Chapter 10
SHIFT WORK
Allene J. Scott
Shift work refers to hours of work occurring outside the regular daytime schedule, that is, work schedules not falling between 6 a.m. and 6 p.m. According to the May 2004 Work Schedules and Work at Home supplement to the Current Population Survey by the US Bureau of Labor Statistics, considering only the primary job of full‐time workers, 19% of male and 16% of female workers are shift workers including 3–4% each reporting working “irregular schedules” (varying with the needs of the business), rotating shifts, or night shifts. Over one half of protective service workers, for example, police and firefighters, are shift workers. Similarly the majority of employees in eating and drinking establishments work nondaytime schedules. One third of transportation workers are shift workers. Industries with 5% or less of employees working shift work schedules include construction, education, finance, and insurance.
Alertness and performance of shift workers, particularly when working schedules involving the night (graveyard) shift, may be compromised. Night workers and rotating shift workers are at increased risk of falling asleep on the job and of falling asleep when driving home after work.1,2 Public safety has been compromised by catastrophes involving chemical plant, nuclear power plant, and transportation accidents attributed to work schedule‐related drowsiness.3,4
Shift work also takes a toll on individuals and their families, due to conflicts between the work schedule and domestic responsibilities. Shift work has been identified as an emerging risk factor for several medical and mental health conditions, which receive extra attention in this chapter.
OCCUPATIONAL SETTING
There are many different types of shift work schedules. The type of schedule is often determined by traditional practices of the industry. For example, the Southern‐Swing schedule, a weekly, backward rotation, has been commonly used for generations in the steel industry. Worker preferences, labor union interests, and job process needs are common factors influencing schedule design.
Common shift work schedule designs include the following:
- Fixed (“permanent”)—Each individual employee works one shift. The number of days worked in a row and the number of days off vary between industries. “Permanent” night work is, in one sense, a misnomer, because almost all workers return to daytime activity on their days off and thus still switch back and forth between night work and daytime activity.
- Rotating—An individual employee regularly changes the shift worked. Rotating schedules vary in the direction and speed of rotation:
- Direction of rotation: This may be clockwise (forward rotation, phase delay) or counterclockwise (backward rotation, phase advance).
- Speed of rotation: This may be slow (greater than weekly), weekly, or rapid. Examples of rapidly rotating systems include the European metropolitan (two identical consecutive shifts are worked consecutively, followed by two of the next shift until all three shifts have been worked, 2‐2‐2) and the continental (same as the metropolitan, except three of one of the shift times are worked, 2‐2‐3) rotation schedules.
- Split shifts—A hiatus of a few hours separates the work hours performed on the same day. For example, this may be done in the restaurant or transportation industries to cover peak business hours.
- Alternative rotations—An increasingly popular 4‐day “compressed workweek” with 10–12 hours shifts used in a single‐, two‐, or three‐shift operation. The 8‐day week with 4 10‐hours work days followed by 4 days off is used primarily in firms operating for 10 hours/day, 7 days/week or operating 20 hours/day in two shifts. Twelve‐hour shift schedules are common in hospital nursing schedules. Schedules with “flexitime” give employees some choice in designing their own personal daily work hours to meet weekly requirements.
MEASUREMENT GUIDELINES
Several factors affect tolerance to night work and rotating shift work, including (i) individual differences in susceptibility to performance and alertness deficits related to altered sleep–wake cycles and to symptomatology from disruption of biological circadian rhythms, (ii) differences in social/family responsibilities and supportiveness, (iii) effectiveness of off‐the‐job and on‐the‐job coping strategies for maximizing sleep and minimizing performance deficits, (iv) the schedule design, and the predictability and flexibility of time off.
In order to evaluate the appropriateness of a particular work schedule for a specific industrial site, information should be gathered from human resources, from safety officers, and directly from the workers. The general categories of necessary information include (i) demographics of the workforce, (ii) frequency of cases of shift work intolerance from medical surveillance programs and/or medical claims, (iii) rates of on‐the‐job fatigue‐related accidents or performance deficits, and (iv) information from confidential worker surveys concerning sleepiness on the job, motor vehicle accidents or near misses driving home from each shift, general well‐being, and worker satisfaction with the schedule.
Tepas et al. have described a survey method for use in designing and evaluating shift work systems tailored to specific workers and plants.5 Their work–sleep survey has demonstrated that there are significant differences between industrial plants with respect to demographics, worker habits, and worker preferences and that “Before recommending a new work‐system shift scheme to a plant, the complexity of shift‐work issues must be recognized by assessing a wide range of personal, social, and health issues.” Education of workers with respect to the reasons for the selection and expectations of how the scheduling system will work is also important. Follow‐up surveys, conducted after the system has been in operation for some time, are recommended to evaluate the effects on the workers’ well‐being and performance. Kogi made similar recommendations based on an extensive survey of male and female shift workers in Japan working various rotating schedules.6 These recommendations included considering other factors, in addition to the shift rotation, when determining ways to minimize the detrimental effects of shift work on the worker and his/her family, such as social and family life responsibilities, worker commuting time, and possibilities for anchor sleep (regularly scheduled sleep periods) during night shifts.
Direct comparisons of studies looking at performance or health outcomes of shift workers are often difficult, due to differences in schedule design. Pertinent shift work exposure parameters include, in addition to the number of years employed as a shift worker, schedule design variables such the direction and frequency of shift rotation, the number of nights worked in a row, the number of days off after night shift assignment, the amount of overtime, and the length of shifts. National data collection is limited in the United States. The United States Bureau of Labor Statistics does annually collect and tabulate occupational injury data available from employers regarding the time of day the injury event occurred and hours on the job worked before the event.7 The Federal Occupational Safety and Health Administration’s (OSHA) Form 301 Incident Report (Rev 01/2004) which employers are required to complete and maintain in their files for 5 years also request this information, if available. The BLS includes this data in some of its published estimates.8 However, OSHA only requires employers to record work site‐wide annual average employment and total hours worked by all payroll employees, and BLS injury statistics do not include data regarding the composition of work force by shift, so shift work hour‐based rates are not determinable from the national labor statistics (Bureau of Labor Statistics, personal E‐mail communication, December 31, 2014).8 Limited opportunities therefore exist to look at US national data concerning on‐the‐job accidents and scheduling factors.
Despite the inherent design problems in shift work research and the limited national data, field and laboratory studies of 24‐hour sleep and performance rhythms, and of health outcomes in shift workers, have provided information that has been used to make reasonable recommendations concerning shift work scheduling. These are presented in the following section.
EXPOSURE GUIDELINES (SCHEDULE DESIGN)
The various combinations of individual susceptibility factors and scheduling designs make it difficult to assess the effective “exposure” to shift work and thus the “risk” that different shift systems may have on health and safety outcomes. Comparisons of groups must account for the type of shift schedule as well as years spent in shift work. Wedderburn has proposed criteria for assessing shift work schedules for potential risk for health and well‐being including the perturbation of the circadian rhythm, performance at work, health, and social life.9 The “Rota Risk Profile Analysis,” developed by Jansen and Kroon utilizes several physiological and psychosocial risk factors associated with the schedule design including regularity of shift timetable, periodicity (the degree to which the “biological clock” is disturbed), shift load (the average length of shifts) and week load (the average length of the work week), opportunities for nighttime sleep (between 11 p.m. and 7 a.m.) and constancy in night rest (variation in the week), predictability of the shift cycles, and opportunities and constancy for household and family tasks, for evening recreation, and for weekend recreation.10
As is true of threshold limit values® (TLVs®) for chemical exposures, which are developed to protect nearly all healthy workers, with the recognition that a small percentage of workers are susceptible to lower levels of exposure,11 recommendations for shift work scheduling design, which control exposure to night work, are applicable to most workers. Shift work scheduling recommendations may not be adequate for workers with greater individual susceptibility to shift work‐related biological rhythm disruption or with family/social situations limiting daytime sleep opportunities. In addition, with respect to performance on the job, the appropriateness of recommendations for the length of shift varies with the type of industry involved and related productivity versus safety and alertness demands.
Chronotoxicologic considerations
OSHA Permissible Exposure Limits (PELs) for occupational exposures to chemicals are determined for an 8‐hour time‐weighted average (TWA). TLVs® calculated by the American Conference of Governmental Industrial Hygienists (ACGIH) for airborne concentrations of chemical exposures are also based on an 8‐hour workday.11 Neither exposure limit adjusts for nocturnal differences in metabolism when working at night. With the exception of the extended shift reduction factor requirement included in the OSHA lead standard, OSHA PELs do not require measurement adjustment for shifts over 8 hours.12 The ACGIH11 suggests that industrial hygienists follow the Brief and Scala model13 for adjusting TLVs® for work shifts over 8 hours. The Brief and Scala model considers the increased uptake due to longer work exposure and adds a second component to account for the decreased metabolic clearance during the shorter time away from the workplace exposure.
In addition, the susceptibility to the adverse affects of the toxic materials may be greater when exposure occurs at night, just as response to medications varies with the time of administration. Smolensky and Reinberg14 have prepared a detailed discussion of chronotoxicology as it relates to biological monitoring of workplace exposures.
Musculoskeletal considerations
Work involving heavy physical labor and/or repetitive motion may need to be adjusted for production speed and number of breaks. Job assignments may need to be rotated to prevent repetitive musculoskeletal stress/injury. The number of hours between shifts worked and the number of sequential days off may be significant factors in the management of acute and chronic strain/sprain injuries. A model for special provisions for night workers who are also exposed to physical and/or toxic stress developed by the Austrian “Night Shift/Heavy Work Law” has been summarized by Koller.15
General scheduling considerations
Ideally, shift work schedules should be designed to minimize the potential negative effects of night work on worker sleep, health, and performance. However, medical and performance/safety considerations are not the only driving force in scheduling design. Economic ramifications including staffing levels and production needs, labor union issues, and worker preferences are important factors in influencing the final outcome. In order to help balance all of these considerations, shift work scheduling consultants are increasingly being utilized to assist companies in evaluating and redesigning schedules.
Advantages and disadvantages of different schedules
Rotating schedules
Entrainment considerations
Differences in recommendations concerning whether permanent night shift schedules are preferable to rotating schedules, and if a rotating schedule is used, the appropriate speed of schedule rotation, reflect differing opinions about the effect of the schedule design on nocturnal adjustment by the night worker and the desirability of circadian re‐entrainment to nocturnal work.16 In order to maximize night work performance, rapid re‐entrainment is desirable. However, minimizing circadian system disruption in order to avoid related deleterious effects on health and well‐being conflicts with this goal and the goal of entraining the night worker to the inverted day–night cycle is difficult to reach. It has been demonstrated that more than a week of consecutively worked nights is needed before complete adjustment of the circadian system begins to occur.17,18 Most workers revert to day activity on nonwork days, and because considerable re‐entrainment to diurnal activity occurs over only a couple of days off, biological rhythm desynchronization can be expected to occur each time the worker starts on the night shift period.19 Weekly and rapidly rotating schedules do not allow enough time for full adjustment to night orientation. When no more than two or three nights in a row are worked, little diurnal shifting of the circadian system will have occurred.20 If the degree of adjustment to night work has been overestimated as has been suggested,21 the rationale is weakened for using “permanent” or slowly rotating shift systems in order to maximize adjustment of the circadian system.
Studies of long‐term tolerance to shift work have suggested that shift workers with large amplitudes in circadian temperature rhythms (indicating resistance to adjustment) have better long‐term tolerance to shift work.22–24 Reinberg et al. have therefore suggested that if tolerance to shift work over the long term is associated with large circadian amplitude of the temperature rhythm and therefore slow adjustment, rapid rotation is preferable to weekly rotation.22 Mills et al. suggested that if re‐entrainment were accomplished, the worker would have trouble functioning in the diurnal world on days off.25
Circadian and sleep debt considerations
The forward (clockwise, phase‐delay) direction for schedule changes is usually considered preferable, since the biological clock is easier to set back than ahead. Jet‐lag and shift work laboratory studies have demonstrated that it is easier to adjust to a phase‐delay than to a phase‐advance time shift.25–28 Shift work field studies also have supported a clockwise rotation over a counterclockwise direction.25,29–31 Folkard has pointed out that when there is not a day off between shift changes, systems that rotate forward allow a break of 24 hours, while many backward rotations have a “quick return” after only an 8‐hour break.32
With respect to the speed of rotation, Vidacek et al., in their study of weekly rotating shift workers, found, for the night shift only, a day‐of‐the‐week effect, with productivity increasing through the third day and then falling over the last two days of the 5‐day shift period, but not to the first‐day low.33 They suggested that a weekly rotation might capitalize on circadian adjustment while minimizing effects of sleep deprivation in comparison to rapidly rotating or more slowly rotating schedules. Wilkinson et al. found, for a slowly rotating system, that compared to the first night worked, performance on tests sensitive to the effects of sleep deprivation was significantly poorer on the seventh work night, but not on the fourth night.34 Other studies have shown evidence of sleep deprivation after only two consecutive nights on the job35,36 and that, for rotating workers, sleep is most disturbed at the beginning of the night shift.37 Czeisler et al. reported that most weekly rotators on a phase‐advance schedule need 2–4 days for their sleep schedule to adjust to shift changes.38
Chronic sleep deprivation has been demonstrated after several nights worked in succession in rotating systems.39–41 Williamson and Sanderson evaluated the effects of switching from a clockwise, slowly rotating (seven straight shifts) to a clockwise, rapidly rotating system with no more than three nights worked in a row.42 After the switch, workers reported improved sleep, and no longer complained of feeling tired and irritable at work. Smith et al. compared a slowly rotating continuous 8‐hour shift system involving seven shifts worked in succession to rapidly rotating continuous 8‐ and 12‐hour shifts.43 After switching to the rapid rotations, day sleep was reported to be improved, fatigue decreased, home and social life improved, and symptoms of circadian disruptions decreased.
Eastman has described a protocol involving worker’s controlling bright light exposure before bed to achieve minimal circadian misalignment when working a three‐shift system with 2‐week slowly rotating schedule in the delaying direction.44 Smith and Eastman have concluded that rapidly rotating shift schedules that include day and night work should be abolished, because the circadian biological clock cannot phase‐shift fast enough to reduce circadian rhythm misalignment (however an effective sleep and light schedule can be applied if the rotation is between evening and night shifts), and that rotation between day and evening shifts also produces less circadian disruption than switching between day and night work.45
Maasen et al. found that good sleep was obtained by workers on a weekly rotating system; however, the schedule provided 6 days off following the week of night work, and the morning shift did not start until 0800.46 Based on health, safety, and productivity evaluations before and after the introduction of a new shift schedule, Moore‐Ede’s group has been successful with a 21‐day slowly rotating schedule which requires workers to maintain nocturnal orientation on their days off during the 3 weeks of night work.47
There are other variables to be considered when contrasting rotating schedules with different speeds of rotation. Providing adequate time off after night shifts is necessary to prevent chronic sleep deprivation. Dahlgren has concluded that “the speed of rotation in itself, without consideration of how the free days are organized is an insufficient criteria for judging the relative merits of different shift systems.”48 Others have recommended that there should not be many night shifts in succession and that workers should have at least 24 hours off after each night shift.35,49 With respect to worker preference, several studies have found rapid rotations to be preferred by workers who have experienced them over weekly and permanent night shifts.39,41,50–53
Permanent shifts
For the permanent night worker, sleep deprivation appears to be persistent. Some research indicates that the sleep reduction associated with night work is greater for rotating workers; however, sleep deprivation performance deficits can be reversed during nonnight shift rotation time.54 Tepas et al. found measures of vigilance to be poorer for permanent night workers than for slowly rotating workers when on the night shift; other evidence of chronic sleep deprivation was found for permanent night workers but not for the rotators.55 The total average duration of sleep appears to be shorter for permanent night workers than for rotating ones, although sleep when on the night shift may be shorter for rotators.56
Length of shifts: 8‐ versus 12‐hour shifts
In addition to the type of rotation, the length of the shift may affect fatigue‐related performance parameters. Several studies have found a negative impact of 12‐hour shifts on performance and alertness parameters. Rosa et al., in a simulated work study, measured several performance parameters and collected worker reports of drowsiness and fatigue; the results were interpreted to support that 12‐hour/4‐day “compressed” weeks were more fatiguing than 8‐hour/6‐day weeks.57 Using the same fatigue test battery in a work site assessment, an overall decrease in performance and alertness as well in total sleep time was observed after a switch from an 8‐hour three‐rotating work shift schedule to a 12‐hour shift schedule.58
There have been reports of fatigue effects on performance toward the end of 10‐ and 12‐hour shifts.59–62 Of special concern is that an increase in fatigue and sleepiness may occur in the last hours of a long shift coinciding with time of the circadian nadir of alertness. In a case–control study of large truck crashes on interstate highways, driving for over 8 hours was associated with nearly a doubling of the risk of crash involvement compared to drivers who had driven fewer hours.63 Lisper et al. found, in a 12‐hour observed driving test, that most episodes of falling asleep occurred after 8 hours of driving.64 Several studies, however, comparing 8‐ and 12‐hour systems have reported improvement or minimal deleterious effects on health, performance, and alertness or sleep parameters with the introduction of a 12‐hour shift.65–70 Mitchell and Williamson71 reported for a sample of electrical power station workers, changing from an 8‐hour slowly backward rotating system to a forward rapidly rotating 12‐hour system resulted in improvements in perceived sleep quality, with less broken sleep patterns being reported, and less use of alcohol as a sleep aid; these workers also reported feeling fresher at the beginning and end of the 12‐hour shifts. In this study, while there were also no ill effects of 12‐hour shifts on four out of five performance measures, performance on a vigilance task declined over 12‐hour shifts, but not over 8‐hour shifts; injury data was similar for the 8‐ and 12‐hour systems (two injuries being reported during the 8‐hour schedule compared to the one during the 12‐hour schedule). In his extensive review of the available research, Tucker concluded that there was little direct evidence of an increase in risk to safety from switching to a compressed workweek; however he also pointed out evidence from field studies suggesting that fatigue increases toward the end of extended shifts in some work settings.72 This observation is consistent with increasing evidence that accident risk increases with the duration of the work shift, and the increase in risk may be dramatic toward the end of shifts longer than 8 hours.
Using compressed workweeks with 12‐hour shifts to cover 24‐hour operations has been recommended by shift work scheduling consultants.73 Although compressed workweeks require working long shifts, they have become popular because they provide several days off in a row allowing workers to have blocks of time off for family/social activity. In addition, some switches to 12‐hour schedules come with less potential for required overtime and working back‐to‐back shifts.
Compared to the estimated 5–7% of US workers in general who work more than one job, about 25% of 12‐hour shift workers report moonlighting. Concern has been raised that these workers may be returning to work, possibly at night, already tired from other work activities.62,74,75
Multifactorial issues contribute to the advantageous and disadvantageous outcomes reported when 12‐hour shifts have been introduced into various industries and worker populations76; and limitations and inconsistencies in the research data published on this topic have been noted.62,76–78 Reasons for conflicting findings and recommendations include differences in the demographic characteristics of workforces, differences in priorities of employees versus employers, differences in production and safety issues for various types of industries, and the inconsistencies in the types of work schedules examined across studies, for example, fixed versus rotating schedules, speed and direction of rotation, number of hours worked per week, number of consecutive days worked, and number of rest days and weekends off. Sirois and Moore‐Ede have pointed out that workplace staffing levels rather than the shift schedule determines the amount of overtime worked and may be the primary determinant of the actual length of shifts, the time employees have off between shifts, and the number of consecutive days worked.79
When considering changing to a 12‐hour shift schedule, in order to avoid fatigue‐related problems, particularly when public safety is a factor, Knauth has suggested that shifts over 8 hours be used only if (i) the nature of work and the workload are suitable, (ii) sufficient time for breaks are provided, (iii) the design of the shift system minimizes the accumulation of fatigue, (iv) coverage for absent workers is provided and overtime is not involved, (v) toxic stressors are limited, and (vi) a complete recovery is possible after the shift.80
Recommendations by Rosa and Colligan for shift work scheduling design involving 12‐hour shifts include the following: only two to three shifts be worked in a row, for night work two is probably best, and a day or two off should follow night shifts.81
Tucker recommended the following fatigue countermeasures for compressed workweek schedules based on several previously published guidelines and additional studies reviewed72:
- Overtime, moonlighting, and long commutes should be avoided where it impacts on recovery.
- Utilize fatigue countermeasures (e.g., liberal rest breaks, job rotation) to minimize the impact of extended shifts to avoid boredom.
- Allow shorter, more frequent breaks within shifts, rather than fewer long ones.
- Ensure adequate recovery between shifts, for example, three successive rest days for long sequences of long shifts and extended recovery periods (3–4 days) after periods of night work due to the likelihood of circadian disruption.
- Take account of changes in risk outside the workplace, such as the risk of fatigue while commuting after a long shift and domestic care responsibilities.
- Readjust margins of error inherent in the work design (e.g., overtime policies) to account for the effects of extended shift length and/or quick returns.
- Ensure availability of sufficient personnel to cover all shifts to avoid increased risk associated with overtime on longer shifts.
- Redistribute workloads to be low at times of high fatigue/low alertness, for example, midafternoon and last few hours of the shift, especially at night.
Other factors related to scheduling decisions
Early morning starting times
Sleep deprivation and related fatigue have been associated with early starting times (before 7–8 a.m.) for the morning shift. In addition to being sleep deprived when working night shifts, rotating shift workers may have their sleep cut short when working first shifts requiring very early rising times; and permanent day workers on early starting 12‐hour shifts have been reported to be more sleepy than their night‐working counterparts.82,83 Day workers, who must begin work early in the morning, may have job/social‐bound sleep restrictions with significant consequences.84,85 Knauth et al. have advised that morning shifts should not begin too early to avoid an accumulation of sleep deficits.35
Schedule predictability and flexibility
One reason for worker preference for a 12‐hour shift system may be that there is less variation in work times compared to 8‐hour three‐shift rotations. In order to maximize participation in family and social activities, work schedule predictability is necessary for making plans and keeping commitments. The Centers for Disease Control and Prevention (CDC) has recommended that rotating shift schedules be stable and predictable.86 Reorganization of the on‐call shifts may minimize the negative effects on social and family well‐being.87 A recent systematic review of 10 controlled before‐and‐after studies of the effects of different types of flexible working arrangement found evidence that shift scheduling interventions, which allow for flexibility in working patterns and give workers more control over working time, improve health and/or well‐being.88 In their recent analysis of sickness absence days in Finnish workers, Nätti et al. found a higher incidence of sickness absence in shift work compared with day work primarily due to less working time control, supporting the conclusion that increased worker control of their work scheduling may counteract the negative health effects of shift work.89
Approaches to making schedule changes
The actual shift system design chosen is not the only factor which will determine the success of a schedule change. The manner in which the decision was made may be equally important. A schedule system is more apt to be well accepted by the workers if their desires with respect to free time and family/social responsibilities are considered in its design.90,91 The acceptance of an ergonomically “good” schedule may reflect whether workers or management initiated the introduction of the new schedule.92 According to Kogi, the trend in scheduling design is to use a participatory process.93 Recommended steps to follow when making shift schedule changes are as follows: (i) carry out a group study of operational needs, worker preferences, health and tolerance issues of the workforce, and potential options; (ii) utilize joint planning to make plans for feasible options and specific measures; (iii) provide for feedback and dialogue to build a consensus and allow for adjustment and training; (iv) implement jointly the new work organization, in a progressive fashion if appropriate; and (v) jointly evaluate the change, taking further action as needed.
The following general guidelines for shift system design, not specific to 12‐hour/compressed workweek schedules, were published by Costa et al.94 and included in Tucker’s 2006 recommendations72:
- Minimize night work.
- Favor quick shift rotation and clockwise rotation.
- Avoid permanent night work except for safety critical conditions, where complete adjustment is necessary.
- Avoid early morning changeovers.
- Maximize regularity, free weekends, minimum of two consecutive days off, and promote flexible work practices.
- Assess working environment for hazards and stressors.
