Chapter 22
BACTERIA
Christopher J. Martin, Aletheia S. Donahue, and John D. Meyer
ACINETOBACTER SPECIES
Common name for disease: None
Occupational setting
Acinetobacter species are ubiquitous in nature. They are commonly isolated from work settings with moist environments and microenvironments (e.g., swine confinement buildings,1 wastewater treatment plants,2 composting plants,3 poultry-processing plants,4 cotton mills,5 metal-working operations,6 and bakeries.7)
Exposure (route)
Inhalation is the main route of exposure in the occupational setting.
Pathobiology
Acinetobacter species, particularly Acinetobacter baumannii and the closely related species A. pittii and A. nosocomialis, are among the most common causes of healthcare-associated pneumonia and other infections, particularly in intensive care unit (ICU) settings and long-term care facilities.8 Patient-to-patient transmission is frequent in such outbreaks, with isolated case reports of transmission to healthcare workers.9 The number of isolates in healthcare settings increases during times of conflict and following natural disasters, with recent large outbreaks involving soft-tissue infections among previously healthy US soldiers wounded in Afghanistan and Iraq.10
These organisms rarely cause infection outside of the clinical setting. Community-acquired pneumonia has been described in persons with cancer and alcoholism, with a preponderance of case reports during warm, humid months and from Asia and Australia.8
An outbreak of pneumonia caused by A. baumannii has been reported among three individuals working in close proximity in a foundry.11 Two of the cases were fatal, and an examination of the lung tissue identified evidence of a mixed dust pneumoconiosis with features compatible with siderosis in both. The concomitant presence of iron may increase the virulence of this microorganism.12A. Iwoffii has been implicated in an outbreak of hypersensitivity pneumonitis in workers in an automobile parts manufacturing plant using metalworking fluids.6
Exposure to water aerosols, such as metalworking fluids, from environments with contamination involving multiple microorganisms, including Acinetobacter, has been associated with a spectrum of respiratory diseases (asthma, hypersensitivity pneumonitis, bronchitis, humidifier fever) in several occupational settings, mostly involving automotive and aeronautical manufacturing.13 Despite extensive investigation, the specific etiology of disease in these outbreaks remains elusive, and therefore the precise role of Acinetobacter is uncertain.
Diagnosis
Infections with this organism are diagnosed using standard isolation and culture methods of appropriately selected clinical specimens. Rapid molecular methods such as polymerase chain reaction-based assays are increasingly available both to detect Acinetobacter directly from patient specimens and to identify the presence of specific antimicrobial resistance genes.14 Genotyping can help to identify the source of the outbreak and guide infection control measures.
Treatment
Since many Acinetobacter strains have developed multidrug resistance, therapy depends on the clinical setting (healthcare-associated versus community) as well as results of susceptibility testing. There are a wide number of options including cefepime, imipenem, meropenem, ampicillin/sulbactam, tigecycline, colistin, and polymyxin B. Acinetobacter species with resistance to all routinely tested antibiotics have been described in healthcare-associated outbreaks.15
Medical surveillance
There are no recommended medical surveillance activities.
Prevention
Engineering controls and work practices should be aimed at reducing microbial contamination of water and other media. Aerosolized processes involving contaminated water are of particular concern. The use of air-purifying respirators may also be appropriate.
References
- 1. Cormier Y, Tremblay G, Meriaux A, et al. Airborne microbial contents in two types of swine confinement buildings in Quebec. Am Ind Hyg Assoc J 1990; 51:304–9.
- 2. Laitinen S, Kangas J, Kotimaa M, et al. Workers’ exposure to airborne bacteria and endotoxins at industrial wastewater treatment plants. Am Ind Hyg Assoc J 1994; 55(11):1055–60.
- 3. Lundholm M and Rylander R. Occupational symptoms among compost workers. J Occup Med 1980; 22:256–7.
- 4. Fallschissel K, Klug K, Kämpfer P, et al. Detection of airborne bacteria in a German turkey house by cultivation-based and molecular methods. Ann Occup Hyg 2010; 54(8):934–43.
- 5. Delucca AJ and Shaffer GP. Factors influencing endotoxin concentrations on cotton grown in hot, humid environments: a two year study. Br J Ind Med 1989; 46:88–91.
- 6. Zacharisen MC, Kadambi AR, Schlueter DP, et al. The spectrum of respiratory disease associated with exposure to metal working fluids. J Occup Environ Med 1998; 40(7):640–7.
- 7. Domanska A and Stroszejn-Mrowca G. Endotoxin in the occupational environment of bakers: method of detection. Int J Occup Med Environ Health 1994; 7(2):125–34.
- 8. Munoz-Price LS and Weinstein RA. Acinetobacter infection. N Engl J Med 2008; 358(12):1271–81.
- 9. Whitman TJ, Qasba SS, Timpone JG, et al. Occupational transmission of Acinetobacter baumannii from a United States serviceman wounded in Iraq to a health care worker. Clin Infect Dis 2008; 47(4):439–43.
- 10. Centers for Disease Control and Prevention (CDC). Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR 2004; 53:1063–6.
- 11. Cordes LG, Brink EW, Checko PJ, et al. A cluster of Acinetobacter pneumonia in foundry workers. Ann Intern Med 1981; 95:688–93.
- 12. Phillips M. Acinetobacter species In: Bennett JE, Dolin R, and Blaser MJ (eds.), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed. Philadelphia: Elsevier Saunders, 2014.
- 13. Burton CM, Crook B, Scaife H, et al. Systematic review of respiratory outbreaks associated with exposure to water-based metalworking fluids. Ann Occup Hyg 2012; 56(4):374–88.
- 14. Denys GA and Relich RF. Antibiotic resistance in nosocomial respiratory infections. Clin Lab Med 2014; 34(2):257–70.
- 15. Pendleton, JN, Gorman, SP, and Gilmore, BF. Clinical relevance of the ESKAPE pathogens. Exp Rev Anti Infect Ther 2013; 11(3):297–308.
BACILLUS SPECIES
Common names for disease: Anthrax, woolsorter’s disease, ragpicker’s disease, splenic fever.
Occupational setting
Anthrax is an enzootic disease with a worldwide distribution transmitted to humans via contact with animals or animal products. In 2001, an outbreak of bioterrorism-related anthrax occurred from contaminated mail sent through the United States Postal Service.1 Eventually, 23 confirmed or suspected cases were documented, including one in a laboratory worker who handled environmental samples from the outbreak.2
Anthrax is most commonly associated with herbivores, especially cattle, which acquire infection through ingestion of endospores on contaminated soil.3 Potential sources of human exposure are raw wool or hair,4 bone,5 meat,5 and hides or skins imported from areas where anthrax is enzootic, especially Africa and Asia.6 Shepherds, farmers, craft workers, and workers in manufacturing plants using the above materials are at highest risk for occupational anthrax7 and, in the past, textile mills that used these animal products presented a significant occupational hazard.8 Rare cases continue to be reported in these settings.9
Exposure (route)
Naturally occurring anthrax is an extremely rare cause of human disease in the United States.6 Transmission occurs via inhalation, cutaneous contact, or ingestion of endospores. Recent large outbreaks of disease among users of contaminated heroin in several European countries have led to the recognition of a distinct presentation from a fourth route of exposure termed “injectional” or “injection” anthrax.10
The portal of entry will also determine the clinical picture. Cutaneous anthrax cases are the most common and result from endospores being introduced through cuts or abrasions in the skin.11 Endospores are within the respirable size range and are, therefore, deposited at the alveolar level following inhalation.12 Ingestion of meat contaminated with endospores results in gastrointestinal anthrax. There are no known cases of person-to-person transmission of anthrax via the inhalation route. However, endospores are produced in cases of cutaneous anthrax, which have caused widespread contamination and secondary transmission of disease, including in a healthcare setting.13
Pathobiology
Anthrax Bacilli are Gram-positive, rod-shaped bacteria, which produce endospores that are not true spores since they are not the product of reproduction but a dormant form of the bacteria. Endospores are the infectious form of the disease, can persist for decades in the environment, and are resistant to environmental extremes of desiccation, heat, freezing, and ultraviolet light as well as many common disinfectants. The species of greatest concern is B. anthracis, although a recent outbreak of disease resembling cutaneous anthrax among shepherds has been attributed to B. pumilus.14
Upon penetration into the host, the endospores either germinate locally or are phagocytized and transported into the lymphatic system to regional lymph nodes with subsequent germination.15 Within hours of germination, the bacilli produce potent exotoxins, which have multiple physiological effects, causing widespread inflammation, edema, necrosis, hypotension, hypoperfusion, congestion, and hemorrhage.15
Anthrax exists in three primary forms depending upon the route of entry: cutaneous, inhalational, and gastrointestinal. Cutaneous anthrax, which accounts for more than 95% of naturally occurring anthrax cases,16 results from the introduction of endospores into the skin, most commonly on the head and neck or upper extremity, via a wound, penetrating animal fiber, or an insect bite. The incubation period is estimated to range from 1 to 12 days. The endospores germinate and multiply in the subcutaneous tissue, with production of exotoxin causing tissue necrosis. A slowly enlarging papule is first noticed, which then vesiculates, eventually bursting to form a black eschar around which smaller vesicles may appear. The lesion is generally painless and may be associated with impressive local edema, regional lymphadenopathy, and septicemia. The disease may be self-limited and mortality is less than 1% with appropriate therapy, although airway compromise from infections involving the face and neck can occur.16 The diagnosis should be considered in any patient with a painless ulcer with vesicles and edema who has a history of exposure to animals or animal products.
Recently, a new type of anthrax infection, termed injection or injectional anthrax, has been described among European heroin users. No cases have been observed to date in North America. It is thought that contamination may occur because the heroin is produced in Afghanistan and transported through Iran and Turkey, all countries where anthrax is enzootic.17 Unlike cutaneous anthrax, papules, vesicles, and eschars are generally not observed and there is an increased risk of shock and death, with mortality reported to be 37%.10
While inhalation anthrax is very rare in the natural setting, it accounted for 11 of the 22 cases in the bioterrorist attack of 2001.17 Following inhalation and alveolar deposition, endospores are rapidly phagocytized in the terminal alveoli by macrophages and carried to mediastinal lymph nodes. There, the endospores germinate and multiply, producing large amounts of exotoxin. The result is a hemorrhagic, edematous mediastinitis, evidenced by the characteristic widening of the mediastinum on imaging studies. The initial prodromal phase of the illness follows an incubation period of 1–5 days and lasts 3–4 days with nonspecific, flu-like symptoms. Without prompt treatment, a second phase with septic shock, meningitis, and gastrointestinal involvement ensues. Death can occur within 24 hours of the onset of this phase. The mortality approaches 100% without treatment, but with early diagnosis, use of multiple antibiotics, and improvements in supportive care, mortality was 46% among the 11 inhalational cases in the outbreak in the United States.16
Gastrointestinal anthrax is extremely rare outside of enzootic regions and occurs after ingestion of contaminated meat. The incubation period has been estimated to be 42 hours.3 The central feature of this form of disease is ulcers, which can occur anywhere along the gastrointestinal tract from the oral cavity to the cecum, depending upon where endospores are deposited. In the oropharyngeal form, symptoms and signs include fever, anorexia, cervical lymphadenopathy, and edema. In the intestinal form, there is mesenteric lymphadenitis with vomiting, anorexia, fever, abdominal pain, hematemesis, and bloody diarrhea. Ascites, septicemia, intestinal perforation, shock, and death may ensue. The case fatality ratio ranges from 25 to 60%.16
Although most commonly associated with inhalational anthrax, all forms of the disease can lead to shock, which is usually fatal despite aggressive supportive measures.16
Diagnosis
The Centers for Disease Control and Prevention (CDC) has provided definitions for suspected, probable, and confirmed cases of cutaneous, inhalational, gastrointestinal, oropharyngeal, and meningeal anthrax as well detailed guidance on diagnosis.18 Bacilli can be cultured and identified from a variety of appropriately collected clinical specimens such as blood, drainage from cutaneous lesions, cerebrospinal fluid, sputum, pleural fluid, and feces. Several techniques are available to directly and rapidly identify the microorganism from samples, such as the polymerase chain reaction (PCR). A variety of immunological-based methods toward components of both the microorganism and the exotoxins have been developed. Isolates should be sent for confirmatory testing to the CDC’s Laboratory Response Network.19
Treatment
Early antimicrobial therapy is essential. During the 2001 outbreak, all cases of inhalational anthrax treated during the prodromal phase survived, while all those treated later died.17
The approach to treating anthrax differs from that of other bacterial infections in several important ways.16 Because of the potential for the persistence of endospores in the body, prolonged therapy (60 days) is often indicated. Since exotoxins rather than the bacilli mediate many of the effects of anthrax infection, antimicrobials that inhibit protein synthesis and exotoxin production (i.e., clindamycin or linezolid) may need to be added to bactericidal agents (fluoroquinolones).
The treatment of choice for cutaneous anthrax is doxycycline or an oral fluoroquinolone such as ciprofloxacin. Penicillin VK or amoxicillin may be used for penicillin-susceptible strains.16 If systemic illness develops or there is a risk of airway compromise, patients should be treated like other forms of anthrax. Gastrointestinal, inhalational, or injectional anthrax should be treated with intravenous ciprofloxacin combined with either clindamycin or linezolid if meningitis has been excluded. If there is the possibility of meningeal involvement, three-drug therapy is recommended with ciprofloxacin, meropenem, and linezolid being the current antibiotics of choice.16 Antitoxin therapy should be added if there is a high index of suspicion of systemic involvement.16 In 2015, the FDA-approved Anthrasil—Anthrax Immune Globulin Intravenous, Human—to treat patients with inhalational anthrax in combination with appropriate antibacterial drugs.20
Medical surveillance
There are no validated measures to monitor individual’s exposure to anthrax. While the results of serological studies and nasal swabs may be useful for epidemiologic purposes, they should not be used for medical surveillance purposes following suspected exposure of workers.21 The Occupational Safety and Health Administration (OSHA) recommends baseline, periodic, and final evaluations of workers potentially exposed to anthrax, which includes an assessment of contraindications or adverse effects from vaccination or antibiotics.22 Medical monitoring of workers with potential anthrax exposure should be performed within the context of a comprehensive occupational health and safety program which includes a risk assessment for various jobs, a health and safety plan, and on-site monitoring for heat, stress, fatigue, and adverse psychological effects associated with the response, including personal protective equipment, to this highly virulent agent.23
Anthrax is a nationally notifiable disease in the United States and a CDC Category A bioterrorism agent; therefore, suspected cases should be immediately reported to local public health authorities.
Prevention
The National Institute of Occupational Safety and Health (NIOSH) has provided detailed recommendations on personal protective equipment for biological agents, including anthrax, with an orientation toward terrorist-related events.23 The specific level of respiratory protection varies depending upon the hazard and suspected level of exposure from a self-contained breathing apparatus to a full facepiece air-purifying respirator.
Historically in the industrial setting, measures such as the washing of potentially contaminated material, mechanization, improved ventilation, and hygiene of work areas have all been successful in greatly reducing occupational cases of anthrax infection.24 OSHA provides detailed recommendations on the management of anthrax in the workplace including sampling, personal protective equipment, and decontamination measures.22 Because of the well-known resistance of Bacillus endospores to many commonly used disinfection measures, careful attention must be paid to the correct choice of agent. Ethylene oxide, chlorine dioxide, paraformaldehyde, and irradiation have appropriate endosporicidal activity.22 Alcohol, alcohol-based sanitizers,25 and ultraviolet irradiation26 are considered ineffective.
In the healthcare setting, standard precautions are recommended with contact precautions for cases of cutaneous anthrax with uncontained drainage.27 In the agricultural setting, anthrax has been successfully mitigated through vaccination of livestock and epidemiologic measures to promptly identify, trace, and dispose of infected animals through incineration.28 Since such measures are not uniformly applied throughout the world, added precautions should be taken when handling animal products imported from higher risk countries.
The Advisory Committee on Immunization Practices (ACIP)’s recommendations for prophylactic anthrax vaccination are summarized in Table 22.1.29
TABLE 22.1 ACIP recommendations for anthrax vaccination.
Source: MMWR Recommendation Report 2010 Jul 23;59(RR-6):1–30.
| Occupation/Group | Vaccine recommendation |
| General population | Not recommended prior to a bioterrorism event. |
| Medical personnel | Not recommended. |
| Persons who handle animals or animal products | Only recommended when other preventive measures deemed insufficient. |
| Persons who routinely have contact with animal hide drums or animal hides | Not recommended. |
| U.S. veterinarians and animal husbandry technicians | Not recommended, unless at higher risk due to potential exposures from work involving enzootic areas or research settings. |
| Laboratory workers | Only recommended for personnel with repeated exposure to endospores, especially with the potential for aerosolization. |
| Workers in postal processing facilities | Not recommended |
| Military personnel | Only when deemed to have a “calculable risk” of exposure to aerosolized endospores. |
| Environmental investigators and remediation workers | Recommended for those who repeatedly enter areas contaminated with endospores. |
| Emergency responders | Only for those whose response activities may involve exposure to endospores. Vaccination should be voluntary and incorporated within a broader occupational health and safety program. |
After exposure to anthrax, post-exposure prophylaxis consisting of ciprofloxacin or doxycycline as well as vaccination is recommended.29 Anthrax immune globulin has also been approved for post-exposure prophylaxis.
References
- 1. Centers for Disease Control and Prevention. Investigation of bioterrorism-related anthrax and adverse events from antimicrobial prophylaxis. JAMA 2001; 286(20):2536–7.
- 2. Centers for Disease Control and Prevention. Public health dispatch: update: cutaneous anthrax in a laboratory worker-Texas, 2002. JAMA 2002; 288(4):444.
- 3. Sweeney DA, Hicks CW, Cui X, et al. Anthrax infection. Am J Respir Crit Care Med 2011; 184(12):1333–41.
- 4. Kissling E, Wattiau P, China B, et al. B. anthracis in a wool-processing factory: seroprevalence and occupational risk. Epidemiol Infect 2012; 140(5):879–86.
- 5. Brandes Ammann A and Brandl H. Anthrax in the canton of Zurich between 1878 and 2005. Schweiz Arch Tierheilkd 2007; 149(7):295–300.
- 6. Nguyen TQ, Clark N, and the 2006 NYC Anthrax Working Group. Public health and environmental response to the first case of naturally acquired inhalational anthrax in the United States in 30 years: infection of a New York City resident who worked with dried animal hides. J Public Health Manag Pract 2010; 16(3):189–200. doi:10.1097/PHH.0b013e3181ca64f2.
- 7. Shafazand S, Doyle R, Ruoss S, et al. Inhalational anthrax: epidemiology, diagnosis and management. Chest 1999; 116:1369–76.
- 8. Stone SE. Cases of malignant pustule. Boston Med Surg J 1868; I:19–21.
- 9. Winter H and Pfisterer RM. Inhalation anthrax in a textile worker: non-fatal course. Schweiz Med Wochenschr 1991; 121(22):832–5.
- 10. Berger T, Kassirer M, and Aran AA. Injectional anthrax – new presentation of an old disease. Euro Surveill 2014; 19(32):pii: 20877.
- 11. Goel AK. Anthrax: a disease of biowarfare and public health importance. World J Clin Cases 2015; 3(1):20–33.
- 12. Duncan EJ, Kournikakis B, Ho J, et al. Pulmonary deposition of aerosolized Bacillus atrophaeus in a Swine model due to exposure from a simulated anthrax letter incident. Inhal Toxicol 2009; 21(2):141–52.
- 13. Yakupogullari Y and Koroglu M. Nosocomial spread of Bacillus anthracis. J Hosp Infect 2007; 66(4):401–2.
- 14. Tena D, Martinez-Torres JA, Perez-Pomata MT, et al. Cutaneous infection due to Bacillus pumilus: report of 3 cases. Clin Infect Dis 2007; 44(4):e40–2.
- 15. Hendricks KA, Wright ME, Shadomy SV, et al. Centers for disease control and prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis 2014; 20(2):e130687. doi:10.3201/eid2002.130687.
- 16. Martin GJ and Friedlander AM. Bacillus anthracis (Anthrax). In Bennett JE, Dolin R, and Blaser MJ (eds.), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed. New York: Saunders, 2014.
- 17. Jernigan DB, Raghunathan PL, Bell BP, et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis 2002; 8(10):1019–28.
- 18. Centers for Disease Control and Prevention, National Notifiable Diseases Surveillance System, Anthrax (Bacillus anthracis): 2010 Case Definition. Available at: http://wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=609&DatePub=1/1/2010%2012:00:00%20AM (accessed on June 1, 2016).
- 19. Centers for Disease Control and Prevention (CDC). The Laboratory Response Network Partners in Preparedness. Available at: http://www.bt.cdc.gov/lrn/ (accessed on June 1, 2016).
- 20. U.S. Food and Drug Administration. FDA Approves Treatment for Inhalation Anthrax. 2015. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm439752.htm (accessed on June 1, 2016).
- 21. Centers for Disease Control and Prevention (CDC). Interim guidelines for investigation of and response to Bacillus anthracis exposures. MMWR 2001; 50(44):987–90.
- 22. Occupational Safety and Health Administration. eTools: Anthrax: How Should I Decontaminate During Response Actions? Available at: https://www.osha.gov/SLTC/etools/anthrax/decon.html (accessed on June 1, 2016).
- 23. National Institute for Occupational Safety and Health (NIOSH), Recommendations for the Selection and Use of Respirators and Protective Clothing for Protection against Biological Agents. 2009. DHHS (NIOSH) Publication Number 2009-132. Available at: http://www.cdc.gov/niosh/docs/2009-132/default.html (accessed on June 30, 2016).
- 24. Brachman PS. Inhalation anthrax. Ann N Y Acad Sci 1980; 353:83–93.
- 25. Weber DJ, Sickbert-Bennett E, Gergen MF, et al. Efficacy of selected hand hygiene agents used to remove Bacillus atrophaeus (a surrogate of Bacillus anthracis) from contaminated hands. JAMA 2003; 289(10):1274–7.
- 26. Spotts Whitney EA, Beatty ME, Taylor TH Jr, et al. Inactivation of Bacillus anthracis spores. Emerg Infect Dis 2003; 9(6):623–7.
- 27. Siegel JD, Rhinehart E, Jackson M, et al. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(10 Suppl 2):S65–164.
- 28. Shadomy SV and Smith TL. Zoonosis update. Anthrax. J Am Vet Med Assoc 2008; 233(1):63–72.
- 29. Wright JG, Quinn CP, Shadomy S, et al. Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2010; 59(RR-6):1–30.
BORRELIA SPECIES
Common names for disease: Lyme disease, Lyme borreliosis
Occupational setting
Lyme disease is a zoonosis and the most common vector-borne disease of humans in temperate regions of the Northern Hemisphere, including the United States. The number of confirmed cases in the United States peaked at 29,959 in 2009 and has been roughly stable over the past several years.1 The disease is found worldwide, with important foci in forested areas of Europe, Asia, and North America. The distribution of the disease parallels the distribution of the vectors.2 In the United States, the vast majority of cases occur within two main geographic regions: the northeastern coastal states from Virginia to Maine and the Upper Midwest, primarily Wisconsin and Minnesota (Figure 22.1).
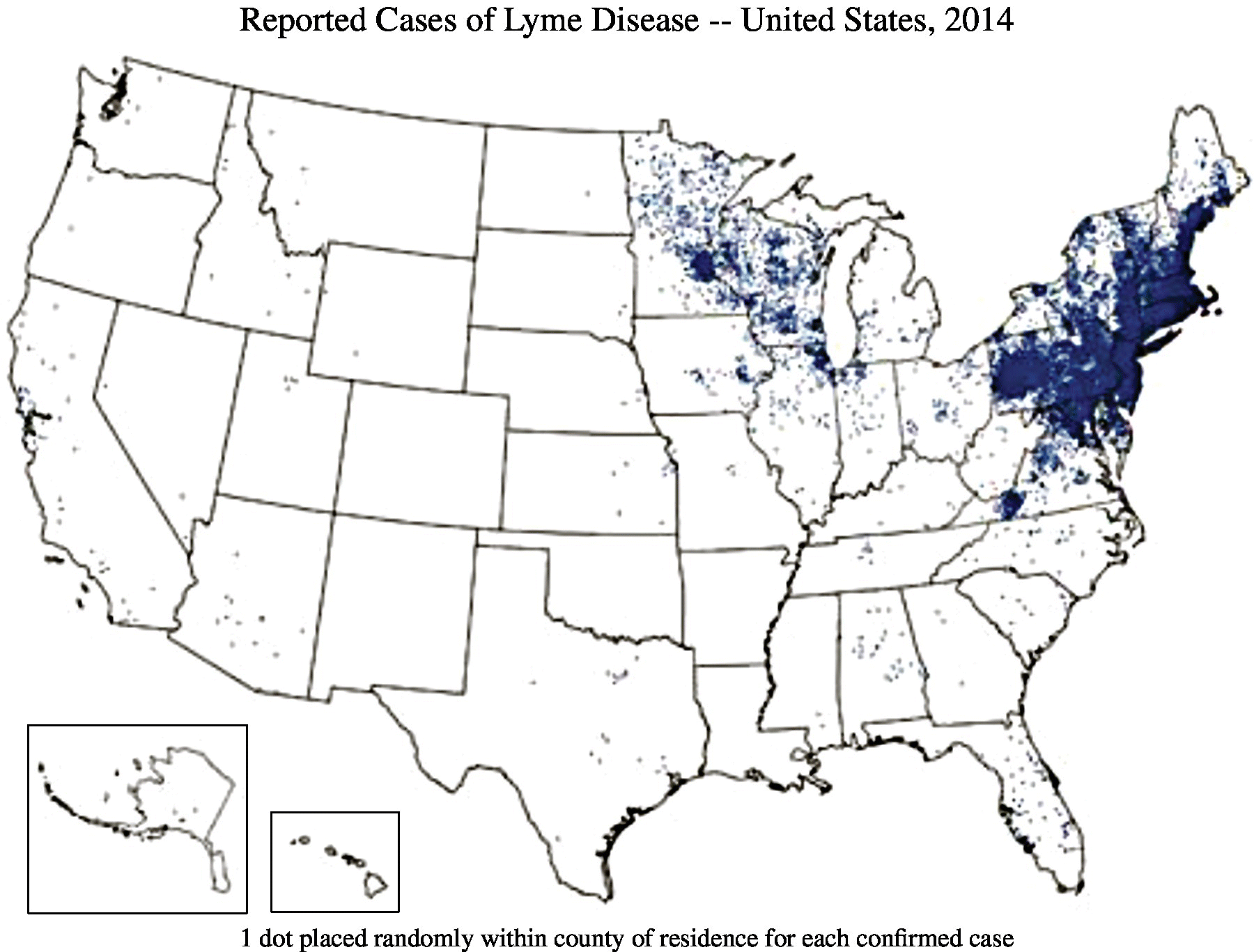
FIGURE 22.1 Reported cases of Lyme disease in USA, 2014.
All outdoor workers in endemic areas should be considered at an increased risk of Lyme disease. Specific occupational groups of concern include forestry and agricultural workers,3 military recruits,4 hunters,5 and urban park workers.6
Exposure (route)
Transmission to humans occurs through the bite of ticks of the Ixodes genus (hard-bodied ticks) infected by Borrelia bacteria. These include I. scapularis (deer tick or blacklegged tick) in the northeastern and upper midwestern United States, I. pacificus (Western blacklegged tick) in the western United States, I. ricinus (castor bean tick) in Europe, and I. persulcatus (taiga tick) in Asia.
The tick vectors of Lyme disease have a 2-year life-span, which takes place in three stages: larva, nymph, and adult. Larval and nymphal ticks feed only once during the season before overwintering and emerging into the next stage the following year. Feeding activity for larvae peaks in the late summer/early fall, for nymphs during the summer, and for adults in the early spring and late fall. Although the subadult stages of Ixodes ticks are not species-specific in seeking hosts for their blood meals, the major reservoirs for Borrelia are small mammals such as chipmunks or mice as well as birds.7Ixodes ticks generally feed on their hosts for 3–5 days, increasing manyfold in size and weight during this time. Studies in rodents suggest that B. burgdorferi transmission does not occur when ticks are feeding on a host for less than 24 hours, with the highest risk being between 48 and 72 hours.8
Only nymphs and adult ticks are infected by Borrelia. However, as adults are much larger, they are usually discovered and removed before transmission can occur. As a result, the vast majority of Lyme disease in humans results from smaller nymph bites during summer months. Often, prolonged tick attachment occurs in less noticeable areas such as hair-covered parts of the body: the axilla and groin.
