications (eds R. Müller and V. Nicolau), Springer, Berlin, pp. 147–179.
54. Allain, L.R. and Vo-Dinh, T. (2002) Surface-enhanced Raman scattering detection of the breast cancer susceptibility gene BRCA1 using a silver-coated microarray platform. Anal. Chim. Acta, 469, 149–154.
55. Scarano, S., Mascini, M., Turner, A.P.F. et al. (2010) Surface plasmon resonance imaging for affinity-based biosensors. Biosens. Bioelectron., 25, 957–966.