- Assess individual workers for health status and individual differences impacting shift work tolerance (e.g., morningness, rigid sleeping habits) and social situation (responsibility for young children, long‐distance commutes)
- Include provision for medical surveillance.
NORMAL PHYSIOLOGY
Numerous psychological and physiologic variables have been documented to have a demonstrable 24‐hour, circadian (Latin: circa = about, and dies = a day)95 rhythm, for example, body temperature, the sleep–wake cycle, cardiovascular parameters, cognitive performance, hormonal and immunologic factors, metabolic responses including to medications and toxins, and psychological variables.96,97
Circadian rhythms do not merely reflected responses to external time cues but also have an endogenous component, creating significant consequences for shift workers. The existence of a biological clock in humans was initially demonstrated over 50 years ago in temporal isolation studies, in which subjects were separated from all environmental and social time cues.98 Under normal nychthermal conditions (daytime activity and nighttime sleep), the circadian system is synchronized with the 24‐hour solar day by external triggers to which the biological clock is responsive. The normal phase relationships of the multitude of biological rhythms are achieved by an orchestrated response to the internal pacemaker.
The time cues, which are capable of entraining the biological clock to an external periodicity, have been termed “zeitgebers” (German: time giver). Zeitgebers allow the biological clock, which typically runs slightly slower than the 24‐hour day, to be reset and entrained to the 24‐hour day.99,100 Various agents have been shown to act as zeitgebers, including light, social factors, and behavioral patterns such as eating schedules and sleep–wake schedules.101,102 Social cues, the sleep–wake schedule and the rest–activity cycle, are relatively weak zeitgebers in comparison to sunlight (or electrical lighting of at least 7 000–13 000 lx).103
The phase‐shifting effect of light on the circadian timing system is secondary to its suppressing action on melatonin secretion by the pineal gland; melatonin induces sleep and depresses the core body temperature.104 Melatonin release is controlled by the suprachiasmatic nuclei (SCN) in the hypothalamus; the SCN is the primary endogenous circadian pacemaker/master biological clock.105,106
The scientific research regarding the molecular level of circadian rhythms has advanced rapidly over the last decade. Genetic researchers have identified clock genes and proteins in mammals that control the intracellular circadian clock via transcriptional modulators that allow the cellular recognition of time of day and preparation for expected stimuli. Circadian rhythm of electrical activity in SCN neurons reflects the expression of clock genes that are ultimately responsible for circadian biological rhythms. The circadian system is complex involving intercellular events and transcriptional–translational feedback loops. The expression of clock genes is not limited to the SCN, having been identified in several organs, for example, vasoactive intestinal peptide‐expressing cells have been shown to play a role in entrainment by light; circadian clocks identified in cardiomyocytes and vascular smooth muscle cells allow the cardiovascular system to anticipate diurnal variations in stimuli.107–114
PATHOBIOLOGY (CIRCADIAN RHYTHMS AND SHIFT WORK)
During night shift work, activity is out of phase with the circadian body temperature and other coupled rhythms. In addition, because individual biological rhythms re‐entrain to a time shift at different rates, each time the work schedule rotates after the time shift, the circadian system will be in a desynchronized state for a period of time. For example, sodium and potassium excretions are closely linked in a stable rhythmic environment but have significant differences in their rate of re‐entrainment to phase shifts.115 The sleep–wake rhythm adjusts faster than the body temperature rhythm, and activity re‐entrains faster than many physiologic functions.116 This desynchronization of re‐entrainment of individual biological rhythms is a reflection of differences in response times of the multiple clock genes involved in maintaining the circadian system; for example, circadian oscillators in the anterior section of the SCN adapt faster than those in the posterior region117; oscillators in the hypothalamus adjust faster than those in peripheral tissues—resulting in temporary loss, after imposed time shifts, of the normal central control of the circadian system.109
Circadian rhythms are more easily re‐entrained after a time shift if all the important zeitgebers, including the light–dark cycle and activity, are synchronously shifted, such as occurs with transmeridian flights. For shift workers, zeitgebers are shifted in a nonsynchronized manner. Knauth and Rutenfranz failed to find complete inversion of the body temperature rhythm in shift workers even after 21 consecutively worked night shifts and concluded that the circadian system never fully adapts to night work.17 Other field studies of shift workers have also found adaptation to night work to be incomplete.118–120
The circadian system is responsible for maintaining the internal sequencing and normal relationship of physiologic events and metabolism. Biological processes are thus coordinated for optional functioning of the organism. In animal studies, circadian system disruption has been shown to result in metabolic dysregulation and misalignment of physiologic parameters and biomarkers and increase progression or susceptibility to disease.121–128 The critical restorative functions of sleep are maximized during the nighttime hours by the normal phase relationship of biological rhythms. Overnight activity results in desynchrony between the circadian biological clock and the sleep–wake cycle. Studies in humans have provided increasing evidence of manifestations of circadian misalignment of sleep and wake activities during night work.129–131
Workers on night shifts experience circadian misalignment of metabolic rhythms and chronic sleep loss; both contribute to the increased risk of symptoms and diseases reported. The International Agency for Research on Cancer (IARC) has recently classified shift work as a Group 2A carcinogen—“probably carcinogenic to humans.”132,133 Other long‐term health risks have been associated with the desynchronization of circadian oscillators (biological clocks) in both central nervous and peripheral tissues, which are experienced by night and rotating shift workers. Increased risk for various medical syndromes and diseases and possible pathophysiologic mechanisms are discussed in later sections in this chapter.
Night work and sleep deprivation
Regular night work is associated with chronic sleep deprivation with sleep after the night shift typically being shortened by 2–4 hours.134,135 The sleep length of night workers is 15–20% that of day and afternoon workers, averaging 4–6 hours compared to 7–9 hours, respectively.85,136 In addition to being shorter than nighttime sleep, day sleep is poor in quality due to frequent awakenings and disruptions of the normal REM/non‐REM sleep stage pattern.137–139
The etiology of the sleep problem of night workers is multifactorial. A major determinant of sleep duration and quality is the endogenous circadian system.139–142 Job schedule requirements, domestic responsibilities, and environmental conditions may also significantly contribute to the sleep problems of night workers.143–145
Sleep deprivation is associated with increased irritability and generalized fatigue that can compromise social and domestic interactions.146–148 In addition to decreasing quality of life and general well‐being, inadequate sleep has been shown to have deleterious effects on metabolism and hormonal functions. Short sleep duration has also been associated with increased risk of obesity, diabetes, cardiovascular disease, and overall mortality.129,149–153
Sleep deprivation associated with night work negatively impacts alertness and job performance. Sleepiness and falling asleep on the job are reported by workers and have been documented by objective measures. In safety‐sensitive industries such as transportation, sleepiness related to work scheduling has had catastrophic consequences.4,154–156
DIAGNOSIS
Jet lag versus shift lag
The signs and symptoms of jet lag are an example of desynchronosis due to desynchronization of the normal phase relationships between biological rhythms within the circadian system and to the external desynchronization between the circadian system and the 24‐hour solar day–night cycle to which the biological clock is normally synchronized (entrained).
Symptoms of jet lag include (in order of frequency as typically reported by frequent jet travelers) daytime sleepiness and fatigue, difficulty sleeping at night, poor concentration, slow physical reflexes, irritability, digestive system complaints, and feelings of depression. Not surprisingly, studies of shift workers have demonstrated that shift workers experience very similar symptoms,36,157 so symptoms of “shift lag”158 are essentially the same as those of jet lag. However, symptoms of shift work‐related desynchronosis, which often go unrecognized, have more significance for long‐term health. Unlike jet‐lag symptoms, which are limited to a few days following travel, shift work‐related desynchronosis is chronic; and the ongoing malalignment between the night work and the predominantly day‐oriented social/business schedule opposes achieving circadian re‐entrainment.
Shift work intolerance
For most shift workers, shift‐lag symptoms are not debilitating, but for a significant minority, the symptoms of desynchronosis are significant. Surveys of former shift workers indicate that, for some, health complaints increase with continued shift work and become severe enough to cause the worker to give up a job, often following medical advice.159,160
Up to 20% of night or rotating shift workers have a disproportionate amount of symptoms of illness when assigned to chronobiologically poorly designed schedules.96,161 Clinical intolerance to night work has been defined by the presence and intensity of the following set of medical complaints: (i) sleep alterations, (ii) persistent fatigue (not disappearing after time off to rest), (iii) changes in behavior, (iv) digestive system problems, and (v) the regular use of sleeping pills (near pathognomonic of shift work intolerance).162,163 Askenazi et al. consider the presence of the symptoms in categories (i), (ii), and (v) to be essential to classify a worker as shift work intolerant.163
The term shift work maladaptation syndrome (SMS) has been used to refer to the typical constellation of signs and symptoms seen in shift work‐intolerant workers. Symptoms are pronounced and worsen with continued exposure to shift work. The longer the worker stays on shift work, the worse the symptoms become, and eventually the worker may be fired, quit his/her job, or be involved in an accident. Inability to adjust family/social life to the work schedule and poor schedule design may significantly contribute to the degree of intolerance.164
Circadian rhythm sleep disorders recognized in the International Classification of Sleep Disorders (ICSD‐2) include shift work disorder and jet‐lag disorder.165,166 Essential features of these two disorders include a misalignment between endogenous circadian rhythm and exogenous factors affecting the sleep period timing or duration. Criteria for the diagnosis of shift work sleep disorder (SWSD), also referred to as shift work disorder (SWD), are:
- Complaints of insomnia or excessive sleepiness temporally associated with a recurring work schedule that overlaps the usual sleep period
- Symptoms associated with the shift work schedule for at least 1 month
- Circadian and sleep‐time misalignment as demonstrated by sleep log or actigraphy for at least 7 days
- Sleep disturbance that is not explained by another sleep disorder, medical or mental disorder, or medication or substance abuse disorder
Estimates of the prevalence of shift workers with SWD are similar to that reported for shift work intolerance and SMS. Using study instruments to diagnose SWD in accordance with the ICSD‐2 criteria, Waage et al. found that the prevalence of SWD in offshore oil riggers was 23%.167 Significant differences found in subjective health complaints between workers with and without SWD included, but were not limited to, gastrointestinal (GI) complaints and depression.
Some individuals are apparently relatively asymptomatic during circadian misalignment.166 Individual factors that predispose to shift work intolerance are not fully understood. In general, age over 40–50 years, extreme morningness, and rigid sleep requirements are characteristics that have been associated with decreased tolerance for night work.22,168–171 A review of research assessing circadian factors and shift work tolerance16 suggests that (i) individuals with small amplitudes of certain circadian rhythms, for example, body temperature, may be more prone to desynchronization of rhythms when subjected to time shifts; (ii) some individuals are more likely to experience desynchronization of biological circadian rhythms unrelated to zeitgeber manipulation; and (iii) certain individuals are particularly sensitive to rhythm desynchronization manifesting clinically significant symptomatology. Roden et al.172 found that night workers with high work satisfaction tended not to lose diurnal orientation of melatonin rhythms and suggested factors other than resynchronization of the circadian systems may be important for shift work tolerance.
In addition to individual biological susceptibility, factors affecting shift work tolerance include social and family situations, working conditions, and shift work schedule arrangements. Support at home and from coworkers and supervisors at work facilitate adjustment and tolerance to shift work.173,174 Shift work disorders must be assessed in the complex framework of interrelationships of these factors.
Shift work and specific medical disorders
Gastrointestinal (GI) disorders
GI dysfunction is common in shift workers.175–177 Gastritis or other digestive disorders have been an explanation frequently given by shift workers for absenteeism and for switching to day work for health reasons.178 While some studies have not found an increased incidence of peptic ulcer disease (PUD) in shift workers, the majority of studies investigating PUD have found shift workers to at greater risk for the disease than day workers.179,180
The etiology of GI symptoms and disorders in shift workers is probably multifactorial, involving dietary factors, psychosocial stress, sleep loss, as well as circadian disruption. Night workers’ mealtimes are in conflict with the circadian rhythms of gastric acidity and gastric emptying.181,182 Shift workers may alter their diet due to lack of eating facilities available during the night shift.183–185 In their recent review of GI conditions in shift workers, Knuttson and Bogglid discuss studies suggesting that disturbance of gastrin/pepsin secretion and decreased resistance to Helicobacter pylori infection are contributing factors to PUD in shift workers.180
Metabolic syndrome, cardiovascular disease, and diabetes mellitus
Shift work involving night work has been found to be a risk factor for development of metabolic syndrome (obesity together with dyslipidemia, hypertension, and often impaired glucose tolerance due to insulin resistance). There is also evidence that abnormalities seen in metabolic syndrome are risk factors for development of both cardiovascular disease (CVD) and Type 2 diabetes mellitus (DM).186–189
Results from a cross‐sectional study of 226 female hospital nurses and 134 male workers at a manufacturing firm by Ha and Park190 were consistent with there being an association between metabolic risk factors for CVD or metabolic syndrome and shift work. Regression analyses revealed an association between shift work duration and the metabolic risk factors for cardiovascular disease. Duration of shift work was significantly associated with systolic blood pressure or cholesterol level among male workers aged 30 or more. Body mass index (BMI) was nonsignificantly associated with the duration of shift work in both male workers and female nurses ≥ 30 years of age. Recently Scheer et al. conducted a laboratory protocol study of metabolic measurements during inverted sleep–wake cycles.191 This allowed control of mealtimes. Circadian misalignment, when subjects ate and slept 12 hours out of phase from their habitual times, systematically decreased leptin, increased glucose (despite increased insulin), reversed the daily cortisol rhythm, and increased mean arterial blood pressure. In three of eight subjects, during circadian misalignment, postprandial glucose responses were in the prediabetic range. Several studies have found increased risk of hypertension, undesirable lipoprotein profiles, elevated triglyceride levels, and obesity in shift workers compared to day workers.192–200
Cardiovascular disease
Well‐designed epidemiological studies have found an increased risk of CVD associated with shift work of, on average, around 40%.201 Increased risk of stroke has also been reported in shift workers. Analysis of cohort study data from the Nurses’ Health Study looked at the risk of ischemic stroke related to the total number of years nurses had worked rotating night shifts.202 After adjusting for multiple vascular risk factors, of the 80 108 study participants available for analysis, 60% reported working at least 1 year of rotating night work. Rotating night shift work was associated with a 4% increase in risk of ischemic stroke for every 5 years of shift work exposure (hazard ratio, 1.04; 95% CI: 1.01–1.07; ptrend = 0.01). The increased risk may be limited to women with a history of shift work for ≥ 15 years.
Prospective and historical prospective studies have found a dose–response relationship between shift work exposure and cardiovascular disease. In a small historical prospective study of rotating shift workers, Knutsson et al. found an increased risk of CVD in shift workers.203 The relative risk of ischemic heart disease (IHD) increased with increasing years of exposure to shift work (6–10 years, relative risk (RR) = 2.0; 11–15 years, RR = 2.2; 16–20 years, RR = 2.8; combined RR = 1.4). This dose–response relationship continued for up to 20 years of exposure ( p < 0.05). (Twenty‐one or more years of shift work had a RR = 0.4, attributed to the healthy worker effect). Based on multiple logistic regression analysis, the association between shift work and an increased risk of IHD was independent of age and smoking habits. Subsequently, analysis of data from the ongoing prospective Nurses’ Health Study during 4 years of follow‐up of 79 109 nurses between 1988 and 1992 found evidence that ≥ 6 years of night shift work exposure may increase the RR of CHD, consistent with the previous findings in the paper mill workers.204 This large study allowed for control of more confounders, in addition to age and smoking, including body mass index (BMI), hypertension, diabetes, hypercholesterolemia, physical activity, and alcohol use. The multivariate adjusted RR was 1.21 for women reporting < 6 years and 1.5 for women reporting ≥ 6 years of rotating night work. In 2005, Karlsson et al. reported findings for a total and cause‐specific mortality in pulp and paper workers in Sweden in a historical cohort between 1952 and 2001.205 Plant records provided accurate shift work exposure information to rotating night work shifts; mortality data were obtained from the national cause of death register. A longer duration of shift work was associated with increased risk of CHD. Shift workers with > 30 years of shift work exposure had the highest risk (SRR, 1.24, 95% CI: 1.04–1.49).
Additional analysis of data from the Helsinki Heart Study by Tenkanen et al. demonstrated an interaction of shift work exposure with lifestyle factors known to increase the risk of coronary heart disease (CHD).206 For shift workers, the relative risk of CHD rose gradually with increasing numbers of adverse lifestyle factors, but for day workers, no clear dose–response pattern was found. Overall the results of this study were consistent with the notion that shift work may have a triggering effect on other lifestyle factors that can increase the risk of CHD, and that active preventive medicine intervention is particularly important for shift workers.
There is evidence that chronobiological factors independently contribute to the increased risk of CVD reported in shift workers. Young has provided a discussion of evidence supporting that notion that disruption of the normal diurnal sleep–wake activity which occurs during night work results in desynchrony of circadian clocks within the cardiovascular system interfering with its ability to anticipate the variations in neurohormonal stimuli, which may contribute to the development of cardiovascular disease in shift workers.207 Shift work has been shown in studies controlled for differences in work demands and work stress to be an independent risk factor for CVD.208
Peter et al. studied the association between shift work and cardiovascular risk factors of hypertension and elevated blood lipids, in the context of the psychosocial work environment.209 In addition to finding direct effects of shift work on cardiovascular risk, mediating effects of psychosocial work factors (effort–reward imbalances) were found, supportive of the hypothesis that a stressful work environment can act as a mediator of adverse effects of shift work on hypertension and partly on atherogenic lipid levels.
Boggild and Knutsson201 have previously presented evidence for a complex mechanistic model involving interdependent pathways involving circadian system disruption, behavioral lifestyle changes, disruption of social interactions, as well as changes in biomarkers of CVD, for example, development of dyslipidemia and hypertension. Most recently, Puttonen et al.210 reviewed current research information regarding mechanisms between shift work and CVD finding sufficient epidemiological evidence for several possible disease mechanisms. They described an updated model of interrelated pathways stemming from “circadian” stress brought on by shift work. The model describes mechanisms whereby the interaction of psychosocial, behavioral, and physiologic stress factors may contribute to cardiovascular disease outcomes (see Figure 10.1).
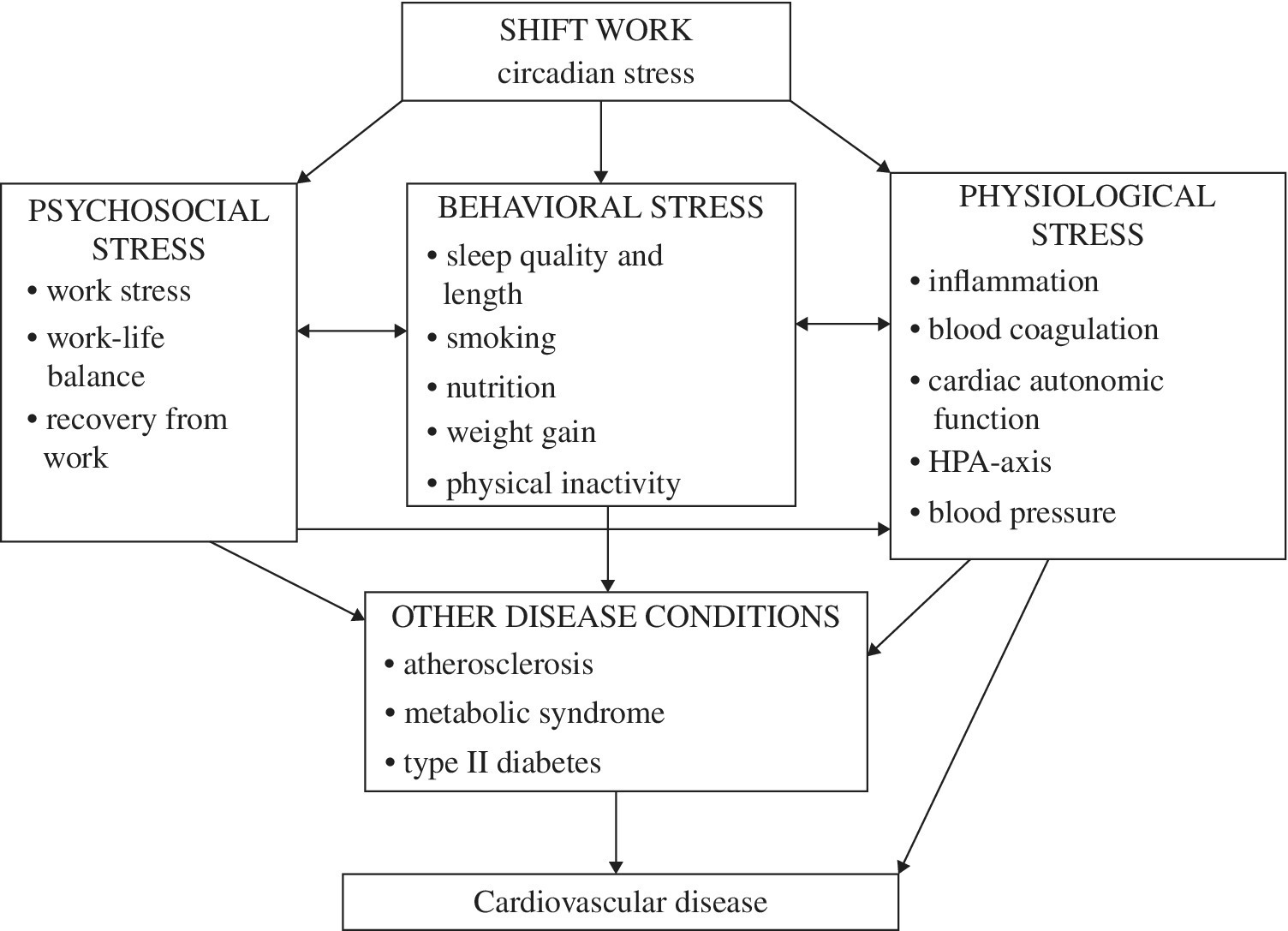
FIGURE 10.1 Model of interrelated pathways from shift work “circadian stress” to cardiovascular disease outcomes.
Used with permission from Puttonen S, Hãrmã M, Hublin C. Shift work and cardiovascular disease – pathways from circadian stress to morbidity. Scan J Work Environ Health 2010; 36(2):96–108.210
Diabetes mellitus (DM) Type 2
As noted above, changes in glucose tolerance with postprandial glucose levels in prediabetic ranges have been demonstrated in laboratory studies of circadian misalignment of sleep–wake cycle. Several studies have supported an association of night work and an increased risk of obesity and metabolic syndrome, conditions known to be related to Type 2 DM.187,188,211–214
In addition to the increased risk of CHD reported in 2005 by Karlsson et al., the risk of diabetes was also increased as the number of years of shift work exposure increased.205 Kroenke et al. analyzed data over 6 years regarding work characteristics and incidence of Type 2 diabetes mellitus (DM), from the prospective Nurses’ Health Study (NHS) II.215 They found a positive association (ptrend < 0.001) between years in rotating night shift work and diabetes, mediated by BMI. Subsequently, Pan et al. performed an updated analysis of cohort data from the Nurses’ Health Study I (1988–2008, 69 269 women) and Nurses’ Health Study II (1989–2007, 107 915) studies; statistical analysis adjusted for diabetes risk factors revealed a monotonic association with duration of rotating night shift work and increased risk of Type 2 DM in both cohorts.216 Compared to women with no shift work exposure, the hazards ratio (95% CI) for women with 1–2 years of shift work was 1.05 (1.00–1.11), for 3–9 years 1.20 (1.14–1.26), for 10–19 years 1.40 (1.30–1.51), and for ≥ 20 years 1.58 (1.43–1.74), ptrend < 0.001. After adjustment for updated BMI, there was attenuation in the association. These findings were consistent with an increased risk of Type 2 DM in women, in part related to body weight; women with > 20 years of shift work exposure still had a 44% increased risk of developing diabetes after the adjustment for BMI. In 2005, Japanese researchers reported the results from a longitudinal study of male blue‐collar and white‐collar workers over 8 years using data from annual health examinations and glycosylated hemoglobin levels.217 Relative risk of DM for two‐shift workers and three‐shift workers, compared with fixed daytime workers, was 1.73 and 1.33, respectively, after adjustment for confounding factors, but these increases were not statistically significant. Using white‐collar workers as the reference group, there was a significantly increased risk of DM for the two‐shift workers (RR = 2.01), but not for three‐shift rotators, or daytime blue‐collar workers. Another Japanese longitudinal study, a year later, of 5629 male steelworkers compared onset of DM in day workers with alternating shift workers over a 10‐year period using data from annual health examinations.218 Analysis of this data revealed an increased odds ratio (OR) for alternating shift workers compared to day workers of 1.35 (95% CI: 1.05–1.75); OR for BMI was also increased 1.28 (1.23–1.33).