There is no evidence of person-to-person, airborne, waterborne, or foodborne transmission of disease. Although concern has been raised about the possibility of in utero transmission based on case reports, no convincing evidence of adverse effects in pregnancy among infected mothers has been demonstrated in large studies.9
Pathobiology
In the United States, Lyme disease is only caused by Borrelia burgdorferi sensu stricto, whereas in Europe and in Asia, B. afzelii, B. garinii as well as at least nine other species are additional causes of disease.7Borrelia are fastidious spirochetes that are difficult to culture from infected patients, and hence diagnosis is based most frequently on clinical and serologic criteria rather than isolation of the organism from cases.
After a tick bite that results in the transmission of spirochetes, the most common sign of Lyme disease is a distinctive rash at the site of attachment known as erythema migrans, which develops after 3–32 days.7 Although this rash is classically described as having an erythematous border surrounding a clear center with the appearance of a “bull’s-eye” in an archery target, most cases have either enhanced central or uniform erythema. The lesion is usually solitary and can expand to reach more than 60 cm in diameter. Erythema migrans may be asymptomatic, mildly pruritic, or painful.
This early, or localized, infection may be accompanied by more generalized symptoms of fever, headache, arthralgias, malaise, and fatigue. In untreated patients, erythema migrans can resolve within 3–4 weeks. Approximately 20% of patients will present with these nonspecific symptoms only without cutaneous findings.10
About 2–3% of patients will go on to develop disseminated disease within days or weeks.10 The earliest sign of systemic involvement is multiple, smaller lesions of erythema migrans. Patients at this stage are generally ill with severe malaise, fatigue, headaches, and arthralgias. The manifestations of the disseminated stage can be protean, but several syndromes are of interest. Carditis usually presents as conduction abnormalities (the most characteristic being atrioventricular nodal block), which can be asymptomatic but can also result in sudden death.11 The nervous system is also an important site of early disseminated infection, with meningitis, neuropathy of several different cranial nerves (most commonly a Bell’s palsy), peripheral neuropathy, encephalitis, radiculoneuritis, and myelitis all being described. Arthritis, which most commonly affects the knee, is an additional complication of late-stage, disseminated disease. Without therapy, many of these manifestations can last weeks or months, recur, or become chronic.
In a small number of cases, subjective symptoms persist for months or years after completion of appropriate antimicrobial therapy and resolution of objective abnormalities. This condition has been labeled by some as “chronic” Lyme disease and has been an area of controversy. However, multiple, well-designed clinical trials have shown no benefit from prolonged antimicrobial treatment in ameliorating these subjective symptoms, which are highly nonspecific.7
Diagnosis
Lyme disease is a clinical diagnosis based on compatible symptoms and signs as well as a history of a possible bite by the appropriate type of tick.
As noted previously, Borrelia can be difficult to culture from patients, so serologic tests are the mainstay to confirm the diagnosis. CDC recommends a two-step approach with initial testing using enzyme immunoassay or immunofluorescence assay, followed by testing with the more specific Western blot test to confirm positive results or further test equivocal results as summarized in Figure 22.2.12
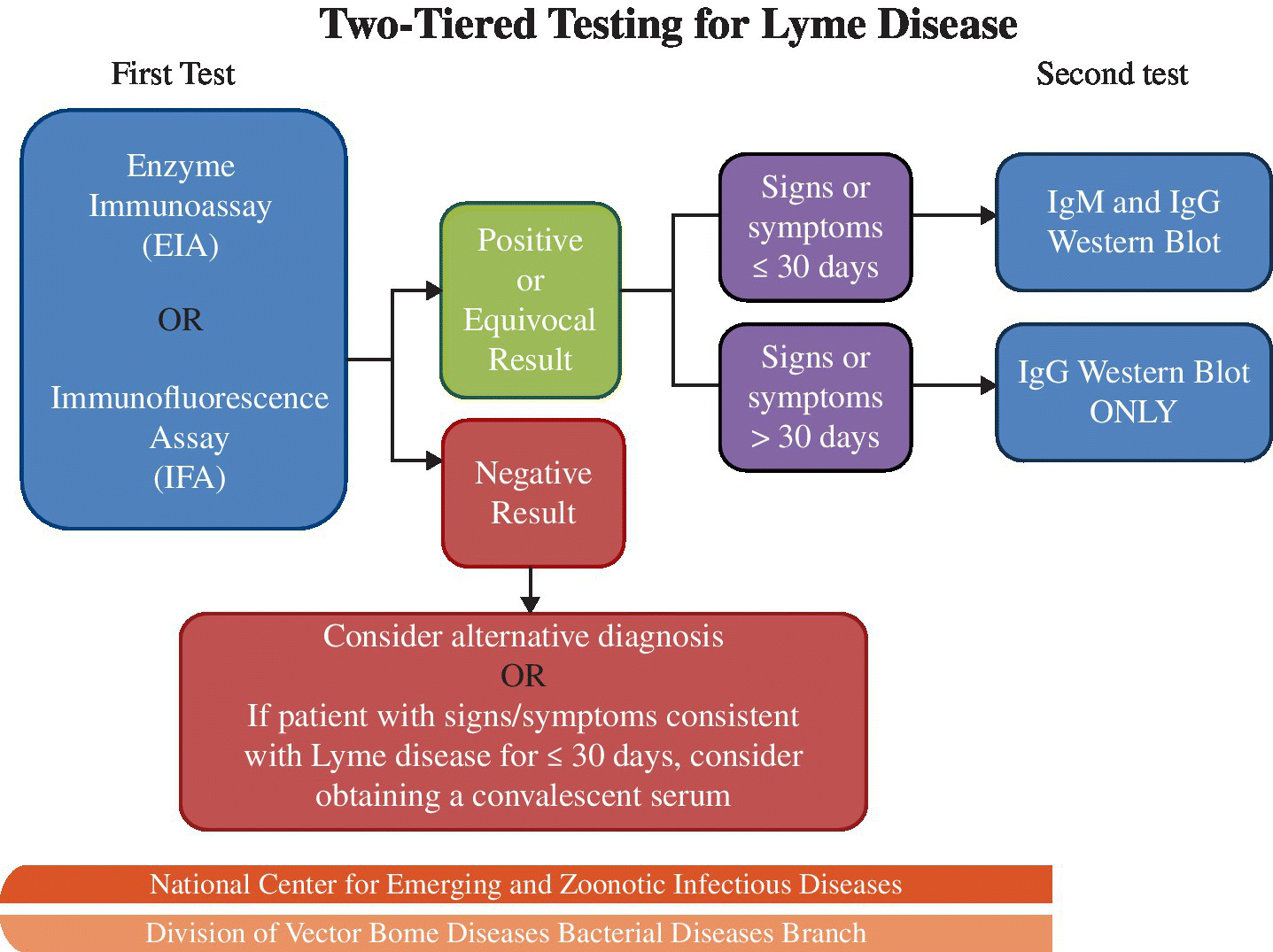
FIGURE 22.2 CDC diagnostic testing algorithm for Lyme disease.
Source: MMWR 1995; 44:590–1.
Diagnostic difficulties associated with Lyme disease have been well reported in the lay and scientific literatures. Although sensitivity increases over time following infection and with disseminated disease, it is poor throughout all phases, including convalescence.7
Treatment
Early treatment is important to prevent the complications from disseminated disease. Doxycycline, amoxicillin, and cefuroxime are appropriate agents administered orally for 14 days for the treatment of erythema migrans, all with reported cure rates of approximately 90%.13 This course should be extended to 28 days for cases with arthritis. Parental therapy with the same agents is indicated when carditis is present, while ceftriaxone or cefotaxime are recommended when there is meningeal involvement.
Approximately 15% of patients will develop worsening cutaneous findings and abrupt onset of arthralgias, myalgias, and increased fever usually within 24 hours of antimicrobial treatment as a result of endotoxins released from bacteriolysis (the Jarisch–Herxheimer reaction). This condition is usually self-limited within 48 hours and can be treated symptomatically with nonsteroidal anti-inflammatory drugs.7 However, delayed onset and more severe reactions necessitating a discontinuation of antimicrobial therapy have also been reported.14
Medical surveillance
There are no currently recommended medical screening activities for Lyme disease. The use of anti-Borrelia antibody screening is not recommended both because of low sensitivity in detecting early infection and lack of specificity in distinguishing past exposure from current infection. Lyme disease is a nationally notifiable disease in the United States and cases must be reported to public health authorities.
Prevention
Tick populations can be controlled for a short time in the environment through the application of commercially available pesticides and removal of leaf litter.15 Recent studies have shown reductions by about two-thirds in the prevalence of infected ticks following use of an oral vaccine targeting a mouse reservoir.16 However, effectiveness in decreasing disease transmission to humans has not yet been demonstrated.
Personal preventive measures are the preferred means to reduce disease transmission. Workers should be advised to avoid contact with areas of heavy undergrowth and leaf litter wherever possible. Other preventive behaviors—wearing light-colored clothing for easier visualization of ticks, tucking trousers into socks, and wearing long-legged trousers and long-sleeved shirts—are recommended for their ease and low cost.
Insect repellents containing concentrations of at least 20% DEET (N,N-diethyl-m-toluamide) are effective after application to the skin. Clothing, tents, and footwear purchased pre-treated or treated with 0.5% permethrin provide protection from questing ticks even after several launderings because of binding of the insecticide to the fibers. Whereas DEET repels, permethrin rapidly kills ticks, and field studies have revealed decreases in the number of tick bites among outdoor workers who wear permethrin-treated clothing.17 All gear, clothing, and any pets should be examined for the presence of ticks on a daily basis. Ticks on clothing can be killed in a laundry drier at high heat for 1 hour.
It is important to emphasize that studies in animals suggest that the spirochete is not transmitted from tick to host until after 24 hours of feeding.8 Thus, workers should be advised to conduct a careful head-to-toe inspection of the body each day. Bathing or showering as soon as possible after work can both aid in identifying and washing away ticks.
Attached ticks should be removed with fine-tipped tweezers using slow, upward pressure, attempting to avoid breaking off the mouth parts.18 After removal of the tick and any broken-off mouth parts, the area should be disinfected or washed with soap and water and the tick disposed by flushing down the toilet, submerging in alcohol, or sealing in plastic. The tick should not be crushed by hand.18
The use of prophylactic doxycycline for asymptomatic individuals with a history of tick bite in an endemic area remains controversial. Although effective in preventing Lyme disease, the low risk of acquiring infection after a single tick bite (from 1 to 3% in highly endemic regions) and high cure rate of symptomatic cases indicate to many that prophylactic therapy is not warranted.7 The number needed to treat for people bitten by a deer tick has been calculated to be 50 to prevent one case of erythema migrans.7 Vigilance for early symptoms and signs in persons who have recently sustained a tick bite or who have removed a tick is recommended in place of tick-bite prophylaxis in most instances. However, an exception may be made when there is evidence of prolonged tick attachment, such as a history of removal of an engorged nymphal tick.7
Manufacture of a Lyme disease vaccine for use in humans was discontinued in 2002 due to poor sales and perceptions of safety concerns. Since protection declines over time, workers reporting prior vaccination should not be regarded as immune. New vaccines are under development.19
References
- 1. Centers for Disease Control and Prevention. Lyme Disease: Lyme Disease Data. Available at: http://www.cdc.gov/lyme/stats/index.html (accessed on June 1, 2016).
- 2. Centers for Disease Control and Prevention. Appendix methods used for creating a national lyme disease risk map. MMWR, 1999; 48(RR07):21–24. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4807a2.htm (accessed on June 30, 2016).
- 3. Tokarska-Rodak M, Plewik D, Kozioł-Montewka M, et al. Risk of occupational infections caused by Borrelia burgdorferi among forestry workers and farmers. Med Pr 2014; 65(1):109–17.
- 4. Oksi J, Viljanen MK. Tick bites, clinical symptoms of Lyme borreliosis, and Borrelia antibody responses in Finnish army recruits training in an endemic region during summer. Mil Med 1995; 160(9):453–6.
- 5. Cetin E, Sotoudeh M, Auer H, et al. Paradigm Burgenland: risk of Borrelia burgdorferi sensu lato infection indicated by variable seroprevalence rates in hunters. Wien Klin Wochenschr 2006; 118(21–22):677–81.
- 6. Krstić M and Stajković N. Risk for infection by lyme disease cause in green surfaces maintenance workers in Belgrade. Vojnosanit Pregl 2007; 64(5):313–8.
- 7. Shapiro ED. Lyme disease. N Engl J Med 2014; 370:1724–31.
- 8. des Vignes F, Piesman J, Heffernan R, et al. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J Infect Dis 2001; 183(5):773–8.
- 9. Lakos A, Solymosi N. Maternal Lyme borreliosis and pregnancy outcome. Int J Infect Dis 2010; 14(6):e494–8.
- 10. Steere AC and Sikand VK. The presenting manifestations of Lyme disease and the outcomes of treatment. N Engl J Med 2003; 348:2472–4.
- 11. Centers for Disease Control and Prevention. Three sudden cardiac deaths associated with Lyme carditis – United States, November 2012–July 2013. MMWR Morb Mortal Wkly Rep 2013; 62(49):993–996. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6249a1.htm (accessed June 30, 2016).
- 12. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR 1995; 44:590–1.
- 13. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–134.
- 14. Kadam P, Gregory NA, Zelger B, et al. Delayed onset of the Jarisch-Herxheimer reaction in doxycycline-treated disease: a case report and review of its histopathology and implications for pathogenesis. Am J Dermatopathol 2015; 37(6):e68–74.
- 15. Hayes EB and Piesman J. How can we prevent Lyme disease? N Engl J Med 2003; 348:2424–30.
- 16. Richer LM, Brisson D, Melo R, Ostfeld RS, et al. Reservoir targeted vaccine against Borrelia burgdorferi: a new strategy to prevent Lyme disease transmission. J Infect Dis 2014; 209(12):1972–80.
- 17. Richards SL, Balanay JAG, and Harris JW. Effectiveness of permethrin-treated clothing to prevent tick exposure in foresters in the central Appalachian region of the USA. Int J Environ Health Res 2014; 7:1–10.
- 18. Centers for Disease Control and Prevention. Lyme Disease: Tick Removal. Available at: http://www.cdc.gov/lyme/removal/index.html (accessed on June 1, 2016).
- 19. Wressnigg N, Pöllabauer EM, Aichinger G, et al. Safety and immunogenicity of a novel multivalent OspA vaccine against Lyme borreliosis in healthy adults: a double-blind, randomised, dose-escalation phase 1/2 trial. Lancet Infect Dis 2013;13(8):680–9.
BRUCELLA SPECIES
Common names for the disease: Brucellosis, Bang’s disease, Mediterranean fever, undulant fever, Neapolitan fever, Malta fever, Gibraltar fever, Cyprus fever.
Occupational setting
Brucellosis is the most common zoonotic infection globally and is caused by transmission of several species of the genus Brucella from different animals (Table 22.2).1 As such, a high index of suspicion is warranted in travelers or military personnel returning from endemic areas, especially the Middle East.2
TABLE 22.2 Host animals for Brucella species causing disease in humans (adapted from CDC: http://www.cdc.gov/brucellosis/veterinarians/host-animals.html)
| Species | Main Host(s) | Less Common Host(s) |
| B. abortus | Cattle, water buffalo, bison | Pigs, elk, horses |
| B. melitensis | Goats, sheep, camel | |
| B. suis | Pigs, feral swine, boar | Cattle, horses, caribou, reindeer and hares |
| B. canis | Dogs | Foxes |
| B. pinnipedialis also known as B. pinnipediae | Pinnipeds (seals, sea lions, walruses) | |
| B. ceti also known as B. cetaceae | Cetaceans (dolphins, porpoises, whales) |
Species well recognized to cause disease in humans include B. abortus, B. melitensis, B. canis, and B. suis. Of these, B. melitensis is the most virulent species and causes the majority of cases diagnosed in humans globally. B. abortus is a common species implicated in occupational infections acquired in the agricultural setting, while B. abortus RB51 (RB51) and B. abortus S19 (S19) are attenuated strains used in vaccines for cattle, which can cause brucellosis in humans.3 More recently identified species from marine mammals (B. pinnipedialis and B. ceti) have been identified very rarely as causes of disease in humans.4,5
Reported cases of brucellosis in humans in the United States have declined from a peak of 6341 in 1947 to the current plateau of about 100 per year,6 primarily as a result of animal control methods, including vaccination, inspection, and prompt segregation of diseased animals (Figure 22.3). Brucellosis has been eradicated in all cattle herds in the United States apart from areas of Idaho, Wyoming, and Montana, adjacent to the Grand Teton National Park and Yellowstone National Park as a result of spillover from elk.7

FIGURE 22.3 Number of reported cases of brucellosis – United States and U.S. territories, 2010.
Source: http://www.cdc.gov/brucellosis/resources/surveillance.html.
Brucellosis can be transmitted by consumption of unpasteurized milk or milk products such as cheese8 as well as raw or undercooked meat.9 This mode of transmission is still the most frequent source worldwide. Occupational activities with particularly high risk of exposure include animal slaughter, meat processing, meat-packing, hunting, and milking, or handling of semen, aborted animal fetal tissue, placentas, laboratory specimens, and Brucella vaccines. Occupations with the highest risk of exposure are livestock handling and slaughterhouse workers,10 veterinarians,11 meat-packers,12 farmers, dairy workers,13 and hunters.14 Brucellosis is considered to be the most common laboratory-acquired infection15 with outbreaks also described following exposure to attenuated vaccines.16 Marine species of Brucella have been implicated in potential occupational exposures among university and laboratory employees performing a rescue and subsequent necropsy of an infected porpoise.17
Exposure (route)
Occupational infection usually occurs through direct contact or inhalation. Because the bacteria are easily aerosolized and have a low infectious dose, inhalational exposure is a significant concern, especially among slaughterhouse workers18 and laboratory personnel as a result of mouth pipetting and sniffing of cultures.19 The bacteria also enter the body by penetrating the mucosa of the mouth, or throat, or through the conjunctiva when infected material is splashed or sprayed. Transmission through the skin, particularly in slaughterhouse, abattoir workers, and veterinarians, may occur through cuts, abrasions, or percutaneously via injuries from sharps.
Ingestion is less likely in the occupational setting but remains an important route of exposure in cases transmitted by infected dairy products or meat products.9 Transmission has rarely been reported from person to person, breast-feeding, sexual activity, blood transfusion, and tissue transplantation.
Pathobiology
Brucella organisms are small, nonmotile, aerobic, Gram-negative rods. They are facultative intracellular parasites, a property that allows these bacteria to evade the immune system. They can survive phagocytosis by neutrophils and macrophages and spread hematogenously throughout the host once within the bloodstream.
The clinical course is variable with an incubation period ranging from 1 week to several months. Although frequently described as having protean manifestations, fever, usually accompanied by chills, is always present.20 Malodorous perspiration is regarded as being almost pathognomonic and additional constitutional symptoms are usually present. Any organ system can be involved, with the most common being the musculoskeletal (usually as arthritis of knees, hips, ankles, or wrists) reproductive system (causing loss of pregnancy in women or epididymo-orchitis in men), or liver (hepatitis with mild elevations in transaminases).20 Marine species seem to have a predilection for central nervous system (CNS) involvement.5 Although rare, endocarditis is the most common cause of death.
Approximately 10% of cases will relapse, often as a result of inadequate antimicrobial therapy. Such relapses are usually milder than the initial presentation and can be treated with appropriate antibiotics.20 Overall, the prognosis with treatment is considered excellent, with mortality less than about 2–5%.
Diagnosis
Infection with Brucella species can be determined by standard laboratory methods. A definitive diagnosis is made by isolating Brucella organisms in cultures from blood or other specimens. Culturing is often difficult, given the slow growth and fastidious nature of these bacteria. As a result, the laboratory should be alerted when Brucella infection is suspected in order to prolong the length of culture time and ensure that laboratory staff takes appropriate protective measures.
Agglutination tests for Brucella antigen can detect infections arising from B. abortus, B. melitensis, and B. suis. Two serum samples are preferred for serological testing: the first drawn when the patient is acutely ill (within the first 7 days) and the second 2–4 weeks later. A fourfold or greater rise in antibodies is considered positive for brucellosis infection.
ELISA and polymerase chain reaction (PCR) tests are also available.
Treatment
Prolonged treatment is indicated, often to include an agent with intracellular activity (i.e., doxycycline). A variety of regimens have been recommended, which usually include a combination of doxycycline, rifampin, or trimethoprim-sulfamethoxazole, although it is not clear which regimen is most effective. A recent systematic review concluded that 6 weeks of doxycycline plus 2–3 weeks of streptomycin was more effective than a 6-week course of doxycycline plus rifampin.21
Medical surveillance
Brucellosis is a nationally notifiable disease in the United States, and B. abortus, B. melitensis, and B. suis are select agents requiring prompt reporting to the Federal Select Agent Program when isolation or release occurs.22 Brucellosis toxin is a CDC Category B bioterrorism disease.
Laboratory workers exposed to B. abortus should have blood drawn for serological studies at 0, 6, 12, 18, and 24 weeks post exposure. Serological testing is not available for exposures to the RB51 vaccine.
Prevention
The most important preventive measures, which have resulted in a dramatic decline in human brucellosis in the United States, are the vaccination and careful inspection of animals at risk, along with immunologic testing of cows’ milk and blood for evidence of Brucella infection. Diseased animals are segregated or slaughtered. Despite these eradication measures, work practice measures are essential for protection against remaining diseased animals. Kill floors should be isolated from other areas of the slaughterhouse and be under negative-pressure ventilation with entry restricted to essential personnel. All workers handling animal products, including milk, and especially placenta, uterine discharges, and blood, should wear heavy gloves, aprons, and goggles.23 The use of high-top boots should be considered as well.
Areas where exposure is likely should be posted with information about brucellosis, including routes of exposure, disease symptoms, and preventive activities. Work sites should have accessible handwashing facilities, first aid kits for prompt treatment of wounds, and separate areas, isolated from animal work, for eating and drinking. Such activities should be prohibited in work areas.
CDC has provided detailed preventive recommendations for both laboratory personnel and those exposed to the RB51 vaccine.24 Work with Brucella should be performed in a class II biosafety cabinet using biosafety level 3 precautions. Post-exposure prophylaxis of doxycycline and rifampin for at least 21 days is recommended following high-risk exposures.
Pasteurization of milk and thorough cooking of meat (especially from game) are important general preventive measures.
There is no vaccine available for use in humans.25
References
- 1. Centers for Disease Control and Prevention. Host Animals for Brucella Species. Available at: http://www.cdc.gov/brucellosis/veterinarians/host-animals.html (accessed on June 1, 2016).
- 2. Bechtol D, Carpenter LR, Mosites E, et al. Brucella melitensis infection following military duty in Iraq. Zoonoses Public Health. 2011; 58(7):489–92.
- 3. Ashford D, di Pietra J, Lingappa J, et al. Adverse events in humans associated with accidental exposure to the livestock brucellosis vaccine RB51. Vaccine 2004; 22:3435–9.
- 4. Sohn AH, Probert WS, Glaser CA, et al. Human neurobrucellosis with intracerebral granuloma caused by a marine mammal Brucella spp. Emerg Infect Dis 2003; 9:485–8.
- 5. Whatmore AM, Dawson CE, Groussaud P, et al. Marine mammal Brucella genotype associated with zoonotic infection. Emerg Infect Dis 2008; 14:517–8.
- 6. Centers for Disease Control and Prevention. Brucellosis Surveillance. Available at: http://www.cdc.gov/brucellosis/resources/surveillance.html (accessed on June 1, 2016).
- 7. Rhyan JC, Nol P, Quance C, et al. Transmission of brucellosis from elk to cattle and bison, Greater Yellowstone Area, USA, 2002–2012. Emerg Infect Dis 2013; 19(12):1992–5. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3840865/ (accessed on June 30, 2016). doi:10.3201/eid1912.130167.
- 8. Castell-Monsalve, J, Rullán JV, Peiró Callizo EF, et al. 1996. Epidemic outbreak of 81 cases of brucellosis following the consumption of fresh cheese without pasteurization. Rev Esp Salud Publica; 70(3):303–11.
- 9. Chen S, Zhang H, Liu X, et al. Increasing threat of brucellosis to low-risk persons in urban settings, China. Emerg Infect Dis 2014; 20(1):126–30.
- 10. Buchanan TM, Hendricks SL, Patton CM, et al. Brucellosis in the United States, 1960–1972: an abattoir-associated disease. Medicine 1974; 53:427–39.
- 11. Centers for Disease Control and Prevention (CDC). Human exposure to Brucella abortus strain RB51—Kansas, 1997. MMWR Morb Mortal Wkly Rep 1998; 47(9):172–5.
- 12. Landau Z, Green L. Chronic brucellosis in workers in a meat-packing plant. Scand J Infect Dis 1999; 31(5):511–2.
- 13. Trunnell TN, Waisman M, and Trunnell TL. Contact dermatitis caused by Brucella. Cutis 1985; 35(4):379–81.
- 14. Simoes EM and Justino JD. Brucellosis infection in a feral swine hunter. Nurse Pract 2013; 38(7):49–53.
- 15. Traxler RM, Guerra MA, Morrow MG, et al. Review of brucellosis cases from laboratory exposures in the United States in 2008 to 2011 and improved strategies for disease prevention. J Clin Microbiol 2013; 51(9):3132–6.
- 16. Wallach JC, Ferrero MC, Victoria Delpino M, et al. Occupational infection due to Brucella abortus S19 among workers involved in vaccine production in Argentina. Clin Microbiol Infect 2008; 14(8):805–7.
- 17. Centers for Disease Control and Prevention. Human exposures to marine Brucella isolated from a Harbor Porpoise – Maine, 2012. MMWR 2012; 61(25):461–3.
- 18. Trout D, Gomez TM, Bernard BP, et al. Outbreak of brucellosis at a United States pork packing plant. J Occup Environ Med 1995; 37(6):697–703.
- 19. Centers for Disease Control and Prevention. Overview of Laboratory Risks. Available at: http://www.cdc.gov/brucellosis/laboratories/risks.html (accessed on June 1, 2016).
- 20. Pappas G, Akritidis N, Bosilkovski M, et al. Brucellosis. N Engl J Med 2005; 352(22):2325–36.
- 21. Yousefi-Nooraie R, Mortaz-Hejri S, Mehrani M, et al. Antibiotics for treating human brucellosis. Cochrane Database Syst Rev 2012; 10:CD007179.
- 22. Centers for Disease Control and Prevention. Federal Select Agent Program. Available at: http://www.selectagents.gov (accessed on June 1, 2016).
- 23. Kligman EW, Peate WF, and Cordes DH. Occupational infections in farm workers. Occup Med 1991; 6(3):429–46.
- 24. Centers for Disease Control and Prevention. Brucellosis Homepage: Laboratory Personnel. Available at: http://www.cdc.gov/brucellosis/laboratories/index.html (accessed on June 1, 2016).
- 25. Oliveira SC, Giambartolomei GH, and Cassataro J. Confronting the barriers to develop novel vaccines against brucellosis. Exp Rev Vac 2011; 10(9):1291–305.
CAMPYLOBACTER SPECIES
Common name for disease: Campylobacteriosis
Occupational setting
Although Campylobacter is the most common bacterial cause of gastroenteritis, only a small proportion of cases are linked to outbreaks.1 Point-source outbreaks in the workplace have been related to both food2 and water3 contamination. Direct, occupationally acquired infections have not only been described in poultry processing workers most frequently4 but also farmworkers on dairy farms5 and zoo workers.6 Outbreaks have also been documented among occupants of child day care centers7 and prisons,8 raising the possibility of illness being acquired by workers in these sectors. As with other enteric pathogens, international travel is a well-known risk factor for illness from Campylobacter.9
Exposure (route)
Spread usually occurs through fecal–oral transmission. Since the infectious dose is very low, meat is easily contaminated during processing and cross-contamination creating other potential sources for infection is common. Poultry and, to a lesser extent, swine are the most common animal reservoirs, although an increasingly wide variety of animals are recognized as potential sources, including household pets such as dogs10 as well as reptiles.11 Animals infected with Campylobacter are not symptomatic.