Reproductive health
Preterm delivery (Ptd), small for gestational age (Sga), and low birth weight (Lbw)
McDonald and colleagues conducted a large cross‐sectional study in Montreal, in which 22 761 live births were assessed for maternal employment risk factors for LBW and preterm delivery (PTD).219 Observed‐to‐expected (O/E) ratios for LBW and PTD were increased significantly for long hours of work (46 hours/week or more), with ratios of 1.34 (p < 0.01) and 1.24 (p = 0.03), respectively. Rotating shift work was also associated with LBW, O/E = 1.38 (p < 0.01). A significant association was found in the services sector between rotating shift work and PTD, O/E = 1.88 (p < 0.01). Analysis of prematurity factors from the Montreal study, in which gestational age was allowed for in the analysis of birth weight data, done by Armstrong et al. suggested that shift work might slow fetal growth and increase the risk of PTD.220
In a retrospective cohort study, Axelsson et al. looked at the effect of night work, evening work, working irregular hours outside the period of 0645–1745, and rotating shift work compared to permanent day work on pregnancy outcome.221 They found an association between irregular working hours and an increased risk of LBW. For nonsmoking mothers with infants of birth order 2+, an increased risk of LBW was associated with irregular hours (p < 0.01) or rotating shift work (p < 0.05).
Xu and colleagues found higher proportions of preterm birth and LBW for rotating shift workers compared to regular day workers, with adjusted OR of 2.0 for PTD and 2.1 for LBW.222 Subsequently Fortier et al. reported the results of a Canadian study of 4390 women who had recently delivered live‐born singleton infants.223 No deleterious effect was found for those doing shift work (defined as occasional evening or night work), OR = 1.03. No increase in the risk of delivering a small‐for‐gestational‐age (SGA) baby was found related to working shift work or regular evenings or nights. Interestingly, long work hours (40 or more) were also not related to PTD or SGA outcomes.
Nurimen found a small association between shift work (varying types of nonregular day work including two‐ and three‐shift rotations) and SGA infants in a study comparing 1475 mothers of infants with certain structural malformations to mothers of babies without malformations.224 Mothers who had worked shift work schedules during most of their pregnancy had a slightly increased risk of having SGA infants compared to day‐working mothers (adjusted rate ratio = 1.4).
Research data is limited for permanent night work. Saurel‐Cubizolles and Kaminski did not find an association between night work and preterm delivery or low birth weight.225 However, only 4% of the female hospital employees interviewed were night workers, and some of them worked nights only occasionally. In another study, no increased risks were found for pregnancy outcome of permanent night workers; however, 91% of the night workers questioned were part‐time employees.221 Fortier et al. reported an increased OR of 1.45 for delivering preterm for women working regular evening or night shifts who continued to do so after 23 weeks of pregnancy.223
Croteau et al. conducted a case–control study by evaluating the relationship of occupational conditions during pregnancy and the increased risk of delivering a small‐for‐gestational‐age (SGA) infant and whether taking measures to eliminate these conditions decreases that risk.226 1 536 cases and 4 441 controls were selected from 43 898 women who had single live births between January 1997 and March 1999 in Québec. The women were interviewed by telephone after delivery. The risk of having an SGA infant increased with an irregular or shift work schedule alone; and there was a cumulative index of at least two of the following occupational conditions: night work, irregular or shift work schedule, standing, lifting loads, noise, and high job strain with low social support. As the number of job conditions that were not eliminated during pregnancy increased, the risk increased ( ptrend = 0.004; ORs = 1.00, 1.08, 1.28, 1.43, and 2.29 for 0, 1, 2, 3, and 4–6 conditions, respectively). Elimination of the conditions before 24 weeks of pregnancy brought the risks close to those of unexposed women.
Bonzini et al. reported the results of a systematic review of the epidemiological research between 1966 and February 2010 relating shift work and pregnancy outcomes including PTD, LBW, small for gestational age (SGA), and preeclampsia.227 Twenty‐three relevant studies were retrieved. Preterm delivery was consistently defined as birth at < 37 weeks of gestation. LBW was defined as a birth weight < 2500 g, except for one study that reported risk estimates for birth weight < 3000 g. SGA was defined as birth weight < 10th percentile for gender and gestational age, with one study including infants < 5th percentile. Pooled estimates of relative risk (RR) were calculated in random‐effects meta‐analyses. Their search identified 17 original studies that investigated the association between shift work and PTD: 10 studies concerning SGA and 6 studies concerning LBW. The pooled estimate of RR (16 studies) for PTD was 1.16 (95% CI: 1.00–1.33); when five reports of poorer methodological quality were excluded, there was no longer statistical significance. Increased RRs for LBW were also observed (RR 1.27, 95% CI: 0.93–1.74) and for SGA (RR 1.12, 95% CI: 1.03–1.22). Estimates of risk for PTD tended to reduce with inclusion of more recent studies in meta‐analyses. The authors pointed out that one explanation of this time trend could be that in most countries, precautionary legislation had been introduced, allowing pregnant workers to be assigned to nonshift work schedules, or to take earlier prenatal leave, so that fewer women continued shift work during the later stages of pregnancy. Night shifts may have been voluntarily suspended if women suspected that it could be detrimental to their pregnancy, and thus risks in women who continue to work shifts late in pregnancy might be underestimated due to a healthy pregnant worker effect (healthier women with uncomplicated pregnancies being less likely to change work schedules). Two studies were noted to support the notion that the risk of PTD associated with shift work was higher in women whose work conditions did not change during the pregnancy226,228; and a study comparing European countries found that significant associations of PTD with shift work were mainly observed in countries where long prenatal leaves were infrequent and legislative support for preventive measures was weaker.229 Ten studies were analyzed looking at the association between shift work and the risk of delivering an SGA baby, including five prospective cohort investigations. With the exception of one, the studies tended to rule out a more than moderate effect (RRs ranging from 0.8 to 1.5). The pooled risk estimate was 1.12 (95% CI: 1.03–1.22, test for heterogeneity p = 0.39). When a poor quality study was removed from the analysis, the RR was 1.10 (95% CI: 1.00–1.20). Six studies were analyzed looking at the association between shift work and risk of delivering an LBW infant. One of the three cohort studies showed a significantly elevated risk. In the pooled meta‐analysis, the combined risk estimate was 1.27 (95% CI: 0.93–1.74, test for heterogeneity p = 0.39). Overall, the authors concluded their findings suggest that the risk of preterm delivery, low birth weight, or small for gestational age from working shift work in pregnancy is small; and the available data does not make a compelling case for mandatory restrictions on shift working in pregnancy. Pooled estimates of risk for specific patterns of work schedules were not calculated because of the small number of studies with sufficient information. The authors point out that further studies are needed to address the question of whether adverse birth outcomes are related to different types of rotating work schedules or to fixed night work and suggest, as circumstances permit, pregnant women who wish to reduce their exposure to shift and night work be allowed to do so.
Fetal loss and spontaneous abortion
In a 1988 cross‐sectional analysis of 22 613 previous pregnancies, in 56 067 Montreal women, McDonald et al. found a small but significant increase in spontaneous abortion associated with working 46 hours or more per week (O/E 1.19, p < 0.01) and for rotating shift work (O/E 1.25, p < 0.01).230 Axelsson et al. studied the relation between shift work and spontaneous abortion in a cross‐sectional study of 3358 Swedish midwives.231 Hours of work information was divided into day workers, permanent night work, and two‐ or three‐shift rotators. The OR was increased for night work and three‐shift work (OR = 1.63 and 1.49, respectively) for women who worked during the first trimester. After restricting analysis to first pregnancies, a significant increased risk was also found for night work (OR = 6.89). The OR was significantly elevated for two‐shift workers (OR = 2.70), but not significantly increased for three‐shift schedules. When analysis was done separately for early and late spontaneous abortions, night work, but not rotating shift work, was associated with an elevated risk of late (beyond the 12th week of pregnancy) abortions (OR = 3.33).
In a case–control study, Infante‐Rivard et al. compared the work schedules of 331 women who had experienced pregnancy loss to 993 pregnant women matched for gestational age. For fixed evening schedules, the adjusted OR was substantially elevated at 4.17, and for fixed nights the adjusted OR was also elevated, but to a lesser degree (OR = 2.68).232 Axelsson et al. reported the results from their retrospective cohort study included a slight, but not statistically significant, increase in the risk for miscarriage (RR = 1.44; 95% CI: 0.83–2.51) associated with irregular working hours including rotating shift work.221 Axelsson and Molin found an increased miscarriage rate of borderline significance in shift workers (OR = 2.07; 95% CI: 0.98–4.34).233 In a retrospective cohort study of laboratory workers, a significantly increased risk of miscarriage was reported for shift workers (RR = 3.2; 95% CI: 1.36–7.47).234 Two other studies, as reviewed by Nurimen, also found an increased risk of miscarriage in shift workers.235–237
Zhu et al. reported the results of a prospective cohort study using the Danish National Birth Cohort. Fixed night work was associated with fetal loss.238 Over 33 000 pregnancies were identified in day workers and over 8 000 pregnancies in shift workers between 1998 and 2001. Fetal loss included spontaneous abortion and stillbirth. The hazard ratios for fetal loss compared to day workers, adjusted for potential confounders, was increased for fixed night shift work: for all fetal loss, HR = 1.85 (CI: 1.00–3.42); for spontaneous abortion, HR = 1.81 (0.88–3.72); and for stillbirth, HR = 1.92 (0.59–6.24). No high risk was found for rotating shifts.
Two case–control studies of spontaneous abortion and shift work did not find an increased risk.239,240 As pointed out by Nurimen, in these two negative studies, rotating shifts were part of broad‐based exposure categories and were not analyzed explicitly.237
Bonde et al. recently conducted a systematic review and meta‐analysis of published studies between 1996 and 2012, which looked at the risk of miscarriage related to shift work, working hours, and physical stressors.241 Working fixed nights was associated with a moderately increased risk of miscarriage (pooled RR 1.51; 95% CI: 1.27–1.78), as determined by the fixed model OR in five better quality studies reporting RRs for fixed night work compared with day work. Pooled fixed meta‐OR for studies (n = 7) reporting risk of miscarriage for three‐shift schedules or evening/night shifts compared to day or two‐shift workers was associated with a small increased risk (OR 1.12; 95% CI: 0.96–1.42).
Shift work and subfecundity
Ahlborg et al. studied subfertility in Swedish midwives in a survey sent to 3985 midwives (84% response rate).242 Those who worked rotating shifts or permanent nights had decreased fertility compared to day workers. Bisanti et al. surveyed women during prenatal clinic visits or after giving birth to determine the time of unprotected intercourse prior to conception.243 Rotating shift work was associated with an increased risk of subfecundity (OR = 2.0). Spinelli et al. interviewed new mothers who had delivered within the preceding week. After adjustment for confounders, fertility was not significantly reduced in shift‐working mothers [fecundability (conception rate) ratio = 0.9; p > 0.10].244 A Japanese questionnaire study on working conditions found pregnancy rates lower for women doing shift work (10.0%) compared to day workers (18.1%) (p < 0.01).236
Tuntiseranee et al. recently reported no association between shift work and subfecundity, but did find long hours of work to increase time to pregnancy.245 This effect was greatest when both partners were working over 70 hours/week (OR = 4.1 in primigravid women and 2.0 for all pregnant subjects). There was no increased OR when only the male partner worked long hours.
Zhu et al. analyzed data from the Danish National Birth Cohort (DNBC), including 39 913 pregnant women who were enrolled from March 1, 1998, to May 1, 2000, to examine whether shift work was associated with reduced fecundity as estimated by time to pregnancy (TTP).246 Data on job characteristics and TTP (0–2, 3–5, 6–12, and > 12 months) were used for 17 531 daytime workers and 3 907 shift workers who had planned pregnancies. Fecundity odds ratios were calculated (OR > 1 indicating a shorter TTP). Fixed evening workers and fixed night workers had a longer TTP. Compared with daytime workers, the adjusted ORs were 0.80 (95% CI: 0.70–0.92) for fixed evening workers, 0.80 (95% CI: 0.63–1.00) for fixed night workers, 0.99 (95% CI: 0.91–1.07) for rotating shift (without night) workers, and 1.05 (95% CI: 0.97–1.14) for rotating shift (with night) workers. The proportions of unplanned pregnancies and contraceptive failures were higher among fixed evening and fixed night workers. The researchers concluded that the slightly reduced fecundity among fixed evening workers and fixed night workers may be mediated by pregnancy planning bias or differential options for sexual contacts; and there was no unequivocal evidence of a causal association between shift work and subfecundity.
Cancer
Taylor and Pokock were the first to report an increased risk of cancer in shift workers in a 1972 mortality study.247 A 1990 mortality study by Raffnsson and Gunnarsdottir also reported an increased risk of cancer associated with night work.248
A 1996 Norwegian nested case–control study of breast cancer incidence in radio and telegraph operators, working at sea, followed 2619 mostly postmenopausal women for about 30 years.249 Cancer cases were identified from National Norwegian Cancer Registry. Job histories of work on ships were collected for shift work, as well as travel through time zones classified for each ship mentioned in the job histories to define shift work. After controlling for duration of employment and after adjustment for age and year of birth of first child, there was an excess risk of breast cancer associated with exposure to night shift work (SIR, 1.5; 95% CI: 1.1–2.0). Breast cancer risk was highest in women with the highest cumulative shift work history compared to no shift work, RR = 6.1(95% CI: 1.5–24.2). There appeared to be an increased risk of breast cancer in women ≥ 50 years of age with increasing cumulative exposure to shift work compared to no shift work (low exposure 0–3.1 years, adjusted for duration of employment, RR = 3.2, 95% CI: 0.6–17.3; high exposure 3.1–20.7 years, adjusted for duration of employment, RR = 4.3, 95% CI: 0.7–26.0; ptrend = 0.13).
Subsequent studies looking at the risk of breast cancer in shift workers followed between 2001 and 2007 includes the following:
- A 2001 case–control study by Davis et al. looked at sleep patterns and habits, lighting in bedrooms, and shift work history in 813 cancer cases (identified by the Cancer Surveillance System of Seattle) and 793 age‐matched controls.250 Information about sleeping habits, light‐at‐night exposure, and lifetime occupational history was obtained from in‐person interviews. Night work was defined as at least one “graveyard” (beginning after 19:00 and ending before 09:00) shift/week in the 10 year prior to diagnosis. After adjusting for parity, family history of breast cancer, contraceptive use, and history of hormone replacement therapy, there were an increased risk for breast cancer associated with graveyard shift work (OR 1.6, 95% CI: 1.0–2.5), a 13% increase in risk for additional year of having worked at least one such shift per week (OR 1.13; 95% CI: 1.01–1.27), and a 14% increase risk in women reporting, for any reason, frequently not sleeping during the middle of the night (when melatonin levels are typically highest) (OR, 1.14; 95% CI: 1.01–1.28, per night/week; OR 1.7 for the group with at least 2.6 nights/week of interrupted sleep).
- A population‐based case–control study nested in a cohort of all female employees in Denmark based on national pension fund data found that women who worked at night for at least 6 months had a 50% greater risk for breast cancer compared to age‐matched controls (OR, 1.5; 95% CI: 1.2–1.7).251 A positive trend was noted with increasing duration of work at night. 7035 women with incident breast cancer were identified in the Danish Cancer Registry. Individual employment histories were reconstructed using information from the pension fund. Night work definition was based on information from the nationwide interview survey of living and working environment conditions; trades with at least 60% of female responders working at night were considered to have a predominant nighttime schedule; trades with < 40% reporting nighttime schedules were classified as day workers. The RR of breast cancer was 1.5 (95% CI: 1.3–1.7; 434 cases) among women who worked in night work trades at least a half year, at least 5 years before diagnosis. Analysis was done after controlling for age, social class, parity, and age at birth of first child and last child. For the subgroup of women with more than 6 years predominantly working at night, the RR was 1.7 (95% CI: 1.3–1.7; 117 cases). In a subanalysis of nurses (41% having predominantly night work), the RR of breast cancer was also significantly evaluated (RR, 1.3; 95% CI: 1.1–1.4).252
- Two large prospective cohort studies of night shift work and breast cancer risk used data from the Nurses’ Health Study cohorts (Nurses’ Health Study I and Nurses’ Health Study II).253,254 The Nurses’ Health Study began in 1976, when 121 701 registered nurses in the United States, 30–55 years of age, were enrolled and completed a questionnaires about their health status, medical history, and known or suspected risk factors for cancer. Since baseline, questionnaires have been mailed biannually with the exception of lifetime history of night work (total number years working rotating night shifts at least three nights per month), which was assessed in 1988. In 2001 Schernhammer et al. reported the results of follow‐up of 78 562 women in the Nurses’ Health Study who answered the 1988 questionnaire and were cancer‐free at baseline over 10 years (1988–1998).253 2441 incident breast cancer cases were documented during that time. After adjustment for known breast cancer risk factors, their analysis revealed a significantly increased risk of breast cancer in women who had worked ≥ 30 years of rotating night work compared to women who never worked rotating night shifts (RR 1.36; 95% CI: 1.04–1.78). The risk increased with numbers of years of shift work (test for trend, p = 0.02). The similarly designed Nurses’ Health Study II study was established in 1989, when the prospective cohort of 116 671 registered female nurses (no overlap with Nurses’ Health Study) were enrolled and completed the baseline questionnaire, including questions on total months worked on rotating night shifts at least three nights per month. Information was updated in 1991, 1993, 1997, and 2001. In 2006, Schernhammer et al. reported, in 115 022 mostly premenopausal women, an elevated breast cancer risk of 1.79 (95% CI: 1.06–3.01; p = 0.65) among those who worked ≥ 20 years of rotating night shift work compared with women with no work history of rotating night shifts.254
- In 2006, Lie et al.255 reported the results of a nested case–control study within a cohort of 44 835 Norwegian nurses. Using the nationwide cancer registry, 537 breast cancer diagnoses during 1960–1982 were identified. Work history was reconstructed from the nurses’ registry (self‐report of work history periodically updated) and census information. It was assumed that nurses doing clinical work at infirmaries worked at nights (with the exception of physiotherapy and outpatient departments); it was assumed that work sites other than infirmaries involved day work only. An association was found between duration of night work and breast cancer risk (ptrend = 0.01). The RR associated with > 30 years of night work was 2.21.
- In 2006, O’Leary et al. published results of their case–control study in New York—the Electromagnetic Fields and Breast Cancer on Long Island Study (EBCLIS).256 They did not observe an association between night work and breast cancer risk. To evaluate the effects of electromagnetic frequency, women from within this case–control study were selected according to their degree of residential stability (EBCLIS component). EBCLIS comprised 576 breast cancer cases and 585 matched population‐based controls. Occupational history was obtained in personal interviews as well as history of residential light‐at‐night exposures. Shift work was defined as “ever” working in at least one job during the past 15 years that included evening shifts (could start in the afternoon and end as late as 02:00) and overnight shifts (starting as early as 19:00 and continuing until the next morning). Results were adjusted for age, parity, education, family history of breast cancer, and history of benign breast disease.
- Results of a retrospective registry‐based cohort study were reported in 2007 comprising all 1 148 661 Swedish women active in the workforce per 1960 and 1970 census reports.257 Workers were followed up for breast cancer morbidity using the Swedish Cancer Registry. Annual surveys of living conditions among 46 438 randomly selected subjects who participated in a personal interview were used for assessing night work. Shift workers were defined as those who reported their workplace had a rotating schedule with at least three possible shifts per day or had any night work hours (between 01:00 and 04:00) at least 1 day during the week preceding the interview. Participants working in job titles and industry combinations (from census data regarding workers’ industry and socioeconomic status) with at least 40% shift work were classified as shift workers. The reference group comprised subjects in occupation–industry combinations in which < 30% reported being shift workers. In analyses using the census information for the definition of exposure, no increase in risk was found for women classified as shift workers.
In October 2007, a panel of 24 scientists who reviewed the epidemiological, experimental, and mechanistic research for International Agency for research on Cancer (IARC) concluded that there is “limited evidence in humans for the carcinogenicity of shift work that involves night work”; “sufficient evidence in experimental animals for the carcinogenicity of light during the daily dark period (biological night)”; and “shiftwork that involves circadian disruption is probably carcinogenic to humans (Group 2A).”132 The research included in the IARC panel analysis is reviewed in the subsequent IARC Monograph published in 2010.258 The epidemiological data was primarily based on the seven breast cancer studies reviewed above.249–257 The IARC panel discussion noted that each of the studies used different definitions of shift work; that six of the eight breast cancer studies, including the two prospective cohort studies from the Nurses’ Health Study, “consistently pointed towards a modestly increased risk of breast cancer among long‐term employees who performed night shiftwork, defined in different ways”; and that one of the two negative studies had important limitations in design. Other points included the following: there were a relatively limited number of studies, most focusing on the single profession of nursing; there was some potential for confounding by unknown risk factors, and inconsistent and inaccurate exposure assessments of shift work, which may have biased the results toward the null; the evidence for an association with breast cancer and night work was consistent in the studies specifically designed to address this question; and studies of the incidence of breast cancer in female flight cabin crews provided additional support.
The conclusions of the IARC working group drew attention to the evidence of an association of night work with risk for developing cancer, prompting additional reviews, meta‐analysis of the existing data of the breast cancer studies, and new research.
Costa et al.259 reviewed the epidemiologic studies included in the IARC report and a recent case–control study, published in 2010, using data from the German Gene–Environment Interaction and Breast Cancer (GENICA) study260 and concluded that the loose definitions of exposure to night work in the published studies did not allow for proper assessment of the risk of cancer associated with circadian disruption. In the GENICA study, shift work exposure (defined as working the full period between 24:00 and 05:00) was based on personal interview information; cases experience about 800 night shifts compared to 300 among controls. Long‐term night work, ≥ 20 years, was associated with a statistically nonsignificant increase in breast cancer risk, OR 2.48 (95% CI: 0.62–9.99). Limitations of the GENICA study include low prevalence of night work, especially long‐term exposure, and the retrospective assessment of work history.
In 2011 Hansen and Stevens published results from a Danish case–control study of breast cancer in nurses (identified from the national cancer registry) and collected detailed information on lifetime shift work history.261 Overall, nurses working rotating shifts after midnight had significantly increased risk of developing breast cancer compared to permanent day‐working nurses (OR 1.8, 95% CI: 1.2–2.8). The greatest increased risk was seen associated with long‐term day–night rotating shift schedules (OR 2.6, 95% CI: 1.8–3.8).
Three meta‐analysis of breast cancer studies in shift workers were published in 2013. Kamdar et al. identified 15 studies published by 2012 that met their inclusion criteria.262 Using a random‐effects model, they found the pooled RR for women with ever night shift exposure to be 1.21 (95% CI: 1.00–1.27, p = 0.056). For long‐term night work, defined as ≥ 8 years, RR was 1.04 (95% CI: 0.92–1.18). Subgroup analysis for nurses with long‐term exposure was reported to suggest an increased risk of breast cancer. Jia et al. reported, in their analysis of 13 studies through 2012, a pooled adjusted RR of 1.20 (95% CI: 1.08–1.33) for risk of breast cancer associated with ever working versus never working night shift work.263 Later, in August 2013, Wang et al. published results of their meta‐analysis of cohort and case–control studies published in May 2013.264 They summarized evidence by frequency and duration of cumulative exposure to night shift work and used a dose–response regression model to evaluate the relationship between exposure to night work and risk of breast cancer. Ten studies were included. The pooled adjusted RR for association between “ever exposed to night shift work” and breast cancer was 1.19 (95% CI: 1.05–1.35). Analysis of the dose–response relationship revealed a 3% increase in risk of breast cancer for every 5 years of exposure to night work (pooled RR 1.03 (95% CI: 1.01–1.05) pheterogeneity < 0.001). This meta‐analysis also suggested that an increase of 500‐night shifts increases the RR 13%.
Also, in August 2013, results of an Australian case–control study were published by Fritschi et al. Women diagnosed with breast cancer between 2009 and 2011 were identified from the Western Australian Cancer Registry (mandatory reporting of invasive cancer); randomly selected controls were from the Western Australian electoral roll (compulsory adult enrollment). 1202 cases and 1785 controls completed the study.265 Questionnaires followed by telephone interviews were used to obtain work history. A statistically significant association was found between breast cancer and phase shift caused by night work (OR 1.22, 95% CI: 1.01–1.47). There was evidence of a dose–response relationship, but no duration–response relationship.
In 2014, Hansen looked at the influence of night shift work on the survival of breast cancer.266 Women participating in two separate Danish case–control studies of breast cancer were interviewed by phone to obtaining information including night shift work history and known risk factors for breast cancer. The national cause of death register was used to follow up for death. Time‐to‐event analyses were done using Cox proportional hazards models and Kaplan–Meier survival plots. There was a significant tendency of decreasing survival in fixed and rotating night shift workers compared to day workers and by increasing years of prior nondaytime work (p = 0.04).
The current research provides support for biological plausibility and potential mechanisms for an association of long‐term exposure to night shift work with increased risk of cancer. For example, animal studies have demonstrated that the development of neoplasias has been associated with disruption of the rhythmicity of the circadian period 2 gene.267 Additional findings have been reviewed in detail by Costa et al.259 and in the IARC monograph258 including alteration of endocrine rhythms and immune system function due to sleep deprivation, nocturnal suppression of melatonin by light at night (LAN), and circadian disruption at the cellular and molecular level. The phase shifts experienced by night and rotating shift workers result in molecular level desynchronization in circadian oscillators in peripheral tissues, as well as the CNS, which provides a pathophysiological basis for the reported increased risk for shift workers of developing cancers. Immune responses may be suppressed via circadian disruption of the central oscillator in the SCN. Immune suppression reflects multiple events including decreased number of natural killer (NK) cells, cytotoxic lymphocytes, and proinflammatory cytokines, interferon, and tumor necrosis factor (TNF). Suppression of melatonin may lead to estrogen elevation with clinical significance in endocrine‐dependent tumors; melatonin counteracts the enhancing effects of estradiol on breast cancer cell activity; melatonin can act as a free radical scavenger by activation of antioxidative pathways and has antiproliferative effects on human cancer cells in vitro. Melatonin has been shown to be oncostatic in certain tumor cells; exogenous melatonin has been shown to have anti‐initiating and oncostatic activity on chemically induced cancers.268–272 Clinical trials in humans have also demonstrated favorable responses to melatonin in cancer patients, alone or in combination with standard treatment regimens.273 Chronobiologists have also noted the effects of loss of normal diurnal rhythm in cortisol levels associated with sleep disruption which support a mechanistic shift work role in the observed association between shift work and increased risk of breast cancer.274 The circadian clock may act as a tumor suppressor at systemic and molecular levels via clock‐controlled genes involved in controlling cell cycles and tumor suppressor genes. Processes relevant to carcinogenesis, for example, DNA repair and cellular proliferation, have circadian variation.
In the very recent study of molecular mechanisms, Liu et al. followed up on previous findings indicating that long‐term exposure to LAN in night workers may result in dysregulated patterns of methylation, inducing alteration of microRNAs relevant to cancer.275 They found a 49% increase in miR‐34b promotor methylation in shift workers—with results suggesting that long‐term shift work may increase the risk of breast cancer via methylation‐based suppression of miR‐34b, with a related reduction in immune‐mediated antitumor capacity.
In another recent study, Monsees et al. hypothesized that circadian genes influence breast cancer risk in women and investigated gene–environment interactions in nurses working rotating shift work.276 Using blood samples collected from > 29 000 cancer‐free participants in the Nurses’ Health Study II study between 1996 and 1999, the researchers looked at variants of genes relevant to the circadian system in women in the Nurses’ Health Study II cohort. They tested for associations between certain genotypes and breast cancer risk and potential interactions between genotype and rotating shift work in a subset. Data was available for cumulative exposure to rotating shift work prior to the last blood sample collection on the subset (438 cases; 880 controls). Analysis of interactions between clock genes and shift work was limited to incident cases as diagnosis of cancer may influence work schedule. The researchers tagged genes with known key roles in circadian regulation. The results provided evidence that a coding single‐nucleotide polymorphism (SNP) in NPAS2 (Ala394Thr;rs2305160) may modify the influence of shift work on risk of breast cancer. None of the selected genetic variants were significantly associated with breast cancer risk; however rs23051560 (Ala394Thr), in the largest circadian gene, was most strongly associated with breast cancer risk (p = 0.0005). Among women with minimal (< 2 years) exposure to rotating night work, NPAS2Ala394Thr variant Thr genotypes (Ala/Thr and Thr/Thr) were associated with significantly less risk of breast cancer compared to the Ala/Ala genotype. Women homozygous for the minor allele, Thr/Thr genotype, who had ≥ 2 years of rotating shift work exposure had 2.83 times higher risk for breast cancer compared to women of the same genotype with < 2 years exposure (95% CI: 1.47–5.56). In their discussion of their findings, the authors pointed out that Asian populations have lower frequencies of Thr compared to Europeans, and if the interaction observed is causal, a weaker effect of shift work would be expected in populations with a lower prevalence of Thr genotype at Ala394Thr. They also noted that the prospective Shanghai Women’s Health Study observed no association between lifetime history of shift work and risk of breast cancer and that genetic interactions modifying the effects of night work may explain the lack of association of shift work with risk of breast cancer observed in the Asian population.277
Overall there are increasing epidemiologic support and molecular research evidence that a history of working several years of shift work involving nights increases a woman’s risk of developing breast cancer and the magnitude of the risk is similar to other known risk factors for breast cancer. There is also some epidemiological evidence of increased risk of developing other cancers in women. In the Nurses’ Health Study, the RR for colorectal cancer in women working rotating nights 15 years or more compared to women who had never worked rotating night shifts was 1.35 (95% CI: 1.03–1.77; ptrend = 0.04).278 602 incident cases of colorectal cancer were documented among 78 586 women followed from 1988 to 1998. Study participants were asked how many years in total they had worked in rotating night shifts, at least three nights per month in addition to working days or evenings. Working rotating night shifts for 20 or more years has also been found to be associated with a significant increase in risk for endometrial cancer, especially in obese women (multivariate relative risk = 1.47 (95% CI: 1.03–1.14)).279 Stratified analysis of obese rotating night workers found a doubled risk of endometrial cancer (MVRR = 2.09, 95% CI: 1.24–3.52). A nonsignificant increase was found in obese women who did not do rotating night work.
There are also epidemiological studies implicating that shift work is a risk factor for cancer in men. Limitations of the epidemiological studies available in 2010, looking at shift work‐related circadian disruption and the risk of cancer in men and women, were critically reviewed by Costa et al.259 The issues highlighted were mostly related to the diversity of shift work ascertainment methods, varying exposure durations, different definitions of exposure windows, assessment of changes in night shift schedules during the working lifetime, and adjustment for confounders. Parent et al. specifically addressed some of these issues in their recent report of a population‐based case–control study of night work and cancer in men conducted in Canada, in the 1980s, by (i) including the period between 1:00 and 2:00 a.m. in the definition of night work precluding evening shift work being considered night work exposure; (ii) calculating a cumulative index of night work exposure; and (iii) incorporating adequate analytic control for several potential confounders.280 Cases were male patients, 35–70 years old, diagnosed at any of the 18 major Montreal hospitals with incident, pathologically confirmed cancer. Participation of all large hospitals in this area ensured a nearly complete (97%) population‐based identification of cases. Between 1979 and 1985, 4576 eligible cancer patients were accrued; 3730 patients (82%) were successfully interviewed. Controls were recruited from the general population using electoral lists (a nearly complete list of voting age citizens in Quebec). Interviews were conducted from 1979 to 1986. For each job held, history of shift work was obtained including the start and finish times of shifts. The results suggest that night work may increase cancer risk at several anatomic sites in men. Compared with men who never worked at night, the adjusted ORs among men who ever worked at night were 1.76 (95% confidence interval (CI): 1.25, 2.47) for lung cancer, 2.03 (95% CI: 1.43, 2.89) for colon cancer, 1.74 (95% CI: 1.22, 2.49) for bladder cancer, 2.77 (95% CI: 1.96, 3.92) for prostate cancer, 2.09 (95% CI: 1.40, 3.14) for rectal cancer, 2.27 (95% CI: 1.24, 4.15) for pancreatic cancer, and 2.31 (95% CI: 1.48, 3.61) for non‐Hodgkin’s lymphoma. The significant increased risks of cancer in the prostate and colon were irrespective of the timing of night work. There was an effect of long‐term night work (> 10 years) for cancers of the prostate, colon, and bladder and for non‐Hodgkin’s lymphoma; the evidence was weaker for other cancer types. There were too few men in the study who had worked in jobs involving night work for over 20 years to conduct analyses for longer exposure. As the authors point out, the findings of increased risks across a wide array of cancer types suggest a common underlying mechanism, with the anticancer effects of melatonin being the most often evoked theory.
Lahti et al. reported the results of a retrospective cohort study in 2008 showing a modest increased risk of non‐Hodgkin’s lymphoma in men with high shift work exposure based on a job‐exposure matrix.281 Cancer cases were identified from the Finnish Cancer Registry. 6307 cases were identified diagnosed between 1971 and 1995. Exposure to night work for 10 years was associated with an RR of 1.10 (95% CI: 1.03–1.19).
Three of the four studies looking at the risk of prostate cancer in shift workers have found an elevated risk for prostate cancer associated with shift work exposure. In 2006, Kubo et al. reported the results of a prospective cohort study of Japanese shift workers.282 Individual questionnaires were used to collect shift work history. The RR for fixed night work was 2.3 (95% CI: 0.6–9.2) and 3.0 for rotating shifts (95% CI: 1.2–7.7). The following year, Conlon et al. reported the results of a Canadian case–control study.283 Study participants completed a questionnaire including what was their “usual work time (daytime, evening/nightshift, rotating shift, other).” OR for rotating shift workers with ≤7 years’ shift work exposure was 1.44 (95% CI: 1.10–1.87); for 7.1–22 years, 1.14 (95% CI: 0.86–1.52); for 22.1–34 years, 0.93 (95% CI: 0.70–1.23); and for > 34 years, 1.30 (95% CI: 0.97–1.74). The Parent et al. study, discussed above, also found a significantly increased risk of prostate cancer.281 A retrospective cohort study by Schwartzbaum et al., which included job sectors with 40% rotating shift workers, did not identify a statistically significant elevated risk, reporting an SIR of 1.04 for shift work in 1970 (CI: 0.99–1.10) and 1.02 for shift work in 1960 and 1970 (CI: 0.95–1.10).257
Flynn‐Evans et al. reported a strong positive association with shift work and elevated prostate‐specific antigen (PSA) level, supporting the hypotheses that sleep or circadian disruption is associated with elevated PSA and that shift‐working men are at some increased risk of developing prostate cancer.284 Shift work and PSA test data was obtained as part of the National Health and Nutrition Examination Survey (NHANES) study. Data was combined from three NHANES surveys (2005–2010) to obtain current work schedule among employed men aged 40–65 years with no prior history of cancer (except nonmelanoma skin cancer). Men who reported working regular night shifts or rotating shifts were considered shift workers. Using multivariable logistic regression models, PSA levels were compared among current shift workers and nonshift workers. Statistical analysis revealed a statistically significant age‐adjusted association between current shift work and elevated PSA ≥ 4.0 ng/mL (odds ratio = 2.48, 95% CI: 1.08–5.70; p = 0.03). The confounder‐adjusted odds ratio was 2.62 (95% CI: 1.16–5.95; p = 0.02). The confounder‐adjusted odds ratio for those with total PSA ≥ 4.0 ng/mL and free PSA ≤ 25% was 3.13 (95% CI: 1.38–7.09; p = 0.01).
Aggravation or exacerbation of medical disorders
Circadian variation in individual physiological parameters is discussed in the preceding section “Normal Physiology.” Symptom manifestation of numerous medical conditions and response to prescribed medications also follow circadian rhythms and may be altered by the reversal of the sleep‐activity period experienced when doing night work and the associated phase shifts in various metabolic parameters. Differences in the rate of absorption, distribution, and elimination of a drug can be seen depending on the timing of the drug administration. There are a physiological coupling between the circadian activity–rest cycle and the chronopharmacological mechanism and a related predictable time‐of‐day variation with respect to best time for administration of some medications. Chronotherapeutic schedules used in the treatment of human cancer are based on predictable circadian rhythms in tolerance to chemotherapy and tumor responsiveness; and changing the time of day of ingestion of certain blood pressure‐lowering medications alters the effectiveness on blood pressure control.285–287 Thorough reviews of these medical chronobiology and chronopharmacology issues have been provided in reviews by Smolensky and D’ALonzo288 and Smolensky et al.289
Diabetes mellitus
Regularity of timing of meals and administration of antiglycemic medication are important in the management of diabetes mellitus. The type and amount of food eaten are also essential elements in the control of glucose levels. Plasma glucose levels in diabetics are higher in the morning than at night, and studies have shown that insulin response to a glycemic stimulus follows a circadian rhythm and that blood glucose control may vary with the time of day. Diurnal variation has been demonstrated in the effect of the type of meal (simple vs. carbohydrates) on blood glucose control in insulin‐dependent diabetics. Gastric emptying response limits the rate of digestion and absorption of nutrients, and there is a circadian rhythm to the gastric emptying response; diets that slow the gastrointestinal digestion of carbohydrates have been used successfully in controlling glucose levels in diabetic patients. Studies in rats suggest that the timing of meals, relative to the gastric emptying response, may induce changes in the number and affinity of insulin receptors and thus affect responsiveness to insulin.182,290–294
Shift work may interfere with the timing and type of meals eaten by workers, which make compliance with dietary recommendations more difficult. Circadian rhythm disruption inherent in night work may alter the response to pharmacological control of blood glucose levels. Provision for counseling and medical surveillance in cooperation with recommendations from treating providers is essential for night workers with diabetes. However an a priori restriction for diabetics from working shift work is not supported by the available medical evidence. A study by Poole et al. of automobile workers found that insulin‐dependent DM is not necessarily a contraindication for night work, with the availability of newer insulin regimens and glucose monitoring meters.295 However the type of rotation did adversely impact control; very rapid rotations were not used. Compared to day workers, glucose control for insulin‐dependent diabetic workers was not significantly better for day workers than for shift workers. More slowly rotating shifts (2‐week rotations) were associated with better control than weekly rotating shifts.
Epilepsy
There are circadian time‐of‐day patterns in the frequency of epileptic seizure occurrences. Partial seizures have been found to demonstrate different peaks in frequency depending on what lobe the seizure arises from. Temporal lobe seizures are more likely to occur in the late afternoon and early evening.296,297
The onset of seizures has also been related to changes in corticosteroid levels, which become desynchronized with phase inversions of the sleep–wake cycle.298 Recently van Campen et al. studied the relationship between circadian rhythm of cortisol and time of epileptic seizure occurrence.299 They used a systematic literature search to identify relevant reports with 24‐hour data of seizure occurrence and then combined and related the data to a standard circadian cortisol rhythm. The occurrence of generalized seizures and focal seizures originating from the parietal lobe in particular followed the circadian rhythm of cortisol with a sharp rise in the early morning, followed by a gradual decline. The results support the hypothesis that changes in cortisol (stress hormone) level influence the occurrence of epileptic seizures.
Sleep deprivation techniques are used to provoke epileptic electroencephalographic discharges in patients under diagnostic evaluation for epilepsy. Sleep deprivation of 24–26 hours can cause electroencephalogram (EEG) activation in epileptics. In patients in whom this has been observed, seizure episodes have been related to loss of sleep.300,301 Bergonzi et al. found that REM sleep stage deprivation activates EEG epileptic activity and sometimes clinical seizures in persons with generalized or focal epilepsy.302
Tarp’s review concluded that a lack of sleep leads to an increased frequency of seizures in some epileptics.303 Kendris et al. recently found that primary generalized epilepsy patients were five times more likely to have a late (evening‐type) chronotype compared to healthy controls and generalized epilepsy patients were more likely to be evening types compared to study participants with focal epilepsy or those without epilepsy.304 These researches point out that late chronotype is a risk factor for circadian misalignment which can impact seizure control in patients with epilepsy and that preventing sleep deprivation and integrating chronotype evaluations and chronotherapy are important in comprehensive care of patients with epilepsy.
Asthma
Circadian rhythms of airway resistance have been demonstrated by pulmonary function testing in both normal and asthmatic subjects. Dyspnea in symptomatic asthmatics is greatest, and peak expiratory flow rate (PEFR) lowest, in the early morning, with the reverse occurring in the early afternoon. A threefold difference in amplitude has been found for dyspnea, and the amplitude for PEFR was found to be 20% of the mesor.305–307
Nonnocturnal asthmatic subjects showed an increase frequency and severity of response to environmental exposures when challenged in the evening in comparison to a morning exposure. A relationship between the nocturnal propensity for respiratory symptoms in asthmatics and circadian rhythms is demonstrated by (i) coincidentally decreased levels of circulating cortisol or epinephrine, or urinary levels of adrenocorticosteroid hormones and catecholamines; (ii) decreased dynamic lung compliance; (iii) decreased airway patency; and (iv) increased bronchial reactivity to allergen triggers such as house dust and to histamine and acetylcholine.308–310
The pharmacokinetics and effectiveness of bronchodilators vary with the time of administration in a circadian fashion. Chronobiological considerations have been used to optimize the effectiveness of bronchodilators and steroids in controlling asthma.311–313 The time of administration of steroidal anti‐inflammatory and beta‐agonist (bronchodilator) medications can be critical in achieving control of respiratory symptoms and minimizing side effects. Treatment schedules are however based on regular diurnal activity by the patient. For regular night workers, it may be possible to adjust the recommended dosing schedule to fit the reversal in the activity–sleep cycle, if the worker is able to maintain a consistent activity–rest pattern. Chronotherapeutic applications may be limited and consistent control of symptoms difficult in an individual engaged in rotating shift work with irregular sleep–wake schedules. On the other hand, it has been suggested that for unmanageable asthmatics, irregular schedules might actually reduce bronchospastic episodes by decreasing the amplitude of the PEFR rhythm.314 It remains to be determined to what extent the severity of asthma is affected by being awake and active through the night and sleeping during the day. Research is needed for studying the effects of shift work on the control of asthmatic symptoms and on effectiveness of chronotherapeutic interventions for asthmatic shift workers.
Psychosocial disruption and depression
Shift work increases social and family stress. Night work, evening work, and irregular schedules often conflict with family life and social events. Social and family life factors may interfere with good sleep hygiene and other chronobiological coping strategies. This disruption may contribute significantly to the shift work intolerance seen in individuals with desynchronosis which often includes depressive symptomatology.96,161,315,316
Shift systems that usually keep the worker away from home during the afternoon and evening are most disruptive for family interaction.317 The significance of the stress that shift work may impose on marriages is reflected in the results of studies reporting higher divorce rates for shift workers and for shift work dropouts than for day workers.318–320
Smith and Folkard surveyed the wives of nuclear power plant operators concerning the impact of their husbands’ shift work on themselves and their family.321 Over 70% believed there were occasional or frequent marital conflicts related to shift work. Approximately one third of the spouses had tried to persuade their husbands to quit shift work. Wyatt and Marriott reported that night workers blamed their shift schedule for broken or strained marriages.322 These research findings are consistent with the observation of Mott and colleagues who pointed out in their extensive studies of the effects of shift work on families that “… the schedules of husbands and wives are so closely interwoven that it would be a serious mistake to consider the effects of shift work upon only one of the marriage partners.”323
Although it is recognized that shift workers report decreased well‐being related to shift work schedules, the role shift work may have in causing specific psychiatric illnesses is unclear.324 However, chronobiological observations raise concern about the mental health of shift workers—particularly about their risk of developing depression. As discussed in the preceding section “Jet Lag versus Shift Lag,” time shifts imposed by transmeridian jet travel or by shift work produce internal rhythm desynchronization. Related symptoms of desynchronosis can include psychophysiological disturbances of well‐being, resembling symptoms of affective disorders.315,324–326 In addition, changes in mood have been shown to be associated with irregularity of sleep patterns and changes in attitude with sleep deprivation.327,328
Studies of patients diagnosed with depressive disorder have demonstrated that these patients typically have a dysfunction of the circadian system. This may reflect an abnormal functioning of the endogenous biological clock and/or an abnormal response to external zeitgebers.329–331
Hypotheses proposed to explain the abnormalities in circadian rhythms seen in depression have been reviewed by Monk.332 Despite the uncertainties, it is clear that clinical depression is often associated with circadian dysfunction. This dysfunction has most consistently been evidenced by reduced amplitudes of circadian rhythms in depressed patients and phase changes, such as the early morning awakening characteristic of this disorder. The typical early morning awakening seen in endogenous depression suggests phase advancement of the circadian rhythm, and it has been suggested that depressed patients suffer from a phase disruption of the sleep–wake and “awakening–readiness” rhythms. Certain antidepressant medications, such as tricyclic antidepressants, monoamine oxidase inhibitors, and lithium, cause a phase delay of several circadian rhythms.333,334 In temporal isolation studies, shifts in the light–dark cycle were associated with an increase in depressive symptoms.335 Not surprisingly appropriately timed bright light therapy can be an effective antidepressant.336
Depressive patients have also been found to have a shorter REM sleep latency and to experience more REM sleep in the first third of the night and less in the last third than controls.335–337 Phase advancing the time of sleep with respect to the REM–temperature–cortisol circadian rhythm leads to a remission of symptoms in some depressed patients.338
The converse, that is, delaying the time of sleep would precipitate depression in susceptible persons, may have been observed. Two weeks following a study where four subjects underwent a 12‐hour sleep delay, one of the subjects committed suicide. Retrospectively, it was observed that the subject’s circadian temperature rhythm was phase‐advanced with respect to the other three subjects’ and did not re‐entrain to the schedule shift, but remained advanced relative to the shift.337 There are also studies which support the notion that imposed time shifts may lead to the development of clinical depression in vulnerable individuals. Healthy subjects were observed to have an increase in depressive symptoms after experiencing a phase‐delay time shift.339 In bipolar patients, episodes of mania have been triggered by time zone changes and by a night of sleep deprivation and have then been successfully managed by regularization the manic patient’s schedule.340 A review of psychiatric incidents at London’s Heathrow Airport revealed the direction of air travel to predict the type of affective disorder experienced, with mania primarily occurring after eastbound flights (phase advance) and depression mainly after westbound travel (phase delay).341
A model proposed by Ehlers and colleagues to explain the relationship between imposed circadian rhythm disruption and the occurrence of depression involves a cascade of effects linking adverse life events with the onset or recurrence of depression in vulnerable individuals.342,343 The life event is proposed to result in a change in the individual’s daily social routines (analogous to the effect of shift work schedules), leading to circadian rhythm maladaptation. The resulting desynchronosis includes depressive symptomatology, and in vulnerable individuals, a major depressive episode may result. The term zeitstörers (German: time disrupter) was coined to describe these agents/events, including shift work, that disrupt the circadian system.343
As a group, shift workers report excessive symptoms of depressive illness, suggesting that shift work may predispose vulnerable individuals to affective disorders. Increased psychological symptoms and increased scores for depression on mood profiles have been reported in nurses during their first months of working starting shift work.344,345 Costa et al. found shift workers to have a 5–15% increase in a “neurotic disorders” category, which included depression.346 In a comparative study of retired day workers and retired shift workers, cases of depression as identified by a neuropsychiatrist were more frequent in the retired shift workers.347 Results from a pilot study of the prevalence of major depressive disorder (MDD), defined by the Structured Clinical Interview for DSM‐III‐R criteria, in 100 current and former shift workers found a monotonic trend of increasing prevalence of MDD as years of exposure to shift work increased up to 20 years of exposure.348 The rate decreased after 20 years, likely reflecting a healthy worker effect. Overall lifetime prevalence of MDD was 15%, compared with the prevalence in the general population, which is estimated to be around 10%.349
TREATMENT (COUNTERMEASURES)
In order for individual coping strategies to be effective, families must be involved. The shift worker needs to be aware of the toll that the shift work schedules may take on the family, and the family to be aware of the effect of the shift work schedule on the worker. The provision of educational programs for both the worker and family is essential for employees to successfully cope with shift work schedules in terms of performance at work, responsibilities at home, and health considerations. Educational materials addressing shift work issues, including countermeasures published in laymen’s terms, are available which will assist employers and employees in this endeavor.81,350,351
Diet and exercise
Good dietary habits and regular exercise are recommended in general for preventive health reasons. As noted in the above section “Shift Work and Specific Medical Disorders,” shift working is associated with metabolic disorders, particularly related to hyperlipidemia, glucose intolerance, and metabolic syndrome. Dietary habits may be even more important for shift workers’ preventive health to reduce the risk of risk of coronary artery disease, diabetes, and obesity than for workers in general.206 Shift work and the time of the shift worked appear to affect the amount and quality of food eaten and the energy distribution over the day. Lowden et al., in their comprehensive review of the available studies of shift work‐related dietary issues, observed that factors such as time availability and social context are important in determining food intake at work, particularly at night, and that a case can be made that shift workers need to be provided with both the opportunity and the appropriate facilities to maintain healthy eating habits in the workplace.352
Sleep deprivation and circadian disruption can affect the endogenous signals and disrupt the homeostatic control of food intake; and moderate sleep deprivation has been shown to be associated with an increase in consumption of energy from snacks with a higher carbohydrate content.353,354 It has been proposed that disruption of these peripheral circadian oscillators may be involved in the development of obesity, Type 2 diabetes, and metabolic syndrome355; if this is so, it supports the argument that circadian disruption should be minimized when working at nights by keeping the same mealtimes across the shift cycle to maintain a relatively diurnal dietary rhythm356 and avoiding eating, or restricting energy intake, between midnight and 0600 hours.