Campylobacter is highly prevalent and widespread in poultry processing. The prevalence of colonization in flocks increases from 5–10% to 25–40% in the summer, with a corresponding seasonal increase in human infections.12 Asymptomatic infections in humans are very common, among both experienced and new workers.13 Several case reports have implicated airborne transmission of droplets as an additional route of infection, including an occupational setting in a poultry worker.14
In the general population, disease is most frequently caused when meat or dairy products are consumed following improper preparation and storage. Recent large outbreaks of disease have resulted from consumption of unpasteurized milk15 and exposure to muddy surface water through participation in an obstacle course at a cattle ranch.16 Person-to-person17 transmission can occur but is considered uncommon and is only a risk from symptomatic cases, especially young children. Rare cases of sexual transmission have been reported.18
Pathobiology
Campylobacteria are motile, Gram-negative, curved rods. C. jejenui is the species responsible for most Campylobacter illness. The major reservoir for C. jejuni is poultry, particularly chickens. A closely related species more commonly found in swine, C. coli, produces an illness clinically indistinguishable from that produced by C. jejuni. C. upsaliensis is rarely implicated as a cause of gastroenteritis,19 while C. fetus has additionally caused bacteremia and infections, such as endocarditis, abscesses, septic arthritis, and abortions, with pericarditis reported in a slaughterhouse worker.20 Recently, several emerging Campylobacter species have been isolated from environmental, food, animal, or human clinical isolates: C. hyointestinalis, C. lanienae, C. sputorum, C. concisus, and C. curvus.21
Acute enterocolitis is caused by C. jejuni, in about 90–95% of cases, C. coli in about 5–10% of cases, and other species for less than 1% of cases. Following an incubation period of 1–10 days, the most common symptoms are diarrhea (with or without blood), abdominal pain, and fever. However, this represents the midpoint on a continuum of presentations, which may range from an asymptomatic carrier state to severe, prolonged diarrhea. Abdominal pain may be very prominent, mimicking a surgical abdomen or inflammatory bowel disease. Symptoms are typically self-limited and resolve within 2–5 days.
C. jejuni gastroenteritis may be followed by the development of Guillain–Barré syndrome, usually within 12 weeks of the infection, which has also been reported following large outbreaks.22 This association may also exist for C. coli.23 Reactive arthritis or Reiter syndrome, most frequently involving the knee, may also follow Campylobacter infection in less than 5% of those infected.24
Diagnosis
The presence of the bacteria with a characteristic “gull wing” appearance on microscopic examination of stool specimens supports the diagnosis, and isolation of the organism in culture confirms it. A variety of rapid testing methods based on PCR with improved sensitivity and specificity are under development that may allow more rapid and convenient testing.25 Serologic testing is not recommended for routine clinical use.
Treatment
In the vast majority of cases, the disease is self-limited. In more severe cases of enteritis, supportive therapy consisting of fluid and electrolyte replacement is the primary consideration. Antibiotic therapy is generally not recommended, as it only shortens the duration of symptoms minimally and may result in further antibiotic resistance.26 However, antibiotics may be indicated in select patients with high fever, bloody or profuse diarrhea, or protracted illness. Erythromycin and azithromycin are the antibiotics of choice. Ciprofloxacin and tetracycline resistance is now widespread as a result of use in the agriculture setting.27 Antibiotic treatment eliminates excretion of the bacteria.
Medical surveillance
No specific surveillance measures are recommended. Campylobacteriosis is a nationally notifiable disease in the United States and cases must be reported to the local health authorities.
Prevention
Proper preparation and storage of food at the workplace will prevent this and other causes of infectious gastroenteritis. Work practices should emphasize good hygiene with strict handwashing after contact with potentially infected materials. Eating and smoking should not occur in work areas and gloves should be worn. In slaughterhouses, work practices and engineering controls should be directed toward minimizing fecal contamination.
While bacteria can be shed for days to weeks, the risk of person-to-person transmission is considered to be low. Employees in jobs with a high risk of transmission (e.g., food handlers, healthcare workers, and childcare workers) should be vigilant and promptly report any signs or symptoms compatible with gastroenteritis from any infectious cause to allow removal from high-risk activities while symptomatic. Travelers should take appropriate preventive precautions common to all enteric pathogens, with particular attention to avoiding undercooked poultry.
No vaccine is available.
References
- 1. Taylor EV, Herman KM, Ailes EC, et al. Common source outbreaks of Campylobacter infection in the USA, 1997–2008. Epidemiol Infect 2013; 141(5):987–96.
- 2. Murphy O, Gray J, Gordon S, and Bint AJ. An outbreak of campylobacter food poisoning in a health care setting. J Hosp Infect 1995; 30(3):225–8.
- 3. Rautelin H, Koota K, von Essen R, et al. Waterborne Campylobacter jejuni epidemic in a Finnish hospital for rheumatic diseases. Scand J Infect Dis 1990; 22(3):321–6.
- 4. de Perio MA, Niemeier RT, Levine SJ, et al. Campylobacter infection in poultry-processing workers, Virginia, USA, 2008–2011. Emerg Infect Dis 2013; 19(2):286–8.
- 5. Gilpin BJ, Scholes P, Robson B, et al. The transmission of thermotolerant Campylobacter spp. to people living or working on dairy farms in New Zealand. Zoonoses Public Health 2008; 55(7):352–60.
- 6. Forsyth MB, Morris AJ, Sinclair DA, et al. Investigation of zoonotic infections among Auckland Zoo staff: 1991–2010. Zoonoses Public Health 2012; 59(8):561–7.
- 7. Goosens H, Giesendorf BA, Vandamme P, et al. Investigation of an outbreak of Campylobacter upsaliensis in day care centers in Brussels: analysis of relationships among isolates by phenotypic and genotypic typing methods. J Infect Dis 1995; 172(5):1298–305.
- 8. Fernandez-Martin JI, Dronda F, Chaves F, et al. Campylobacter jejuni infections in a prison population coinfected with the human immunodeficiency virus. Rev Clin Esp 1996; 196(1):16–20.
- 9. Ricotta EE, Palmer A, Wymore K, et al. Epidemiology and antimicrobial resistance of international travel-associated Campylobacter infections in the United States, 2005–2011. Am J Public Health 2014; 104(7):e108–14.
- 10. Mughini Gras L, Smid JH, Wagenaar JA, et al. Increased risk for Campylobacter jejuni and C. coli infection of pet origin in dog owners and evidence for genetic association between strains causing infection in humans and their pets. Epidemiol Infect 2013; 141(12):2526–35.
- 11. Patrick ME, Gilbert MJ, Blaser MJ, et al. Human infections with new subspecies of Campylobacter fetus. Emerg Infect Dis 2013; 19(10):1678–80.
- 12. Jore S., Viljugrein H., Brun E., et al. Trends in Campylobacter incidence in broilers and humans in six European countries, 1997–2007. Prev Vet Med 2010; 93:33–41.
- 13. Ellström P, Hansson I, Söderström C, et al. A prospective follow-up study on transmission of campylobacter from poultry to abattoir workers. Foodborne Pathog Dis 2014; 11(9):684–8.
- 14. Wilson IG. Airborne Campylobacter infection in a poultry worker: case report and review of the literature. Commun Dis Public Health 2004; 7(4):349–53.
- 15. Centers for Disease Control and Prevention. Recurrent outbreak of Campylobacter jejuni infections associated with a raw milk dairy—Pennsylvania, April–May 2013. MMWR Morb Mortal Wkly Rep 2013; 62(34):702.
- 16. Zeigler M, Claar C, Rice D, et al. Outbreak of campylobacteriosis associated with a long-distance obstacle adventure race—Nevada, October 2012. MMWR Morb Mortal Wkly Rep 2014; 63(17):375–8.
- 17. Rotariu O, Smith-Palmer A, Cowden J, et al. Putative household outbreaks of campylobacteriosis typically comprise single MLST genotypes. Epidemiol Infect 2010; 138(12):1744–7.
- 18. Gaudreau C, Helferty M, Sylvestre JL, et al. Campylobacter coli outbreak in men who have sex with men, Quebec, Canada, 2010–2011. Emerg Infect Dis. 2013; 19(5):764–7.
- 19. Couturier BA, Hale DC, and Couturier MR. Association of Campylobacter upsaliensis with persistent bloody diarrhea. J Clin Microbiol 2012; 50(11):3792–4.
- 20. Ganeshram KN, Ross A, Cowell RP, et al. Recurring febrile illness in a slaughterhouse worker. Postgrad Med J 2000; 76(902):790–1.
- 21. Miller WG, Chapman MH, Yee E, et al. Multilocus sequence typing methods for the emerging Campylobacter species C. hyointestinalis, C. lanienae, C. sputorum, C. concisus, and C. curvus. Front Cell Infect Microbiol 2012; 2:45.
- 22. Jackson BR, Zegarra JA, López-Gatell H, et al. Binational outbreak of Guillain-Barré syndrome associated with Campylobacter jejuni infection, Mexico and USA, 2011. Epidemiol Infect 2014; 142(5):1089–99.
- 23. van Belkum A, Jacobs B, van Beek E, et al. Can Campylobacter coli induce Guillain-Barré syndrome? Eur J Clin Microbiol Infect Dis 2009; 28(5):557–60.
- 24. Porter CK, Choi D, Riddle MS. Pathogen-specific risk of reactive arthritis from bacterial causes of foodborne illness. J Rheumatol 2013; 40(5):712–4.
- 25. Van Lint P, De Witte E, De Henau H, et al. Evaluation of a real-time multiplex PCR for the simultaneous detection of Campylobacter jejuni, Salmonella spp., Shigella spp./EIEC, and Yersinia enterocolitica in fecal samples. Eur J Clin Microbiol Infect Dis 2015; 34(3):535–42.
- 26. Ternhag A, Asikainen T, Giesecke J, et al. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis 2007; 44(5):696–700.
- 27. Melero B, Juntunen P, Hänninen ML, et al. Tracing Campylobacter jejuni strains along the poultry meat production chain from farm to retail by pulsed-field gel electrophoresis, and the antimicrobial resistance of isolates. Food Microbiol 2012; 32(1):124–8.
CLOSTRIDIUM BOTULINUM (INCLUDING C. ARGENTINENSE, C. BARATII, AND C. BUTYRICUM)
Common names for diseases: Botulism, infant botulism, wound botulism
Occupational setting
A toxin formed by the bacterium Clostridium botulinum (or, more rarely, C. argentinense, C. butyricum, and C. baratii) causes botulism. These organisms are ubiquitous in most soils, have also been found in agricultural products, and in a diverse array of animals, including marine animals.1 Type A and B botulinum toxins are commercially available for cosmetic and therapeutic use, including the treatment of a variety of conditions involving involuntary muscle spasm, such as cervical dystonia, blepharospasm, and strabismus.
To date, only one report of nonfatal botulism acquired in the occupational setting has been documented among three veterinary lab workers.2 They became ill 3 days after inhaling botulinum type A toxin while performing necropsies on guinea pigs and rabbits whose fur had been covered with aerosolized toxin.
Although botulism has been reported in patients receiving therapeutic injections3 and intravenous drug users,4 no cases have been reported among healthcare providers to date. In theory, laboratory workers in research or public health facilities or those involved in the manufacture of botulinum toxin are also at risk.
Exposure (route)
Several forms of botulism are recognized. Foodborne, wound, and intestinal botulism (which is further subdivided as infant or adult) are the natural forms of disease. Inhalational botulism requires aerosolization, while iatrogenic botulism occurs from overdosing of the injected toxin. In the United States in 2012, 160 laboratory-confirmed cases of botulism were reported to CDC. A total of 122 cases were of the infant form, 25 were foodborne, 8 were wounds, and 5 were cases of unknown or other etiology.5
Since the toxin is readily inactivated by heat, uncooked or improperly cooked foods are the source of disease. Although commonly associated with home-canned foods, almost any food can cause botulism and most cases in the United States involve vegetables. The bacteria cannot penetrate intact skin and person-to-person transmission has not been described.
Inhalational exposure is a significant concern in the context of bioterrorism,6 although, as noted previously, only three human cases have been described from this route.2
Pathobiology
C. botulinum is a spore-forming, obligate anaerobic bacillus. Disease is caused by the toxin, which is regarded as the most toxic substance known. Foodborne, inhalational, and iatrogenic forms result from exposure to preformed, externally derived toxin, whereas the toxin in intestinal and wound botulism originates from Clostridia bacteria that have colonized these sites in the host.
In a conducive environment, such as the hypoxic atmosphere produced by canning, in a deep wound, or in the intestine, clostridial spores germinate. The growing bacterial colonies release a potent neurotoxin, which is taken up in the circulation and acts at peripheral cholinergic synapses to block the release of acetylcholine, causing multiple cranial nerve palsies and subsequent diffuse muscular weakness. In untreated cases, death is usually due to respiratory failure from paralysis of respiratory muscles. There are seven types of toxin, designated A–H, which may be elaborated by the bacillus, but most human cases are caused by types A, B, and E, with rare cases due to type F. In 2014, a novel-type H toxin was identified from a case of infant botulism.7
Diagnosis
A high level of clinical suspicion is needed to make this diagnosis, which is frequently missed.8 The paralysis initially affects bulbar musculature and subsequently descends to a generalized weakness. Since disease results from intoxication rather than infection, constitutional signs and symptoms such as fever are not seen.
Often, extensive investigations are needed to rule out other neurological causes of paralysis. The diagnosis of botulism is supported by demonstrating the toxin in serum, stool, or in the suspected food source. C. botulinum can sometimes be cultured from the stool in cases among infants or those with intestinal anomalies. The presence of C. botulinum spores in the implicated food is less helpful than finding toxin, as the spores are ubiquitous and are not themselves harmful. In cases of suspected wound botulism, serum should be tested for toxin and the wound cultured for the organism. However, since the sensitivity of the toxin assay is only 33–44% and takes 4 days to yield results,9 the diagnosis should be made on clinical grounds. The presence of bilateral cranial-nerve palsies with a subsequent descending paralysis should raise the suspicion of botulism, regardless of the exposure history.10
Treatment
After collection of serum for specific toxin identification, all suspected cases of botulism should be treated as soon as possible with antitoxin. The only available antitoxin in the United States is a heptavalent botulinum antitoxin, which covers toxin types A–G and can be obtained from CDC through referral from state health departments.10 Since patient outcomes are much better with early antitoxin therapy (ideally within 24 hours), administration should not be withheld while awaiting laboratory confirmation of botulism.
Supportive treatment, which may include ventilation, is the second cornerstone of botulism management. Such care may be required for months, especially when antitoxin treatment has been delayed.
Cases of wound botulism should be treated with antitoxin as well as wound debridement or drainage, and antibiotics, with Penicillin G as the preferred agent.
Medical surveillance
There are no recommended medical screening activities for botulism. Botulism is a nationally notifiable disease in the United States and all cases, confirmed or suspected, must be reported immediately to the local public health authorities. Botulinum toxin is a CDC Category A bioterrorism agent.
Prevention
Appropriate food handling can prevent the majority of cases. Public notification and recall of tainted products are essential after identification of commercial food sources of poisoning. Tracing of others who may have consumed contaminated food is important when botulism has been identified in commercially prepared or distributed foods. The public should be educated about the risk of botulism being present in bulging containers, such as cans, but they should be aware that this sign of contamination is often absent. Those involved in home canning should be educated about the proper time, temperature, and pressure needed to destroy spores. Uneviscerated fish products should be avoided because of the risk of contamination. Prompt cleaning of wounds and careful attention (including irrigation or debridement) to wounds that are not healing may prevent wound botulism. Botulism associated with toxin manufacture and use is, to date, only a theoretical risk and should be preventable by maintaining strict containment procedures in handling or production of the toxin.
No vaccine is available. A toxoid vaccine was withdrawn in the United States in 2011 due to concerns about declining immunogenicity and adverse local reactions from boosters.11
References
- 1. From the Centers for Disease Control and Prevention. Outbreak of botulism type E associated with eating a beached whale—western Alaska, July 2002. JAMA 2003; 289(7):836–8.
- 2. Holzer VE. Botulism from inhalation [in German]. Med Klin 1962; 57:1735–8.
- 3. Coban A, Matur Z, Hanagasi HA, et al. Iatrogenic botulism after botulinum toxin type A injections. Clin Neuropharmacol 2010; 33(3):158–60.
- 4. Yuan J, Inami G, Mohle-Boetani J, et al. Recurrent wound botulism among injection drug users in California. Clin Infect Dis 2011; 52(7):862–6.
- 5. Centers for Disease Control and Prevention. Botulism Annual Summary, 2012. Atlanta, GA: US Department of Health and Human Services, CDC, 2014.
- 6. Arnon SS, Schechter R, Inglesby TV, et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA 2001; 285(8):1059–70.
- 7. Barash JR and Arnon SS. A novel strain of Clostridium botulinum that produces type B and type H botulinum toxins. J Infect Dis 2014; 209:183–91.
- 8. St Louis ME, Peck SH, Bowering D, et al. Botulism from chopped garlic: delayed recognition of a major outbreak. Ann Intern Med 1988; 108(3):363–8.
- 9. Vasa M, Baudendistel TE, Ohikhuare CE, Clinical problem-solving. The eyes have it. N Engl J Med 2012; 367(10):938–43.
- 10. Rao AK, Jackson KA, and Mahon BE. The eyes have it. N Engl J Med 2013; 368(4):392.
- 11. Centers for Disease Control and Prevention. Notice of CDC’s Discontinuation of Investigational Pentavalent (ABCDE) Botulinum Toxoid Vaccine for Workers at Risk for Occupational Exposure to Botulinum Toxins. MMWR 2011; 60(42):1454–5.
CLOSTRIDIUM DIFFICILE
Common names for disease: C. difficile colitis, pseudomembranous colitis, antibiotic-associated colitis
Occupational setting
Although generally associated with individual antibiotic use, healthcare-associated infection from Clostridium difficile has been documented in nurses1 and laboratory workers.2 However, such reports are rare, and, in some cases, the workers had been prescribed antibiotics prior to acquiring the infection.3
Exposure (route)
C. difficile is a ubiquitous bacterium, widely found in soil and forming part of the normal colonic flora in many healthy adults.
Person-to-person transmission of C. difficile spores occurs through the fecal–oral route. Spores are resistant to gastric acid and subsequently germinate upon reaching the large intestine. Most infections are healthcare-associated, although there is increasing evidence that the sources of infection are more complex than previously recognized. One large study reported that only 35% of cases in a hospital could be traced to a symptomatic patient.4 Asymptomatic carriers and spore-contaminated surfaces may represent additional sources of infection.5
In addition, community-acquired infections may now account for at least 20% of infections.6 There is evidence that C. difficile is a zoonotic infection implicating new modes of transmission such as foodborne.6
Pathobiology
C. difficile is an anaerobic, spore-forming, Gram-positive bacillus, which produces a variety of toxins, the most important of which are denoted as toxins A and B.
Colitis due to C. difficile is more common in elderly and debilitated patients, the immunocompromised, and those taking antibiotics. Antibiotic-associated colitis occurs when alteration of the normal intestinal flora disrupts competitive inhibition and allows overgrowth of C. difficile with elaboration of toxins into the intestinal lumen. Clinical effects arise from the toxins, as the bacteria themselves are rarely invasive.
There is a wide range of symptoms arising from C. difficile infection, ranging from an asymptomatic carrier state to life-threatening colitis with the characteristic “pseudomembrane” of yellowish exudate. In antibiotic-associated cases, symptoms usually occur during treatment or within 1–2 weeks of completion but can begin as long as 12 weeks after therapy. A typical patient with C. difficile colitis presents with profuse, foul-smelling diarrhea, which may be watery or green and mucoid. There is usually crampy abdominal pain, with fever and abdominal tenderness on examination. Reactive arthritis may develop after C. difficile infection.
Diagnosis
The diagnosis should be suspected in anyone with three or more diarrheal stools within 1 day who received antibiotics within the previous 12 weeks.
Laboratory confirmation of C. difficile colitis can be challenging. The most widely available test is to detect toxins A and B in the stool using an enzyme immunoassay (EIA). However, not all labs test for both toxins and the sensitivity is limited. A variety of more rapid tests with higher sensitivity are increasingly available, some based on PCR and others on EIA.
Routine stool cultures have poor specificity because of the widespread presence of nonpathogenic strains of C. difficile. However, a culture followed by an assay for toxin performed on isolates, known as a toxigenic culture, solves this problem. Although currently regarded as the gold standard, this method is both labor intensive and requires 2–4 days to provide results. Therefore, authorities currently recommend either a PCR test for toxigenic strains or a two-step approach using a rapid EIA-based test followed by confirmatory testing.7
Empiric treatment should be given when clinically appropriate while awaiting test results. Testing should only be performed on diarrheal stool and repeat testing is discouraged, as it may yield falsely positive test results due to asymptomatic colonization, which can occur for a prolonged period following infection.
Treatment
Mild cases of antibiotic-associated colitis may be treated with discontinuation of antibiotics and supportive therapy only. Metronidazole is the first-line antibiotic for mild to moderate cases, and vancomycin for severe cases.7 A recent meta-analysis has concluded that short-term use of probiotics in conjunction with antibiotics is effective and safe.8 Antiperistaltic agents should not be used, since they may cause toxin retention and obscure symptoms. Recurrence occurs in 20–30% of patients.
Colectomy may be indicated for severe cases. Fecal microbiota transplantation shows considerable promise as a safe new therapy, although randomized controlled clinical trials are limited and this approach is not yet fully standardized.9
Medical surveillance
There are no recommended medical surveillance activities beyond those for healthcare-associated infections.
Prevention
Some preventive measures are common to many bacterial infections and include judicious use of antibiotics, prompt identification of cases, and contact precautions for cases of diarrhea.
A significant challenge in controlling C. difficile infection is that the spores are resistant to alcohol, handwashing, and usual cleaning measures. Therefore, universal glove use, enhanced cleaning measures using known sporicidal agents, and avoidance of shared equipment should be considered in high-risk healthcare areas.10
References
- 1. Strimling MO, Sacho H, and Berkowitz I. Clostridium difficile infection in health-care workers. Lancet 1989; 2(8667):866–7.
- 2. Bouza E, Martin A, Van den Berg RJ, et al. Laboratory-acquired Clostridium difficile polymerase chain reaction ribotype 027: a new risk for laboratory workers? Clin Infect Dis 2008; 47(11):1493–4.
- 3. Hell M, Indra A, Huhulescu S, et al. Clostridium difficile infection in a health care worker. Clin Infect Dis 2009; 48(9):1329.
- 4. Eyre DW, Wilcox MH, and Walker AS. Diverse sources of C. difficile infection. N Engl J Med 2014; 370(2):183–4.
- 5. Ali S, Manuel R, and Wilson P. Diverse sources of C. difficile infection. N Engl J Med 2014; 370(2):182.
- 6. Hoover DG and Rodriguez-Palacios A. Transmission of Clostridium difficile in foods. Infect Dis Clin North Am 2013; 27(3):675–85.
- 7. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31(5):431–55.
- 8. Goldenberg JZ, Ma SSY, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Datab Syst Rev 2013, 5, art. no.: CD006095.
- 9. Kapel N, Thomas M, Corcos O, et al. Practical implementation of faecal transplantation. Clin Microbiol Infect 2014; 20(11):1098–105. Available at: http://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)65300-3/fulltext (accessed on June 30, 2016).
- 10. Hsu J, Abad C, Dinh M, et al. Prevention of endemic healthcare-associated Clostridium difficile infection: reviewing the evidence. Am J Gastroenterol 2010; 105(11):2327–39.
CLOSTRIDIUM PERFRINGENS (also C. SEPTICUM, C. NOVI)
Common names for diseases: Gas gangrene, myonecrosis, enteritis necroticans, necrotic enteritis, Darmbrand, pigbel.
Occupational setting
As is the case with other Clostridium species, Clostridium perfringens bacteria are ubiquitous. C. perfringens is considered to be one of the most widely distributed disease-causing bacteria, commonly found in soils and as an intestinal inhabitant in both animals and humans.
C. perfringens causes both a wide spectrum of gastrointestinal disease and a necrotic infection of tissue. Outbreaks of the common foodborne illnesses (food poisoning and diarrhea) occur most commonly in restaurants.1 However, large outbreaks have also been reported in institutional settings, which included staff who ate at the cafeteria.2 Some of these cases were fatal, due to a much rarer foodborne illness, variously known as necrotic enteritis, necrotizing colitis, enteritis necroticans, or “pigbel.”2
C. perfringens can also cause myonecrosis or gas gangrene, which usually occurs after traumatic injury or surgery. Other clostridial species less commonly associated with gas gangrene include C. septicum3 and C. novyi.4
While case reports are rare, occupations at risk are those with high risk of traumatic injury with the potential for contamination, such as agricultural workers5 and military personnel.6
Exposure (route)
In cases of foodborne illness, the route of infection is ingestion. In gas gangrene caused by C. perfringens, bacteria are introduced into a wound from an external source such as soil, or the wound may be seeded from the patient’s own colonic flora such as with penetrating abdominal injuries. C. novyi has only been implicated in cases of gas gangrene among injection drug users4, while C. septicum can cause spontaneous gas gangrene without antecedent trauma, thought to arise from hematogenous seeding from the patient’s own gastrointestinal flora.3
Person-to-person transmission has not been described.
Pathobiology
C. perfringens is an anaerobic, spore-forming, Gram-positive bacillus. It is also considered to be the most rapidly growing microorganism, with a generation time of less than 7 minutes under ideal conditions. Five different strains are recognized according to the toxin produced, denoted as types A–E. Only types A and C have been associated with disease in humans. While a variety of toxins are produced, the three clinically relevant forms are alpha, which causes gas gangrene; beta, which causes necrotic enteritis; and C. perfringens enterotoxin (CPE), which causes food poisoning.
Diseases caused by C. perfringens result from the effects of toxins produced after germination and replication of the spores in a hospitable anaerobic environment. The most common, but least serious, syndrome is food poisoning, caused by C. perfringens type A. When spore-contaminated food (usually meat) is inadequately heated, or inadequately reheated after slow cooling, spores germinate and produce CPE, which is then ingested with the contaminated food. The circumstances under which the toxin is produced are common in institutional kitchens preparing food in large batches. After an average incubation period of 8–12 hours, diarrhea and abdominal cramps develop. Fever and vomiting are uncommon. Symptoms are self-limited and usually resolve within 24 hours.
Enteritis necroticans is caused by the beta toxin produced by C. perfringens type C and is most frequently associated with the ingestion of undercooked pork.7 The bacteria multiply in the small intestine and release beta toxin, which causes intestinal necrosis. Segmental intestinal gangrene and other severe complications may occur. This very serious disease is rare in industrialized countries. Children with protein malnutrition, diabetics, or those who have had pancreatic or gastric resection are at increased risk, possibly due to deficiencies in pancreatic proteases, which break down beta toxin.7
Gas gangrene is a rare but devastating syndrome caused by alpha toxin, which is produced by C. perfringens as well as C. novyi and C. septicum. It usually occurs after traumatic injury or surgery, but spontaneous cases have been described, especially as a result of C. septicum.3C. perfringens is a common contaminant of open wounds. Factors that are thought to promote clostridial replication in a wound are foreign bodies, vascular insufficiency, and concurrent infection with other bacteria. The incubation period is usually 2–3 days. The first symptom is usually sudden pain at the wound site, which may be pale, edematous, and tender. Crepitus may be palpated and gas from bacterial metabolism may be seen on radiographic studies. The skin color progresses from pale to magenta or bronze, and hemorrhagic bullae with a thin brown serosanguinous discharge, which has a characteristic offensive, sweet odor, may develop. Necrosis of muscle is an associated finding.
Other soft-tissue infections due to C. perfringens include uncomplicated polymicrobial abscesses, crepitant cellulitis, suppurative myositis, emphysematous cholecystitis, anaerobic pulmonary infections (especially empyema), and, rarely, after penetrating head trauma, brain abscess.
Diagnosis
Clostridial food poisoning is diagnosed by recovery of C. perfringens organisms from suspected food, or stool from patients collected within 48 hours of symptom onset. Cultures with greater than 106 colony-forming units (CFUs)/g and demonstration of the CPE gene are considered diagnostic.
Diagnosis of enteritis necroticans is primarily clinical and should be suspected in a patient with the risk factors outlined above, in the setting of anorexia, vomiting, abdominal pain, and bloody diarrhea. Absence of colonic involvement and rapid progression of the illness to sepsis and shock favor the diagnosis.
Diagnosis of gas gangrene is primarily clinical but is supported by evidence of myonecrosis seen at surgery. Wound exudates may reveal Gram-positive or Gram-variable rods with a typical “box-car” appearance and few white blood cells. This may be the earliest laboratory confirmation of this disease. Spores are not seen on Gram stain. Only 15% of gas gangrene cases have bacteremia.