Carbohydrate‐rich meals produce greater decrements in mental performance (in contrast to physical performance) and increase sleepiness as compared to fat‐rich meals; although compared to circadian effects on sleep and performance, the effects of meal differences and the postprandial response are relatively small. Regarding whether night workers should fast or feed during the night shift, laboratory studies have shown subjective ratings of sleepiness and energy levels to be lower at night in the fasting condition.357–360
In their review, Lowden et al. discuss the limitations of study designs and contradictory findings and the complexity of eating habits of shift workers that limit conclusions regarding dietary recommendations for shift workers.352 They note in particular that additional research is needed to identify when shift workers eat, with respect to their work hours and circadian rhythms, in order to answer the question of when and what night workers should eat to avoid inducing metabolic disturbances and optimize wakefulness and performance. Acknowledging the gaps in the current research, they have identified some broad guidelines that may be included in nutrition management strategies—noting that these guidelines are to be considered in parallel with appropriate fatigue management strategies, are targeted directly at the individual or the employer, and are appropriate not only for shift workers but also other populations:
General guidelines
- Avoid eating, or at least restrict energy intake, between midnight and 0600 hours.
- Try to eat at the beginning and end of the shift.
- Avoid “large meals” (> 20% of daily energy intake) 1–2 hours before the main daily sleep episode.
- Provide a variety of food choices: complete or vegetarian meals and high‐quality snacks are recommended.
- Avoid foods and beverages classified as low‐quality snacks.
- Provide appropriate dining facilities in as pleasant a surrounding as possible for works to eat with coworkers, or allow meals to be eaten away from the workplace.
- Maintain a healthy lifestyle with exercise, regular mealtimes, and good sleep hygiene when not working.
Specific guidelines for shift work
- Eat breakfast before day sleep to avoid wakening due to hunger.
- Stick as closely as possible to a normal day and night pattern of food intake. See, for example, the Nordic Nutrition Recommendations (Nordic Council of Ministers. Nordic Nutrition recommendations. Copenhagen: Nordic Council of Ministers; 2004).
- Divide the 24‐hour intake into eating events with three satiating meals each contributing 20–35% of 24‐hour intakes. Higher energy needs require more frequent eating.
- Avoid overreliance on (high‐energy content) convenience foods and high‐carbohydrate foods during the shift. Select vegetable soups, salads, fruit, yogurt, whole grain sandwiches, cheese or cottage cheese (topped with fruit), boiled egg, nuts, and green tea (for antioxidant activity)—noting that this may not be palatable for some workers.
- Design shift schedules which allow adequate time between shifts for sleep and meal preparation, avoid quick returns.
- Avoid sugar‐rich products such as soft drinks, bakery items, sweets, and nonfiber carbohydrate foods (high glycemic load), for example, white bread.
Physical exercise has been demonstrated to reduce general fatigue in shift workers and sleepiness at work, increase sleep duration and the quantity of slow‐wave sleep which is vital to the restorative functions of sleep, and decrease musculoskeletal symptoms. Physical exercise can cause circadian rhythm phase advances and delays. Overall the research supports that there is benefit for shift workers from appropriately timed, regular physical exercise on sleep and performance outcomes. Recommendations for exercise for shift workers include the following: (i) moderate physical exercise is preferred over intensive training, (ii) exercise should be done a few hours before the main sleep period, and (iii) for morning or day shifts, the best exercise time is after the shift. After night shifts, the exercise should be done before an evening nap.169,361–363
Maximizing sleep
The most significant factor interfering with sleep for night workers is daytime noise. Even if the worker is not aware of actually being awakened by noise, sleep quality may be compromised.364 Actions should be taken to soundproof the bedroom as much as possible. In addition to utilizing sound damping materials, for example, ceiling tiles and carpeting, white noise from a fan or air conditioner may be helpful. Family and neighbor cooperation may be needed to control noisy activities near the night worker’s sleeping quarters. The phone and doorbell should not be audible in the bedroom. Comfortable earplugs can also be used to attenuate noise.
Light exposure should be limited to as close to nighttime conditions as possible. Lined drapery and window blinds or dark room shades are suggested. Eyeshades are another option for decreasing light exposure.
Applying “sleep hygiene,” a technique initially developed to help patients with insomnia, is also a recommended coping strategy for shift workers. Sleep hygiene is a program applying regular procedures and following behavioral rules that enhance the ability to fall asleep and stay asleep.365
Although the regular use of sleeping pills is contraindicated, short‐acting hypnotics such as triazolam have been shown to improve quality and duration of daytime sleep. Intermittent use for a day or two, under a physician’s care, may be useful when beginning a run of night shifts or following a transmeridian flight. However, caution must be exercised regarding the timing of administration of even short‐acting hypnotics, as impaired cognition may linger 8 hours after administration.366–368
Caffeine and other alertness‐enhancing drugs
Caffeine belongs to the xanthine class of drugs, which have been shown to cause phase shifts of the temperature rhythm in animals. Caffeine is an effective countermeasure for night workers due to its stimulant effect in counteracting sleepiness and to its ability to delay sleep onset at night. Caffeine has been shown to have beneficial effects on alertness and performance and to decrease sleep tendency as measured by multiple sleep latency tests.369–372
The dose of caffeine should be limited to avoid undesirable side effects, such as heart palpitations. Caffeine’s effect in increasing alertness is most apparent after a time of abstinence, and with repeated doses, and the effect may diminish with repeated doses. Caffeine disrupts daytime sleep more than nocturnal sleep, and consumption should be avoided closer than around 5 hours before bedtime.373–375 Shift workers should limit use to the first half of night or evening shifts. It is important to avoid caffeine during the last half of the evening shift or night shift, since the worker’s bedtime will come soon after getting home. Fruit juice is good alternative drink for the second half of the shift.
Amphetamines and stimulant diet pills should never be used to treat shift work‐related sleepiness due to adverse side effects and potential for abuse. Newer alertness‐enhancing drugs may have some usefulness for occasional alertness promotion.376 Research on the wake‐promoting medication, modafinil, has demonstrated its ability to improve performance and decrease extreme sleepiness in night workers.377 Modafinil has been approved by the US Food and Drug Administration for use in increasing alertness in night workers; however it may also increase insomnia and is not a substitute for adequate sleep. As pointed out by the American Academy of Sleep Medicine (AASM), caffeine is a readily available, inexpensive alternative.378 The AASM guidelines do include modafinil as an indicated medication to enhance alertness during the night shift for patients diagnosed with SWD (shift work disorder, i.e., shift work‐related sleep disturbances and impairment of waking alertness and performance). However, ethical concerns are raised with the use of medication rather than changing the work schedules for workers who have been determined to be shift work intolerant.376,379
Bright light and melatonin
In addition to its sleep‐inducing property, animal and human laboratory studies have shown that melatonin effects phase shifts of circadian rhythms when administered with appropriate timing.380,381 Several field studies have demonstrated melatonin to be useful for ameliorating jet‐lag symptoms.104,382 Five milligrams daily, taken orally, is the typical dose used in research protocols, although lower doses may also be effective.383 Specific instructions (and side effect warnings) for taking the hormone for eastbound and westbound flights, as given to subjects participating in jet‐lag studies, are included in the review by Arendt and Deacon.104 The American Academy of Sleep Medicine (AASM) has recommended the use of melatonin at appropriate times to reduce the symptoms of jet lag and improve sleep following transmeridian flights and suggests that immediate‐release formulations in doses from 0.5 to 5 mg may be effective.378
Shift workers have anecdotally reported benefits from using over‐the‐counter preparations of melatonin for shift‐lag symptoms.384 There is limited research available on the use of melatonin in real shift work situations. Beneficial effects on sleep and alertness have been reported associated with bedtime administration, but some performance measures may be adversely affected.385 Sharkey et al. found that in laboratory‐simulated night shifts, melatonin was effective in preventing decreased sleep time during daytime sleep only on the first day of administration and had no effect on alertness (assessed with the multiple sleep latency test) or on performance measures or mood during the night shift.384 Inappropriately timed administration may be dangerous due to its sedative effect. Effects of long‐term, regular usage of melatonin are not known. In addition to its sleep‐inducing role, melatonin may influence blood pressure regulation, immune modulation, control of tumor growth, and antioxidant action on free radicals.105
The AASM has recommended the administration of melatonin as a guideline, prior to day sleep, for shift workers diagnosed with SWD. The report also points out, however, that there is mixed evidence supporting the use of melatonin, that it is difficult to draw firm conclusions from the current research due to variability in shift schedules and dosage and timing, and that subjects have seldom been diagnosed according to SWD criteria.378
Exposure to bright light, 7 000–12 000 lx (comparable to sunlight), has been demonstrated to result in phase shifts of the circadian timing system. The timing of the exposure determines the direction of the shifts, that is, either a phase‐advance or phase‐delay response.44,386,387 Although appropriately timed bright light exposure can enhance adjustment to night shifts, practical application for shift workers is a different matter. Not only is the timing of the light exposure critical, but also prevention of outdoor sunlight exposure at times is necessary (e.g., on the commute home after dawn). In addition, there is considerable individual variation in the degree of phase‐shift response. The American Academy of Sleep Medicine report points out that although circadian realignment has been achieved with light exposure in simulated shift work situations, larger studies are needed to determine the clinical utility of timed light therapy for the treatment of SWD.378
The use of both bright light and melatonin together for readaptation from night work to a daytime schedule has been studied. Specific protocols have been described in detail for the timing of light and medication following long‐term and short‐term night work by Pallesen et al.388 Assistance from a chronobiologist is probably needed to make practical, understandable schedules for a worker. The goal is to provide predictability of the shift‐work schedule without unexpected overtime, and commitment from the worker and employer for successful application. A final consideration is the concern that has been raised regarding use of bright light at night related to the possible oncogenic risk for estrogen‐sensitive breast cancers associated with long‐term bright light exposure during the night shift; as discussed in the preceding “Cancer,” section, melatonin suppresses estrogens, and light exposure decreases melatonin secretion.
Naps
About one‐third of night workers take a nap for about an hour in the late afternoon before night shifts.389,390 Although not usually allowed in the United States, provision for on‐the‐job naps during night shifts is not uncommon in Japan. Scheduled nap times during the first night shift are effective in counteracting the extreme decrease during the early morning circadian trough in alertness.391 Field study evidence that scheduled napping at work improves performance has been reported in aviation studies of 30 minutes cockpit naps.392. Other studies have shown that naps taken during the night shift can increase alertness and performance.393,394 Napping in on call rooms is a standard practice for medical interns. Napping during the night shift has been shown to be beneficial for counteracting some effects of sleep deprivation in nurses.395 Overall, it appears that naps can be an effective countermeasure against on‐the‐job sleepiness. Allowance should be planned for the initial 5–15 minutes period of sleep inertia after awakening. Short naps of less than 15 minutes do not appear to have significant risk of sleep inertia.
MEDICAL SURVEILLANCE
Although there are no US federally mandated requirements for medical evaluations for night work exposure, the International Labor Organization (ILO) 1990 Convention (No. 171) includes provisions for a health assessment for workers before beginning their night work and health assessments of night workers at regular intervals as well as for work‐related problems that may be secondary to the work schedule.396 In addition, the European Directive No. 93/104/EC, “Concerning certain aspects of the organization of working time,” also considers it a right of workers to have a free health assessment before beginning their first assignment to the night shift.397
Recommendations for medical evaluations of workers before they begin night work assignments have been made by occupational medicine practitioners and by chronobiologists who have studied health effects of shift work. Identification of individual characteristics that are associated with poor tolerance of night work is recommended, not with the goal of disqualifying workers for night work but with the recognition that, for some, night work may medically not be advisable. In most situations, the preplacement examination will provide an opportunity to make susceptible workers aware of their individual risks and plan appropriate medical supervision and develop coping strategies.15,96,97,173,398,399
The frequency of medical surveillance examinations is somewhat arbitrary. However, recommendations are consistent in advising evaluation during the first few months after beginning shift work and at regular but less frequent intervals, depending on the work schedule and the age of the worker. A reasonable schedule has been outlined by Harma which includes the following: the first follow‐up health check scheduled no later than 2 months after night/shift work has begun; subsequently, for workers between 25 and 45 years of age, 3–5 year intervals; for those under 25 or over 45 years of age, 2‐year intervals; and for those over 60, 1‐year intervals are advised.169 More frequent evaluations may be needed for individuals with underlying conditions that may be aggravated by shift work. Costa has made more general recommendations for the first medical surveillance health check to be during the first year of shift or night work, for those under 45 years successive evaluations to be at least every 3 years, and for those over 45 successive checkups to be every 2 years.400 Ongoing medical surveillance programs, including periodic medical screening examinations and appropriate laboratory testing, have been recommended for rotating and permanent night workers. In addition, follow‐up evaluations of day workers who have left shift work for medical reasons have also been advised.15,173
Common conditions that may be exacerbated by shift work have already been reviewed in this chapter. Smolensky et al. have recently reviewed numerous other medical and psychiatric conditions exhibiting circadian fluctuation in symptoms and response to external temporal triggers and pharmacological treatments.289
Potential contraindications to working shift work involving night shifts are summarized in Table 10.1. For example, due to the recognized increase in likelihood of seizure events in epileptic individuals associated with circadian rhythm disruption and sleep deprivation, clearance from the neurologist managing a potential shift worker with epilepsy before initial assignment to a night work schedule is a reasonable requirement. For asthmatics, medical surveillance with involvement of the treating physician is essential for monitoring any changes in frequency of bronchoconstriction and response to prescribed medications. Both rotating and permanent night workers with diabetes should be monitored for changes in response to dietary and pharmacological management of glucose control. Circadian disruption in glucose tolerance has been noted. Studies of permanent night workers have shown only partial adjustment of glucose and insulin rhythms after 2 years of regular night work.401 Sleep restriction can also lower glucose tolerance.402,403 Changes in the timing and quality of meals and metabolism related to working nights also necessitate monitoring for increased levels of undesirable triglycerides and lipoproteins.
TABLE 10.1 Potential contraindications for working night or rotating shifts.
| Condition | Examples | Comments |
| Asthma | Poor control or increasing use of rescue inhalers after starting shift work. The time of administration of steroidal anti‐inflammatory and beta‐agonist (bronchodilator) medications can be critical in achieving control of respiratory symptoms and minimizing side effects | Medical surveillance indicated to monitor for changes in frequency of bronchoconstriction and response to prescribed medications |
| Cancer | Endocrine‐sensitive cancers | Avoid work schedules interfering with chronotherapy considerations of timing of chemotherapy or radiation treatments or associated with sleep deprivation |
| Other cancers in treatment involving chronotherapeutic schedules | ||
| Cardiovascular disease | Ischemic heart disease; poorly controlled hypertension; high‐risk/multiple risk factors for acute myocardial infarction | Shift work has been shown to be an independent risk factor for CVD. Shift work may have a triggering effect on lifestyle factors that can increase the risk of CHD; active preventive medicine intervention is important for shift workers. Medical surveillance lab work should include lab work for monitoring for increased levels of undesirable triglycerides and lipoproteins |
| Diabetes mellitus | Poorly controlled diabetes; changes in control on rotating shifts. Regularity of timing of meals and administration of insulin and antiglycemic medication are important in the management of diabetes mellitus | Monitor for changes in response to dietary and pharmacological management of glucose control. Medical surveillance programs should include lab work for monitoring for prediabetes |
| Epilepsy | Generalized or partial seizures. Clearance should be obtained from the neurologist managing a potential shift worker with epilepsy before initial assignment to a night work schedule | Sleep deprivation associated with night work and circadian rhythm misalignment may increase frequency of seizures in some epileptics |
| Gastrointestinal disorders | Chronic peptic ulcer disease; symptomatic inflammatory bowel diseases | Uncontrolled with standard treatments; history of exacerbations related to work schedule changes; work schedules not allowing for regular timing of meal break |
| Psychiatric disorders | Bipolar disorder—irregular schedule may trigger manic behavior | Treatment involving timed light therapy may not be compatible with the shift work schedule. Shift work intolerance seen in individuals with desynchronosis often includes depressive symptomatology |
| Other diagnoses as determined by the treating psychiatrist | ||
| Pregnancy | Increased risk or history of preterm delivery, miscarriage, or low birth weight while working a shift work schedule | Pregnant shift workers should advise their obstetrician regarding their hours of work. Do not increase shift length or initiate shift work during a pregnancy |
| Prescription medications | If time of dosing affects drug effectiveness. For example, changing the time of day of ingestion of certain blood pressure‐lowering medications reduces effectiveness | Differences in the rate of absorption, distribution, and elimination of a drug can be seen depending on the timing of the drug administration |
| Sleep disorders | Narcolepsy, shift work disorder, and other circadian rhythm sleep disorders, long or rigid sleep requirements, uncontrolled sleep apnea | Treatment involving timed light, melatonin, and/or planned sleep schedule exposure may not be compatible with the shift work schedule |
Based on the available epidemiological evidence, medical conditions exacerbated by shift work may be absolute or relative contraindications to shift work.96,173,402 Identification of individual characteristics that are associated with poor tolerance of night work is recommended not with the goal of disqualifying workers for night shifts but providing appropriate medical counseling and medical surveillance for those for whom night work may not be medically advisable. Depending on the severity of the condition and stability of treatment needed, the individual’s overall tolerance to shift work, and the particular shift work schedule involved, temporary or permanent restriction from night work may be in the best medical interest of the worker. Occupational health physicians should remember that shift work intolerance is a manifestation of complex medical and psychosocial interactions and individual worker responses will vary in terms of severity and timing of onset of clinical signs and symptoms.403
While medical surveillance programs for shift workers are important for early detection of shift work‐related health problems, Kogi has pointed out that medical surveillance examinations alone cannot adequately meet the health needs of shift workers.93 The medical surveillance program for shift workers should include educational/counseling opportunities related to the assessment and optimization of shift work coping strategies.169,173 Joint efforts by occupational health and safety teams, in conjunction with working with supervisors and workers, are necessary to provide necessary preventive medicine programs and address scheduling considerations. Adjustments may be needed in medical surveillance schedules for chemical exposures to account for quick turnover times or extended hours of work.
PREVENTION AND ADMINISTRATIVE CONTROLS
Scheduling decisions
Scheduling decisions should be made with the goal of minimizing the potential negative impact of shift work on worker sleep, health, and performance. Although scheduling designs understandably reflect business needs and employee preferences, sleep and health considerations should not be secondary concerns. Before making schedule changes, the demographics of the workforce, including lifestyles, sleep habits, common medical problems, and shift scheduling preferences, as well as the type of work and the environment in which it is performed, should be assessed.86,404
Recognizing that “tailor‐made” shift systems need to involve compromises between conflicting interests of employees and employers and ergonomic considerations, Knauth has recently provided detailed practical recommendations for achieving ergonomically sound shift schedule systems.405 Four general categories of factors important in the evaluation of the degree of shift system compliance with ergonomic recommendations are reviewed: (i) the sequence of shifts, including the speed and direction of rotation and special cases; (ii) the duration and distribution of working time, including the number of consecutive working days, shift duration, and time off; (iii) the position of the working time, including the start of the morning shift and the end of the evening and night shift and number of free weekends; and (iv) short‐term deviations from the established shift schedule resulting from wishes of the employees or from requirements of the employer.
It is clear from the above discussions that preparing schedule designs for shift systems is a complex matter. In addition, there may be individual workers with medical restrictions that need to be worked into particular rosters. Fortunately, computer software programs have been developed to assist in the process.406,407
Occupational health programs
Workplace facilities and environmental conditions can impact on tolerance to shift work and shift worker performance. In order to assist employees in dietary countermeasures, equivalent canteen/eating facilities should be provided for night workers as for day workers. At a minimum, a microwave, refrigerator, and vending machines with low‐fat nutritious foods should be available, including dairy products and fruit juices.
Other environmental factors should be assessed which can increase alertness on the job and help prevent episodes of falling asleep. For instance, bright, uniform lighting will enhance alertness. Nonvariable background noise which promotes boredom may be replaced with judiciously selected music and, if appropriate, social interactions between workers. Keeping room temperatures below 70°F and providing opportunities for physical activity have been recommended to maximize alertness on the night shift.350,408
Educational programs should be provided for workers and their families that provide information for shift work coping strategies. In addition, workers should be advised of the increased risk of motor vehicle accidents on the drive home when working night shifts. Provision of sleeping facilities for workers who need to sleep before driving home should be considered. The degree of driving risk for the individual workforce should be assessed, and the aggressiveness of preventive measures taken based on the findings. Some proactive companies, having recognized the difficulty that night workers with families face in obtaining childcare, have established 24‐hour childcare facilities for their workers.409 Monk and Folkard have recommended that employers develop a “Shift Work Awareness Program” for coordinating educational and social support programs.351
References
- 1. Coleman RM, Demant WC. Falling asleep at work: a problem for continuous operations. Sleep Res 1986; 15:265.
- 2. Novak RD, Novak SE. Focus group evaluation of night nurse shiftwork difficulties and coping strategies. Chronobiol Int 1996;13(6):457–63.
- 3. Scott AJ. Chronobiological considerations in shiftworker sleep and performance and shiftwork scheduling. Hum Perform 1994; 7(3):207–33.
- 4. Scott AJ. Sleepiness and fatigue: risks for the transportation industry. Clin Occup Environ Med 2003; 3:81–108.
- 5. Tepas DI, Armstrong DR, Carlson ML, et al. Changing industry to continuous operations: different strokes for different plants. Behav Res Methods Instrum Comput 1985; 17(6):670–6.