Treatment
For C. perfringens food poisoning, rehydration, and other supportive measures are required in exceptional cases of severe diarrhea. Otherwise, the illness resolves without treatment. Treatment for enteritis necroticans includes chloramphenicol or penicillin G, supportive care, and bowel decompression. Small bowel resection may be required for persistent paralytic ileus, septicemia, peritonitis, persistent pain, or a palpable mass lesion.
The cornerstone of treatment of gas gangrene is early and extensive surgical debridement, with wide excision for abdominal wall involvement, and usually amputation if an extremity is involved. Penicillin remains the antibiotic of choice, although clindamycin, metronidazole, and the carbapenems are acceptable alternatives.
The use of hyperbaric oxygen in the treatment of necrotic tissue infections generally is controversial8 but may be considered as an additional therapy. Surgery should not be delayed. The mortality rate of gas gangrene treated with surgery and antibiotics still approaches 25%.
Medical surveillance
There are no recommended medical screening activities for C. perfringens. Outbreaks of Clostridia food poisoning should be reported promptly to the local health authorities.
Prevention
Foodborne clostridial disease can be prevented by adequate cooking temperatures and rapid cooling and by adequate reheating of foods. Division of large batches of food into smaller units facilitates rapid cooling, which prevents germination of clostridial spores. A vaccine against C. perfringens beta toxin is in use in previously endemic countries, where it has been quite effective in preventing enteritis necroticans.9 Cleaning grossly contaminated wounds may help prevent gas gangrene, but most cases are not easily prevented. Early detection and treatment may mitigate some of the more severe manifestations and reduce mortality.
References
- 1. Grass JE, Gould LH, and Mahon BE. Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998–2010. Foodborne Pathog Dis 2013; 10(2):131–6.
- 2. Centers for Disease Control and Prevention. Fatal foodborne Clostridium perfringens illness at a state psychiatric hospital—Louisiana, 2010. MMWR Morb Mortal Wkly Rep 2012; 61(32):605–8.
- 3. Wu YE, Baras A, Cornish T, et al. Fatal spontaneous Clostridium septicum gas gangrene: a possible association with iatrogenic gastric acid suppression. Arch Pathol Lab Med 2014; 138(6):837–41.
- 4. Palmateer NE, Hope VD, Roy K, et al. Infections with spore-forming bacteria in persons who inject drugs, 2000–2009. Emerg Infect Dis 2013; 19(1):29–34.
- 5. Demianchuk AV and Vanat IM. Gas gangrene caused by agricultural trauma. Klin Khir 1974 (10):71–2.
- 6. Rudge FW. The role of hyperbaric oxygenation in the treatment of clostridial myonecrosis. Mil Med 1993; 158(2):80–3.
- 7. Gui FL, Subramony C, Fratkin J, et al. Fatal enteritis necroticans (pigbel) in a diabetic adult. Mod Pathol 2002; 15(1):66–70.
- 8. Willy C, Rieger H, and Vogt D. Hyperbaric oxygen therapy for necrotizing soft tissue infections: contra. Chirurg. 2012; 83(11):960–72.
- 9. Lawrence GW, Lehmann D, Anian G, et al. Impact of active immunization against enteritis necroticans in Papua New Guinea. Lancet 1990; 336:1165–7.
CLOSTRIDIUM TETANI
Common names for disease: Tetanus, lockjaw
Occupational setting
Tetanus is caused by a toxin released by Clostridium tetani, a ubiquitous bacterium found in greatest numbers in soil, especially soil rich in fecal matter such as manure. It is a potential problem in any outdoor job, especially work in which minor skin trauma is frequent. Most occupational cases occur following minor injuries of the hands or fingers in the agriculture and forestry sectors.1 Epidemics have occurred following natural disasters.2 In the United States, there has been a progressive and dramatic decline in tetanus with 19 reported cases and two deaths in 2009 (Figure 22.4).3
FIGURE 22.4 Tetanus – number of reported cases and deaths, United States, 1900–2010.
Source: Centers for Disease Control and Prevention. Chapter 16: Tetanus. Manual for the surveillance of vaccine-preventable diseases. Centers for Disease Control and Prevention, Atlanta, GA, 2011. Available at http://www.cdc.gov/vaccines/pubs/surv-manual/chpt16-tetanus.html.
Exposure (route)
Spores of C. tetani reproduce after inoculation into traumatized skin. Recent surveillance data from the United States indicate that only 71.7% of cases had antecedent acute trauma, and, of these, only 36.5% sought medical attention.3 For 13% of cases, the entry was a chronic wound.3 Therefore, minor or even unnoticed trauma can be a portal of entry.
Pathobiology
C. tetani is an anaerobic, Gram-positive rod that produces hardy spores found in large numbers in soil. When spores are inoculated into a wound, they germinate and produce exotoxins, including tetanospasmin, responsible for the signs and symptoms of tetanus. Following hematogenous spread, the toxin travels via motor nerve axons and binds to receptors in muscle and the central nervous system, replicating the actions of neurotransmitters. The effects of the toxin last several weeks and recovery probably requires the growth of new synapses.
The nervous system effects vary, likely as a result of both the size of the inoculum and the degree of existing immunity. Effects can be classified into one of the following two categories: central motor control effects in which poisoning of inhibitory neuronal cells causes unopposed motor activity leading to rigidity and spasms, and autonomic instability, the leading cause of death in tetanus, which is manifested by increased sympathetic tone with massive catecholamine release from disinhibition of the sympathetic system neurons of the autonomic system. Occasionally, parasympathetic disinhibition is seen causing hypertension and cardiac dysrhythmias.
The incubation period varies from 1 day to several months, but the average is approximately 8 days. The shorter the period of time between spore inoculation and symptom onset, the poorer the prognosis. Other predictors of poor prognosis are autonomic dysfunction at presentation and burn or surgical site as the portal of entry.
Tetanus is divided clinically into a variety of presentations including localized, generalized, cephalic, and neonatal tetanus, which will not be discussed here. Localized tetanus is the least severe form and occurs when muscle at the site of the injury is fixed in spasm. Progression to generalized tetanus may occur. Generalized tetanus is the most common form and usually starts with nonspecific symptoms, such as malaise, restlessness, headache, insomnia, irritability, and profuse sweating, followed by stiffness, twitching and pain at the wound site, and fever. These progress to the classic signs of tetanus: trismus or lockjaw, risus sardonicus, the straightening of the upper lip which has reminded some of a sardonic smile, and opisthotonic posturing, in which spasm of the back muscles occur such that a patient placed supine would rest on his heels and the back of his head only. There is no loss of consciousness and the condition is extremely painful. In generalized tetanus, there may be airway compromise, diaphragmatic dysfunction, and autonomic dysfunction. Clinical progression can occur for up to 2 weeks despite administration of antitoxin, and full recovery may take months. The cephalic form of tetanus occurs following head trauma or ear infections and affects cranial nerves.
Diagnosis
Diagnosis is on clinical grounds only, based on the history and characteristic physical examination findings. Vaccination status should be established to increase the level of suspicion, since 92% of cases of tetanus among those with known vaccination status occurred in un- or under-vaccinated persons in the United States.3
Cultures lack both sensitivity and specificity. Laboratory measurement of tetanus antibodies can be performed prior to tetanus immune globulin (TIG) administration. However, there are numerous problems with this test, including disagreement on what levels are considered protective, limited availability, and numerous case reports of tetanus in those considered to have protective levels of antibodies.4 Early diagnosis is essential to ensure prompt treatment.
Treatment
Passive immunotherapy with intramuscular human TIG is the standard therapy. TIG binds only free toxin and not that already bound, which explains the progression of symptoms once therapy has begun and the long recovery period. If human TIG is not available, equine antitoxin may be given intravenously after testing has ruled out hypersensitivity to horse serum. Tetanus toxoid should also be administered, as clinical tetanus does not confer subsequent immunity. Wounds should be cleaned and debrided. Full recovery may take a month or longer.
Patients with tetanus should be carefully monitored for signs of airway compromise from laryngospasm and may require extensive supportive therapy. Contractions can be severe enough to cause long-bone or spinal fractures. Because of prolonged debilitation, secondary complications are common and include pulmonary emboli and secondary healthcare-associated infections.
Medical surveillance
There are no recommended medical screening activities for tetanus. Tetanus is a nationally notifiable disease in the United States and cases must be reported to local public health authorities. Aggressive case finding has been recommended to ensure up-to-date boosters and reduce the size of the unvaccinated population.
Prevention
Active immunization with tetanus toxoid prevents disease. A three-dose schedule is part of the primary series for all infants and children with booster doses every 10 years. All adults with an unknown vaccination status should receive the three-dose series of vaccines. In cases of tetanus-prone injuries, passive immunization with human TIG should be given to patients with incomplete or unknown vaccine history. The current CDC recommendations for wound management are shown in Table 22.3.3
TABLE 22.3 CDC recommendations for tetanus wound management.
Source: MMWR 2011 Apr 1;60(12):365–9.
| History of adsorbed tetanus toxoid (doses) | Clean minor wounds Tdap or Td† | Clean minor wounds TIG§ | All other wounds* Tdap or Td† | All other wounds* TIG§ |
| <3 or unknown | Yes | No | Yes | Yes |
| ≥3¶ | No** | No | No†† | No |
* Such as (but not limited to) wounds contaminated with dirt, feces, soil, and saliva; puncture wounds; avulsions; and wounds resulting from missiles, crushing, burns, and frostbite.
† For persons >10 years, Tetanus and diphtheria toxoids and acellular pertussis (Tdap) is preferred to tetanus and diphtheria toxoids (Td) if the patient has never received Tdap and has no contraindication to pertussis vaccine. For persons 7 years of age or older, if Tdap is not available or not indicated because of age, Td is preferred to TT.
§ TIG is human tetanus immune globulin. Equine tetanus antitoxin should be used when TIG is not available.
¶ If only three doses of fluid toxoid have been received, a fourth dose of toxoid, preferably an adsorbed toxoid, should be given. Although licensed, fluid tetanus toxoid is rarely used.
** Yes, if it has been 10 years or longer since the last dose.
†† Yes, if it has been 5 years or longer since the last dose. More frequent boosters are not needed and can accentuate side effects.
References
- 1. Luisto M and Seppäläinen AM. Tetanus caused by occupational accidents. Scand J Work Environ Health 1992;18(5):323–6.
- 2. Jeremijenko A, McLaws ML, and Kosasih H. A tsunami related tetanus epidemic in Aceh, Indonesia. Asia Pac J Public Health 2007;19:Spec No:40-4.
- 3. Centers for Disease Control and Prevention. Tetanus surveillance—United States, 2001–2008. MMWR Morb Mortal Wkly Rep 2011; 60(12):365–9.
- 4. Vollman KE, Acquisto NM, and Bodkin RP. A case of tetanus infection in an adult with a protective tetanus antibody level. Am J Emerg Med 2014; 32(4):392.e3–e4.
CORYNEBACTERIUM SPECIES
Common name for disease: Diphtheria
Occupational setting
Corynebacterium diphtheriae, the most important of the Corynebacteria, causes diphtheria. Diphtheria has been virtually eliminated in the working populations of many countries because of immunization. From 1980 to 2010, 55 cases of diphtheria were reported in the United States.1 Most of these cases were in adults.
Nevertheless, a large population of susceptible adults due to declining immunity without booster doses, together with importation of cases from travelers to endemic countries has heightened concern about a possible resurgence of this disease in the United States.2
C. ulcerans, frequently present in farm animals, may also cause diphtheria in humans.3 A variety of other species, several of which are zoonoses, have been described as sources of infection in occupational settings, but are very rare. These include C. striatum causing septic arthritis following a scalpel injury in a surgeon,4C. aquaticum infection of a high-pressure injection injury,5 and C. pseudotuberculosis (also known as C. ovis) among those occupationally exposed to large animals.6
Corynebacteria have also been cultured from the smoke plume of an operating room laser.7
Exposure (route)
Person-to-person transmission occurs through inhalation of airborne respiratory droplets. Transmission from contact with infected skin lesions or fomites is unusual.
Pathobiology
Corynebacteria are pleomorphic, Gram-positive, aerobic bacilli. By far, the most pathogenic species is C. diphtheriae, for which humans are the only known reservoir. Most infections are asymptomatic.
Signs and symptoms of infection develop after an incubation period of 2–5 days, locally at either mucous membranes (respiratory, ocular, or genital diphtheria) or the superficial layers of the skin through pre-existing skin breaks (cutaneous diphtheria). C. diphtheria may or may not produce an exotoxin, depending on whether or not the bacterium has itself been infected by a bacteriophage containing the gene mediating toxin production. The toxin causes both local tissue necrosis and systemic effects with absorption. In addition to nonspecific signs of shock (tachycardia, stupor), the most common systemic effects include myocarditis and neuritis.
Local infection with toxigenic diphtheria is followed by hyperemia, edema, and development of the characteristic gray exudative pseudomembrane. Although virtually any mucous membrane can be infected, the most frequent and well-known sites are the pharynx and tonsils. Fever is low-grade and a classic finding is of a “bull neck” appearance from a combination of submandibular edema and lymphadenopathy. Extensive membrane formation may lead to respiratory obstruction.
Cutaneous diphtheria is less severe than infection of the respiratory tract. Lesions usually occur in the setting of primary infection with other organisms (typically, Staphylococcus aureus and group A streptococci). The characteristic lesion is a non-healing ulcer with a gray membrane.
Infections caused by non-toxigenic diphtheria are milder and confined to local effects. The usual picture is pharyngitis and tonsillitis, although endocarditis has also been described.8
Diagnosis
In endemic areas, toxigenic diphtheria may be diagnosed on clinical grounds alone based on the relatively specific clinical picture. Definitive diagnosis requires selective culture from nasal and throat swabs. Because this procedure may not be routinely performed in some laboratories, communication with laboratory personnel regarding a suspicion of diphtheria is advisable to ensure that appropriate isolation and identification techniques are applied. An immunodiffusion test for the toxin (known as the Elek test) as well as a PCR-based assay for the toxin gene can also be used. Direct examination through microscopy of stained samples may be unreliable because of the presence of commensals with a similar appearance.
Treatment
Treatment of active diphtheria consists of diphtheria antitoxin, antibiotics directed against the organism, and supportive care. Diphtheria antitoxin is no longer licensed for use in the United States and must be obtained through an Investigational New Drug protocol by contacting the CDC Emergency Operations Center (770-488-7100). Penicillin and erythromycin are the drugs of choice, although antibiotic resistance is an increasing concern. Supportive therapy should pay particular attention to maintenance of airway.
Medical surveillance
Although cases of respiratory diphtheria only are nationally notifiable in the United States, prompt involvement of public health authorities is advisable in any suspected case of infection with toxigenic diphtheria. Respiratory cases should be reported promptly by telephone to the CDC Emergency Operations Center (770-488-7100).
Some have advocated the routine culturing of all throat swabs for C. diphtheria, since non-toxigenic strains have been isolated with increasing frequency and the potential exists for conversion of these organisms to toxigenic forms.8 Such a program identified three cases of non-toxigenic C. diphtheria tonsillitis in British military personnel.9
Prevention
Active immunization prevents disease. A three-dose schedule is part of the primary series for all infants and children with booster doses every 10 years. All adults with an unknown vaccination status should receive the three-dose series of vaccines. Since infection may not confer immunity, vaccination is also recommended for those with a history of the illness. Travelers should ensure that vaccinations are up-to-date prior to departure.
Patients with respiratory diphtheria should be strictly isolated. After 48 hours of antibiotic therapy, the disease is no longer contagious. Close contacts should be traced and receive a booster (or full series of the vaccine if unimmunized or vaccine status is unknown) and antibiotic prophylaxis. Antitoxin is reserved for use at early signs of illness. Two consecutive negative cultures following therapy of cases and carriers should be obtained to document elimination of the organism.
References
- 1. Tiwari TSP. Diphtheria. In Roush SW, Baldy LM, Centers for Disease Control and Prevention (eds). Manual for the Surveillance of Vaccine-Preventable Diseases, 5th ed. Centers for Disease Control and Prevention, Atlanta, GA, 2011. Available at: http://www.cdc.gov/vaccines/pubs/surv-manual/chpt01-dip.html (accessed on June 30, 2016).
- 2. Centers for Disease Control and Prevention. Fatal respiratory diphtheria in a U.S. traveler to Haiti—2003. MMWR 2003; 52:1285–6.
- 3. Sangal V, Nieminen L, Weinhardt B, et al. Diphtheria-like disease caused by toxigenic Corynebacterium ulcerans strain. Emerg Infect Dis 2014; 20(7):1257–8.
- 4. Cone LA, Curry N, Wuestoff MA, et al. Septic synovitis and arthritis due to Corynebacterium striatum following an accidental scalpel injury. Clin Infect Dis 1998; 27(6):1532–3.
- 5. Larsson P, Lundin O, and Falsen E. “Corynebacterium aquaticum” wound infection after high-pressure water injection into the foot. Scand J Infect Dis 1996; 28(6):635–6.
- 6. Peel MM, Palmer GG, Stacpoole AM, et al. Human lymphadenitis due to Corynebacterium pseudotuberculosis: report of ten cases from Australia and review. Clin Infect Dis 1997; 24(2):185–91.
- 7. Capizzi PJ, Clay RP, and Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med 1998; 23:172–4.
- 8. Wilson AP. The return of Corynebacterium diphtheriae: the rise of non-toxigenic strains. J Hosp Infect 1995; 30(suppl):306–12.
- 9. Sloss JM and Faithfull-Davies DN. Non-toxigenic Corynebacterium diphtheriae in military personnel. Lancet 1993; 341(8851):1021.
ERYSIPELOTHRIX RHUSIOPATHIAE
Common names for diseases: Erysipeloid, Erysipelothricosis, fish poisoning, fish-handler’s disease, seal finger, crab dermatitis, Baker–Rosenbach syndrome, Klauder disease.
Occupational setting
Erysipelothrix rhusiopathiae has been termed an “occupational pathogen” since the majority of infections occur through work with a wide variety of animals, including mammals, birds, fish, and crustaceans.1 Those at increased risk include fishermen,2 farmers,3 and meat processors.4 Human infection has also occurred in a laboratory setting.5 An outbreak occurred among workers at a shoe factory, with E. rhusiopathiae isolated from washings of leather and casein glue.6 The organism is found in animal waste and can remain viable in the environment for several months, thus providing an additional source of exposure in farm workers.7
Exposure (route)
Cutaneous inoculation of bacteria from a contaminated source is the usual mode of transmission when scales, shell, or bone fragments puncture the skin surface. Exposure following an animal bite has also been documented.8
Pathobiology
E. rhusiopathiae is a nonmotile, non-sporulating, Gram-positive rod found as a commensal or pathogen in many animal species. Swine are the primary reservoir and are also particularly susceptible to disease. The organism is harbored in the pharynx and excreted in feces. Other potential reservoirs include turkeys, chickens, ducks, deer, emus, sheep, crab, and fish.1
Infection can result in three clinical entities in humans.1 The most common is erysipeloid, a localized skin infection at the site of contact (generally the back of the hand or fingers). The incubation period is 2–7 days. The involved area is a clearly demarcated, edematous, violaceous lesion that fades centrally as it spreads peripherally. Swelling can be extensive. Lesions can be asymptomatic, painful, or mildly pruritic. Fever and arthralgia occur in ~10% of cases, and lymphangitis and lymphadenopathy can be associated as well.1 Cellulitis from E. rhusiopathiae can be distinguished from that commonly caused by streptococci or Staphylococcus aureus by the absence of pitting on pressure and lack of suppuration in the lesion.
The diffuse cutaneous form is an unusual presentation in which multiple skin lesions occur in a generalized pattern. The individual lesions are similar in appearance to those seen in localized presentations. Although patients may have constitutional symptoms, blood cultures are negative.
The third form is a rare, severe systemic illness consisting of sepsis with seeding of infection in a variety of sites, most commonly acute or subacute endocarditis. In a review of 45 E. rhusiopathiae endocarditis cases, 36% were accompanied by the characteristic skin lesion, 89% had an identifiable occupational association, and the aortic valve was preferentially affected in 61%.9
Diagnosis
The diagnosis is largely clinical, based on a history of trauma involving the typical sources of infection such as fish or animal bones together with the characteristic skin lesions.
The organism can be cultured from skin biopsies of the lesions or from blood cultures in patients with endocarditis. Identification can be difficult, since the organism resides deep in skin lesions, is difficult to culture, and may be incorrectly identified.10 A PCR-based assay offers a more rapid means of identification, but experience on the performance of this test is limited.10
Treatment
Penicillin is the recommended therapy for both localized and systemic infections.10 The bacterium is not sensitive to vancomycin, which is commonly used to treat endocarditis due to Gram-positive organisms. Therefore, in patients with endocarditis and a history of compatible occupational exposure, empirical antibiotic therapy should include coverage of this organism until the bacterial etiology can be established.
Medical surveillance
There are no specific medical screening or surveillance activities for this pathogen.
Prevention
E. rhusiopathiae is readily killed by commonly available disinfectants that can be used to clean contaminated surfaces.11
Containment and control measures should be in place wherever potentially infected animals are kept, slaughtered, or processed, or where animal waste is used.1 Prevention efforts should also focus on avoidance of skin inoculation and worker education about the bacterium and its clinical presentations. Guards for cutting instruments and gloves with metal mesh or other reinforcement are useful in preventing skin abrasions and lacerations. Work practices designed to reduce contact with bone fragments, fish scales, and knife tips should be encouraged. Handwashing after contact with infected animals and patients or their bacteriologic specimens is essential.
References
- 1. Brooke CJ and Riley TV. Erysipelothrix rhusiopathiae: bacteriology, epidemiology and clinical manifestations of an occupational pathogen. J Med Microbiol 1999; 48(9):789–99.
- 2. Rocha MP, Fontoura PR, Azevedo SN, et al. Erysipelothrix endocarditis with previous cutaneous lesion: report of a case and review of the literature. Rev Inst Med Trop Sao Paulo 1989; 31(4):286–9.
- 3. Andrychowski J, Jasielski P, Netczuk T, et al. Empyema in spinal canal in thoracic region, abscesses in paravertebral space, spondylitis: in clinical course of zoonosis Erysipelothrix rhusiopathiae. Eur Spine J 2012; 21(Suppl 4):S557–63. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3369048/pdf/586_2012_Article_2289.pdf (accessed on June 30, 2016).
- 4. Hill DC and Ghassemian JN. Erysipelothrix rhusiopathiae endocarditis: clinical features of an occupational disease. South Med J 1997; 90(11):1147–8.
- 5. Ajmal M. A laboratory infection with Erysipelothrix rhusiopathiae. Vet Rec 1969; 85(24):688.
- 6. Popugaĭlo VM, Podkin IuA, Gurvich VB, et al. Erysipeloid as an occupational disease of workers in shoe enterprises. Zh Mikrobiol Epidemiol Immunobiol 1983 (10):46–9 [Russian].
- 7. Chandler DS and Craven JA. Persistence and distribution of Erysipelothrix rhusiopathiae and bacterial indicator organisms on land used for disposal of piggery effluent. J Appl Bacteriol 1980; 48(3):367–75.
- 8. Abedini S and Lester A. Erysipelothrix rhusiopathiae bacteremia after dog bite. Ugeskr Laeger 1997; 159(28):4400–1 [Danish].
- 9. Gorby GL and Peacock JE. Erysipelothrix rhuriopathiae endocarditis: microbiologic, epidemiologic, and clinical features of an occupational disease. Rev Infect Dis 1988; 10:317–25.
- 10. Veraldi S, Girgenti V, Dassoni F, et al. Erysipeloid: a review. Clin Exp Dermatol 2009; 34(8):859–62.
- 11. Fidalgo SG, Longbottom CJ, and Rjley TV. Susceptibility of Erysipelothrix rhusiopathiae to antimicrobial agents and home disinfectants. Pathology 2002; 34(5):462–5.
ESCHERICHIA COLI
Common names for disease: Traveler’s diarrhea, turista, food poisoning
Occupational setting
Escherichia coli is a normal commensal of the human intestinal tract. Disease is caused by novel or particularly virulent strains transmitted primarily by the fecal–oral route. Occupations with particular risk for symptomatic E. coli infection include workers in long-term care facilities, childcare centers, hospitals, and schools.1 Jobs requiring travel to other countries, especially low-income countries, put workers (including military personnel) at risk for traveler’s diarrhea due to E. coli.2 The gastrointestinal tract of animal handlers will be colonized with the E. coli strains in the feces of the animals they handle, with evidence of an acquired protective immunity.3 Large outbreaks have occurred among visitors at farms from animal contact.4
Numerous outbreaks of serious diarrheal disease have been caused by E. coli transmitted by consumption of undercooked meats, especially improperly handled and prepared ground beef.5 This may be a risk for workers eating in institutional cafeterias.6 Like agricultural workers, food handlers have been found to be colonized with potentially pathogenic E. coli,7 although the risk of disease in this group is unclear. Finally, laboratory8 and healthcare-associated9E. coli infections, presumably due to failure to follow standard precautions, have been documented.
Exposure (route)
The primary route of exposure is fecal–oral. A review of outbreaks from E. coli O157:H7 over a 20-year period in the United States reported that 52% of cases were foodborne, 14% person-to-person, 9% waterborne, 3% from animal contact, and 0.3% laboratory-related.5 Approximately 20% of cases are thought to arise from secondary transmission.10
Pathobiology
E. coli is an aerobic, non-spore-forming Gram-negative rod that has hundreds of serotypes classified by various antigens. Although many of these serotypes can cause a wide variety of diseases, certain types have been implicated more frequently in specific diarrheal syndromes. There are six major categories of diarrheagenic E. coli, each producing disease through a different mechanism as follows:
- Enterotoxigenic E. coli (ETEC) is implicated in most cases of traveler’s diarrhea. It is also an important cause of childhood diarrhea in low-income countries. Infection results from consumption of contaminated food or water. Bacteria adhere to the intestinal mucosa and produce an enterotoxin that causes massive fluid secretion into the gut. Disease can range from an asymptomatic carrier state to severe, watery diarrhea with cramping abdominal pain following an incubation period of hours to a few days. Symptoms last for 3–5 days and usually resolve without specific treatment. The adult resident population is not affected, because regular exposure leads to the development of immunity to the bacterial adhesive factor.
- Another group variously referred to as enterohemorrhagic E. coli (EHEC), Shiga toxin-producing E. coli (STEC), or verocytotoxic E. coli (VTEC) is typified by the strain O157:H7, which causes regular foodborne outbreaks in the United States (Figure 22.5).5
Following ingestion of contaminated food, usually undercooked ground beef, there is an incubation period of 1–10 days, followed by abdominal cramping and frequently bloody diarrhea without fever or fecal leukocytes lasting less than 7 days. The course may be complicated by hemolytic-uremic syndrome with subsequent renal failure, especially in children, or by thrombotic thrombocytopenic purpura.
- Enteroaggregative E. coli (EAEC) is becoming increasingly implicated in cases of traveler’s diarrhea, acute diarrhea among children in both low- to high-income countries as well as chronic diarrhea, especially among the immune-compromised. The precise mode of transmission is not known. The mechanism of disease is not well understood, although it involves damage to colonic mucosa with loss of microvilli. Diarrhea is mucoid and may be protracted.
- Enteropathogenic E. coli (EPEC) is the major cause of infantile diarrhea in low-income countries. Transmission is from person-to-person. These bacteria disrupt the protective mucous gel coating the intestinal cells, bind to the cells, and cause characteristic mucosal lesions. After a 2–6 day incubation period, the clinical response is acute watery diarrhea with vomiting lasting 1–3 weeks. Dehydration can be severe enough to be fatal.
- Enteroinvasive E. coli (EIEC) is similar to ETEC with invasion of intestinal cells, evoking an inflammatory response that destroys the intestinal mucosa. Transmission is from contaminated food and this class is implicated in large outbreaks in low-income countries. The clinical syndrome includes watery diarrhea or a more severe form resembling bacterial dysentery and is rare in the United States. The incubation period is usually 2–3 days, followed by fever and diarrhea, which can be bloody with numerous fecal leukocytes lasting 1–2 weeks.
- Diffusely-adhering E. coli (DAEC) has been identified in cases of diarrhea among infants and children in both low- and high-income countries. The mode of transmission, clinical features, and mechanism of disease are not well-known.
FIGURE 22.5 E. coli O157 outbreaks in the United States, 1982–2002 (N = 350).
Source: Emerg Infect Dis 2005 Apr;11(4):603–9.