- 6. Kogi K. Comparison of resting conditions between various shift rotation systems for industrial workers. In: Reinberg A, Vieux N, Andlauer P, eds. Night and shift work: biological and social aspects. Oxford: Pergamon Press, 1981:417–24.
- 7. U.S. Department of Labor, Bureau of Labor Statistics. Survey of Occupational Injuries and Illnesses, 2014 OMB No. 1220‐0045. Page 6 of the survey instrument. Available at http://www.bls.gov/respondents/iif/forms/soii2014.pdf (accessed May 3, 2016).
- 8. Bureau of Labor Statistics. Case and Demographic Characteristics for Work‐related Injuries and Illnesses Involving Days Away From Work Resource Table Categories: Calendar Year 2013 Survey Results Tables R76 and R83. Available at http://www.bls.gov/iif/oshcdnew2013.htm#13 first link (accessed May 3, 2016).
- 9. Wedderburn A. Instruments for designing, implementing and assessing working time arrangements. Bulletin of European Studies on Time, 7. Luxembourg: Office for Official Publications of the European Communities, 1994
- 10. Jansen B, Kroon H. Rota‐risk‐profile‐analysis. Work Stress 1995; 9:245–55.
- 11. American Conference of Governmental Industrial Hygienists. 2014 TLVs and BEIs, threshold limit values for chemical and physical agents biological exposure indices. Cincinnati: ACGIH, 2014.
- 12. Occupational Safety and Health Administration (OSHA). Standard Interpretation 11/10/1999 [corrected 6/21/2007]: OSHA policy regarding PEL adjustments for extended work shifts. Available at https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=INTERPRETATIONS&p_id=22818 (accessed May 3, 2016).
- 13. Brief RS, Scala RA. Occupational exposure limits for novel work schedules. Am Ind Hyg Assoc J1975 36:467–9.
- 14. Smolensky M, Reinberg A. Clinical chronobiology: relevance and applications to the practice of occupational medicine. Shiftwork Occup Med State Art Rev 1990; 5(2):239–72.
- 15. Koller M. Occupational health services for shift and night workers. Appl Ergon, 1996; 27(1):31–7.
- 16. Monk TH, Folkard S. Circadian rhythms and shift work. In: Hockey GRS, ed. Stress and fatigue in human performance. Chichester: John Wiley & Sons, Ltd, 1983:97–121.
- 17. Knauth P, Rutenfranz J. Experimental shift work studies of permanent night and rapidly rotating shift systems: 1. Circadian rhythm of body temperature and re‐entrainment at shift change. Int Arch Occup Environ Health 1976; 37:125–37.
- 18. Knauth P, Emde E, Rutenfranz J, et al. Re‐entrainment of body temperature in field studies of shiftwork. Int Arch Occup Environ Health 1981; 49:137–49.
- 19. Van Loon JH. Diurnal body temperature curves in shiftworkers. Ergonomics 1963; 6:267–73.
- 20. Costa G, Ghirlanda G, Tarondi G, et al. Evaluation of a rapidly rotating shift system for tolerance of nurses to nightwork. Int Arch Occup Environ Health 1994; 65:305–11.
- 21. Folkard S. The pragmatic approach to masking. Chronobiol Int 1989; 6(1):55–64.
- 22. Reinberg A, Vieux N, Andlauer P, et al. Tolerance of shift work, amplitude of circadian rhythms, and aging. In: Reinberg A, Vieux N Andlauer P, eds. Night and shift work: biological and social aspects. Oxford: Pergamon Press, 1981:341–54.
- 23. Leonard R. Night‐ and shift‐work. In: Reinberg A, Vieux N, Andlauer P, eds. Night and shift work: biological and social aspects. Oxford: Pergamon Press, 1981:323–9.
- 24. Reinberg A, Motohashi Y, Bourdeleau P, et al. Alteration of period and amplitude of circadian rhythms in shift workers. Eur J Appl Physiol 1988; 57:5–25.
- 25. Mills JN, Minors DS, Waterhouse JM. Exogenous and endogenous influences on rhythms after sudden time shift. Ergonomics 1978; 21:755–61.
- 26. Klein KE, Wegman HM, Hunt BI. Desynchronization as a function of body temperature and performance circadian rhythm as a result of outgoing and homecoming transmeridian flights. Aerosp Med 1972; 43:119–32.
- 27. Klein KE, Herrmann R, Kuklinski P, et al. Circadian performance rhythms: experimental studies in air operations. In: Mackie R, ed. Vigilance: theory, operational performance, and physiological correlates. New York: Plenum Press, 1977:111–32.
- 28. Bodanowitz M. The change of circadian rhythms of psychomotor performance after transmeridian flights. Translation of DLR‐FB 73‐52, Die veranderung tagesperiodischer schwankungen der psychomotorischen leistung nach transmeridian flugen. Bonn: Deutsche Forschungs‐ und Versuchsanstalt fur Luft‐ und Raumfahrt, Institut fur Flugmedizin, 1973.
- 29. Orth‐Gomer K. Intervention on coronary risk factors by changing working conditions of Swedish policemen. In: Harvath M, Frankth E, eds. Psychophysiologic risk factors of cardiovascular diseases. International Symposium, Suppl. 3. Prague: Avicenum‐Czechoslovak Medical Press, 1982.
- 30. Orth‐Gomer K. Intervention on coronary risk factors by adapting a shiftwork schedule to biologic rhythmicity. Psychosom Med 1983; 45:407–15.
- 31. Lavie P, Tzischinsky O, Epstein R, et al. Sleep–wake cycle in shift workers on a “clockwise” and “counter‐clockwise” rotation system. Isr J Med Sci 1992; 28(8–9):636–44.
- 32. Folkard S. Shift work: a growing occupational hazard. Occup Health 1989; 41:182–6.
- 33. Vidacek S, Kaliterna L, Radosevic‐Vidack B, et al. Productivity on a weekly rotating shift system: circadian adjustment and sleep deprivation effects? Ergonomics 1986; 29(12):1583–90.
- 34. Wilkinson R, Allison S, Feeney M, et al. Alertness of night nurses: two shift systems compared. Ergonomics 1989; 32(3):281–92.
- 35. Knauth P, Landau K, Droge C, et al. Duration of sleep depending on the type of shift work. Int Arch Occup Environ Health 1980; 46:167–77.
- 36. Wojtczak‐Jaroszowa J. Circadian rhythm of biological functions and night work. In: Wojtczak‐Jaroszowa J, ed. Physiological and psychological aspects of night and shift work. Cincinnati: National Institute for Occupational Safety and Health, 1977:3–12.
- 37. Dahlgren K. Adjustment of circadian rhythms and EEG sleep functions to day and night sleep among permanent nightworkers and rotating shiftworkers. Psychophysiology 1981; 18(4):381–91.
- 38. Czeisler CA, Weitzman ED, Moore‐Ede MC, et al. Human sleep: its duration and organization depend on its circadian phase. Science 1980; 210:1254–67.
- 39. Walker J. Frequent alternation of shifts on continuous work. Occup Psychol 1966; 40:215–25.
- 40. Kiesswetter E, Knauth P, Schwarzenau P. Daytime sleep adjustment of shiftworkers. In: Koella WP, Ruther E, Schulz H, eds. Sleep ’84. New York: Gustav Fischer Verlag, 1985:273–5.
- 41. Kogi K. Estimation of sleep deficit during a period of shift rotation as a basis for evaluating various shift systems. Ergonomics 1978; 21(10):861–74.
- 42. Williamson AM, Sanderson JW. Changing the speed of shift rotation: a field study. Ergonomics 1986; 29(9):1085–96.
- 43. Smith PA, Wright BM, Mackey RW, et al. Change from slowly rotating 8‐hour shifts to rapidly rotating 8‐hour and 12‐hour shifts using participative shift roster design. Scand J Work Enviorn Health 1998; 24(S3):55–6.
- 44. Eastman CI. Circadian rhythms and bright light: recommendations for shift work. Work Stress 1990; 4:245–60.
- 45. Smith MR, Eastman CI. Shift work: health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat Sci Sleep 2012; 4:111–32.
- 46. Maasen A, Meers A, Verhagen P. Quantitative and qualitative aspects of sleep in four shift workers. Ergonomics 1978; 21(10): 861–74 (abstract).
- 47. Circadian Technologies, Inc. Improving human performance and health in round‐the‐clock operations. Seminar, Pittsburgh, PA, November 1987.
- 48. Dahlgren K. Adjustment of circadian rhythms to rapidly rotating shift work: a field study of two shift systems. In: Reinberg A, Vieux N, Andlauer P, eds. Night and shift work: biological and social aspects. Oxford: Pergamon Press, 1981:357–65.
- 49. Rutenfranz J, Knauth P. Hours of work and shiftwork. Ergonomics 1976; 19(3):331–40.
- 50. Ghata J, Reinberg A, Vieux N, et al. Adjustment of the circadian rhythm of urinary 17‐OHCS, 5‐HIAA, catecholamines, and electrolytes in oil refinery operators to a rapidly rotating shift system. Ergonomics 1978; 21(10):61–874 (abstract).
- 51. Wedderbrun AAI. How important are the social effects of shiftwork? In: Johnson LC, Tepas DI, Colquhoun WP, Colligan MJ, eds. Biological rhythms, sleep, and shift work. Advances in Sleep Research, Vol 7. New York: Spectrum Publications, 1981:257–69.
- 52. Conroy RT, Mills JN. Human circadian rhythms. London: J & A Churchill, 1970.
- 53. Reinberg A. Clinical chronopharmacology. In: Reinberg A, Smolensky M, eds. Biological rhythms and medicine: cellular, metabolic, physiopathologic, and pharmacologic aspects. New York: Springer‐Verlag, 1983:211–57.
- 54. Tepas DI, Monk TH. Work schedules. In: Salvendy G, ed. Handbook of human factors. New York: John Wiley & Sons, Inc., 1987:819–43.
- 55. Tepas DI, Walsh JK, Moss PD, et al. Polysomnographic correlates of shiftworker performance in the laboratory. In: Reinberg A, Vieux N, Andlauer P, eds. Night and shift work: biological and social aspects. Oxford: Pergamon Press, 1981:179–86.
- 56. Tepas DI, Walsh JK, Armstrong DR. Comprehensive study of the sleep of shift workers. In: Johnson LC, Tepas DI, Colquhoun WP, Colligan MJ, eds. Biological rhythms, sleep and shift work. Advances in Sleep Research, Vol 7. New York: SP Medical & Scientific Books, 1981:347–55.
- 57. Rosa RR, Wheeler DD, Warm JS, et al. Extended workdays: effects on performance and ratings of fatigue and alertness. Behav Res Methods Instrum Comput 1985; 17(1):6–15.
- 58. Rosa RR, Colligan MJ, Lewis P. Extended workdays: effects of 8‐hour and 12‐hour rotating shift schedules on performance, subjective alertness, sleep patterns, and psychosocial variables. Work Stress 1989; 3(1):21–32.
- 59. Mills DQ. Does organized labor want the 4‐Day week? In: Poor R, ed. 4 days, 40 hours. Cambridge, MA: Bursk and Poor Publishing, Inc., 1970:61–9.
- 60. Steele JL, Poor R. Work and leisure: the reactions of people at 4‐day firms. In: Poor R, ed. 4 days, 40 hours. Cambridge, MA: Bursk and Poor Publishing, Inc., 1970:105–22.
- 61. Brief RS, Scala RA. Occupational health aspects of unusual work schedules: a review of Exxon's experiences. Am Ind Hyg Assoc J 1986; 47(4):199–202.
- 62. Colligan MS, Tepas DI. The stress of hours of work. Am Ind Hyg Assoc J 1986; 47:686–95.
- 63. Jones IS, Stein HS. Effect of driver hours of service on tractor‐trailer crash involvement. Arlington: Insurance Institute for Highway Safety, 1987.
- 64. Lisper HO, Laurell H, van Loon J. Relation between time to falling asleep behind the wheel on a closed track and changes in subsidiary reaction time during prolonged driving on a motorway. Ergonomics 1986; 29(3):445–53.
- 65. Laundry BR, Lees RE. Industrial accident experience of one company on 8‐and 12‐hour shift systems. J Occup Med 1991; 33(8):903–6.
- 66. Tucker P, Smith L, MacDonald I, et al. Shift length as a determinant of retrospective on‐shift alertness. Scand J Work Environ Health 1998; 24(S3):49–54.
- 67. Smith PA, Wright BM, Mackey RW, et al. Change from slowly rotating 8‐hour shifts to rapidly rotating 8‐hour and 12‐hour shifts using participative shift roster design. Scand J Work Environ Health 1998; 24(S3):55–61.
- 68. Lowden A, Kecklund G, Aselsson J, et al. Change from an 8‐hour shift to a 12‐hour shift, attitudes, sleep, sleepiness, and performance. Scand J Work Environ Health 1998; 24(S3):69–75.
- 69. Williamson AM, Gower CG, Clarke BC. Changing the hours of shiftwork: a comparison of 8‐ and 12‐hour shift rosters in a group of computer operators. Ergonomics 1994; 37(2):287–98.
- 70. Johnson MD, Sharit J. Impact of a change from an 8‐h to a 12‐h shift schedule on workers and occupational injury rates. Int J Ind Ergon, 2001; 27(5):303–19.
- 71. Mitchell RJ, Williamson AM. Evaluation of an 8‐hour versus a 12‐hour shift roster on employees at a power station. Appl Ergon 2000; 31(1):83–93.
- 72. Tucker, P. Compressed working weeks. Conditions of Work and Employment Series, No. 12. Geneva: International Labour Organization, 2006. Available at http://www.ilo.org/wcmsp5/groups/public/—ed_protect/—protrav/—travail/documents/publication/wcms:travail_pub_12.pdf (accessed May 4, 2016).
- 73. Mardon S, ed. Shiftwork alert, 2(7) and (8) circadian information. Cambridge, MA: Shiftwork Newsletter, 1997.
- 74. Finn P. The effects of shift work on the lives of employees. Monthly Labor Rev 1981; 104(10):31–5.
- 75. Krell, E. Second jobs: blessing or curse? HR Mag 2010; 55(3):57.
- 76. Smith L, Folkard S, Tucker P, et al. Work shift duration: a review comparing eight hour and 12 hour shift systems. Occup Environ Med 1998; 55:217–29
- 77. Smith L, Hammond T, Macdonald I, et al. 12‐hr shifts are popular but are they a solution? Int J Ind Ergon 1998; 21:323–31.
- 78. Caruso CC, Hitchcock EM, Dick RB, et al. Overtime and extended work shifts: Recent findings on illnesses, injuries, and health behaviors. DHHS (NIOSH) Publication, No. 2004‐143. Cincinnati: Department of Health and Human Services, Centers for Disease Control and Prevention, 2004.
- 79. Sirois WG, Moore‐Ede M. Staffing Levels: Key to managing risk in 24/7 operations. Circadian White Paper. LP 2013. Circadian Information, Stoneham.
- 80. Knauth P. Extended work periods. Ind Health 2007; 45:125–36.
- 81. Rosa R, Colligan M. Plain language about shiftwork. NIOSH Publication, No. 97–145. Cincinnati: U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health, 1997.
- 82. Folkard S, Arendt J, Clark M. Sleep and mood on a “weekly” rotating (7‐7‐7) shift system: some preliminary results. In Costa G, Cesana G, Kogi K, Wedderburn A, eds. Shiftwork: health, sleep, performance. Frankfurt: Peter Lang, 1989:484–9.
- 83. Gillberg M. Subjective alertness and sleep quality in connection with permanent 12‐hour day and night shifts. Scand J Work Environ Health 1998; 24(S3):76–81.
- 84. Folkard S, Barton J. Does the ‘forbidden zone’ for sleep onset influence morning shift sleep duration? Ergonomics 1993; 36:85–9.
- 85. Akerstedt T. Work schedules and sleep. Experientia 1984 40:417–22.
- 86. Center for Disease Control. Leading work‐related diseases and injuries. MMWR 1986; 35(39):613–4; 619–21.
- 87. Imbernon E, Warret G, Roitg C, et al. Effects on health and social well‐being of on‐call shifts: an epidemiologic study in the french national electricity and gas supply company. J Occup Med 1993; 35(11):1131–7.
- 88. Joyce K, Pabayo R, Critchley JA, et al. Flexible working conditions and their effects on employee health and wellbeing. Cochrane Database Syst Rev 2010; (2):CD008009. Available at http://summaries.cochrane.org/CD008009/PUBHLTH_flexible‐working‐conditions‐and‐their‐effects‐on‐employee‐health‐and‐wellbeing#sthash.LiB1suFd.dpuf (accessed May 4, 2016). 10.1002/14651858.CD008009.pub2.
- 89. Nätti J, Oinas T, Härmä M, et al. Combined effects of shiftwork and individual working time control on long‐term sickness absence: a prospective study of Finnish employees. J Occup Environ Med 2014; 56(7):732–8.
- 90. Ernst G, Rutenfranz J. Flexibility in shiftwork—some suggestions. Ergonomics 1978; 21(10):861–74 (abstracts).
- 91. Smith P. A study of weekly and rapidly rotating shift workers. Ergonomics 1978; 21(10):861–74 (abstracts).
- 92. Northrup HR, Wilson JT, Rose KM. The twelve‐hour shift in the petroleum and chemical industries. Ind Labor Relat Rev 1979; 32(3):312–26.
- 93. Kogi K. Improving shift workers’ health and tolerance to shiftwork: recent advances. Appl Ergon 1996; 27(1):5–8.
- 94. Costa G, Folkard S, Harrington JM. Shift work and extended hours of work. In Baxter PJ, Adams PH, Caw T‐C, et al., eds. Hunter’s diseases of occupation, 9th edn. London: Arnold, 2000:581–9.
- 95. Halberg F, Halberg E, Barnum CP, et al. Circadian rhythm: coined. In: Withrow RB, ed. Photoperiodism and related phenomenon in plants and animals. Washington, DC: AAAS, 1959:803–78.
- 96. Scott AJ, Ladou J. Shiftwork: effects on sleep and health. In: Scott AJ, ed. Shiftwork: State of the art reviews. Occupational Medicine, State of the Art Reviews, 5(2). Philadelphia: Hanley & Belfus, 1990:273–99.
- 97. Scott AJ. Shift work and health: primary care, clinics in office practice. Occup Environ Med 2000; 27(4):1057–78.
- 98. Wever R. Man in temporal isolation: basic principles of the circadian system. In: Folkard S, Monk TH, eds. Hours of work: temporal factors in work scheduling. Chichester: John Wiley & Sons, Ltd, 1985:15–28.
- 99. Aschoff J. Circadian rhythms in man. Science 1965; 148:1427–32.
- 100. Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near 24‐hour period of the human circadian pacemaker. Science 1999; 284(5423):2177–81.
- 101. Vernibos‐Danelles J, Winget CN. The importance of light, postural, and social cues in the regulation of the plasma cortisol rhythm in man. In: Reinberg A, Halberg F, eds. Chronopharmacology: Proceedings of the 7th International Congress of Pharmacology, Paris 1978. New York: Pergamon Press, 1979:101–6.
- 102. Webb WB, Agnew HW Jr. The effects of a chronic limitation of sleep length. Psychophysiology 1974; 11(5):265–74.
- 103. Duffy JF, Kronauer RE, Czeisler CA. Phase‐shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. J Physiol 1996; 95(pt 1):289–97.
- 104. Arendt J, Deacon S. Treatment of circadian rhythm disorders: melatonin. Chronobiol Int 1997; 14(2):185–204.
- 105. Benarroch, EE. Suprachiasmatic nucleus and melatonin: reciprocal interactions and clinical correlations. Neurology 2008; 71:594–8.
- 106. Vela‐Bueno A, Olavarrieta‐Bernardino S, Fernández‐Mendoza J, et al. Melatonin, sleep, and sleep disorders. Sleep Med Clin 2007; 2:303–8.
- 107. Gekakis N, Staknis D, Nguyen HB, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998; 280(5369):1564–8.
- 108. Darlington TK, Wager‐Smith K, Ceriani MF, et al. Closing the circadian loop: CLOCK‐induced transcription of its own inhibitors per and time. Science 1998; 280(5369):1599–603.
- 109. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 2002; 418:935–41.
- 110. Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Invited review. Am J Physiol Heart Circ Physiol 2006; 290:H1–16.
- 111. Davidson AJ, London B, Block GD, et al. Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens 2005; 27:307–11.
- 112. Durgan DJ, Hotze MA, Tomlin TM, et al. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol 2005; 289:H1530–41.
- 113. McNarmara P, Seo SP, Rudic RD, et al. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a hormonal mechanism to reset a peripheral clock. Cell 2001; 105:877–89.
- 114. Nonaka Y, Emoto N, Ikeda K, et al. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation 2001; 104:1746–8.
- 115. Webb WB. Sleep and biological rhythms. In: Webb WB, ed. Biological rhythms, sleep, and performance. Chichester: John Wiley & Sons, Ltd, 1982:87–141.
- 116. Wever R. Phase shifts of human circadian rhythms due to shifts of artificial zeitgebers. Chronobiologia 1980; 7:303–27.
- 117. Nagano M, Adachi A, Nakaham K, et al. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J Neurosci 2003; 23:6141–51.
- 118. Folkard S, Monk TH, Lobban MC. Short and long‐term adjustment of circadian rhythms in permanent night nurses. Ergonomics 1978; 21:785–99.
- 119. Akerstedt T, Patkai P, Dahlgren K. Field studies of shift work: II. Patterns in psychophysiological activation in workers alternating between night and day work. Ergonomics 1977; 20:849–56.
- 120. Reinberg A, Andlauer P, DePrins J, et al. Desynchronization of the oral temperature circadian rhythm and intolerance to shiftwork. Nature 1984; 308:272–4.
- 121. Martino TA, Tata N, Belsham DD, et al. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension 2007; 49:1104–13.
- 122. Martino TA, Oudit GY, Herzenberg AM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol 2008; 294:R1675–83.
- 123. Penev PD, Kolker DE, Zee PC, et al. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol 1998; 275:H2334–7.
- 124. Karatsoreos IN, Bhagat S, Bloss EB, et al. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A 2011; 108:1657–62.
- 125. Varcoe TJ, Wight N, Voultsios A, et al. Chronic phase shifts of the photoperiod throughout pregnancy programs glucose intolerance and insulin resistance in the rat. PLoS One 2011; 6:e18504.
- 126. Fonken LK, Workman JL, Walton JC, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A 2010; 107:18664–9.
- 127. Lee S, Donehower LA, Herron AJ, et al. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One 2010; 5:e10995.
- 128. Logan RW, Zhang C, Murugan S, et al. Chronic shift‐lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol 2012; 188:2583–91.
- 129. Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 2012; 4:129ra43. 10.1126/scitranslmed.3003200.
- 130. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science 2010; 330(6009):1349–54.
- 131. McHill AW, Melanson EL, Higgins J, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A 2014; 111(48):17302–7.
- 132. Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift‐work, painting, and fire‐fighting. Lancet Oncol 2007; 8:1065–6.
- 133. International Agency for Research on Cancer. Painting, firefighting, and shiftwork. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol 98. Lyon Cedex: WHO, Published by the International Agency for Research on Cancer, 2010.
- 134. Akerstedt T. Shift work and disturbed sleep/wakefulness. Occup Med 2003; 53:89–94.
- 135. Tepas DI, Maham RP. The many meanings of sleep. Work Stress 1989; 3:93–102.
- 136. Tepas DI, Stock CG, Maltese JW, et al. Reported sleep of shift workers: a preliminary report. In: Chase M, Mitler, M, Walter P, eds. Sleep research, Vol. 7. Los Angeles: Brain Information Research Institute, University of California, 1978.
- 137. Smith MJ, Colligan MJ, Tasto DL. Health and safety consequences of shift work in the food processing industry. Ergonomics 1982; 25(2):133–44.
- 138. Tilley AJ, Wilkinson RT, Drud M. Night and day shifts compared in terms of the quality and quantity of sleep recorded in the home and performance measured at work: a pilot study. In: Reinberg A, Vieux N, Andlauer P, eds. Night and shift work: biological and social aspects. Oxford: Pergamon Press, 1981:187–96.