E. coli is also the most frequent cause of urinary tract infections and healthcare-associated bacteremia. It is a common cause of healthcare-associated pneumonia in severely ill patients.
Diagnosis
In cases of diarrhea, stool samples should be obtained for culture and sensitivity, toxin identification, and smear for fecal leukocytes.
Various immunoassays, bioassays, and DNA probes are used to differentiate among the serotypes. Many of these specialized tests are only available through local health department laboratories. When a specific E. coli serotype is suspected, the laboratory should be informed so that appropriate tests may be run. Diagnosis of E. coli infection in other sites, such as blood or urinary tract, is made by culture and Gram stain of the appropriate samples.
Treatment
The first consideration in treating all forms of E. coli diarrhea should be supportive care consisting of electrolyte and fluid replacement to prevent dehydration. Severe cases of traveler’s diarrhea can be presumed to be due to ETEC and may be treated with a short course of antibiotics such as trimethoprim–sulfamethoxazole, doxycycline, rifaximin, or a fluoroquinolone, which shorten the duration of illness by 1–2 days. Anti-motility agents are useful for symptomatic relief but are contraindicated when diarrhea is bloody due to an increased risk of hemolytic-uremic syndrome. Similar choices of antibiotics can be used, as appropriate, in protracted cases of EPEC and EAEC diarrhea. Antibiotics are currently considered contraindicated for EHEC diarrhea as they may also increase the risk of hemolytic-uremic syndrome and enhance toxin production.11
Medical surveillance
There are no recommended medical screening activities for infections due to E. coli. STEC is a nationally notifiable disease in the United States. Foodborne outbreaks of diarrhea due to E. coli should be reported to the local public health authorities.
Prevention
Meticulous handwashing with strict avoidance of high-risk foods and untreated water is the mainstay of prevention of traveler’s diarrhea. Unfortunately, because of transmission from food handlers serving travelers, cases will still occur despite these measures.
Bismuth subsalicylate has been shown to be effective in preventing traveler’s diarrhea, although the four times a day dosing with blackening of the tongue and stool make this option unappealing to many. Prophylaxis should not be extended beyond 3 weeks due to the risk of salicylate toxicity.
Various antibiotics have also been shown to be effective. However, they are generally not recommended prophylactically, due to the cost, the brief, self-limited nature of most cases, the increasing emergence of resistant bacteria, and the potential for serious complications such as pseudomembranous colitis. An antibiotic with poor oral bioavailability called rifaximin has been shown to be effective in preventing traveler’s diarrhea but is currently only FDA-approved for treatment.12
Proper preparation and storage of food at the workplace will prevent this and other causes of infectious gastroenteritis. Work practices should emphasize good hygiene with appropriate glove use, strict handwashing after contact with potentially infected material, and disinfecting potentially contaminated surfaces. Unpasteurized dairy products and juices as well as undercooked meats should always be avoided. There is evidence that diarrheal disease can be reduced among both children and staff in childcare centers through use of handwashing, proper diaper changing, and food preparation equipment specifically designed to reduce the transmission of enteric infection.13
References
- 1. Wikswo ME, Hall AJ, and Centers for Disease Control and Prevention. Outbreaks of acute gastroenteritis transmitted by person-to-person contact—United States, 2009–2010. MMWR Surveill Summ 2012; 61(9):1–12.
- 2. Nada RA, Armstrong A, Shaheen HI, et al. Phenotypic and genotypic characterization of enterotoxigenic Escherichia coli isolated from U.S. military personnel participating in Operation Bright Star, Egypt, from 2005 to 2009. Diagn Microbiol Infect Dis 2013; 76(3):272–7.
- 3. Quilliam RS, Chalmers RM, Williams AP, et al. Seroprevalence and risk factors associated with Escherichia coli O157 in a farming population. Zoonoses Public Health 2012; 59(2):83–8.
- 4. Wise J. Outbreak of E. coli O157 is linked to Surrey open farm. BMJ 2009; 339:b3795.
- 5. Rangel JM, Sparling PH, Crowe C, et al. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg Infect Dis 2005; 11(4):603–9.
- 6. Welinder-Olsson C, Stenqvist K, Badenfors M, et al. EHEC outbreak among staff at a children’s hospital—use of PCR for verocytotoxin detection and PFGE for epidemiological investigation. Epidemiol Infect 2004; 132(1):43–9.
- 7. Oundo JO, Kariuki SM, Boga HI, et al. High incidence of enteroaggregative Escherichia coli among food handlers in three areas of Kenya: a possible transmission route of travelers’ diarrhea. J Travel Med 2008; 15(1):31–8.
- 8. Spina N, Zansky S, Dumas N, et al. Four laboratory-associated cases of infection with Escherichia coli O157:H7. J Clin Microbiol 2005; 43(6):2938–9.
- 9. Burke L, Humphreys H, and Fitzgerald-Hughes D. The revolving door between hospital and community: extended-spectrum beta-lactamase-producing Escherichia coli in Dublin. J Hosp Infect 2012; 81(3):192–8.
- 10. Snedeker KG, Shaw DJ, Locking ME, et al. Primary and secondary cases in Escherichia coli O157 outbreaks: a statistical analysis. BMC Infect Dis 2009; 9:144.
- 11. Wong CS, Jelacic S, Habeeb RL, et al. The risk of the hemolytic uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med 2000; 342:1930–6.
- 12. Alajbegovic S, Sanders JW, Atherly DE, et al. Effectiveness of rifaximin and fluoroquinolones in preventing travelers’ diarrhea (TD): a systematic review and meta-analysis. Syst Rev 2012; 1:39.
- 13. Kotch JB, Isbell P, Weber DJ, et al. Hand-washing and diapering equipment reduces disease among children in out-of-home child care centers. Pediatrics 2007; 120(1):e29–36.
FRANCISELLA TULARENSIS (INCLUDING F. NOVOCIDA)
Common names for disease: Tularemia, rabbit fever, deer fly fever
Occupational setting
Tularemia was first described as a zoonotic disease, resembling plague, in ground squirrels in Tulare County, California, from which the disease and species names are derived. Classically, tularemia is described as a disease of small-game hunters.1 Cases have also been described among trappers,2 farm workers,3 professional landscapers,4 and laboratory workers.5
Approximately 125 cases are reported in the United States per year.6 Most cases are in males, children, and the elderly, and reported in summer, corresponding to increased human activity outdoors.6 Cases have been reported from almost every state, with a large proportion from Missouri, Arkansas, Oklahoma, Massachusetts, South Dakota, and Kansas (Figure 22.6).6

FIGURE 22.6 Reported cases of tularemia — United States, 2001–2010.
Source: MMWR 2013 Nov 29; 62(47):963-6.
Exposure (route)
The organism is both highly infectious and transmissible to humans through a wide variety of routes. Direct transmission occurs through handling of infected animal carcasses, hides, or fur, through non-intact skin or after bites by infected animals.6 Inhalation is an important route of exposure in outbreaks from cleaning and handling dead animals1 and for laboratory technicians. Although a large number of arthropods transmit disease between various animal hosts, hard ticks, deer flies, horse flies, and mosquitoes are thought to be the principle vectors for humans.7 Disease can also arise from ingestion of contaminated food or water. Person-to-person transmission has not been reported.
Pathobiology
Francisella tularensis is a Gram-negative, pleomorphic, nonmotile, non-spore-forming bacterium that has been found in more than 100 species of animals.8 Two tularemia strains (types A and B) have been identified on the basis of virulence. Type A is highly virulent, is found only in North America, and is most often associated with lagomorphs (hares or rabbits).7 Type B tularemia is less infectious, found throughout Europe, Asia, and North America, is associated with rodents as well as aquatic environments, and causes mild or even subclinical human diseases.7
A common source outbreak of disease from the closely related species, F. novicida, thought to be due to contaminated ice in a prison, has recently been reported.9
The incubation period is usually 3–7 days. As many as six different clinical syndromes with overlapping features are recognized, all of which include a high fever, each arising from different portals of entry for the bacteria. Ulceroglandular tularemia is the most common form, accounting for approximately 75% of cases with initial symptoms consisting of headaches, myalgias, and rigors.10 Skin inoculation of the organism from an arthropod bite or infected wound produces a cutaneous ulcer with a depressed, blackened center, and well-demarcated, elevated margins. Proximal to this lesion, painful lymphadenopathy may ensue. If there is no cutaneous ulcer, the condition is simply referred to as glandular tularemia. Oculoglandular tularemia consists of conjunctivitis with pre-auricular lymphadenopathy and arises from splashes or direct inoculation with infected material into the eyes. Oropharyngeal tularemia involves a non-exudative pharyngitis, oral ulcers, and tonsillitis with cervical lymphadenopathy following ingestion of contaminated food or water. The mortality rate for these untreated forms of tularemia is less than 5%, but fever can last for weeks, the ulcer heals slowly over weeks to months, and lymphadenopathy can persist for months.
Typhoidal tularemia (also known as septicemic tularemia) is the second most common form with hepatosplenomegaly in the absence of cutaneous or lymph node involvement and a heart rate lower than expected given the high fever (pulse temperature disassociation).10 The mode of transmission is uncertain but is thought to be from ingestion.
Pneumonic tularemia is a severe, atypical pneumonia that has a high case fatality ratio when untreated, approaching 60% in the pre-antibiotic era. Appropriate therapy has decreased the case fatality ratio to less than 1%. This form arises either from primary inoculation of the lungs by inhalation of infected aerosols or after hematogenous dissemination.
Diagnosis
Tularemia should be suspected in patients with a compatible exposure history, especially children and men over age 55 years, with acute fever and regional lymphadenopathy.6
Because of the risk of transmission in the laboratory, notification of all personnel handling specimens sent for testing is essential. Cultures from appropriately collected clinical specimens are considered definitive.11 However, blood cultures are usually negative. A variety of rapid testing methods using PCR, direct fluorescent antibody, or immunohistochemical-based assays can be used but are not widely available.
Treatment
Fluoroquinolones are the antibiotics of choice for mild to moderate cases, while severe cases should receive intravenous streptomycin or gentamicin.12 Tetracyclines such as doxycycline are an alternative therapy, but are less desirable due to the common contraindications, need for more prolonged therapy, and increased frequency of relapses. Treatment for up to 3 weeks may be indicated depending upon the stage of disease and choice of antibiotic.
Relapses are common and are more likely to occur following treatment that is delayed or of insufficient duration.12
Medical surveillance
There are no recommended medical screening activities. Tularemia is a nationally notifiable disease in the United States and cases must be reported to public health authorities. Because tularemia is a CDC category A bioterrorism agent, immediate notification is recommended for cases caused by suspected intentional release.
Prevention
An attenuated live vaccine was withdrawn in the United States due to concerns about variable efficacy and reversion to wild-type virulence. The use of protective clothing and gloves is recommended during skinning or handling of potentially infected animals. Particular attention should be given to avoid inhalation of aerosols when carcasses are rinsed.1 Prevention of arthropod-borne disease includes strategies to decrease bites with protective clothing, repellent use, and careful inspection of the skin. Laboratories should be alerted about samples from suspected cases and apply biosafety level 2 conditions.
CDC has provided detailed guidance on managing suspected laboratory exposures.13 Antibiotic prophylaxis with either doxycycline or ciprofloxacin can be given to exposed workers.
References
- 1. Hauri AM, Hofstetter I, Seibold E, et al. Investigating an airborne tularemia outbreak, Germany. Emerg Infect Dis 2010; 16(2):238–43.
- 2. Lévesque B, De Serres G, Higgins R, et al. Seroepidemiologic study of three zoonoses (leptospirosis, Q fever, and tularemia) among trappers in Québec, Canada. Clin Diagn Lab Immunol 1995; 2(4):496–8.
- 3. No authors listed. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 14-2000. A 60-year-old farm worker with bilateral pneumonia. N Engl J Med 2000; 342(19):1430–8.
- 4. Hofinger DM, Cardona L, Mertz GJ, et al. Tularemic meningitis in the United States. Arch Neurol 2009; 66(4):523–7.
- 5. Lawler A. Biodefense labs. Boston University Under Fire for Pathogen Mishap. Science 2005; 307(5709):501.
- 6. Centers for Disease Control and Prevention. Tularemia – United States, 2001–2010. MMWR Morb Mortal Wkly Rep 2013; 62(47):963–6.
- 7. Petersen JM, Mead PS, and Schriefer ME. Francisella tularensis: an arthropod-borne pathogen. Vet Res 2009; 40(2):7.
- 8. Hopla CE. The ecology of tularemia. Adv Vet Sci Comp Med 1974; 18:25–53.
- 9. Brett ME, Respicio-Kingry LB, Yendell S, et al. Outbreak of Francisella novicida bacteremia among inmates at a Louisiana correctional facility. Clin Infect Dis 2014; 59(6):826–33.
- 10. Nigrovic LE and Wingerter SL. Tularemia. Infect Dis Clin North Am 2008; 22(3):489–504.
- 11. Centers for Disease Control and Prevention. Tularemia: for Clinicians: Diagnostic Testing. Available at: http://www.cdc.gov/tularemia/clinicians/index.html (accessed on June 6, 2016).
- 12. Boisset S, Caspar Y, Sutera V, et al. New therapeutic approaches for treatment of tularaemia: a review. Front Cell Infect Microbiol 2014; 4:40.
- 13. Centers for Disease Control and Prevention. Tularemia Fact Sheet. Available at: http://www.cdc.gov/tularemia/resources/lab/TularemiaLabExposureFactSheet.pdf (accessed on June 6, 2016).
HAEMOPHILUS DUCREYI
Common names for disease: Chancroid, soft chancre
Occupational setting
In the United States, there was a steady decline in cases of chancroid, a sexually transmitted infection, between 1987 and 2001, with 15 cases reported in 2012.1 However, due to the difficulty in culturing Haemophilus ducreyi, cases may be underreported.
Outbreaks occur in association with high-risk behavior, often involving sex trade workers, and in conjunction with other sexually transmitted infections, most notably HIV.2
Exposure (route)
Chancroid is sexually transmitted.
Pathobiology
H. ducreyi is a small, pleomorphic coccobacillus that causes genital and perianal ulcerative lesions. After sexual exposure to an infected person, there is a variable incubation period of a day to several weeks. The chancroidal lesion begins as a tender erythematous papule, which becomes pustular and then ulcerates, and lesions are often multiple. The ulcers are usually painful and ragged in appearance, with easy bleeding upon manipulation. Tender inguinal adenopathy is common and may progress to abscess (bubo), which often spontaneously drains.
Diagnosis
Presumptive diagnosis on clinical grounds without laboratory confirmation is often inaccurate, since other ulcerating sexually transmitted diseases, including syphilis and genital herpes simplex, may mimic chancroid.2 Material taken from the base of an ulcer, or aspirated from a bubo, may be cultured and identified using special techniques to isolate H. ducreyi. However, the appropriate culture media is not widely available and the sensitivity of this method is less than 80%.3 There is no FDA-approved PCR-based test in the United States, although laboratories may develop their own assay. A presumptive diagnosis can be made on clinical grounds in a patient with the characteristic ragged, purulent, painful genital ulcers and tender inguinal lymphadenopathy after ruling out (or concomitantly treating for) syphilis and Herpes simplex.
Treatment
Recommended antibiotics include oral azithromycin or IM ceftriaxone, both of which offer the advantage of being effective in a single dose. HIV coinfected patients should be carefully monitored due to an increased risk of treatment failure with single dose regimens. Oral ciprofloxacin and erythromycin are alternatives.
Fluctuant nodes should be drained by needle aspiration to prevent rupture and fistula formation.
Medical surveillance
There are no recommended medical screening activities. Chancroid is a nationally notifiable disease in the United States and cases must be reported to the local health department. Partners should be identified and treated, even if asymptomatic.
Prevention
Practicing safe sexual practices can prevent chancroid. Male circumcision has been shown to reduce the risk of transmission.4 Identification and treatment of partners, who are often asymptomatic carriers, may help curtail outbreaks.
References
- 1. Centers for Disease Control and Prevention. 2012 Sexually Transmitted Diseases Surveillance: Other Sexually Transmitted Diseases. Available at: http://www.cdc.gov/std/stats12/other.htm (accessed on June 30, 2016).
- 2. Mertz KJ, Weiss JB, Webb RM, et al. An investigation of genital ulcers in Jackson, Mississippi, with use of a multiplex PCR assay: high prevalence of chancroid and human immunodeficiency virus infection. J Infect Dis 1998; 178(4):1060–6.
- 3. Lockett AE, Dance DA, Mabey DC, et al. Serum free media for the isolation of Haemophilus ducreyi. Lancet 1991; 338:326.
- 4. Weiss HA, Thomas SL, Munabi SK, et al. Male circumcision and risk of syphilis, chancroid, and genital herpes: a systematic review and meta-analysis. Sex Transm Infect 2006; 82(2):101–9.
HAEMOPHILUS INFLUENZA
Common name for disease: None
Occupational setting
Invasive disease due to Haemophilus influenzae is more common in young children, among whom nasopharyngeal carriage rates are high. When disease occurs in adults, it is usually among those with impaired immune function or other chronic medical conditions. Outbreaks of disease have occurred in childcare centers (even among fully vaccinated populations),1 long-term care facilities,2 and hospitals.3
Exposure (route)
Person-to-person transmission occurs through airborne droplets or by direct contact with infectious secretions.
Pathobiology
H. influenzae is a pleomorphic, Gram-negative coccobacillus found only in humans, and there are no other known hosts. H. influenzae is dichotomized into encapsulated and nonencapsulated (also known as nontypeable) strains. Encapsulated bacteria are further divided into the following types: a–f. While all six types are capable of causing human disease, the most clinically relevant is type b, which produces a polysaccharide capsule, a polymer of polyribitol ribose phosphate (PRP) allowing bacteria to evade opsonization and spread systemically.
Nontypeable strains are commonly part of the normal flora in the upper respiratory tract, with colonization occurring shortly after birth. The prevalence of colonization declines into adulthood. Nontypeable H. influenzae is responsible for many mild illnesses in adults, including sinusitis, conjunctivitis, otitis media, and exacerbation of COPD.
Encapsulated H. influenzae is more likely to cause invasive disease, through hematogenous spread to distant sites. The most common form of invasive disease is meningitis, which is associated with antecedent head trauma, sinusitis, otitis, or cerebrospinal fluid leak. The clinical course resembles other forms of purulent meningitis. Epiglottitis is also unusual in adults, but it is the only invasive H. influenzae disease to affect healthy adults without underlying or preceding illness.4 It presents with sore throat, fever, and dyspnea, progressing to dysphagia, drooling, and an upright posture with the neck extended and chin protruding to maintain airflow. Death may result due to airway obstruction. Pneumonia due to H. influenzae, usually nontypeable, may occur in adults with lung disease or alcoholism. The radiographic picture varies, but a pleural effusion, usually sterile, is common. Septic arthritis occurs on occasion, usually in adults with impaired immunity or a pre-existing arthritic condition. Bacteremia may accompany invasive disease in adults.
Diagnosis
A diagnosis of H. influenzae invasive disease is made by confirmatory Gram stain and culture of appropriately collected clinical samples. All isolates should be serotyped, in view of the important distinction between type b and other serotypes. A variety of rapid testing methods are available to detect the PRP capsular polysaccharide found on the type b strain.
Identifying nontypeable H. influenza as the cause of noninvasive disease is challenging, as it is often present as a colonizer in the setting of polymicrobial growth. Therefore, these diagnoses are usually made on clinical grounds. Blood cultures in cases of pneumonia are usually negative.
Treatment
Invasive infections should be treated with a cephalosporin such as ceftriaxone or cefotaxime. Steroids are indicated to reduce neurological sequelae in cases of meningitis. Epiglottitis is a medical emergency requiring airway maintenance.
Medical surveillance
There are no recommended medical screening activities for diseases due to H. influenzae. Cases of invasive H. influenzae disease should be reported to the local public health authorities.
Prevention
Invasive H. influenzae type b disease has almost disappeared in those countries incorporating the vaccine into the primary childhood schedule.5 Vaccination of adults is not routinely indicated because of high rates of natural immunity but should be considered in special populations with underlying conditions at high risk for infection.6 The vaccine for H. influenzae type b does not confer protection against other encapsulated or nontypeable strains.
In cases of H. influenzae type b outbreaks, rifampin chemoprophylaxis is indicated for all household contacts of persons less than 4 years who are not fully vaccinated or those less than 18 years who are immunocompromised, irrespective of vaccination status.6 In childcare centers with two or more cases of invasive disease within 60 days and unimmunized/underimmunized children, prophylaxis is indicated for all attendees, regardless of age or vaccine status, and all childcare providers.6
N95 respirators have been shown to be protective against bacterial colonization among healthcare workers.7
References
- 1. McVernon J, Morgan P, Mallaghan C, et al. Outbreak of Haemophilus influenzae type b disease among fully vaccinated children in a day-care center. Pediatr Infect Dis J 2004; 23(1):38–41.
- 2. Van Dort M, Walden C, Walker ES, et al. An outbreak of infections caused by non-typeable Haemophilus influenzae in an extended care facility. J Hosp Infect 2007; 66(1):59–64.
- 3. Yang CJ, Chen TC, Wang CS, et al. Nosocomial outbreak of biotype I, multidrug-resistant, serologically non-typeable Haemophilus influenzae in a respiratory care ward in Taiwan. J Hosp Infect 2010; 74(4):406–9.
- 4. Takala AK, Eskola J, and Van Alphen L. Spectrum of invasive Haemophilus influenzae type b disease in adults. Arch Intern Med 1990; 150:2573–6.
- 5. Bisgard KM, Kao A, Leake J, et al. Haemophilus influenzae invasive disease in the United States, 1994–1995: near disappearance of a vaccine-preventable childhood disease. Emerg Infect Dis 1998; 4(2):229–37.
- 6. Briere EC, Rubin L, Moro PL, et al. Prevention and control of Haemophilus influenzae type b disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-01):1–14.
- 7. MacIntyre CR, Wang Q, Rahman B, et al. Efficacy of face masks and respirators in preventing upper respiratory tract bacterial colonization and co-infection in hospital healthcare workers. Prev Med 2014; 62:1–7.
HELICOBACTER PYLORI
Common name for disease: None
Occupational setting
Numerous studies are available that have examined occupational risk factors for Helicobacter pylori infection. However, since the vast majority of the studies are cross-sectional and the risk of infection also varies markedly with numerous other factors, such as geographic location, socioeconomic status, age, and gender, occupational studies must be interpreted with caution due to the possibility of confounding.
The evidence for infection among those in gastroenterology departments has recently been reviewed and provides a mixed picture.1 While a statistically significant increased overall risk was reported, this was only observed when compared to nonmedical controls rather than hospital staff from other departments. Further, a consistent increase risk was only seen in studies from Asia, not Europe or America/Australia. Therefore, there may be a modest increased risk of infection from those in the healthcare setting generally, rather than from exposure to specific activities such as endoscopy. Healthcare workers in long-term care facilities2 and laboratory workers handling specimens3 may also be at increased risk. There is concern that infection can occur from contaminated water, and one study has reported an increased prevalence of seropositivity among fish handlers.4 No association has been found for dentists5 or sewage workers.6
Exposure (route)
Humans are the only important host for H. pylori and are estimated to have been infected for approximately 100 000 years or earlier.7 It is considered to be the most common human chronic infection, with an overall global prevalence of approximately 50%.
While the reservoir is the stomach, person-to-person transmission occurs by routes that are incompletely understood. The fecal–oral route is considered to be more important in low-income countries than the oral–oral route in high-income countries.8 Most individuals acquire infection in childhood. Although the prevalence of infection increases with age, this is not because of new infections in adults (which is rare) but a birth cohort effect reflecting declines in infection thought to be due to improved sanitation and hygiene. Much research has focused on contaminated water as a vehicle for infection, but studies have been hampered by difficulties in culturing the organism from this source.
Pathobiology
H. pylori is a Gram-negative, spiral-shaped rod found within the gastric mucosal layer or adhering to the epithelium of the stomach. Although chronic superficial gastritis occurs in essentially all those infected, less than 15% go on to develop an associated disease due to a variety of environmental, host, and bacterial strain-related factors.
H. pylori infection has been causally associated with peptic ulcer disease, gastric adenocarcinoma, and mucosa-associated lymphoid tissue (MALT) lymphoma. Infection appears to reduce the risk of gastroesophageal reflux disease. It is now understood that H. pylori infection also has profound systemic effects. Accordingly, the list of associated conditions continues to grow and includes immune thrombocytopenic purpura, idiopathic sideropenic anemia, vitamin B12 deficiency, diabetes mellitus, cardiovascular disease, hepatobiliary disease, and neurologic disease.9
Diagnosis
The gold standard approach to diagnosis includes histologic examination and culture of gastric biopsy specimens for H. pylori. Additional non-endoscopic tests include the urea breath test, serology for IgG, and the fecal antigen test.
Treatment
For many years, triple therapy consisting of a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole was standard. However, increasing antibiotic resistance, especially to clarithromycin, has resulted in unacceptably high failure rates. As a result, therapy based on susceptibility testing is preferred. Empiric therapy should be guided by local resistance patterns.10 There are now concomitant therapies, sequential therapies, and hybrid therapies with four drug combinations.
Medical surveillance
There are no recommended medical screening or surveillance activities.
Prevention
In view of the many unresolved issues concerning transmission of H. pylori, specific preventive recommendations cannot be provided. General good hygiene precautions, applicable to prevent the spread of many different organisms, should be in place. Efforts to develop a vaccine have been unsuccessful to date.11
References
- 1. Peters C, Schablon A, Harling M, et al. The occupational risk of Helicobacter pylori infection among gastroenterologists and their assistants. BMC Infect Dis 2011; 11:154.
- 2. De Schryver A, Cornelis K, Van Winckel M, et al. The occupational risk of Helicobacter pylori infection among workers in institutions for people with intellectual disability. Occup Environ Med 2008; 65(9):587–91.
- 3. Matysiak-Budnik T, Briet F, Heyman M, et al. Laboratory-acquired Helicobacter pylori infection. Lancet 1995; 346(8988):1489–90.
- 4. Ullah SS, Shamsuzzaman SM, Ara MN, et al. Seropositivity of Helicobacter pylori among the fish handlers. Mymensingh Med J 2010; 19(2):219–24.
- 5. Lin SK, Lambert JR, Schembri MA, et al. The prevalence of Helicobacter pylori in practising dental staff and dental students. Aust Dent J 1998; 43(1):35–9.
- 6. Tschopp A, Joller H, Jeggli S, et al. Hepatitis E, Helicobacter pylori and peptic ulcers in workers exposed to sewage: a prospective cohort study. Occup Environ Med 2009; 66(1):45–50.
- 7. Moodley Y, Linz B, Bond RP, et al. Age of the association between Helicobacter pylori and man. PLoS Pathog 2012; 8(5):e1002693.
- 8. Bruce MG and Maaroos HI. Epidemiology of Helicobacter pylori infection. Helicobacter 2008; 13(Suppl 1):1–6.
- 9. Roubaud Baudron C, Franceschi F, Salles N, et al. Extragastric diseases and Helicobacter pylori. Helicobacter 2013; 18(Suppl 1):44–51.
- 10. Graham DY and Shiotani A. Which therapy for Helicobacter pylori infection? Gastroenterology 2012; 143(1):10–2.
- 11. Koch M, Meyer TF, and Moss SF. Inflammation, immunity, vaccines for Helicobacter pylori infection. Helicobacter 2013; 18(Suppl 1):18–23.
LEGIONELLA SPECIES (LEGIONELLA PNEUMOPHILA, LEGIONELLA LONGBEACHAE)
Common names for disease: Legionellosis, Legionnaires’ disease, Pontiac fever
Occupational setting
Legionella organisms are ubiquitous in natural aquatic sources and proliferate easily in water supply systems, cooling towers, evaporative condensers, and distribution lines. Disease caused by Legionella pneumophila has been reported in many diverse settings including workers cleaning steam turbine condensers1 and cooling towers,2 from aerosols generated by a leaking coolant system,3 a water tank cooling system for welding,4 in sewage treatment workers,5 crews6 or those repairing ships7, from showers in long-distance truck drivers,8 in well excavators,9 in workers at an automobile engine manufacturing plant,10 and in workers at an offshore drilling facility.11
In the healthcare setting, outbreaks in hospitals12 and long-term care facilities13 are well known. Although the vast majority of cases are reported among patients, especially the immunocompromised including neonates, employees have also been affected.12 Contamination of dental water supplies has been extensively documented but case reports of disease among staff are limited.14
More than 20% of cases of Legionnaire’s disease reported to CDC are travel associated, usually from cruise ships or hotels.15
Pontiac fever and Legionnaire’s disease can also be caused by L. longbeachae. Unlike other Legionella species, this organism is found in natural soil, commercial potting soil, and compost, especially in New Zealand and Australia, although disease occurs globally. Outbreaks have occurred among gardeners16 and nursery workers.17
In a study of Legionnaires’ disease in New York City, there was a 230% increase in reported cases from 2002 to 2009. Cases followed a socioeconomic gradient, with the highest incidence occurring in the highest poverty areas.18 Work in transportation, repair, protective services, cleaning, or construction was associated with a significantly higher risk for Legionnaires’ disease compared with the general working population.18
Exposure (route)
Transmission of L. pneumophila occurs through inhalation of aerosols or aspiration of water droplets, while transmission of L. longbeachae is through inhalation of soil dust. Person-to-person transmission does not occur.
Pathobiology
Organisms in the Legionellaceae family are aerobic, Gram-negative rods, which do not grow in routine culture media. L. pneumophila, responsible for approximately 90% of infections, exists in 16 different serogroups, with serogroups 1, 4, and 6 most frequently causing infection. Almost 20 other Legionella species, most notably L. longbeachae, have also been identified as human pathogens.
Following aspiration or inhalation, bacteria use pili to adhere to the epithelial lining of the respiratory tract. Those reaching the lungs are phagocytized by alveolar macrophages. However, they are able to block mechanisms that cause intracellular killing, multiply in these cells resulting in lysis, and release of more bacteria.
Legionella species cause two distinct clinical syndromes. The first, Pontiac fever, is a flu-like illness that occurs after a short incubation period of 1–2 days. The attack rate is over 90%, but the illness is usually mild and self-limited with a rapid recovery within about a week. Symptoms consist of fever, myalgias, headache, chills, and fatigue.
The other syndrome is Legionnaires’ disease, a pneumonia with high morbidity and mortality rates. The incubation period is longer, varying from 2 to 10 days, and the attack rate is less than 5%. There is a prodrome similar to Pontiac fever, but nonproductive cough is more prominent and lung examination and chest radiographs may reveal evidence of consolidation. Myalgias and a fever greater than 40°C are almost universally present. Chest pain and hemoptysis, which can be confused with a pulmonary embolus, may occur. Watery diarrhea is common. A number of neurologic abnormalities may be present but altered mental status is the most common. Hyponatremia occurs more frequently in pneumonia due to Legionella species than in pneumonia due to other pathogens. Although there is no classic radiographic presentation, the chest x-ray may worsen during the initial treatment and take several months to resolve.
Diagnosis
For Legionnaire’s disease, culture from appropriately collected respiratory specimens, ideally from bronchoalveolar lavage, is the gold standard diagnostic test. However, Legionella organisms are particularly difficult to grow in the laboratory and require selective media and specialized techniques. Results are not available for 3–5 days. Bacteria cannot be isolated in cases of Pontiac fever.
A urinary antigen assay is inexpensive, has high sensitivity and specificity with the additional advantage of same day results. However, it is only available for L. pneumophila serogroup 1, which nevertheless causes the majority of cases. CDC recommends both culture and urinary antigen testing as the preferred diagnostic approach.
Acute and convalescent antibody titers can be drawn, which require paired blood samples to be taken at 3 and 6 weeks postexposure. A fourfold or greater rise in titers is diagnostic. Single titers must be interpreted with caution due to the lack of specificity. Positive serologies indicate exposure to the organism only, which may or may not be associated with the disease. Clinical correlation is therefore essential.
Additional tests that are less widely available include PCR and direct fluorescent antibody testing of biological specimens.
Treatment
Pontiac fever is self-limited and requires no specific treatment. Azithromycin is the treatment of choice for Legionnaires’ disease. Other macrolides, quinolones, or erythromycins are also effective.
Medical surveillance
Legionellosis is a nationally notifiable disease in the United States and other countries.
Prevention
For the prevention of L. pneumophila, proper maintenance of water storage, distribution, and coolant systems is essential. The American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ASHRAE) provides detailed guidance for water systems available at ashrae.org. Routine testing of hospital water supplies is recommended, and facilities with known contamination should have the specialized testing needed for Legionella available for cases of healthcare-associated pneumonia.
Respiratory protection during manual cleaning operations of water systems may be helpful. The prevention of L. longbeachae, especially in the higher risk countries of Oceania, includes use of gloves, a disposable N95 respirator, keeping potting soil damp while in use, and handwashing.
References
- 1. Fraser DW, Deubner DC, Hill DL, et al. Nonpneumonic, short-incubation-period legionellosis (Pontiac fever) in men who cleaned a steam turbine condenser. Science 1979; 205:690–1.
- 2. Girod JC, Reichman RC, Winn WC Jr, et al. Pneumonic and nonpneumonic forms of legionellosis. The result of a common-source exposure to Legionella pneumophila. Arch Intern Med 1982; 142(3):545–7.
- 3. Allen KW, Prempeh H, and Osman MS. Legionella pneumonia from a novel industrial aerosol. Commun Dis Public Health 1999; 2(4):294–6.
- 4. O’Keefe NS, Heinrich-Morrison KA, and McLaren B. Two linked cases of legionellosis with an unusual industrial source. Med J Aust 2005; 183(9):491–2.
- 5. Gregersen P, Grunnet K, Uldum SA, et al. Pontiac fever at a sewage treatment plant in the food industry. Scand J Work Environ Health 1999; 25(3):291–5.
- 6. Rowbotham TJ. Legionellosis associated with ships: 1977 to 1997. Commun Dis Public Health 1998; 1(3):146–51.
- 7. Caylà JA, Maldonado R, González J, et al. A small outbreak of Legionnaires’ disease in a cargo ship under repair. Eur Respir J 2001; 17(6):1322–7.
- 8. Public Health Laboratory Service (UK). Legionnaires’ disease in long distance lorry drivers. Commun Dis Rep 1998; 10:13–4.
- 9. Miragliotta G, Del Prete R, Sabato R, et al. Legionellosis associated with artesian well excavation. Eur J Epidemiol 1992; 8(5):748–9.
- 10. Fry AM, Rutman M, Allan T, et al. Legionnaires’ disease outbreak in an automobile engine manufacturing plant. J Infect Dis 2003; 187(6):1015–8.
- 11. Lapiński TW and Kruminis-Lozowski J. Infection with Legionella pneumophila among workers of Polish sea drilling platforms. Wiad Lek1997; 50(1–3):11–5.
- 12. Ozerol IH, Bayraktar M, Cizmeci Z, et al. Legionnaire’s disease: a nosocomial outbreak in Turkey. J Hosp Infect 2006; 62(1):50–7.
- 13. Trop Skaza A, Beskovnik L, Storman A, et al. Epidemiological investigation of a legionellosis outbreak in a Slovenian nursing home, August 2010. Scand J Infect Dis 2012; 44(4):263–9.
- 14. Chikte UM, Khondowe O, and Gildenhuys I. A case study of a dental receptionist diagnosed with Legionnaires’ disease. SADJ 2011; 66(6):284–7.
- 15. de Jong B, Payne Hallström L, Robesyn E, et al. Travel-associated Legionnaires’ disease in Europe, 2010. Euro Surveill 2013; 18(23):pii: 20498.
- 16. Potts A, Donaghy M, Marley M, et al. Cluster of Legionnaires disease cases caused by Legionella longbeachae serogroup 1, Scotland, August to September 2013. Euro Surveill 2013; 18(50):20656.
- 17. Cramp GJ, Harte D, Douglas NM, et al. An outbreak of Pontiac fever due to Legionella longbeachae serogroup 2 found in potting mix in a horticultural nursery in New Zealand. Epidemiol Infect 2010; 138(1):15–20.
- 18. Farnham A, Alleyne L, Cimini D, et al. Legionnaires’ disease incidence and risk factors, New York, New York, USA, 2002–2011. Emerg Infect Dis 2014; 20(11):1795–1802.
LEPTOSPIRA INTERROGANS
Common names for disease: Leptospirosis, Weil’s disease or syndrome (name applied to severe, icteric disease), milker’s fever
Occupational setting
Leptospirosis is an enzootic infection that is ubiquitous in nature. Important reservoirs include cattle, swine, dogs, rodents, and fish. Many infections in animals are not clinically apparent, and prolonged urinary shedding of the organism can occur. In an Australian study of 208 laboratory-confirmed cases of leptospirosis, 56% had a clear association with occupational exposure.1 High-risk groups include farmers,1 sanitation and sewage workers,2 rodent control workers,3 laboratory animal handlers,4 forestry workers,5 trappers,5 zoo workers,6 veterinarians,7 slaughterhouse and other meat workers,1 and fish farmers.8
Dairy farmers are at high risk during milking. Aerosols from bovine urination may contain leptospires that can infect humans through inhalation or entry through the eyes, nose, or throat.9
More recent case series suggest that multiuse land development, with water from farmlands draining into recreational bodies of water, may be contributing to an increasing proportion of cases from nonoccupational exposures. Waterborne disease is the single most important source of infection.10 Widespread epidemics of leptospirosis have been described following floods.11
Exposure (route)
Humans contract the infection from contaminated fluids, tissues, or waters through direct contact with breaks in skin or mucous membranes. Urinary shedding of organisms from infected animals is the most common source of these pathogens, but meat handling is also an important route of exposure. Although person-to-person transmission is generally not thought to occur, a recent case series raise the possibility of disease transmission to healthcare workers.12
Pathobiology
The organism, a spirochete that is an obligate aerobe, is easily visualized by phase contrast and dark-field microscopy but grows slowly in culture. Leptospira consists of several species, only one of which, L. interrogans, is pathogenic in humans. L. interrogans consists of almost 300 serovars, arranged into 25 major related serogroups. This classification is important, since the animal reservoirs, clinical picture of infection, and geographic distribution vary between serogroups. Active and passive immunity is also serovar-specific. The icterohaemorrhagiae serogroup is usually carried by rats and is associated with the more severe, classic form of leptospirosis known as Weil’s disease. The Australis serogroup is a common cause of infection in many parts of the world such as Australia, New Zealand, and Asia. Serogroup Pomona is frequently found in pigs and cattle. The Canicola serogroup causes canine leptospirosis.
Untreated leptospirosis is most often a self-limited illness. Because of the nonspecific and often mild presentation, the disease is underdiagnosed and underreported. The incubation period is usually about 10 days but can vary from 2 days to 4 weeks. The clinical presentation is variable, but common symptoms include the abrupt onset of fever, headache, muscular pain, nausea, vomiting, and diarrhea. Conjunctival suffusion is more specific than other findings and should raise concern for leptospirosis.
Leptospirosis is a biphasic illness. The first phase is a flu-like illness as described above. A minority of patients will go on to develop a more serious illness. The second phase of the disease is characterized by both liver and kidney failure (Weil’s disease). Pulmonary hemorrhage and cardiac arrhythmia (most commonly AV nodal block) are also seen in more severe disease. The overall case fatality rate as reported by the CDC is 1–5%. However, in patients that go on to develop end organ damage, case fatality rate may be as high as 15%.13
Diagnosis
Leptospirosis should be considered in any patient with fever, myalgias, headache, and nausea or vomiting. Culture of leptospires from blood and cerebrospinal fluid during the first 10 days of the illness, and of urine beyond the first week can aid diagnosis, but the organism grows slowly and cultures may take up to 8 weeks to become positive. The only screening test approved for use in the United States is the indirect hemagglutination assay (IHA), which utilizes pooled antigens from all serogroups of leptospirosis, and is broadly available. However, since the prevalence of different serovars varies geographically, this test may not be sufficiently sensitive in some regions.14 The microscopic agglutination test (MAT) requires paired sera and is generally only available in reference laboratories. ELISA-based screening tests are also available. They have shown satisfactory sensitivity but results should be confirmed with MAT.15 A dipstick assay for serum has been developed which is suitable for widespread field use.16 PCR and multiplex PCR tests have also been developed.17,18 Other laboratory results will show signs of systemic infection with possible renal and liver dysfunction.
Treatment
When given within the first 4 days of infection, penicillin and doxycycline are both effective in shortening the duration of illness and decreasing symptoms of fever, headache, and myalgias. Supportive therapy and careful management of renal, hepatic, hematologic, and central nervous system complications are also important. Therapy has been reported to be infective when administered after day 4 of infection, and the efficacy of other antimicrobial agents has not been rigorously studied in randomized trials. The Jarisch–Herxheimer reaction, an inflammatory reaction induced during antibiotic treatment as a result of rapid release of antigen, is commonly observed during treatment.
Medical surveillance
Screening is not recommended. In the United States, leptospirosis has been reinstated as a nationally notifiable disease as of January 2013.
Prevention
Primary prevention strategies should focus on both animal reservoirs and humans. Animal preventive activities mainly consist of vaccines that are available for cattle and pigs. Vaccines are serovar-specific and are only useful where a small number of serovars are prevalent. Rodent control is important.
Strategies to prevent leptospirosis in humans have included environmental control measures, protective clothing, and antibiotic prophylaxis. A dramatic decline in L. icterohaemorrhagiae infections in Great Britain between 1978 and 1985 was attributed to vigorous rodent control programs, protective clothing use, attention to personal hygiene, and worker education in coal workers, sewer workers, and fish workers.19 Personal protective equipment consisting of gloves and boots, together with careful work practices around domestic animals to avoid contact with potentially contaminated tissues and fluids (particularly urine), are recommended.
A randomized trial of chemoprophylaxis in military personnel at high risk for leptospirosis in Panama revealed that 200 mg of doxycycline administered once weekly was 95% effective in preventing the disease.20 This strategy would seem useful only for populations at high risk for relatively brief periods, such as travelers. There are no currently available human vaccines.
References
- 1. Swart KS, Wilks CR, Jackson KB, et al. Human leptospirosis in Victoria. Med J Aust 1983; 14:460–3.
- 2. De Serres G, Levesque B, Higgins R, et al. Need for vaccination of sewer workers against leptospirosis and hepatitis A. Occup Environ Med 1995; 52(8):505–7.
- 3. Demers RY, Frank R, Demers P, et al. Leptospiral exposure in Detroit rodent control workers. Am J Public Health 1985; 75(9):1090–1.
- 4. Natrajaseenivasan K and Ratnam S. An investigation of leptospirosis in a laboratory animal house. J Communicable Dis 1996; 28(3):153–7.
- 5. Moll van Charante AW, Groen J, Mulder PG, et al. Occupational risks of zoonotic infections in Dutch forestry workers and muskrat catchers. Eur J Epidemiol 1998; 14(2):109–16.
- 6. Anderson DC, Geistfeld JG, Maetz HM, et al. Leptospirosis in zoo workers associated with bears. Am J Trop Med Hyg 1978; 27(1 Pt 1):210–1.
- 7. Kingscote BF. Leptospirosis in two veterinarians. CMAJ 1985; 133(9):879–80.
- 8. Gill ON, Coghlan JD, and Calder IM. The risk of leptospirosis in United Kingdom fish farm workers. Results from a 1981 serological survey. J Hyg (Lond) 1985; 94(1):81–6.
- 9. Skilbeck NW and Miller GT. A serological survey of leptospirosis in Gippsland dairy farmers. Med J Aust 1986; 144:565–7.
- 10. Ciceroni L, Stepan E, Pinto A, et al. Epidemiological trend of human leptospirosis in Italy between 1994 and 1996. Eur J Epidemiol 2000; 16(1):79–86.
- 11. Trevejo RT, Rigau-Perez JG, Ashford DA, et al. Epidemic leptospirosis associated with pulmonary hemorrhage—Nicaragua, 1995. J Infect Dis 1998; 178(5):1457–63.
- 12. Ratnan S and Seenivasan N. Possible hospital transmission of leptospiral infection. J Commun Dis 1998; 30(1):54–6.
- 13. Bharti, AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 2003; 3(12):757–71.
- 14. Effler PV, Domen HY, Bragg SL, et al. Evaluation of the indirect hemagglutination assay for diagnosis of acute leptospirosis in Hawaii. J Clin Microbiol 2000; 38(3):1081–4.
- 15. Winslow WE, Merry DJ, Pirc ML, et al. Evaluation of a commercial enzyme-linked immunosorbent assay for detection of immunoglobulin M antibody in diagnosis of human leptospiral infection. J Clin Microbiol 1997; 35(8):1938–42.
- 16. Smits HL, Hartskeerl RA, and Terpstra WJ. International multi-centre evaluation of a dipstick assay for human leptospirosis. Trop Med Int Health 2000; 5(2):124–8.
- 17. Ahmed SA, Sandai DA, Musa S, et al. Rapid diagnosis of leptospirosis by multiplex PCR. Malays J Med Sci 2012; 19(3):9–16.
- 18. Backstedt BT, Buyuktanir O, Lindow J, et al. Efficient detection of pathogenic leptospires using 16S ribosomal RNA. PLoS One 2015; 10(6):e0128913. doi:10.1371/journal.pone.0128913.
- 19. Waitkins SA. Leptospirosis as an occupational disease. Br J Ind Med 1986; 43:721–5.
- 20. Takafuji ET, Kirkpatrick JW, Miller RN, et al. An efficacy trial of doxycycline chemoprophylaxis against leptospirosis. N Engl J Med 1984; 310:497–500.
LISTERIA MONOCYTOGENES
Common names for disease: Listeriosis
Occupational setting
Listeria monocytogenes is a common environmental bacterium, and has been recovered from soil, dust, and water, and from mammals, birds, fish, ticks, and crustaceans. Most cases of listeriosis in the United States affect urban dwellers without specific occupational exposures to the bacterium; and many are linked to the ingestion of contaminated food, particularly in pregnant women, newborns, and those with compromised immunity1. Veterinarians handling infected calves have developed skin infections, as have laboratory personnel following accidental direct skin inoculation.2,3 Mild cases of listeria conjunctivitis occur occasionally in laboratory and poultry workers.4 Although slaughterhouse workers have been found to have five times the normal fecal carriage rate of L. monocytogenes (5 versus 1%), an increased risk of disease among animal handlers other than veterinarians has not been identified.
Exposure (route)
Exposure occurs by ingestion of contaminated food, by direct skin or eye inoculation, and by transplacental transmission from an infected mother to her fetus. Foods that have been implicated are unpasteurized dairy products, undercooked meats, and vegetables grown in fields fertilized with manure from infected animals.5
Pathobiology
L. monocytogenes is a Gram-positive non-spore-forming aerobic rod, which can cause a variety of clinical syndromes. Transient asymptomatic carriage in the stool is common. Serious symptomatic infection occurs almost exclusively in neonates and immunocompromised adults.1 Infection during pregnancy (a state of relative immunodeficiency) is often unrecognized and may lead to preterm labor, intrauterine fetal demise, or a critically ill baby.
In adults, L. monocytogenes infection can present as sepsis of unknown origin. Symptomatic illness in adults usually occurs in those who are immunosuppressed, including those with acquired immune deficiency syndrome (AIDS) and malignancies. Bacteremia may lead to seeding of the meninges or brain. L. monocytogenes is the leading cause of meningitis in immunosuppressed adults and should be considered in the differential diagnosis of any adult with meningitis. The onset is usually subacute, with low-grade fever and personality changes. Infrequently, there are focal neurologic findings; typical meningeal signs are usually absent. Cerebritis may present with headache and fever or as a paresis resembling a cerebrovascular accident.
In cases of direct inoculation, ulcerating skin lesions have occurred, as well as purulent conjunctivitis, and, rarely, acute anterior uveitis.2 Focal internal infections, which may arise from dissemination, include lymphadenitis, subacute bacterial endocarditis, osteomyelitis, spinal abscess, peritonitis, cholecystitis, and arthritis. Disseminated listeriosis may be accompanied by hepatitis.
Diagnosis
Diagnosis is made by isolation of the organism from cultures of blood, cerebrospinal fluid, skin ulcer, conjunctival pus, or other specimens from an infected site. Presumptive diagnosis may be made pending culture results if a Gram-stained specimen reveals Gram-positive rods resembling diphtheroids (or sometimes diplococci). Large samples of infected fluid are required (at least 10 mL of cerebrospinal fluid) because the bacteria are often sparse and are difficult to isolate. A direct fluorescent antigen test is available but is difficult to interpret and so has little practical use in most laboratories. Cerebritis is diagnosed with CT or MRI scans showing focal areas of increased uptake, without ring enhancement, and a positive blood culture; cerebrospinal fluid culture is usually negative.
Treatment
There have been no controlled studies of the efficacy of various treatment regimens, but clinical experience with penicillin and ampicillin has shown these to be usually effective, although there have been rare cases of resistance to each. Because of the refractory nature of Listeria to the action of most antibiotics, an aminoglycoside, usually gentamicin, is added for synergy.6 For patients with penicillin allergy, the best alternative is probably trimethoprim–sulfamethoxazole, although erythromycin, tetracycline, and chloramphenicol have all been used successfully. If gentamicin is used for central nervous system infection, it should be administered both intravenously and intrathecally. There may be progression of disease despite appropriate antibiotic therapy, and the optimum duration of therapy is unknown. Although 2 weeks of therapy is usually effective, there have been relapses in immunosuppressed patients, who may require 3–6 weeks of treatment. Effective treatment remains difficult in the immunocompromised patient, and mortality remains high (approximately 30%) despite appropriate choice of therapy.
Medical surveillance
There are no recommended medical screening activities for this disease. Cases are required to be reported to local health authorities in many parts of the United States and in some other countries. Prompt reporting of outbreaks is also required.
Prevention
Animal handlers, including veterinarians, should wear gloves and splash goggles, and should wash their hands frequently. Avoiding unpasteurized dairy foods and undercooked meats can prevent foodborne listeriosis. Vegetables and fruits grown near the ground should be washed thoroughly before consumption. Uncooked meats should not be stored near vegetables or ready-to-eat foods. Hands, knives, and cutting boards should be washed after handling uncooked foods.5
References
- 1. Centers for Disease Control and Prevention. Update: multistate outbreak of listeriosis—United States, 1998–1999. MMWR 1999; 47:1117–8.
- 2. McLauchlin J and Low JC. Primary cutaneous listeriosis in adults: an occupational disease of veterinarians and farmers. Vet Rec 1994; 135:615–7.
- 3. Zelenik K, Avberšek J, Pate M, et al. Cutaneous listeriosis in a veterinarian with the evidence of zoonotic transmission—a case report. Zoonoses Public Health 2014; 61(4):238–41. doi:10.1111/zph.12075.
- 4. Jones D. Foodborne illness: foodborne listeriosis. Lancet 1990; 336:1171–74.
- 5. Centers for Disease Control. Update: Foodborne listeriosis—United States, 1988–1990. MMWR 1992; 41:251–8.
- 6. Jones EM and MacGowan AP. Antimicrobial chemotherapy of human infection due to Listeria monocytogenes. Eur J Clin Microbiol Infect Dis 1995; 14:165–75.
MYCOPLASMA PNEUMONIAE
Common names for disease: Mycoplasma pneumonia, atypical pneumonia, walking pneumonia
Occupational setting
Transmission of Mycoplasma pneumoniae is thought to require close, prolonged contact. Therefore, closed populations such as those in military barracks1 or on college campuses are subject to M. pneumoniae infections.2
Although epidemiologic data are limited and somewhat inconsistent, outbreaks in hospitals have been described.3 Since many cases in such instances are quite mild, it is possible that many outbreaks go unrecognized.3 A cluster of cases among workers in a prosthodontics laboratory was suspected to be caused by an aerosol generated during abrasive drilling on the false teeth of a patient who was diagnosed with M. pneumoniae 11 days later.4 Since the organism also causes respiratory disease in animals, veterinarians, animal handlers, and farmers may be exposed if contact is prolonged.5
Exposure (route)
Transmission occurs through contact with infected respiratory secretions.
Pathobiology
Mycoplasma are the smallest free-living organisms. They are neither true bacteria nor viruses, because, unlike the former, they lack a cell wall, and, unlike the latter, they do not require other cells to grow. M. pneumoniae is the most important species in this group and is a frequent cause of respiratory disease. Other pathologic mycoplasmas such as M. hominis and the closely related Ureaplasma urealyticum are common etiologic agents in infections of the urogenital tract. Infections at other sites by M. hominis are rare but have been described in the immunocompromised.6 Occult infection with M. fermentans has been proposed as a cause of illness in Persian Gulf War veterans, but this association has not been confirmed in serologic studies.7
M. pneumoniae is a common cause of respiratory infections. Illness develops gradually over a period of several days following an incubation period of 1–4 weeks. Symptoms are flu-like and include fever, malaise, headache, sore throat, and cough. Children and young adults are the most frequently affected age groups. Most infections result in relatively benign illness that cannot be distinguished from viral etiologies and may include tracheobronchitis, pharyngitis, and otitis. However, 3–10% of M. pneumoniae infections result in pneumonia.8 Because infection with this organism is so common, its contribution to community-acquired pneumonia is significant, causing an estimated 500 000 cases per year.8
M. pneumoniae pneumonia classically presents with a non-productive cough, fever, and upper respiratory tract symptoms such as sore throat and rhinitis. Pleuritic chest pain, dyspnea, and rigors are less common than in other bacterial pneumonias, and the white blood cell count is often normal. Chest radiographic findings are variable, but lower lobe involvement, patchy infiltrates, and pleural effusions are common. Approximately 5–10% of patients require hospitalization.8
These cases can be severe, resulting in respiratory insufficiency and a number of extrapulmonary complications ranging from otitis media and bullous myringitis to significant neurologic and cardiac disease. Central nervous system involvement occurs in up to 7% of hospitalized patients and includes meningitis, meningoencephalitis, and neuritis.9 Cardiac manifestations due to myocarditis or pericarditis may result in arrhythmias and heart failure. Nausea, vomiting, or diarrhea is reported in 14–44% of patients.9 Skin rashes are common. This infection is commonly believed to be associated with erythema multiforme; however, a systematic review of the case literature found that the association is with Stevens–Johnson syndrome and not erythema multiforme.10
Autoantibodies that agglutinate red blood cells at 40°C (cold agglutinins) can result in hemolytic anemia. Mycoplasma infections have also been associated with a variety of other autoimmune disorders, including rheumatoid arthritis and Guillain–Barré syndrome. A link between infection with M. pneumoniae and asthma has recently been established.11
Diagnosis
Three traditional methods have been used to diagnose M. pneumoniae infections. The organism can be cultured from biological specimens, but since growth is slow, 14–21 days may be needed before Mycoplasma species can be detected. The cold hemagglutinins test detects the IgM autoantibody responsible for the agglutination of red blood cells, but it is neither sensitive nor specific. The complement fixation test measures antibody production to the mycoplasma lipid membrane but is only positive late in the course of illness. Rapid PCR-based assays can be performed on respiratory tract samples and are reported to be both sensitive and specific.12,13
Treatment
Erythromycin and tetracycline are traditionally used in treating M. pneumoniae respiratory infections. However, tetracycline resistance has been described with increasing frequency. The newer macrolides and quinolones have been shown to be effective.14 Supportive care may be needed in the setting of complications.
Medical surveillance
Outbreaks of Mycoplasma infection should be promptly reported to local public health authorities. There are no recommended medical screening activities.
Prevention
Transmission of the organism generally involves prolonged close contact. Therefore, the risk is greatest in households, barracks, and dormitories. Healthcare workers and those exposed to infected animals should practice good hygiene with strict handwashing after contact. Gloves and respiratory protection may be useful if contact is prolonged. In a study of health care workers in Beijing, China, N95 respirators showed a significant reduction in relative risk of mycoplasma pneumonia over medical masks.15
One study has concluded that antibiotic prophylaxis of contacts with azithromycin during outbreaks may significantly reduce secondary attack rates.16 An infected person is generally not regarded as contagious beyond 3 weeks.
References
- 1. Gray GC, Callahan JD, Hawksworth AW, et al. Respiratory diseases among US military personnel: countering emerging threats. Emerg Infect Dis 1999; 5(3):379–85.
- 2. Feikin DR, Moroney JF, Talkington DF, et al An outbreak of acute respiratory disease caused by Mycoplasma pneumoniae and adenovirus at a federal service training academy: new implications from an old scenario. Clin Infect Dis 1999; 29(6):1545–50.
- 3. Kleemola M and Jokinen C. Outbreak of Mycoplasma pneumoniae infection among hospital personnel studied by a nucleic acid hybridization test. J Hosp Infect 1992; 21(3):213–21.