- 139. Weitzman ED, Godmacher D, Kripke D, et al. Reversal of sleep–waking cycle: effect on sleep stage pattern and certain neuroendocrine rhythms. Trans Am Neurol Assoc 1968; 93:153–7.
- 140. Akerstedt T, Gillberg M. Sleep disturbances and shiftwork. In: Reinberg A, Vieux N, Andlauer P, eds. Night and shift work: biological and social aspects. Oxford: Pergamon Press, 1981:127–37.
- 141. Gillberg M, Akerstedt T. Body temperature and sleep at different times of day. Sleep 1982; 5(4):378–88.
- 142. Walsh JK, Tepas DI, Moss PD. The EEG sleep of night and rotating shiftworkers. In: Johnson LC, Tepas DI, Colquhoun WP, Colligan MJ, eds. The twenty‐four hour workday: Proceedings of a Symposium on Variations in Work–Sleep Schedules. Washington, DC: US Government Print Office, 1981:81–127.
- 143. Tepas DI, Sullivan PJ. Does body temperature predict sleep length, sleepiness, and mood in a lab‐bound population? Sleep Res 1982; II:42.
- 144. Knauth P, Rutenfranz J, Schulz H. Experimental shift work studies of permanent night and rapidly rotating shift systems, II. Behavior of various characteristics of sleep. Int Arch Occup Environ Health 1980; 46:111–25.
- 145. Gadbois C. Women on night shift: interdependence of sleep and off‐the‐job activities. In: Reinberg A, Vieux N, Andlauer P, eds. Night and shift work: biological and social aspects. Oxford: Pergamon Press, 1981:223–7.
- 146. Johnson LC, MacLeod WL. Sleep and awake behavior during gradual sleep reduction. Percept Mot Skills 1973; 36:87–97.
- 147. Grandjean E. Fitting the task to the man, an ergonomic approach. London: Taylor & Francis, 1982.
- 148. Cameron C. A theory of fatigue. Ergonomics 1973; 16(5):633–48.
- 149. Speigel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999; 354:1435–9.
- 150. Grandner MA, Hale L, Moore M, et al. Mortality associated with short sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev 2010; 14(3):191–203.
- 151. Ayas NT, Whit DP, Mason JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med 2003; 27(3):205–9.
- 152. Lyytikainen P, Rakonen O, Lahelma E, et al. Association if sleep duration with weight and weight gain: a prospective follow‐up study. J Sleep Res 2011; 20:298–302.
- 153. Morselli, LL. Sleep and metabolic function. Pflugers Arch 2012; 463(1):139–60.
- 154. Torsvall L, Akerstedt T, Gillander K, et al. Sleep on the night shift: 24‐hour EEG monitoring of spontaneous sleep/wake behavior. Psychophysiology 1989; 26(3):352–8.
- 155. Kecklund G, Akerstedt T. Sleepiness in long distance truck driving, an ambulatory EEG study of night driving. Ergonomics 1993; 36:1007–17.
- 156. Winget CM, DeRoshia CW, Markley CL, et al. A review of human physiological and performance changes associated with desynchronosis of biological rhythms. Aviat Space Environ Med 1984; 55(12):1085–93.
- 157. Kogi K. Introduction to the problems of shiftwork. In: Folkard S, Monk TH, eds. Hours of work. New York: John Wiley & Sons, Inc., 1985:165–84.
- 158. Smith MJ, Colligan MJ, Hurrell JJ Jr. A review of the psychological stress research carried out by NIOSH: 1971–1976. In: New developments in occupational stress. Cincinnati: US. Department of Health and Human Services, 1980:1–9.
- 159. Frese M, Okonek K. Reasons to leave shiftwork and psychological and psychosomatic complaints of former shiftworkers. J Appl Psychol 1984; 69(3):509–14.
- 160. Verhaegan P, Maasen A, Meers A. Health problems in shiftworkers. In: Johnson L, Colquhoun WP, Tepas D, eds. Biological rhythms, sleep, and shift work. Advances in Sleep Research, Vol 7. New York: Spectrum Publications, 1981:271–87.
- 161. Moore‐Ede MC, Richardson GS. Medical implications for shift work. Annu Rev Med 1985; 36:607–17.
- 162. Reinberg A, Motohashi Y, Bourdeleau P, et al. Internal desynchronization of circadian rhythms and tolerance of shiftwork. Chronobiologia 1989; 16:21–34.
- 163. Askenazi IE, Reinberg AE, Motohashi Y. Interindividual differences in the flexibility of human temporal organization: pertinence to jet lag and shiftwork. Chronobiol Int 1997; 14(2):99–113.
- 164. Coleman RM. Shiftwork scheduling for the 1990s. Personnel 1989; 66(1):10–5.
- 165. American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual, 2nd ed. Westchester: American Academy of Sleep Medicine, 2005.
- 166. Sack RL, Auckley D, Auger R, et al. Circadian rhythm sleep disorders: Part I. basic principles, shift work, and jet lag disorders: an American Academy of Sleep Medicine review. Sleep 2007; 30(11):1460–83.
- 167. Waage JS, Moen BE, Pallesen S, et al. Shift work disorder among oil rig workers in the North Sea. Sleep 2009; 32(4):558–65.
- 168. Graeber RC, Lauber JK, Connel LJ, et al. International aircrew sleep and wakefulness after multiple time‐zone flights: a cooperative study. Aviat Space Environ Med 1986; 57(12):B3–9.
- 169. Hãrmã M. Aging, physical fitness and shiftwork tolerance. Appl Ergon 1996; 27(1):25–9.
- 170. Akerstedt T, Froberg J. Interindividual differences in circadian patterns of catecholamine excretion, body temperature, performance and subjective arousal. Biol Psychol 1976; 4:277–92.
- 171. Costa G, Lievore F, Casaletti G, et al. Circadian characteristics influencing interindividual differences in tolerance and adjustment to shiftwork. Ergonomics 1989; 32:373–85.
- 172. Roden KM, Koller M, Pirich K, et al. The circadian melatonin and cortisol secretion pattern in permanent night shift workers. Am J Physiol 1993; 265:R261–7.
- 173. Costa G. Guidelines for the medical surveillance of shiftworkers. Scand J Work Environ Health 1998; 24(S3):151–5.
- 174. Loudon R, Bohle P. Work/non‐work conflict and health in shiftwork: relationships with family status and social support. Int J Occup Environ Health 1997; 3:S71–7.
- 175. Angersbach D, Knauth P, Loskant H, et al. A retrospective cohort study comparing complaints and diseases in day and shift workers. Int Arch Occup Environ Health 1980; 45:127–40.
- 176. Minors DS, Scott AR, Waterhouse JM. Circadian arrhythmia: shiftwork, travel, and health. J Soc Occup Med 1986; 36(2):39–44.
- 177. Colligan MJ, Frock IJ, Tasto D. Shift work: the incidence of medication use and physical complaints as a function of shift. In: Occupational and health symposia, 1978. NIOSH Publication, No. 80‐105. Cincinnati: US Department of Health, Education, and Welfare, 1980:47–57.
- 178. Walker J, De la Mare G. Absence from work in relation to length and distribution of shift hours. Br J Ind Med 1971; 28:36.
- 179. Costa G. The impact of shift and night work on health. Appl Ergon 1994; 27(1):9–16.
- 180. Knutsson A, Bogglid H. Gastrointestinal disorders among shift workers. Scand J Work Environ Health 2010; 36(2):85–95.
- 181. Moore JC, Englert E. Circadian rhythms of gastric acid secretion in man. Nature 1970; 226:1261–2.
- 182. Goo RH, Moore JG, Greenberg E, et al. Circadian variation in gastric emptying of meals in humans. Gastroenterology 1987; 93:515–8.
- 183. Reinberg A, Migraine A, Apfelbaum C. Circadian and ultradian rhythms in the eating behavior and nutrient intake of oil refinery operators (Study 2). Chronobiologia 1979; 6(suppl 1):89–102.
- 184. Stewart AJ, Wahlquist ML. Effect of shiftwork on canteen food purchase. J Occup Med 1985, 27(8):552‐4.
- 185. Tepas DI. Do eating and drinking habits interact with work schedule variables? Work Stress 1990; 4(3):203–11.
- 186. Knutson A, Boggild H. Shiftwork, risk factors and cardiovascular disease: review of disease mechanisms. Rev Environ Health 2000; 15:359–72.
- 187. De Bacquer D, Van Risseghem M, Clays E, et al. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol 2009; 38:848–54.
- 188. Lin YC, Hsiao TJ, Chen PC Persistent rotating shift‐work exposure accelerates development of metabolic syndrome among middle‐aged female employees: a five‐year follow‐up. Chronobiol Int 2009; 26:740–55.
- 189. Haffner SM. Epidemiology of insulin resistance and its relation to coronary artery disease. Am J Cardiol 1999; 84:11J–4.
- 190. Ha M, Park J. Shiftwork and metabolic risk factors of cardiovascular disease. J Occup Health 2005; 47(2):89–95.
- 191. Sheer FA, Hilton MF, Mantzoros CS, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 2009; 106 (11):4453–8.
- 192. Orth‐Gomér K. Intervention on coronary risk factors by changing working conditions of Swedish policemen. In: Harvath M., Frankth E., eds. Psychophysiologic risk factors of cardiovascular diseases, International Symposium, Suppl. 3, Actio. Nerv. Sup. (Praha) Avicenum‐Czechoslovak Med. Prague: Avicenum Czechoslovak Medical Press, 1982.
- 193. DeBacker G, Kornitzer M, Peters H, et al. Relation between work rhythm and coronary risk factors. Eur Heart J 1984; 5(suppl 1):307 (abstract).
- 194. Knutsson A. Relationships between serum triglycerides and gamma‐glutamyltransferase among shift and day workers. J Intern Med 1989; 226:337–9.
- 195. Lavie P, Chillag N, Epstein R, et al. Sleep disturbances in shift‐workers: marker for maladaptation syndrome. Work Stress 1989; 3(1):33–40.
- 196. Harenstam A, Theorell T, Orth‐Gomer K, et al. Shiftwork, decision latitude and ventricular ectopic activity: a study of 24‐hour electrocardiograms in Swedish prison personnel. Work Stress 1987; 1(4):341–50.
- 197. Knutsson A, Anderson H, Berglund U, Serum lipoproteins in day and shift workers: a prospective study. Br J Ind Med 1990; 47:132–4.
- 198. Yamasaki F, Schwartz JE, Gerber LM, et al. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension 1998; 32(3):417–23.
- 199. Morikawa Y, Nakagawa H, Miura K. Relationship between shiftwork and onset of hypertension in a cohort of manual workers. Scand J Work Environ Health 1999; 25(2):100–4.
- 200. Karlsson BH, Knutsson AK, Lindahl BO, et al. Metabolic disturbances in male workers with rotating 3 shiftwork. Results of the WOLF study. Int Arch Occup Environ Health 2003; 76:424–30.
- 201. Boggild H, Knutsson A. Shiftwork, risk factors and cardiovascular disease. Scand J Work Environ Health 1999; 25(2):85–99.
- 202. Brown DL, Feskanich D, Sánchez BN, et al. Rotating night shift work and risk of ischemic stroke. Am J Epidemiol 2009; 169(11):1370–7.
- 203. Knutsson A, Akerstedt T, Jonsson BG, et al. Increased risk of ischemic heart disease in shift workers. Lancet 1986; 12(2):89–92.
- 204. Kawachi I, Colditz GA, Stampfer MJ, et al. Prospective study of shiftworkers and risk of coronary heart disease in women. Circulation 1995; 92 (11):3178–82.
- 205. Karlsson B, Alfredsson L, Knutsson A, et al. Total mortality and cause‐specific mortality of Swedish shift‐ and day workers in the pulp and paper industry in 1952–2001. Scand J Work Environ Health 2005; 31(1):30–5.
- 206. Tenkanen L, Sjoblom T, Hãrmã M. Joint effect of shiftwork and adverse life‐style factors on the risk of coronary heart disease. Scand J Work Environ Health 1998; 24(5):351–7.
- 207. Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Phys Heart Circ Phys 2006; 290:H1–16. 10.1152/ajpheart.00582.2205.
- 208. Knutsson A, Hallquist J, Reuterwall C, et al. Shiftwork and myocardial infarction: a case–control study. Occup Environ Med 1999; 56:46–50.
- 209. Peter R, Alfredsson L, Knuttsson A, et al. Does a stressful psychosocial work environment mediate the effects of shift work on cardiovascular risk factors? Scand J Work Environ Health 1999; 25(4):376–81.
- 210. Puttonen S, Hãrmã M, Hublin C. Shift work and cardiovascular disease: pathways from circadian stress to morbidity. Scan J Work Environ Health 2010; 36(2):96–108.
- 211. Barbadoro P, Santarelli L, Croce N, et al. Rotating shiftwork as an independent risk factor for overweight Italian workers: a cross‐sectional study. PLoS One 2013; 8(5):e63289. 10.1371/journal.pone.0063289.
- 212. Antunes IC, Levandovksi R, Dantas G, et al. Obesity and shift work: chronobiological aspects. Nutr Res Rev 2010; 23:155–68.
- 213. Suwazono Y, Dochi M, Sakata K, et al. Longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity 2008; 16:1887–93.
- 214. Zhao I, Bogossian F, Turner C. Does maintaining of changing shift types affect BMI? J Occup Environ Med 2012; 54(5):525–31.
- 215. Kroenke CH, Spiegelman, D, Manson J, et al. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol 2007; 165(2):175–83.
- 216. Pan A, Schernhammer ES, Sun Q, et al. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 2011; 8(12):e1001141. 10.1371/jornal.pmed.1001141.
- 217. Morikawa Y, Nakagawa H, Miura K, et al. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand J Work Environ Health 2005; 31(3):179–83.
- 218. Suwazono Y, Sakata K, Okubo Y, et al. Long term longitudinal study on the relationship between alternating shiftwork and the onset of diabetes mellitus in male Japanese workers. J Occup Envion Med 2006; 48:455–61.
- 219. McDonald A, McDonald J, Armstrong B, et al. Prematurity and work in pregnancy. Br J Ind Med 1988; 45:56–62.
- 220. Armstrong G, Nolin A, McDonald A. Work in pregnancy and birth weight for gestational age. Br J Ind Med 1989; 46:196–9.
- 221. Axelsson G, Rylander R, Molin I. Outcome of pregnancy in relation to irregular and inconvenient work schedules. Br J Ind Med 1989; 46:393–8.
- 222. Xu X, Ding M, Li B, et al. Association of rotating shiftwork with preterm births and low birth weight among never smoking women textile workers in China. Occup Environ Med 1994; 51(7):470–4.
- 223. Fortier I, Marcoux S, Brisson J. Maternal work during pregnancy and the risks of delivering a small‐for‐gestational‐age or preterm infant. Scand J Work Environ Health 1995; 21(6):412–8.
- 224. Nurimen T. Shift work, fetal development and course of pregnancy. Scand J Work Environ Health 1989; 15: 395–403.
- 225. Saurel‐Cubizolles M, Kaminski M. Pregnant women’s working conditions and their changes during pregnancy: a national study in France. Br J Ind Med 1987; 44:236–43.
- 226. Croteau A, Marcoux S, Brisson C. Work activity in pregnancy, preventive measures, and the risk of delivering a small‐for‐gestational‐age infant. Am J Public Health 2006; 96(5):846–55.
- 227. Bonzini M, Palmer KT, Coggon D, et al. Shift work and pregnancy outcomes: a systematic review with meta‐analysis of currently available epidemiological studies. Br J Obstet Gynaecol 2011; 118(12):1429–37.
- 228. Bonzini M, Coggon D, Godfrey K, et al. Occupational physical activities, working hours and outcome of pregnancy: findings from the Southampton Women’s Survey. Occup Environ Med 2009; 66(10):685–90.
- 229. Saurel‐Cubizolles MJ, Zeitlin J, Lelong N, et al. Employment working conditions, and preterm birth: results from the Europop case–control survey. J Epidemiol Community Health 2004; 58(5):395–401.
- 230. McDonald A, McDonald J, Armstrong B, et al. Fetal death and work in pregnancy. Br J Ind Med 1988; 45:148–57.
- 231. Axelsson G, Ahlborg G Jr., Bodin L. Shift work, nitrous oxide exposure, and spontaneous abortion among Swedish midwives. Occup Environ Med 1996; 53:374–8.
- 232. Infante‐Rivard C, David M, Gauthier R, et al. Pregnancy loss and work schedule during pregnancy. Epidemiology 1993; 4(1):73–5.
- 233. Axelson G, Molin I. Outcome of pregnancy among women living near petrochemical industries in Sweden. Int J Epidemiol 1988; 17(2):363–9.
- 234. Axelsson G, Lutz C, Rylander R. Exposure to solvents and outcome of pregnancy in university laboratory employees. Br J Ind Med 1994; 41:305–12.
- 235. Uehata T, Sasakawa N. The fatigue and maternity disturbances of night workwomen. J Hum Ergol (Tokyo) 1982; 11:465–74.
- 236. Hemminki K, Kyyronen P, Lindbohm ML. Spontaneous abortions and malformations in the offspring of nurses exposed to anesthetic gases, cytostatic drugs, and other potential hazards in hospitals, based on registered information of outcome. J Epidemiol Community Health 1985; 39:141–7.
- 237. Nurminen T. Shift work and reproductive health. Scand J Work Environ Health 1998; 24(suppl 3):28–34.
- 238. Zhu JL, Hjollund NH, Andersen AM, et al. Shift work, job stress and late fetal loss: the national birth cohort in Denmark. J Occup Environ Med 2004; 24(11):1144–9.
- 239. Eskenazi B, Fenster L, Wright S, et al. Physical exertion as a risk factor for spontaneous abortion. Epidemiology 1994; 5(1):6–13.
- 240. Bryant E, Love EJ. Effect of employment and its correlates on spontaneous abortion risk. Soc Sci Med 1991; 33:795–800.
- 241. Bonde JR, Jorgensine KT, Bonzinie M, et al. Risk of miscarriage and occupational activity: a systematic review and meta‐analysis regarding shift work, working hours, lifting, standing, and physical work load. Scan J Work Environ Health 2013; 39(4):325–34.
- 242. Ahlborg G Jr., Axelsson G, Bodin L. Shift work, nitrous oxide exposure and subfertility among Swedish midwives. Int J Epidemiol 1996; 25(4):783–90.
- 243. Bisanti L, Olsen J, Basso O, et al. Shift work and subfecundity: a European multicenter study. J Occup Environ Med 1996; 38(4):352–8.
- 244. Spinelli A., Figà‐Talamanca I, Osborn J. Time to pregnancy and occupation in a group of Italian women. Int J Epidemiol 1997; 26(3):601–9.
- 245. Tuntiseranee P, Olsen J, Geater A, et al. Are long working hours and shiftwork risk factors for subfecundity? A study among couples from southern Thailand. Occup Environ Med 1998; 55(2):99–105.
- 246. Zhu JL, Hjollund NH, Boggild H, et al. Shift work and subfecundity: a causal link or an artefact? Occup Environ Med 2003; 60(9):E12.
- 247. Taylor P, Pocock S. Mortality of shift and day workers 1956–68. Br J Ind Med 1972; 29:201–7.
- 248. Rafnsson V, Gunnarsdottir H. Mortality study of fertilizer manufacturers in Iceland. Br J Ind Med 1990; 47:721–5.
- 249. Tynes T, Hannevik M, Andersen A, et al. Incidence of breast cancer in Norwegian female radio and telegraph operators. Cancer Causes Control 1996; 7(2):197–204.
- 250. Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 2001; 93(20):1557–62.
- 251. Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology 2001; 12:74–7.
- 252. Hansen J. Breast cancer among women who work at night. Epidemiology 2001; 12:588–99.
- 253. Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst 2001; 93:1563–8.
- 254. Schernhammer ES, Kroenke CH, Laden F, et al. Night work and risk of breast cancer. Epidemiology 2006; 17:108–11.
- 255. Lie JA, Roessink J, Kjaerheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes Control 2006; 17:39–44.
- 256. O’Leary ES, Schoenfeld ER, Stevens RG, et al. Electromagnetic fields and breast cancer on long Island Study Group. Shift work, light at night, and breast cancer on Long Island, New York. Am J Epidemiol 2006; 164:358–66.
- 257. Schwartzbaum J, Ahlbom A, Feychting M. Cohort study of cancer risk among male and female shift workers. Scand J Work Environ Health 2007; 33:336–43.
- 258. International Agency for Research on Cancer (IARC). Painting, firefighting and shiftwork, IARC Monographs on the Evaluation of Carcinogenic Risk to Humans, Vol 98. Lyon: IARC, 2010.
- 259. Costa G, Hause E, Stevens R. Shift work and cancer‐considerations on rationale, mechanisms, and epidemiology. Scand J Work Environ Health 2010; 36(2):163–79.
- 260. Pesch B, Harth V, Rabstein S, et al. Night work and breast cancer‐results from the German GENICA study. Scand J Work Environ Health 2010; 36(2):134–41.
- 261. Hansen J, Stevens RG. Case‐control study of shift‐work and breast cancer risk in Danish nurses: impact of shift systems. Eur J Cancer 2011; 48:1722–9.
- 262. Kamdar BB, Tergas AI, Mateen FJ, et al. Night‐shift work and risk of breast cancer: a systematic review and meta‐analysis. Breast Cancer Res Treat 2013; 138(1):291–301. 10.1007/s10549‐013‐2433‐1.
- 263. Jia Y, Lu Y, Wu K, et al. Does night work increase the risk of breast cancer? A systematic review and meta‐analysis of epidemiological studies. Cancer Epidemiol 2013; 37:197–203.
- 264. Wang F, Yeung KL, Chan WC, et al. A meta‐analysis on dose–response relationship between night shift work and the risk of breast cancer. Ann Oncol 2013; 24:2724–32.
- 265. Fritschi L, Erren TC, Glass DC, et al. The association between different night shiftwork factors and breast cancer: a case–control study. Br J Cancer 2013; 109:2472–80.
- 266. Hansen J. Night shiftwork and breast cancer survival in Danish women. Occup Environ Med 2014; 71(21):A26.
- 267. Fu L, Pelicano H, Liu J, et al. The circadian gene Period 2 plays an important role in the tumor suppression and DNA damage response in vivo. Cell 2002; 111:41–50.
- 268. Cos S, Fernandez R, Guezmes A, et al. Influence of melatonin on invasive and metastatic properties of MCF‐7 human breast cancer cells. Cancer Res 1998; 58:4383–90.
- 269. Hill SM, Blask DE. Fracture of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF‐seven) and culture. Cancer Res 1998; 48:6121–6.
- 270. Petranka J, Baldwin W, Biermann J, et al. The non‐prostatic action of melatonin in an ovarian carcinoma cell line. J Pineal Res 1999; 26:129–36.
- 271. Anisimov VN, Popovich IG, Zabezhinski MA. Melatonin and colon carcinogenesis: I. Inhibitory effect of melatonin on development of intestinal tumors induced by 1,2‐dimethylhydrazine in rats. Carcinogenesis 1997; 18:1549–53.
- 272. Cini G, Coronnello M, Mini E, et al. Melatonin has growth‐inhibitory effect on hepatoma AHI30 in the rat. Cancer Lett 1998; 125:51–9.
- 273. Vijayalaxmi T, Thomas CR Jr., Reiter RJ, et al. Melatonin: from basic research to cancer treatment clinics. J Clin Oncol 2002; 20:2575–601.
- 274. Haus E, Smolensky M. Biological clocks and shiftwork: circadian dysregulation and the potential long term effects. Cancer Causes Control 2006; 17:489–500.
- 275. Liu R, Jacob DI, Hansen J, et al. Aberrant methylation of miR‐34b is associated with long‐term shiftwork: a potential mechanism for increased breast cancer susceptibility. Cancer Causes Control 2015; 26(2):171–8.