- 4. Sande MA, Gadot F, and Wenzel RP. Point source epidemic of Mycoplasma pneumoniae infection in a prosthodontics laboratory. Am Rev Respir Dis 1975; 112:213–7.
- 5. Jordan FT. Gordon Memorial Lecture: People, poultry and pathogenic mycoplasmas. Br Poult Sci 1985; 26(1):1–15.
- 6. Mattila PS, Carlson P, Sivonen A, et al. Life-threatening Mycoplasma hominis mediastinitis. Clin Infect Dis 1999; 29(6):1529–37.
- 7. Gray GC, Kaiser KS, Hawksworth AW, et al. No serologic evidence of an association found between Gulf War service and Mycoplasma fermentans infection. Am J Trop Med Hyg 1999; 60(5):752–7.
- 8. Mansel JK, Rosenow EC, Smith TF, et al. Mycoplasma pneumoniae pneumonia. Chest 1989; 95:639–46.
- 9. Cassell GH and Cole BC. Mycoplasmas as agents of human disease. N Engl J Med 1981; 304:80–9.
- 10. Tay YK, Huff JC, and Weston WL. Mycoplasma pneumoniae infection is associated with Stevens–Johnson syndrome, not erythema multiforme. J Am Acad Dermatol 1996; 35(5 Pt 1):757–60.
- 11. Daian CM, Wolff AH, and Bielory L. The role of atypical organisms in asthma. Allergy Asthma Proc 2000; 21(2):107–11.
- 12. Abele-Horn M, Busch U, Nitschko H, et al. Molecular approaches to diagnosis of pulmonary diseases due to Mycoplasma pneumoniae. J Clin Microbiol 1998; 36(2):548–51.
- 13. Nilsson AC, Björkman P, and Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol 2008; 8:93.
- 14. Taylor-Robinson D and Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother 1997; 40(5):622–30.
- 15. MacIntyre CR, Wang Q, Rahman B, et al. Efficacy of face masks and respirators in preventing upper respiratory tract bacterial colonization and co-infection in hospital healthcare workers. Prev Med 2014; 62:1–15.
- 16. Klausner JD, Passaro D, Rosenberg J, et al. Enhanced control of an outbreak of Mycoplasma pneumoniae pneumonia with azithromycin prophylaxis. J Infect Dis 1998; 177(1):161–6.
NEISSERIA GONORRHOEAE
Common name for disease: Gonorrhea, clap
Occupational setting
Gonorrhea is primarily a sexually transmitted disease. There are few occupations outside of prostitution where a true occupational risk has been demonstrated, although seafarers are known to be at increased risk of several types of sexually transmitted diseases.1 A theoretical risk exists among dentists caring for patients with oral gonorrhea. Cutaneous infection has been reported in a laboratory worker who cut his finger on a test tube containing Neisseria gonorrhoeae prepared for lyophilization.2
Exposure (route)
Transmission is by direct contact of mucous membranes with the organism, usually during sexual intercourse. Oral or conjunctival splashing, as well as direct inoculation, can transmit the bacteria.
Pathobiology
N. gonorrhoeae is a nonmotile, aerobic, Gram-negative diplococcus. The urethra, endocervix, anal canal, pharynx, and conjunctiva are infected directly by contact with N. gonorrhoeae. The organism penetrates the mucosal epithelium and causes a local inflammatory response within 72 hours (although appearance of clinical symptoms may be delayed). Infection in men results in urethritis, with local extension in the urogenital tract if untreated. Infection in women results in vaginitis, cervicitis, and urethritis, can extend locally to the ducts of Skene, and Bartholin’s glands, and if untreated can cause endometritis, salpingitis, and pelvic inflammatory disease. In both sexes, N. gonorrhoeae can directly infect the anorectal mucosa, pharynx, and conjunctiva, causing symptomatic disease. If untreated, infection can become systemic, resulting in bacteremia, arthritis, tenosynovitis, endocarditis, meningitis, or a disseminated rash.3
Diagnosis
Diagnosis is made by nucleic acid amplification tests from infected body fluid. Gram stain is also used, usually in conjunction with culture. Bacterial culture allows determination of antibiotic resistance patterns, which are increasingly important.
Treatment
Because of widespread resistance, penicillin and oral cephalosporins are no longer the recommended drug of choice for gonorrhea. The current CDC recommendations are for a single dose of ceftriaxone 250 mg intramuscularly and either azithromycin 1 g orally as a single dose or doxycycline 100 mg orally twice daily for 7 days.4
Medical surveillance
There are no recommended medical screening activities for gonorrhea. It is a reportable disease in the United States.
Prevention
Prevention of gonorrhea in the general population consists of treatment of infected individuals and their partners, and the use of condoms or avoidance of sexual contact with infected individuals.4 Individuals who have direct contact with potentially infectious individuals or material, including healthcare and laboratory workers, should use appropriate personal protective equipment such as gloves.
References
- 1. International Labor Organization—World Health Organization. Joint ILO–WHO committee on the hygiene of seafarers. WHO Tech Rep Ser 1961; 224:1–14.
- 2. Collins CH and Kennedy DA. Microbiological hazards of occupational needlestick and ‘sharps’ injuries. J Appl Bacteriol 1987; 62:385–402.
- 3. Cheng DSF. Gonorrhea. In: Parish LC and Gschnait F (eds.), Sexually Transmitted Diseases: A Guide for Clinicians. New York: Springer-Verlag, 1988:59–77.
- 4. Centers for Disease Control and Prevention. Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. Morb Mortal Wkly Rep 2012; 61(31):590–4.
NEISSERIA MENINGITIDIS
Common names for diseases: Meningococcal meningitis, meningococcemia, cerebrospinal fever
Occupational setting
Serious disease due to Neisseria meningitidis, including meningitis and septicemia, usually occurs sporadically. Epidemics usually occur in institutional settings or such places as dormitories, schools, and military barracks. Occupational groups at risk during epidemics would include those who work at close quarters or with institutionalized individuals: military recruits, day care workers, prison personnel, employees of chronic care facilities, and dormitory supervisors. There have also been infections, some fatal, among laboratory personnel working with N. meningitidis.1
Exposure (route)
Spread is by direct contact with the nose, throat, and upper respiratory secretions of persons infected with (or asymptomatically carrying) the bacteria or via inhalation of respiratory droplets from coughing or sneezing carriers. Transmission may be more efficient to persons already suffering with a viral upper respiratory infection.
Pathobiology
N. meningitidis is a Gram-negative diplococcus with a polysaccharide capsule. Capsular antigens form the basis for serogroup typing of the bacteria into different strains. Four serogroups are responsible for the majority of diseases. Serogroups B, C, and Y cause most disease in the United States. However, among those older than 11 years of age, serogroups C, Y, and W cause 73% of disease. These are the serogroups included in available vaccines. Rates of meningococcal disease have been declining in the United States since the late 1990s (Figure 22.7).

FIGURE 22.7 Meningococcal disease – reported cases per 100 000 population, by year, United States, 1970–2011.
Source: MMWR 2013; 62(RR02):1–22.
Asymptomatic carriage of meningococcus in the nose and throat is common and may be chronic, intermittent, or transient. Approximately 10% of the human population harbor meningococci in the nose or throat.2 Nasopharyngeal carriage immunizes the host, with antibody production occurring within 2 weeks. Antibodies are serogroup-specific but confer some cross-immunity against other serogroups. There is also some cross-immunity between Neisseria and other species of bacteria, which probably promotes natural immunity against meningococcus in a population. Serogroup B is poorly immunogenic, possibly because antigens in the polysaccharide capsule resemble neonatal host antigens.
Cases of serious meningococcal disease occur more often in the winter and spring and are more common in very young children. Males are affected more than females.
When transmission of meningococcus occurs, it most commonly results in asymptomatic nasopharyngeal carriage. When illness results from transmission, it usually takes the form of a mild pharyngitis. Pharyngitis may precede serious illness but not reliably. Serious meningococcal infection can progress to death within a few hours of symptom onset, making a high index of suspicion for the disease, and a low threshold to treat essential.
The most common meningococcal syndromes are the following:
- Bacteremia without sepsis. The patient presents with symptoms similar to those of a viral upper respiratory infection or rash and recovers without specific therapy. Diagnosis is sometimes made when blood cultures are later found to be positive.
- Meningococcemia without meningitis. The patient presents with signs and symptoms of bacteremia and sepsis, including fever, chills, malaise, and petechial rash. Leukocytosis and hypotension may be found on examination.
- Meningitis with or without meningococcemia. The patient presents with headache, fever, and meningeal signs; a petechial rash may be present in meningococcemia. Mental status may range from normal to comatose. Reflexes are usually normal. Lumbar puncture will disclose cloudy cerebrospinal fluid.
- Meningoencephalitis. The patient is obtunded, with meningeal signs, abnormal reflexes, and septic cerebrospinal fluid; pathologic reflexes may be present.
Petechial rash is common in meningococcal sepsis and meningitis. The rash is usually predominantly on the trunk and lower body, but petechiae are also often found on mucous membranes, including the palpebral conjunctiva. The lesions sometimes coalesce, especially at pressure points, to form ecchymotic-appearing lesions. A pink papular rash is an infrequent variant, and a vesicular rash is rare.
Endotoxemia may occur as a result of release of bacterial components of the Gram-negative cell wall. Endotoxemia may give rise to disseminated intravascular coagulation, septic shock with heart failure, myocarditis, pericarditis, peripheral hypoperfusion, adult respiratory distress syndrome, and adrenal hemorrhage (Waterhouse–Friderichsen syndrome). Less common manifestations of meningococcal disease include chronic meningococcemia with low-grade fever, rash, and arthritis, recurrent meningococcemia in persons with various complement deficiencies, meningococcal pneumonia, and meningococcal urethritis.
Ten to fifteen percent of cases of meningitis caused by the bacteria are fatal according to CDC. Of those that survive permanent hearing loss, mental retardation, and loss of limbs is not uncommon.3
Diagnosis
Treatment should not be withheld pending definitive diagnosis if meningococcal meningitis or meningiococcemia is suspected as time to antibiotic therapy is lifesaving. Presumptive diagnosis is made based on clinical presentation and by finding typical organisms (Gram-negative diplococci) on Gram-stained smear of cerebrospinal fluid or by recovering meningococcus from cerebrospinal fluid, or blood culture. Occasionally, stained smears from petechiae reveal the organisms. Various antigen detection techniques can be used to identify group-specific polysaccharides. Although these techniques are rapid and specific, they are not sensitive, so they cannot be used to exclude the diagnosis of meningococcal infection. Other findings on cerebrospinal fluid examination vary, although neutrophils predominate in untreated cases. Cerebrospinal fluid chemistry usually reveals low glucose and high protein, findings seen in many infections and not specific to meningococcal meningitis.
Treatment
If the diagnosis is seriously considered, antibiotic therapy should be administered within 30 minutes. In adults, empiric treatment is with a third-generation cephalosporin, ceftriaxone, or cefotaxime administered intravenously. If there is proven susceptibility, penicillin can be used. Dexamethasone is indicated in cases of haemophilus influenza type b and should be administered until that diagnosis is disproved.4 Patients need close monitoring early in the course of meningococcal disease for endotoxin-related complications, including disseminated intravascular coagulation (DIC) and septic shock. Because penicillin does not eradicate nasopharyngeal carriage in patients with meningococcal illness, patients should be given rifampin prior to discharge from the hospital.
Medical surveillance
Household and intimate contacts should be closely watched for signs of meningococcal infection, so that treatment can be started promptly. Surveillance of nasopharyngeal cultures is generally not indicated, since carriage is common, often transient, and not a consistent risk factor for infection. Cases must be reported immediately to local health authorities so that contact tracing can begin.
Prevention
Antibiotic prophylaxis is indicated for household and intimate contacts of cases. Household contact includes contacts in crowded quarters such as day care centers, dormitories, military barracks, prisons, and chronic care facilities. Hospital personnel are not considered to be at increased risk unless there has been intimate contact such as mouth-to-mouth resuscitation.
Rifampin, given as a 600-milligrams dose twice daily for 2 days, is the drug of choice for chemoprophylaxis. If the isolate is appropriately sensitive to sulfadiazine, this may be used as an alternative. Other alternatives for chemoprophylaxis in adults are single-dose ceftriaxone or ciprofloxacin. Improved building ventilation may help prevent or control epidemics in close quarters.
Conjugate and polysaccharide quadrivalent vaccines are currently available against serogroups A, C, Y, and W-135. Vaccination with the conjugate vaccine is recommended for all children aged 11–12 years with a booster at 16 years of age. Patients older than 56 years of age should receive the unconjugated polysaccharide vaccine when they are traveling to an endemic area or if they work with N. meningitides in a laboratory. Travelers to endemic areas, particularly the “meningitis belt” of sub-Saharan Africa extending from Mauritania to Ethiopia, should be vaccinated if their work or journey brings them into prolonged contact with the local populace.5 In 2014, an unvaccinated 25-year-old laboratory worker died after working with N. meningitidis. It was later found that there were several breaches of recommended laboratory safety practices. Work with preparations of meningococcus should only be performed in a BSL 3 laboratory. Laboratory personnel expected to be exposed should be protected by use of standard microbiology laboratory procedures, including double gloves, N-95 fit tested respirator use and closed front laboratory coat, and work under a Class II biological safety cabinet when performing mechanical manipulations with the potential for aerosolization. All laboratory workers should be vaccinated.6 According to CDC, laboratory workers who were infected at work in the United States had a 50% fatality rate.7 Any incident or exposure involving meningococcus should receive prompt medical attention. In cases of percutaneous exposure, penicillin should be used for prophylaxis; for mucosal exposure, rifampin should be used.1
References
- 1. Centers for Disease Control. Laboratory-acquired meningococcemia—California and Massachusetts. MMWR 1991; 40(3):46–47, 55.
- 2. van Deuren M, Brandtzaeg P, and van der Meer JW. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev 2000; 13:144–66.
- 3. Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med 2001; 344:1378–88.
- 4. Brouwer MC, McIntyre P, Prasad K, et al. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 2013; 6:CD004405.
- 5. Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62(RR-2):1–28.
- 6. Centers for Disease Control (CDC). Recommendation of the Immunization Practices Advisory Committee: meningococcal vaccines. MMWR Morb Mortal Wkly Rep 1985; 34(18):255–259. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00020273.htm (accessed on June 30, 2016).
- 7. Centers for Disease Control and Prevention. Biosafety in Microbiological and Biomedical Laboratories (BMBL), 5th ed. Washington, DC: US Government Printing Office, 2009.
PASTEURELLA MULTOCIDA
Common name for disease: Pasteurellosis
Occupational setting
Increased risk of exposure is associated with work involving contact with animals. Occupations with exposure include animal laboratory personnel, veterinarians, pig, cattle and chicken breeders, zoo personnel, and abattoir workers.1
Exposure (route)
The bacteria are usually transmitted through animal bites or scratches, although exposure to animal secretions has been implicated in cases without a history of skin trauma. Pulmonary pasteurellosis is thought to occur via inhalation of aerosolized contaminated secretions; these may be from animals or via nosocomial spread from animals.2,3
Pasteurella multocida infection results in three clinical presentations in humans, all of which are more common in older or immunocompromised individuals. The most common is local infection following a bite or scratch. Evidence of infection develops rapidly and generally can be noted within 24 hours. Local erythema, warmth, and tenderness are accompanied by purulent drainage in about 40% of cases.4 Lymphangitis and lymphadenopathy may be present. Bites or scratches from cats are the most common cause of these infections. Wound cellulitis can be complicated by osteomyelitis, from either local extension to bone or direct inoculation, and septic arthritis. The latter occurs proximal to the wound, often in a joint previously damaged by osteoarthritis or rheumatoid arthritis.
Respiratory tract infections represent the second clinical syndrome caused by P. multocida. The spectrum of disease in respiratory Pasteurella infection ranges from sinusitis to tracheobronchitis to pneumonia with lung abscess or empyema. Pulmonary pasteurellosis is primarily a problem in individuals with underlying lung disease including chronic obstructive pulmonary disease and malignancy. Although unusual, colonization of the respiratory tract has been reported in occupationally exposed individuals without apparent infection or underlying disease.
Third, systemic infections may develop and, as with the other types of P. multocida illnesses, are primarily diagnosed in patients with underlying medical disease. P. multocida meningitis affects the very young and the elderly. Although endocarditis is rare, bacteremia can occur associated with localized sites of infection. Both respiratory and systemic infections can result without a clear history of animal-associated trauma or even documented animal exposure.
Pathobiology
The organism is a small, nonmotile, Gram-negative coccobacillus that frequently exhibits bipolar staining. It is present in the gastrointestinal or respiratory tracts of a number of animal species, including rodents, cats, dogs, and larger domestic and wild animals ranging from cattle to lions. Although it can cause significant disease in animals, it is commonly found in apparently healthy carriers. Approximately 70–90% of oral and nasal secretions in cats have been shown to carry the bacteria.4 Virulence of the organism is thought to be related to a microbial capsule.
Diagnosis
Initial Gram stain of purulent material may suggest the diagnosis; culture of blood, wound, respiratory secretions, or other body fluid is confirmatory. Serologic tests to detect antibody against P. multocida have been used in animals and as research tools in humans.
Treatment
The preferred treatment for P. multocida infection is penicillin; doxycycline is also effective. Other oral therapeutic agents that may be used include amoxicillin, amoxicillin–clavulanate, cefuroxime, tetracycline, and ciprofloxacin.5 Penicillin G and its derivatives or second- and third-generation cephalosporins are effective intravenous therapy.
Medical surveillance
There are no recommended medical screening or surveillance activities.
Prevention
Those working with small animals should wear gloves, use animal-handling techniques designed to avoid bites, and practice careful handwashing after contact. Exposed workers should be educated about the hazards of Pasteurella infection. Bites, if they occur, should be thoroughly cleansed, and attention sought if the worker becomes ill or if the injured site becomes infected. Suturing of bite wounds is controversial, and in general not recommended, especially if the hand is involved, as it may make early detection and management of infection difficult. Prophylactic antibiotics may be beneficial in high-risk wounds where infection is suspected, in hand wounds, in deep puncture wounds, and in immunocompromised patients. Recent microbiological analysis of animal bite wounds found P. multocida to be the most common constituent isolated from cat bites.6 Prophylaxis, if initiated, should usually be with a β-lactam antibiotic plus a β-lactamase inhibitor, such as amoxicillin–clavulinate. Particular care should be taken with the pregnant worker. A 2003 case report described a pregnant veterinarian who presented with fever and vaginal bleeding and was infected with Pasteurella.7
References
- 1. Choudat D, Le Goff C, Delemotte B, et al. Occupational exposure to animals and antibodies against Pasteurella multocida. Br J Ind Med 1987; 44:829–33.
- 2. Beyt BE, Sondag J, Roosevelt TS, et al. Human pulmonary pasteurellosis. JAMA 1979; 242:1647–48.
- 3. Itoh M, Tierno PM, Milstoc M, et al. A unique outbreak of Pasteurella multocida in a chronic disease hospital. Am J Public Health 1980; 70:1170–3.
- 4. Weber DJ, Wolfson JS, Swartz MN, et al. Pasteurella multocida infections: report of 34 cases and review of the literature. Medicine 1984; 63:133–54.
- 5. Weber DJ and Hansen AR. Infections resulting from animal bites. Infect Dis Clin North Am 1991; 5:663–80.
- 6. Talan DA, Citron DM, Abrahamian FM, et al. Bacteriologic analysis of infected dog and cat bites. N Engl J Med 1999; 340:85–92.
- 7. Waghorn DJ and Robson M. Occupational risk of Pasteurella multocida septicaemia and premature labour in a pregnant vet. BJOG 2003; 110(8):780–1.
PSEUDOMONAS AND BURKHOLDERIA SPECIES
Common name for disease: Glanders (B. mallei), melioidosis, Whitmore’s disease, pseudoglanders (B. pseudomallei)
Occupational setting
Pseudomonas and Burkholderia species are free-living, ubiquitous bacteria that are a particularly common contaminant of moist environments and microenvironments. While some species are very important causes of nosocomial infections in immunocompromised patients, these organisms are opportunistic pathogens that rarely cause infection in healthy persons. Therefore, occupationally acquired infection is uncommon.
Nevertheless, Pseudomonas can be a concern either through direct cutaneous infection or indirectly through reactions from exposure to pseudomonas-contaminated media. P. aeruginosa has been associated with skin infections in commercial divers1 and nosocomial keratitis in a nurse.2P. fluorescens has been implicated in an outbreak of hypersensitivity pneumonitis from exposure to contaminated metalworking fluids termed “machine operator’s lung.”3
Although B. mallei and B. pseudomallei are no longer found in North America and Europe, they may be transmitted through laboratory work.4,5 A US Army microbiologist was reported to have acquired B. pseudomallei infection from working in a biological weapons’ defense facility.6B. pseudomallei is well known to occur in immigrants and travelers from endemic areas, including Vietnam veterans, and this infection may be a hazard to workers returning from these regions.7 Unlike many other species in these two genera, B. pseudomallei has been associated with infection in otherwise healthy groups, such as military personnel.8
Exposure (route)
The route of exposure depends on the occupational setting. Transmission through direct contact, often because of improper handwashing, is a well-known means of propagation for nosocomial pathogens in this group.9 Inhalational exposure applies to potential respiratory effects from media contaminated with pseudomonas.
Direct contact of soil onto broken skin is the principal route of exposure for B. mallei and B. pseudomallei. B. pseudomallei may additionally be acquired by inhalation and a recent outbreak in Australia has been attributed to contaminated water.10
Pathobiology
Pseudomonas and Burkholderia are Gram-negative, aerobic, slightly curved, or straight rods. Some species previously grouped under the genus Pseudomonas have been transferred to the genus Burkholderia. These include B. cepacia, B. mallei, and B. pseudomallei.
Community-acquired P. aeruginosa infection is usually localized and occurs as the result of exposure to large number of organisms from a contaminated water source. Examples include folliculitis from swimming pools or hot tubs, otitis externa (“swimmer’s ear”), and eye infections associated with contact lens use. As noted, P. aeruginosa is a common and dreaded nosocomial pathogen infecting multiple sites in immunocompromised hosts.
Exposure to aerosols in contaminated environments has been associated with occupational asthma in several settings. These microenvironments usually have a mixed flora, including Pseudomonas species, and exposure to endotoxin, produced by Gram-negative bacteria, has been implicated as the agent of bronchospasm, as well as symptoms of fever, diarrhea, fatigue, headache, nausea, and eye and nasal irritation.
In 2014, a case of corneal ulcer from P. aeruginosa was described in a 26-year-old nurse anesthetist after work place exposure to body fluids.11 Workplace exposures in the healthcare setting are often multidrug resistant.
B. mallei produces a disease known as glanders. This is primarily an infection of horses, mules, and donkeys. Sporadic cases occur in humans in parts of South America, Africa, and Asia. Three acute clinical pictures are possible: a rapidly fatal sepsis, a pulmonary form, or an ulcerative infection of the mucosa of the nose, mouth, and conjunctiva. A chronic cutaneous form is also recognized. There is concern that B. mallei could be used as an agent of agroterrorism.12
B. pseudomallei causes melioidosis or pseudoglanders, a disease endemic to Southeast Asia and northern Australia. Its range is expanding. The presentation of melioidosis is protean with systemic and localized forms involving virtually any organ system. Most commonly, it presents as pneumonia, mimicking tuberculosis infection, or as an ulcerative skin lesion. The diagnosis is made even more challenging by a variable time course for disease progression. There are acute, subacute, and chronic forms as well as the potential for a latency period lasting several years prior to disease manifestation. It is a category B bioterrorism agent as determined by CDC.
Diagnosis
Standard isolation and identification techniques can be used to diagnose infections with Pseudomonas and Burkholderia species.
Treatment
Therapy may consist of topical antibiotics with systemic antibiotics reserved for use in refractory cases or cases with complicated courses. Because of high rates of drug resistance, nosocomial infections require multiple broad-spectrum antibiotics.
Infections with B. mallei and B. pseudomallei are highly antibiotic resistant. Currently, a 14-day course of ceftazadime combined with either meropenem or imipenem followed by a course of trimethoprim–sulfamethoxazole is recommended.13 Systemic infection has a mortality rate of up to 40%.
Medical surveillance
There are no recommended medical screening or surveillance activities.
Prevention
Engineering controls and work practices should be aimed at reducing microbial contamination of water and other media. Outdoor work practices in endemic regions of B. pseudomallei should avoid soil contact with non-intact skin.
References
- 1. Ahlen C, Mandal LH, Johannessen LN, et al. Survival of infectious Pseudomonas aeruginosa genotypes in occupational saturation diving environments and the significance of these genotypes for recurrent skin infections. Am J Ind Med 2000; 37(5):493–500.
- 2. Bowden JJ and Sutphin JE. Nosocomial pseudomonas keratitis in a critical-care nurse. Am J Ophthalmol 1986; 101:612–3.
- 3. Bernstein DI, Lummus ZL, Santilli G, et al. Machine operator’s lung. A hypersensitivity pneumonitis disorder associated with exposure to metalworking fluid aerosols. Chest 1995; 108(3):636–41.
- 4. Howe C and Miller WR. Human glanders: report of six cases. Ann Intern Med 1947; 26:93.
- 5. Schlech WF 3rd, Turchik JB, Westlake RE Jr, et al. Laboratory-acquired infection with Pseudomonas pseudomallei (melioidosis). N Engl J Med 1981; 305(19):1133–5.
- 6. Centers for Disease Control. Laboratory-acquired human glanders – Maryland. MMWR 2000; 49(24):532–5.
- 7. Koponen MA, Zlock D, Palmer DL, et al. Melioidosis. Forgotten, but not gone! Arch Intern Med 1991; 151(3):605–8.
- 8. Lim MK, Tan EH, Soh CS, et al. Burkholderia pseudomallei infection in the Singapore Armed Forces from 1987 to 1994—an epidemiological review. Ann Acad Med Singapore 1997; 26(1):13–7.
- 9. Doring G, Jansen S, Noll H, et al. Distribution and transmission of Pseudomonas aeruginosa and Burkholderia cepacia in a hospital ward. Pediatr Pulmonol 1996; 21(2):90–100.
- 10. Inglis TJ, Garrow SC, Henderson M, et al. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg Infect Dis 2000; 6(1):56–9.
- 11. Darouiche MH, Baccari T, Hammami KJ, et al. Keratitis after corneal projection of biological fluids: a possible occupational prejudice? Workplace Health Saf 2014; 62:400–2.
- 12. Gill KM. Agroterrorism: the risks to the United States food supply and national security. US Army Med Dep J 2015 (January–March):9–15.
- 13. Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med 2012; 367:1035–44.
RAT-BITE FEVER: STREPTOBACILLUS MONILIFORMIS AND SPIRILLUM MINOR
Common names for diseases: Streptobacillary fever, Haverhill fever, sodoku
Occupational setting
Rat-bite fever results from infection with Streptobacillus moniliformis, most frequently transmitted by the bite of a rat. Although it is rare in North America, cases continue to be reported, mostly in children.1
Work involving exposure to rodents, particularly rats and mice, or small animals that prey on them, such as cats and dogs, confers increased risk. Animal laboratory personnel,2 veterinarians, animal breeders,3 and agricultural workers4 are included in this group. Work in heavily rat-infested areas has also been implicated, either through non-bite trauma or without recognized trauma.
Exposure (route)
Bites from wild or laboratory rats,2 whose oral cavities and upper respiratory tracts are commonly colonized with these organisms, can lead to infection, as can bites or scratches of other animal carriers. In 2004, a 24-year-old pet shop employee died of S. moniliformis endocarditis thought due to a rat scratch.4 Haverhill fever occurs from ingesting food products presumably contaminated with rat excreta containing the organism. Recent outbreaks have implicated contaminated milk.5
Pathobiology
S. moniliformis is a pleomorphic, Gram-negative bacillus that may exhibit branching filaments. Rats are the most common reservoir, shedding the bacteria in saliva and urine.
Rat-bite fever follows an incubation period that can span from 1 to 22 days but is usually less than 10 days.1 The illness consists of a prodrome of relapsing fever, chills, headache, vomiting, myalgias, and polyarthralgia.6 The initial wound often appears to be healed at this point. Within 2–4 days, a rash, usually maculopapular in nature, develops over the extremities. This is followed by polyarthritis. The most frequently described complication in case reports is septic arthritis.7 There are also reports, usually in children, of more serious complications, including septicemia,8 endocarditis,9 localized abscess,10 and death.11
Spirillum minus is a Gram-negative spirochete responsible for rat-bite fever (sodoku) primarily in Asia, although cases are reported in the Americas.12 It has a longer incubation period of 1–3 weeks. The symptoms are similar to those caused by S. moniliformis, except that ulceration at the site of the bite with associated lymphadenopathy and lymphangitis is common, while arthritis is unusual.