- 276. Monsees GM, Kraft P, Hankinson S, et al. Circadian genes and breast cancer susceptibility in rotating shift workers. Int J Cancer 2012; 131(11):2547–52.
- 277. Pronk A, Ji BT, Shu XO, et al. Night‐shift work and breast cancer risk in a cohort of Chinese women. Am J Epidemiol 2010; 171:953–9.
- 278. Schernhammer ES, Laden F, Speizer FE, et al. Night shift work and risk of colorectal cancer in nurses’ health study. J Natl Cancer Inst 2003; 95:825–8.
- 279. Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res 2007; 67:10618–22.
- 280. Parent M‐E, El‐Zein M, Rousseau M‐C, et al. Night work and the risk of cancer among men. Am J Epidemiol 2012; 176(9):751–9.
- 281. Lahti TA, Partonen T, Kyyronen P, et al. Night‐time work predisposes to non‐Hodgkin lymphoma. Int J Cancer 2008; 123:2148–51.
- 282. Kubo T, Ozasa K, Mikami K, et al. Prospective cohort study of the risk of prostate cancer among rotating‐shift workers: findings from the Japan Collaborative Cohort Study. Am J Epidemiol 2006; 164(6):549–55.
- 283. Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology 2007; 18(1):182–3.
- 284. Flynn‐Evans EE, Mucci L, Stevens RG, et al. Shiftwork and prostate‐specific antigen in the National Health and Nutrition Examination Survey. J Natl Cancer Inst 2013; 105(17):1292–7.
- 285. Hrushesky WJ, Bjarnason GA. Circadian cancer therapy. J Clin Oncol 1993; 11(7):1403–17.
- 286. Smolensky MH, Hermida RC, Ayala DE, et al. Administration‐time‐dependent effect of blood pressure‐lowering medications: basis for chronotherapy of hypertension. Blood Press Monit 2010; 15:173–80.
- 287. Hermida RC, Ayala DE, Fernandez JR, et al. Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am J Hypertens 2011; 24:383–91.
- 288. Smolensky MH, D’Alonzo GE. Medical chronobiology: concepts and applications. Am Rev Respir Dis 1993; 147:S2–19.
- 289. Smolensky MH, Portaluppi F, Manfredini R, et al. Diurnal and twenty‐four hour patterning of human diseases: acute and chronic common and uncommon medical conditions. Sleep Med Rev 2014; 21:12–22. 10.1016/j.smrv.2014.06.005.
- 290. Faiman C, Moorehouse JA. Diurnal variation in the levels of glucose and related substances in health and diabetic subjects during starvation. Science 1967; 32:111.
- 291. Mejean L, Bicakova‐Rocher A, Kolopp M. Circadian and ultradian rhythms in blood glucose and plasma insulin of healthy adults. Chronobiol Int 1988; 5:227–36.
- 292. Sensi S, Capani F, Bertini M, et al. Circadian variation in insulin response to tolbutamide and related hormonal levels in fasting healthy subjects. Int J Chronobiol 1976; 3:141–53.
- 293. Jenkins DJA. Lente carbohydrate: a newer approach to the dietary management of diabetes. Diabetes Care 1982; 5:634–41.
- 294. Trout DL, Bhathena SJ, Mobarak M, et al. Evidence that therapeutic alterations of a circadian rhythm for gastric emptying response may be possible. Prog Clin Biol Res 1990; 341B:155–65.
- 295. Poole CJ, Wright AD, Nattrass M. Control of diabetes mellitus in shift workers. Br J Ind Med 1992; 49:513–5.
- 296. Durazzo TS, Spencer SS, Duckrow RB, et al. Temporal distributions of seizure occurrence from various epileptogenic regions. Neurology 2008; 70(15):1265–71.
- 297. Pavolva MK, Shea SA, Bromfield EB. Daylight patterns of focal seizures. Epilepsy Behav 2004; 5(1):44–9.
- 298. Engle R, Halberg F, Gully RJ. The diurnal rhythm in EEG discharge and in circulating eosinophils in certain types of epilepsy. Electroencephalogr Clin Neurophysiol 1952; 4:115–6.
- 299. van Campen JS, Valentijn FA, Jansen FE, et al. Seizure occurrence and the circadian rhythm of cortisol: a systematic review. Epilepsy Behav 2015; 47:132–7. 10.1016/j.yebeh.2015.04.071.
- 300. Ellingson RJ, Wilken K, Bennett DR. Efficacy of sleep deprivation as an activation procedure in epilepsy patients. J Clin Neurophysiol 1984; 1:83–101.
- 301. Pratt KL, Mattson RH, Wubers NJ, et al. EEG activation of epileptics following sleep deprivation: a prospective study of 114 cases. Electroencephalogr Clin Neurophysiol 1968; 24:11–5.
- 302. Bergonzi P, Chuunvilla C, Tempesta E. Selective deprivation of sleep stages in epileptics. In: Koella WP, Levi P, eds. Sleep: physiology, biochemistry, psychology, pharmacology, clinical implications, 1st European Congress on Sleep Research, Basel, 1972. Basil: Kayer, 1973.
- 303. Tarp B. Epilepsy and sleep. In: Guilleminault C, ed. Sleeping and waking disorders: indications and techniques. Menlo Park: Addison‐Wesley Publishing Company, 1982:373–81.
- 304. Kendis H, Baron K, Schuele SU, et al. Chronotypes in patients with epilepsy: does the type of epilepsy make a difference? Behav Neurol 2015; 2105:941354. 10.1155/2015/941354.
- 305. Guberan E, Williams MK, Walford J, et al. Circadian variation of FEV in shift workers. Br J Ind Med 1969; 26:121–5.
- 306. Reinberg A, Guillet P, Gervais P, et al. One‐month chronocorticotherapy (Dutimelan 8–15 mite). Control of the asthmatic condition without adrenal suppression and circadian rhythm alteration. Chronobiologia 1977; 4:295–312.
- 307. Smolensky MH. Aspects of human chronopathology. In: Reinberg A, Smolensky MH, eds. Biological rhythms and medicine: cellular, metabolic, physiopathologic, and pharmacologic aspects. New York: Springer‐Verlag, 1983:131–209.
- 308. De Vries, Goei JT, Booy‐Noord H, et al. Changes during 24 hours in lung function and histamine hyperreactivity of the bronchial tree in asthmatic and bronchitic patients. Int Arch Allergy Appl Immunol 1962; 20:93–101.
- 309. Barnes P, FitzGerald G, Brown M, et al. Nocturnal asthma and changes in circulating epinephrine, histamine, and cortisol. N Engl J Med 1980; 303:263–7.
- 310. Mohiuddin A. Martin RJ. Circadian basis of late asthmatic response. Am Rev Respir Dis 1990; 142:1153–7.
- 311. Smolensky M. The chronobiology and chronotherapy of asthma. Paper presented at Clinical Applied Chronobiology Conference, NIH, Bethesda, MD, June 20, 1989.
- 312. Pincus DJ, Szefler SJ, Ackerson LM, et al. Chronotherapy of asthma with inhaled steroids: the effect of dosage timing on drug efficacy. J Allergy Clin Immunol 1995; 95:1172–8.
- 313. Beam WR, Weiner DE, Martin RJ. Timing of prednisone and alterations of airways inflammation in nocturnal asthma. Am Rev Respir Dis 1992; 146(6):1524–30.
- 314. Hetzel MR, Clark TJ. The clinical importance of circadian factors in severe asthma. In: Reinberg A, Halberg F. eds. Chronopharmacology: Proceedings of the Seventh International Congress of Pharmacology, New York: Pergamon Press, 1982:213–21.
- 315. Reinberg A., Vieux N, Andlauer P, et al. Tolerance to shift work: a chronobiological approach. Adv Biol Psychiatry 1983; 11:35–47.
- 316. Colligan MJ, Rosa RR. Shiftwork: effects of social and family life. In: Scott AJ, ed. Shiftwork: occupational medicine. State of the Art Reviews, Vol 5(2). Philadelphia: Hanley & Belfus, 1990:315–22.
- 317. Nilsson C. Social consequences of the scheduling of working hours. In: Reinberg A, Vieux N, Andlauer P, eds. Night and shiftwork: Biological and social aspects. Oxford: Pergamon Press, 1981:187–96.
- 318. Carpentier J, Cazamian P. Night work: Its effects on the health and welfare of the worker. Geneva: ILO, 1977.
- 319. Koller M, Kundi M, Cervinka R. Field studies of shift work at an Austrian oil refinery: I. Health and psychosocial well‐being of workers who drop out of shiftwork. Ergonomics 1978; 21(10):835–47.
- 320. Simon BL. Impact of shift work on individual and families. Fam Soc 1990; 71:342–8.
- 321. Smith L, Folkard S. The impact of shiftwork upon shiftworkers’ partners. Proceedings of the 10th International Symposium on Night and Shiftwork. Sheffield, September 18–22, 1991, International Commission on Occupational Health.
- 322. Wyatt S, Marriott R. Night work and shift changes. Br J Ind Med 1953; 10:164–72.
- 323. Mott PE. Shiftwork: the social, psychological, and physical consequences. Ann Arbor: University of Michigan Press, 1965.
- 324. Åkerstedt T, Torsvall L. Experimental changes in shift schedules: their effects on well‐being. Ergonomics 1978; 21(10):849–56.
- 325. Meer A, Maasen A, Verhaegaen P. Subjective health after six months and after four years of shift work. Ergonomics 1978; 21(10):857–9.
- 326. Holley DC, Winget CM, DeRoshia CM. Effects of circadian rhythm phase alteration on physiological and psychological variables: Implications to pilot performance. NASA Technical Memorandum, 81277. Washington, DC: National Aeronautics and Space Administration, 1981.
- 327. Friedman RC, Bigger JT, Kornfeld DS. The intern and sleep loss. N Engl J Med 1971; 285(4):201–3.
- 328. Boivin D, Czeisler CA, Dijk DJ. Complex interaction of the sleep–wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry 1997; 54:145–52.
- 329. Healy D, Waterhouse JM. The circadian system and affective disorders: clocks or rhythms? Chronobiol Int 1990; 7(11):5–10.
- 330. Healy D, Waterhouse JM. Reactive rhythms and endogenous clocks (Editorial). Psychol Med 1991; 21:557–64.
- 331. Wehr TA. Reply to D. Healy and J. M. Waterhouse, The circadian system and affective disorders: clocks or rhythms? Chronobiol Int 1990; 7(11):11–4.
- 332. Monk TH. Biological rhythms and depressive disorders. In: Mann JJ, Kupfer DJ, eds. The biology of depressive disorders. New York: Plenum Press, 1993:19–122.
- 333. Wehr TA, Goodwin FK, Biological rhythms and psychiatry. In: Arieti S, Brodie K, eds. American handbook of psychiatry, Vol 7, 2nd ed. New York: Basic Books, 1981:46–107.
- 334. Wirz‐Justice A. Antidepressant drugs: effects on the circadian system. In: Wehr TA, Goodwin FK, eds. Circadian rhythms in psychiatry. Los Angeles: Boxwood Press, 1983:235–43.
- 335. Rockwell DA, Hodgson MG, Beljan JR. Psychologic and psychophysiologic responses to 105 days of social isolation. Aviat Space Environ Med 1976; 10:1087–93.
- 336. Lewy AJ, Sack RL, Miller LS, et al. Antidepressant and circadian phase‐shifting effects of light. Science 1987; 135:352–4.
- 337. Rockwell DA, Winget CM, Rosenblatt LS, et al. Biological aspects of suicide: circadian disorganization. J Nerv Ment Dis 1978; 166(12):851–9.
- 338. Sack DA, Nurnberger J, Rosenthal NE, et al. Potentiation of antidepressant medications by phase advance of the sleep–wake cycle. Am J Psychiatry 1985; 142(5):606–8.
- 339. David MM, MacLean AW, Knowles JB, et al. Rapid eye movement latency and mood following a delay of bedtime in healthy subjects: do the effects mimic changes in depressive illness? Acta Psychiatr Scand 1991; 84(1):33–9.
- 340. Frank E, Kupfer DJ, Ehlers CL, et al. Interpersonal and social rhythm therapy for bipolar disorder: integrating interpersonal and behavioral approaches. Behav Ther 1995; 17:144–9.
- 341. Jauhar P, Weller MPI. Psychiatric morbidity and time zone changes: a study of patients from Heathrow Airport. Br J Psychiatry 1982; 140:231–5.
- 342. Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms: a unified approach to understanding. Arch Gen Psychiatry 1988; 45:948–52.
- 343. Ehlers CL, Kupfer DJ, Frank E, et al. Biological rhythms and depression: the role of zeitgebers and zeitstorers. Depression 1993; 1:285–93.
- 344. Tasto DL, Colligan MJ, Polly SJ. Health consequences of shiftwork. NIOSH. Cincinnati: U.S. Department of Health Education and Welfare, 1978.
- 345. Bohle P, Tilley AJ. The impact of night work on psychological well‐being. Ergonomics 1989; 3:1089–99.
- 346. Costa G., Apostali P, d’Andrea F, et al. Gastrointestinal and neurotic disorders in textile shift workers. In: Reinberg A, Vieux N, Andlauer P, eds. Night and shift work: Biological and social aspects. Oxford: Pergamon Press, 1981:187–96.
- 347. Michael‐Briand C., Chopard JL, Guiot A, et al. The pathological consequences of shiftwork in retired workers. In: Reinberg A, Vieux N, Andlauer P, eds. Night and shiftwork: Biological and social aspects. Oxford: Pergamon Press, 1981:399–406.
- 348. Scott AJ, Monk TH, Brink L. Shiftwork as a risk factor for depression. Int J Occup Environ Health 1997; 3(3):S3–9.
- 349. Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rational and reliability. Arch Gen Psychiatry 1978; 35:733–82.
- 350. Monk TH. How to make shift work safe and productive. Des Plains: American Society of Safety Engineers, 1988.
- 351. Monk TH, Folkard S. Strategies for the employer. In: Making shiftwork tolerable. London: Taylor & Francis, 1992:69–75.
- 352. Lowden A, Moreno C, Holmbäck U, et al. Eating and shift work: effects on habits, metabolism, and performance. Scand J Work Environ Health 2010; 36(2):150–62.
- 353. Rohleder N, Kirschbaum C. Effects of nutrition on neuroendocrine stress responses. Curr Opin Clin Nutr Metab Care 2007; 10(4):504–10.
- 354. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009; 89(1):126–33.
- 355. Zvonic S, Floyd ZE, Mynatt RL, et al. Circadian rhythms and the regulation of metabolic tissue function and energy homeostasis. Obesity (Silver Spring) 2007; 15(3):539–43.
- 356. Duchon JC, Keran CM. Relationships among shiftworker eating habits, eating satisfaction, and self‐reported health in a population of US miners. Work Stress 1990; 4(2):111–20.
- 357. Holmbäck U, Lowden A, Åkerfeldt T, et al. The human body may buffer small differences in meal size and timing during a 24‐h wake period provided energy balance is maintained. J Nutr 2003; 133(9):2748–55.
- 358. Dye L, Lluch A, Blundell JE. Macronutrients and mental performance. Nutrition 2000; 16(10):1021–34.
- 359. Lowden A, Holmbäck U, Åkerstedt T, et al. Performance and sleepiness during a 24 h wake in constant conditions are affected by diet. Biol Psychol 2004; 65:251–63.
- 360. Landström U, Knutsson A, Lennernäs M, et al. Onset of drowsiness and satiation after meals with different energy contents. Nutr Health 2001; 15(2):87–95.
- 361. Hãrmã MI, Ilmarinen J. Physical training intervention in female shift workers: I. The effects of intervention on fitness, fatigue, sleep, and psychosomatic symptoms. Ergonomics 1988; 31(1):39–50.
- 362. Mistlberger RE, Skene DJ. Nonphotic entrainment in humans? J Biol Rhythms 2005; 20:339–52.
- 363. Uroponen H, Vuori I, Partine M. Self‐evaluations of factors promoting and disturbing sleep: an epidemiological survey in Finland. Soc Sci Med 1988; 26:443–50.
- 364. Vallet M, Mouret J. Sleep disturbance due to transportation noise: ear plugs vs. oral drugs. Experientia 1984; 40:429–36.
- 365. Doghramji K, Fredman S. Clinical frontiers in the sleep/psychiatry interface. Satellite Symposium of the 1999 American Psychiatric Association Annual Meeting, Golden, CO. Golden: Medical Education Collaborative, 1999.
- 366. Walsh JK, Muehlbach MJ, Walker PK. Acute administration of triazolam for the daytime sleep of rotating shiftworkers. Sleep 1984; 7:223–9.
- 367. Penetar DM, Belenky G, Garrigan JJ, et al. Triazolam impairs learning and fails to improve sleep in a long‐range aerial deployment. Aviat Space Environ Med 1989; 60:594–8.
- 368. Walsh JK, Muehlbach MJ, Schweitzer PK. Hypnotics and caffeine as countermeasures for shiftwork‐related sleepiness and sleep disturbance. J Sleep Res 1995; 4:80–3.
- 369. Ehret CF, Potter VR, Dobia KW. Chronobiological action of theophylline and of pentobarbital as circadian zeitgebers in the rat. Science 1975; 188:1212–4.
- 370. Dews PB, ed. Behavioral effects of caffeine. In: Caffeine: perspectives from recent research. New York: Springer‐Verlag, 1984:86–103.
- 371. Bonnet MH, Arand DL. The use of prophylactic naps and caffeine to maintain performance during a continuous operation. Ergonomics 1994; 37:1009–20.
- 372. Muehlbach MH, Walsh JK. The effects of caffeine on simulated night‐shift work and subsequent daytime sleep. Sleep 1995; 18(1):22–9.
- 373. Smith AP, Brockman R, Flynn A, et al. Investigation of the effects of coffee on alertness and performance during the day and night. Neuropsychology 1993; 27:217–23.
- 374. Kelly T, Gomez S, Engelland S, et al. Repeated administration of caffeine during sleep deprivation does not affect cognitive performance. Sleep Res 1993; 22:336.
- 375. Carrier J, Fenanndez‐Bolanos M, Robillard R, et al. Effects of caffeine are more marked on daytime recovery sleep than on nocturnal sleep. Neuropsychopharmacology 2007; 32:964–72.
- 376. Akerstedt T, Ficca G. Alertness‐enhancing drugs as a countermeasure to fatigue in irregular work hours. Chronobiol Int 1997; 14(2):145–58.
- 377. Cziesler CA, Walsh JK, Roth T, et al. Modafinil for excessive sleepiness associated with shift‐work sleep disorder. N Engl J Med 2005; 353:476–86.
- 378. Morgenthaler T, Lee‐Chiong T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine Report. Sleep 2007; 30(11):1445–59.
- 379. Basner R. Shift‐work sleep disorder: the glass is more than half empty. N Engl J Med 2005; 353(5):520–1.
- 380. Redman J, Armstrong S, Ng KT. Free‐running activity rhythms in the rat: entrainment by melatonin. Science 1983; 219:1081–9.
- 381. Arendt J, Aldhous M, English J, et al. Some effects of jet lag and their alleviation by melatonin. Ergonomics 1987; 30:1379–93.
- 382. Comperatore CA, Krueger GP. Circadian rhythm desynchronosis, jet lag, shift lag, and coping strategies. Occup Med 1990; 5(2):323–42.
- 383. Suhner A, Schlagenhauf P, Johnson R, et al. Comparative study to determine the optimal melatonin dosage form for the alleviation of jet lag. Chronobiol Int 1998; 15(6):655–66.
- 384. Sharkey KM, Foff LF, Eastman CI. Effects of administration on daytime sleep after simulated night shift work. J Sleep Res 2001; 10(3):181–9.
- 385. Folkard S, Arendt J, Clark M. Can melatonin improve shift workers’ tolerance of the night shift? Some preliminary findings. Chronobiol Int 1993; 10(5):315–20.
- 386. Czeisler CA, Kronauer RE, Allan JS, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science 1989; 244:1328–33.
- 387. Czeisler CA, Johnson MP, Duffy JF, et al. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med 1990; 322(18):1253–9.
- 388. Pallesen S, Bjorvatn B, Magerøy N, et al. Measures to counteract the negative effects of night work. Scand J Work Environ Health 2010; 36(2):109–20.
- 389. Åkerstedt T, Kecklun G, Knuttson A. Spectral analysis of sleep electroencephalography in rotating three‐shift worker. Scand J Work Environ Health 1991; 17:330–6.
- 390. Tepas DI. Shiftworker sleep strategies. J Hum Ergol 1982; 11:325–36.
- 391. Dinges DF, Orne MT, Whithouse WG, et al. Temporal Placement of a nap for alertness: contribution of circadian phase and prior wakefulness. Sleep 1987; 10:313–29.
- 392. Rosekind MR, Graber RC, Dinges DF, et al. Crew factors in flight operations. IX. Effects of planned cockpit rests on crew performance and alertness in long haul operations. Technical Memorandum, A‐94134, Moffet Field: NASA, 1995.
- 393. Kogi K. Should shift workers nap? Spread roles and effects of on‐duty napping. In: Hornberger S, Knauth P, Costa G, Folkard S., eds. Shift work in the 21st century. Frankfurt: Peter Lang, 2000:31–6.
- 394. Tepas D. Should a general recommendation to nap be made to workers? In: Hornberger S, Knauth P, Costa G, Folkard S., eds. Shift work in the 21st century. Frankfurt: Peter Lang, 2000:25–30.
- 395. Ribeiro‐Silva F, Rotenberg L, Soares RES, et al. Sleep on the job partially compensates for sleep loss in night‐shift nurses. Chronobiol Int 2006; 26(3):1389–99.
- 396. International Labour Office (ILO). Night work convention No. 171. Geneva: ILO, 1990. Available at http://www.ilo.org/dyn/normlex/en/f?p=NORMLEXPUB:12100:0::NO::P12100_INSTRUMENT_ID:312316 (accessed on June 28, 2016).
- 397. European Council. Concerning certain aspects of working time. Off J Eur Community 1993; L307:18–24 (Council Directive 93/104/EC).
- 398. Rutenfranz J, Knauth P, Angerback D. Shiftwork research issues. In: Johnson LC, ed. Advances in sleep research. New York: Medical and Scientific Books, 1985:165–96.
- 399. Rutnfranz J, Haider M, Koller M. Occupational health measures for night workers and shift workers. In: Folkard S, Monk TH, eds. Hours of work: temporal factors in work scheduling. Chichester: John Wiley & Sons, Ltd, 1985:199–210.
- 400. Costa G. Shift work and occupational medicine. Occup Med 2003; 53:83–8.
- 401. Simon C, Weibel L, Brandenberger G. Twenty‐four‐hour rhythms of plasma glucose and insulin secretion rate in regular night workers. Am J Physiol Endocrinol Metab 2000; 278(3):E413–20.
- 402. Knuttsson A. Health disorders of shiftworkers. Occup Med 2003; 53:103–8.
- 403. Haider M, Cervinka R, Koller M. A destabilization theory of shiftworkers effects. In: Hekkens JJM, Kerkhof GA, Rietveld WJ, eds. Trends in chronobiology. Oxford: Pergamon Press, 1988:209–17.
- 404. Siwolop S, Therrien L, Oneal M, et al. Helping workers stay awake at the switch. Business Week; December 8, 1986, p. 108.
- 405. Knauth P. Changing scheduling: shiftwork. Chronobiol Int 1997; 14(2):159–71.
- 406. Gartner J, Wahl S. Design tools for shift schedules: empowering assistance for skilled designers & groups. Int J Ind Ergon 1998; 21:221–32.
- 407. Schwarenau P, Knauth P, Keisswetter E, et al. Algorithms for the computerized construction of shift systems which meet ergonomic criteria. Appl Ergon 1986; 17:169–76.
- 408. Circadian Technologies, Inc. Control room operator alertness and health in nuclear power plants. Prepared for Electric Power Research Institute, EPRI Rep. No. NP‐6748—project 2184‐7. Palo Alto CA, 1990.
- 409. Mardon S, ed. Some companies meet shiftworkers’ family needs with 24‐hour child care. Should yours? Shift Work Alert 1996; 1(2):9–11.