Diagnosis
The differential diagnosis for a patient presenting with the signs and symptoms of rat-bite fever is broad. Eliciting a history of bite or rodent exposure is essential in narrowing the possibilities but is not always present.13 Atypical presentations occur, which may delay the diagnosis.14
S. moniliformis is confirmed by culture of biological specimens. The organism has fastidious growth requirements, making culture and isolation difficult. Specific agglutinins appear ~10 days after the onset of illness; a fourfold rise in titer during the following 2 weeks, or an initial titer of 1 : 80 is diagnostic.
Spirillum minus cannot be cultured in vitro and requires inoculation of body fluids intraperitoneally into laboratory animals with subsequent identification of the organism in peritoneal fluid by dark-field microscopy. No serologic test is available for S. minus.
Treatment
Penicillin is the antibiotic of choice.4 Therapy should be IV for at least 7 days depending on response.
Medical surveillance
There are no recommended medical screening or surveillance activities.
Prevention
Control of rat populations at work sites is important. Persons working with small animals should wear gloves and use handling techniques designed to avoid bites. Routine handwashing after contact is essential.
Laboratory rats should be separated from other rodents to reduce the risk of transmission between animals. Bites, if they occur, should be thoroughly cleaned. The utility of prophylactic antibiotics after a bite has not been investigated. Studies of rat bites show that only a small minority becomes infected,13 but some authors recommend a short course of penicillin to reduce the potential morbidity of infection.14
References
- 1. Centers for Disease Control. Rat-bite fever—New Mexico, 1996. MMWR 1998; 47(5):89.
- 2. Anderson LC, Leary SL, and Manning PJ. Rat-bite fever in animal research laboratory personnel. Lab Anim Sci 1983; 33(3):292–4.
- 3. Wilkins EG, Millar JG, Cockcroft PM, et al. Rat-bite fever in a gerbil breeder. J Infect 1988; 16(2):177–80.
- 4. Hagelskjaer L, Sorensen I, and Randers E. Streptobacillus moniliformis infection: 2 cases and a literature review. Scand J Infect Dis 1998; 30(3):309–11.
- 5. Shvartsblat S, Kochie M, Harber P, et al. Fatal rat bite fever in a pet shop employee. Am J Ind Med 2004; 45(4):357–60.
- 6. McEvoy MB, Noah ND, and Pilsworth R. Outbreak of fever caused by Streptobacillus moniliformis. Lancet 1987; 2:1361–3.
- 7. Rumley RL, Patrone NA, and White L. Rat-bite fever as a cause of septic arthritis: a diagnostic dilemma. Ann Rheum Dis 1986; 46(10):793–5.
- 8. Rygg M and Bruun CF. Rat bite fever (Streptobacillus moniliformis) with septicemia in a child. Scand J Infect Dis 1992; 24(4):535–40.
- 9. McCormack RC, Kaye D, and Hook EW. Endocarditis due to Streptobacillus moniliformis. JAMA 1967; 200(1):77–9.
- 10. Vasseur E, Joly P, Nouvellon M, et al. Cutaneous abscess: a rare complication of Streptobacillus moniliformis infection. Br J Dermatol 1993; 129(1):95–6.
- 11. Sens MA, Brown EW, Wilson LR, et al. Fatal Streptobacillus moniliformis infection in a two-month-old infant. Am J Clin Pathol 1989; 91(5):612–6.
- 12. Hinrichsen SL, Ferraz S, Romeiro M, et al. Sodoku—a case report. Rev Soc Bras Med Trop 1992; 25(2):135–8.
- 13. Fordham JN, McKay-Ferguson E, Davies A, et al. Rat bite fever without the bite. Ann Rheum Dis 1992; 51(3):411–72.
- 14. Weber DJ and Hansen AR. Infections resulting from animal bites. Infect Dis Clin North Am 1991; 5:663–80.
RELAPSING FEVER: BORRELIA SPECIES (other than B. burgdorferi)
Common names for disease: Relapsing fever, endemic (or sporadic) and epidemic forms
Occupational setting
Relapsing fever, with few exceptions, occurs in countries throughout the world. Louse-borne, or epidemic, disease is transmitted from person to person by the human body louse (Pediculus humanus). Epidemics are traditionally associated with war, famine, and other catastrophic events; migrant workers and soldiers have been particularly prone to this infection. At present, the epidemic disease is found only in Ethiopia and neighboring countries and occurred in association with the wars there in the 1980s and 1990s.1 Tick-borne, or endemic, disease occurs worldwide, with the largest outbreak in the western hemisphere occurring in 62 campers in Arizona in 1973.2 As in many outbreaks, the disease appeared to be acquired through vacationing in cabins were rodents have nested. Many species of rodents and small mammals including chipmunks, squirrels, rabbits, mice, and rats serve as reservoirs of the infection. The vectors of the disease, ticks of the genus Ornithodoros, are found preferentially in forested mountain habitats, frequently above 3000 ft, especially caves, decaying wood, rodent burrows, and animal shelters.3 Outdoor workers in selected environments, particularly those in remote natural settings, would seem to be at risk for the disease.
B. miyamotoi may be an under-recognized causative agent of a relapsing fever in the United States. The sera of healthy New Englanders were examined between 1991 and 2012, 3.9% were found to be positive for B. miyamotoi antibody. The ixodes tick that carries Lyme disease also carries B. miyamotoi.4
Exposure (route)
The disease is vector-borne, transmitted to humans by the human body louse and ticks of the genus Ornithodoros or Ixodes in the case of B. miyamotoi.
Pathobiology
The relapsing fevers (epidemic and endemic) are caused by several species of Borrelia spirochetes. B. recurrentis is the sole cause of epidemic relapsing fever and is transmitted by the human body louse. At least 15 other species of Borrelia are transmitted by ticks and cause the endemic, or sporadic, variety of the disease; examples include B. turicatae, B. hermsii, B. parkeri, and B. duttonii. The clinical manifestations of louse-borne and tick-borne disease are similar, characterized by acute onset of high fever with rigors, severe headache, myalgias, arthralgias, lethargy, photophobia, and cough, after an incubation period of about 7 days.3 Fever is intermittent, with the initial episode typically lasting 3–6 days, followed by an asymptomatic period of 7–10 days. The patient is often unaware of a tick bite. In the absence of treatment, three to five relapses occur, with the duration and intensity of symptoms decreasing with each relapse of tick-borne disease. A single relapse is characteristic of louse-borne disease.
Diagnosis
Definitive diagnosis requires identification of spirochetes in peripheral blood smears by dark-field microscopy or appropriately stained specimens. Serologic tests are not standardized and are of limited diagnostic value other than to demonstrate rising titers in convalescent sera.
Treatment
As is the case in other Borrelia infections, relapsing fever is best treated successfully with tetracyclines, such as doxycycline. The disease may also respond to erythromycin and chloramphenicol. Penicillin has been associated with an increased rate of relapse. Treatment of tick-borne disease requires 7–10 days. Antibiotic treatment may produce a Jarisch–Herxheimer reaction characterized by fever, chills, tachycardia, and hypotension. This serious complication most likely represents an inflammatory reaction induced during treatment, believed to be due to a rapid release of antigen with associated cytokine or other mediator response. Death is rare in tick-borne relapsing fever and is limited to infants and older individuals. The case fatality ratio of untreated epidemic disease can approach 40%.
Medical surveillance
There are no recommended medical screening or surveillance activities.
Prevention
Prevention of the epidemic form of the disease requires appropriate response to natural and artificial disasters, good personal hygiene, and delousing procedures. In epidemic situations, short-term use of prophylactic antibiotics can contain the spread of infection to persons at high risk.2 Endemic disease can be prevented by activities that limit tick exposure, including vector control, rodent control, and personal protective measures. Environmental application of insecticides and use of tick repellents can also decrease the potential for tick exposure.
References
- 1. Raoult D and Roux V. The body louse as a vector of reemerging human diseases. Clin Infect Dis 1999; 29:888–911.
- 2. Centers for Disease Control. Relapsing fever. MMWR 1973; 22:242–6.
- 3. Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med 1993; 329:936–45.
- 4. Krause PJ, Narasimhan S, Wormser GP, et al. Human Borrelia miyamotoi infection in the United States. N Engl J Med 2013; 17:291–3.
SALMONELLA SPECIES
Common names for disease: Salmonellosis, typhoid fever, paratyphoid fever
Occupational setting
Non-typhoidal Salmonella species can cause infections in most animal species, including poultry, cattle, swine, cats, dogs, and turtles, and individuals in occupations with animal contact are therefore at increased risk. Furthermore, large community outbreaks have occurred in which dairy products or meat from farms has been traced as the primary source (Figure 22.8).1 Such incidents are of particular concern because of the frequency of multiply resistant pathogens due to heavy antibiotic use in animal feeds.1

FIGURE 22.8 Salmonellosis and Shigellosis – reported cases per 100 000 population by year, United States, 1982–2012.
Source: MMWR 2014; 61(53):87.
Food handlers are at risk through contact with contaminated animal products. Organisms causing both non-typhoidal and typhoidal illnesses represent an occupational hazard to healthcare workers. Employees in patient care and laboratory settings experience increased exposure from infected patients, resulting in documented clinical infections of both typhoid fever and enterocolitis.2, 3
In 2006, 21 employees of a facility that made poultry vaccine against Salmonella enteritidis contracted the disease from their workplace.4 Contact with S. typhi in proficiency tests of clinical laboratories and training exercises for medical laboratory technician students has also resulted in typhoid fever.5 International travelers may be exposed to Salmonella species due to poor sanitation.
Exposure (route)
Ingestion of bacteria is the usual route of exposure. This occurs through contamination of food or water with infected fecal material or inadequate handwashing by personnel after contact with infected humans or animals. In 2013, a phlebotomist in Minnesota was infected with salmonella from a patient.6
Transmission has also been described through fomites in a report of laundry workers infected by contact with soiled linen in a nursing home.7
Pathobiology
Salmonellae are Gram-negative, flagellated rods. The nomenclature for this genus has changed as a result of new information gained from DNA studies. Previously distinct species are now all classified under the species S. choleraesuis. There are seven subgroups in this species, with subgroup I containing nearly all human pathogens. The serotypes are generally referred to in shortened form as if they were species instead of the longer, but strictly correct, designation of genus–species–serotype (i.e., S. typhi versus S. choleraesuis (group I) serotype typhi).
A variety of illnesses are caused by Salmonella species; however, they can be divided into two general groups: typhoidal and nontyphoidal. Typhoid fever is caused by S. typhi; the less severe illness, paratyphoid fever, is due to S. paratyphi A, S. paratyphi B (S. schottmuelleri), and S. paratyphi B (S. hirschfeldii). Other Salmonella species are occasionally responsible for this clinical presentation. Humans are the only reservoir for S. typhi and S. paratyphi.
Once ingested, S. typhi penetrates the intestinal wall, causing necrosis and ulceration, and eventually gains access to the bloodstream. After an incubation period of 1–3 weeks, fever, headache, abdominal pain, and constipation or diarrhea may develop. Respiratory symptoms may also be present. Physical examination may reveal intestinal ileus with abdominal tenderness and palpable bowel loops. Rose spots, caused by leakage from capillary endothelial cells due to bacterial infiltration, may be noted on the anterior chest and abdomen. Hepatosplenomegaly is common, and some patients may have decreased levels of consciousness. Laboratory abnormalities include anemia, leukopenia, liver function test alterations, and subclinical clotting abnormalities.
The illness is prolonged in the absence of antibiotic treatment and may be fatal. Regardless of treatment, an extended convalescence is frequently necessary. Complications are numerous including gastrointestinal perforation, hemorrhage, and localized infections, such as pneumonia and meningitis. The chronic carrier state (excretion of bacteria in feces for >1 year) develops in 1–3% of those infected and may not be preceded by a serious initial illness.8 Although typhoid fever has become quite rare in the United States, (Figure 22.9), it should be suspected in recent travelers to undeveloped countries or in exposed healthcare workers.
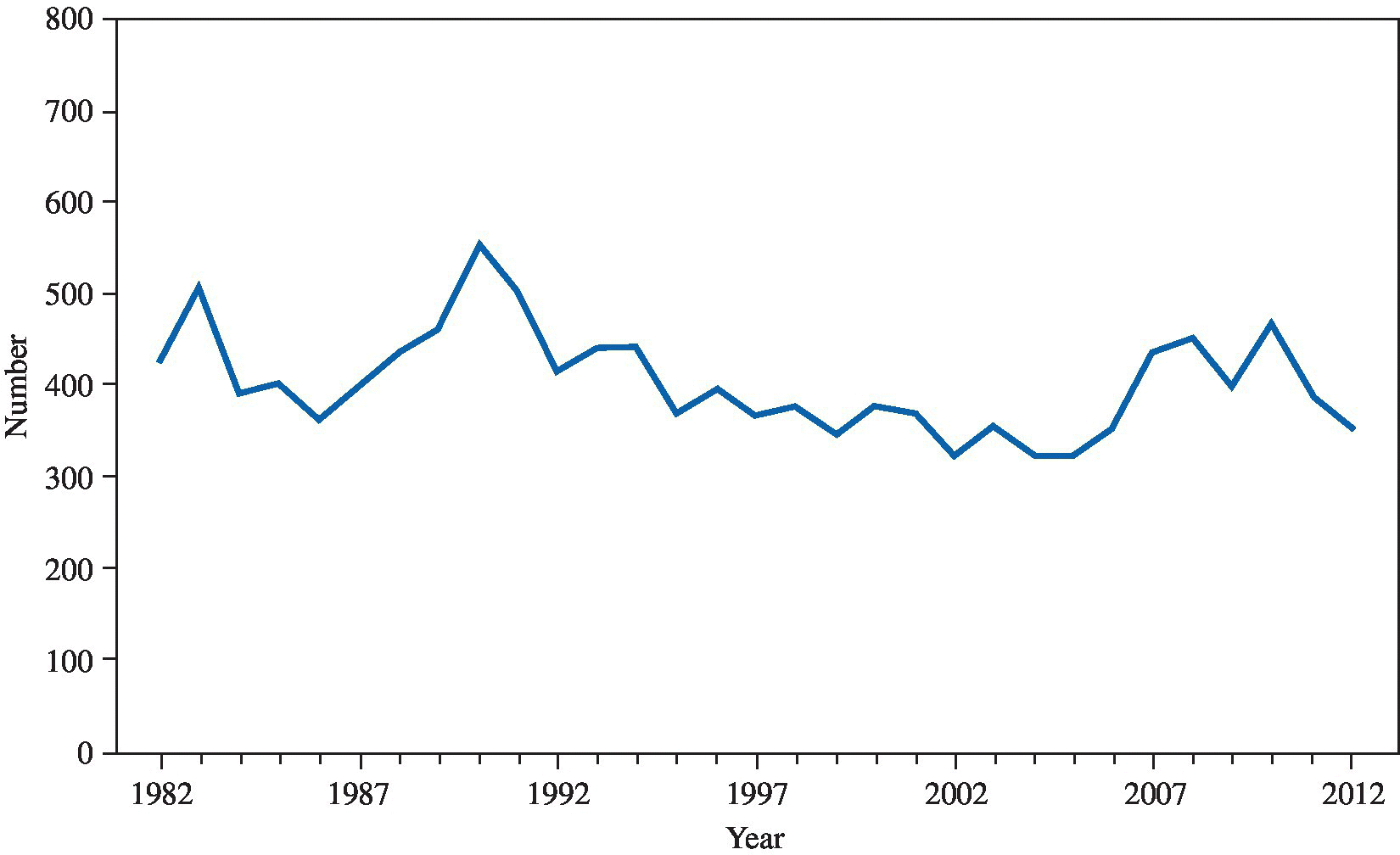
FIGURE 22.9 Typhoid fever – reported cases by year, United States, 1982–2012.
Source: MMWR 2014; 61(53):97.
Non-typhoidal illness, in contrast, is an increasing problem in the United States. From a public health perspective, it is one of the most important zoonotic infections. Most infections are due to contaminated food, especially poultry and their products. The most common presentation is enterocolitis, and S. typhimurium and S. enteritidis are the most frequently responsible serotypes. The incubation period is 6–48 hours after bacterial ingestion. The organisms reach the lower intestinal tract and multiply there. After mucosal invasion, the bacteria may be ingested by macrophages and multiply within these cells. Other factors contributing to bacterial virulence include elicitation of a secretory response, which may be mediated by enterotoxin; tissue destruction, which may be cytotoxin-induced; and antimicrobial resistance, which is increasingly plasmid-related. Host defenses include gastric acidity, which kills bacteria; presence of normal intestinal flora, which decreases bacterial multiplication; and cellular immunocompetence.
Symptoms are fever, nausea, vomiting, and headache, followed by abdominal pain and diarrhea. The diarrhea is generally of moderate volume and nonbloody; it lasts 3–7 days, although bacteria are shed in the stool for an average of 5 weeks. The abdominal pain may localize to the right lower quadrant, mimicking appendicitis. Bacteremia may accompany the illness. Approximately 1% of the infected persons continue to shed bacteria in the stool for over 1 year. Bacteremia without enterocolitis or typhoid fever may occur. Localized abscesses, meningitis, pneumonia, endocarditis, arteritis, and osteomyelitis can result.
Diagnosis
Diagnosis is made by cultures of blood or stool or, in the case of localized infections, specimens from the affected area. Serologic studies are neither sensitive nor specific, although the enzyme-linked immunosorbent assay shows promise. Plasmid and phage typing of the causative organism are useful to determine the source of infection in non-typhoidal illnesses.
Treatment
Multidrug-resistant S. typhi is now widespread. A recent cross-sectional study showed that there is wide spread antibiotic resistance including floroquinolone resistance. Antibiotic choice should be driven by resistance pattern where the patient became infected with S. typhi. Ciprofloxacin is still a reasonable choice for empiric antibiotic therapy but confirmed resistance pattern or treatment failure should prompt reconsideration of antibiotic choice.9
Antibiotic therapy for healthy patients with non-typhoidal salmonella enteroclitis is not indicated10 and, in fact, may prolong fecal excretion of bacteria and increase adverse effects. However, antibiotic therapy can be considered in patients with underlying medical conditions, pediatric patients, elderly, and those requiring hospitalization. Of note was an outbreak with 634 cases of multidrug resistant S. heidelberg in the United States in 2013 and 2014.11 Likely there is emerging widespread antibiotic resistance in non-typhoidal salmonella.
Medical surveillance
Immediate case reporting to the proper health authorities is required in the United States and most other countries.
Prevention
Work practices should emphasize good hygiene with careful handwashing after contact with infected patients, animals, or their feces. Equipment used in patient procedures is potentially infectious as well. Food handlers should wear gloves. Laboratory personnel should utilize good work practices including, in addition to handwashing, the use of gloves, laboratory coats, and mechanical pipetting devices. Eating and drinking in the workplace should be prohibited. Travelers should avoid fresh, peeled fruits and vegetables, and drink only bottled water. Individuals in sensitive jobs who develop infection, such as those in patient care and food handlers, should not return to work until their stool cultures are negative.
Worker education is essential in the management of this occupational hazard. Typhoid vaccines are available for travelers to high-risk areas, individuals persistently exposed to chronic carriers, and laboratory workers having frequent contact with S. typhi. There is an oral live vaccine (Ty21A) and two injectable killed vaccines available (Vi polysaccharide and Vi-rEPA). A Cochrane review showed better protection overall from the two injectable vaccines, with the oral vaccine only protecting against half of the cases.12 New vaccines with fewer side-effects have been developed but do not appear to provide the same level of long-term protection as the older whole-cell vaccine.
The control of foodborne Salmonella requires a number of interventions including the control of bacteria in animal feed, a decrease in the use of antibiotic supplements in animal feed, and proper food-preparation practices. Careful evisceration practices and physical separation of this area from the rest of the slaughterhouse is beneficial.
References
- 1. Molbak K, Baggesen DL, Aarestrup FM, et al. An outbreak of multidrug-resistant, quinolone-resistant Salmonella enterica serotype typhimurium DT104. N Engl J Med 1999; 341(19):1420–5.
- 2. Grist NR and Emslie JAN. Infections in British clinical laboratories, 1984–5. J Clin Pathol 1987; 40:826–9.
- 3. Pike RM. Laboratory-associated infections: incidence, fatalities, causes, and prevention. Annu Rev Microbiol 1979; 33:41–66.
- 4. Centers for Disease Control and Prevention. Salmonella serotype enteritidis infections among workers producing poultry vaccine—Maine, November–December 2006. MMWR Morb Mortal Wkly Rep 2007; 56 (34):877.
- 5. Hoerl D, Rostkowski C, Ross SL, et al. Typhoid fever acquired in a medical technology teaching laboratory. Lab Med 1988; 19:166–8.
- 6. Centers for Disease Control and Prevention. Occupationally acquired Salmonella I 4,12:i:1,2 infection in a phlebotomist—Minnesota, January 2013. MMWR Morb Mortal Wkly Rep 2013; 62(25):525.
- 7. Standaert SM, Hutcheson RH, and Schaffner W. Nosocomial transmission of Salmonella gastro-enteritis to laundry workers in a nursing home. Infect Control Hosp Epidemiol 1994; 15(1): 22–6.
- 8. Ackers ML, Puhr ND, Tauxe RV, et al. Laboratory-based surveillance of Salmonella serotype Typhi infections in the United States: antimicrobial resistance on the rise. JAMA 2000; 283(20):2668–73.
- 9. Lynch MF, Blanton EM, Bulens S, et al. Typhoid fever in the United States, 1999–2006. JAMA 2009; 302:859–65.
- 10. Onwuezobe IA, Oshun PO, and Odigwe CC. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst Rev 2012; 11:CD001167.
- 11. Centers for Disease Control and Prevention. Multistate Outbreak of Multidrug-Resistant Salmonella Heidelberg Infections Linked to Foster Farms Brand Chicken (Final Update). Available at: http://www.cdc.gov/salmonella/heidelberg-10-13/ (accessed on June 6, 2016).
- 12. Anwar E, Goldberg E, Fraser A, et al. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev 2014; 1:CD001261.
SHIGELLA SPECIES
Common names for disease: Shigellosis, dysentery, bacillary dysentery
Occupational setting
Shigella outbreaks have been described in laboratories,1 child day care centers,2 cruise ships,3 primate animal handlers,4 and hospitals.5 Military personnel6 and travelers from endemic areas7 may also be at increased risk. The overall incidence of shigellosis has declined in the United States over the past 5 years, although significant outbreaks continue to occur (Figure 22.8).
Exposure (route)
Transmission is through the fecal–oral route. This occurs most often through direct person-to-person contact. Less commonly, the organism is water- or foodborne.
Pathobiology
Shigellae are nonmotile, nonencapsulated Gram-negative rods of the Enterobacteriaceae family. There are four species that can produce diarrhea. S. sonnei accounts for the overwhelming majority of cases in the United States.8S. dysenteriae produces a toxin (the shiga toxin) and generally results in more severe disease. The shiga toxin is also produced by some serotypes of E. coli, such E. coli O157, and is associated with hemolytic-uremic syndrome in infections from either species of bacteria.9S. flexneri and S. boydii may also cause shigellosis.
Symptoms develop 24–48 hours after infection and include fever, abdominal cramping, tenesmus, and watery diarrhea that may contain blood, pus, and mucus. Mild cases are self-limited and resolve within a few days. Complications are rare and generally occur in children or immunocompromised adults. They include intestinal perforation,10 sepsis,11 and hemolytic-uremic syndrome.9 Delayed sequelae include reactive arthritis and Reiter’s syndrome.12Shigella demonstrates a high degree of pathogenicity; disease may result from an inoculation with as few as 10–100 bacteria. After ingestion and passage through the stomach, the organism invades the mucosa of the colon. Infection is thought to involve primarily local cell invasion and destruction, although the bacilli also produce several toxins. The superficial mucosal layer of the colon then ulcerates and sloughs. Toxins may also contribute to a moderate secretory diarrhea.
Diagnosis
Since bleeding may not be clinically apparent, Shigella should be included within the differential diagnosis of any compatible acute diarrhea. Fecal leukocytes are a frequent finding. A definitive diagnosis is made by culturing the organism from stool samples.
Treatment
Treatment is primarily supportive and consists of oral or, if necessary, intravenous rehydration. Antibiotic therapy may shorten the course of disease and prevent transmission of infection. However, there is growing antibiotic resistance. In 2011, 6% of isolates in the United States were resistant to ciprofloxacin or azithromycin.13 From September 2014 through April 2015, five cases of extremely drug resistant shigellosis were identified in Illinois and Montana residents.14 The isolates were resistant to ampicillin, ciprofloxacin, nalidixic acid, streptomycin, sulfisoxazole, tetracycline, and trimethoprim/sulphamethoxazole; had azithromycin minimum inhibitory concentrations >16 µg/mL; and harbored macrolide resistance genes mphA and ermB.14
Quinolones or cefixime are reasonable choices when empiric antibiotic therapy is indicated.15
Medical surveillance
There are no recommended medical screening activities for infections due to Shigella. Reporting of cases to local public health authorities is required in most jurisdictions.
Prevention
Work practices common to prevent transmission of any enteric pathogen should be in place. These measures include handwashing, glove use, and appropriate preparation and storage of food. Chlorination of water supplies and provision of sanitary facilities are also important.
Persons with active infections can easily transmit the disease, so adequate precautions are essential. These include enteric (or universal) precautions for healthcare workers caring for the infected patient and verification of noninfectious status (with two successive negative stool cultures) before allowing the individual to prepare food or resume child or patient care. In Belgium, an asymptomatic cafeteria worker was thought to be the index case in an outbreak of 52 cases of Shigella.16
Current research offers the hope of a vaccine for Shigella in the future.17
References
- 1. Mermel LA, Josephson SL, Dempsey J, et al. Outbreak of Shigella sonnei in a clinical microbiology laboratory. J Clin Microbiol 1997; 35(12):3163–5.
- 2. Mohle-Boetani JC, Stapleton M, Finger R, et al. Community-wide shigellosis: control of an outbreak and risk factors in child day-care centers. Am J Public Health 1995; 85(6):812–6.
- 3. Centers for Disease Control. Outbreak of Shigella flexneri 2a infections on a cruise ship. MMWR 1994; 43(35):657.
- 4. Kennedy FM, Astbury J, Needham JR, et al. Shigellosis due to occupational contact with non-human primates. Epidemiol Infect 1993; 110(2):247–51.
- 5. Hunter PR and Hutchings PG. Outbreak of Shigella sonnei dysentery on a long stay psychogeriatric ward. J Hosp Infect 1987; 10(1):73–6.
- 6. Mikhail MM, Mansour MM, Oyofo BA, et al. Immune response to Shigella sonnei in US marines. Infect Immun 1996; 64(9):3942–5.
- 7. Aleksic S, Bockemuhl J, and Degner I. Imported shigellosis: aerogenic Shigella boydii 74 (Sachs A 12) in a traveller followed by two cases of laboratory-associated infections. Tropenmed Parasitol 1981; 32(1):61–4.
- 8. Centers for Disease Control. Outbreaks of Shigella sonnei infection associated with eating fresh parsley—United States and Canada, July–August 1998. MMWR 1999; 48(14):285–9.
- 9. Bhimma R, Rollins NC, Coovadia HM, et al. Post-dysenteric hemolytic uremic syndrome in children during an epidemic of Shigella dysentery in Kwazulu/Natal. Pediatr Nephrol 1997; 11(5):560–4.
- 10. Upadhyay AK and Neely JA. Toxic megacolon and perforation caused by Shigella. Br J Surg 1989; 76(11):1217.
- 11. Trevett AJ, Ogunbanjo BO, Naraqi S, et al. Shigella bacteraemia in adults. Postgrad Med J 1993; 69(812):466–8.
- 12. Finch M, Rodey G, Lawrence D, et al. Epidemic Reiter’s syndrome following an outbreak of shigellosis. Eur J Epidemiol 1986; 2(1):26–30.
- 13. Centers for Disease Control and Prevention. Antibiotic Resistance Threats 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on June 6, 2016).
- 14. Replogle ML, Fleming DW, and Cieslak PR. Emergence of antimicrobial-resistant shigellosis in Oregon. Clin Infect Dis 2000; 30(3):515–9.
- 15. Centers for Disease Control. Ciprofloxacin- and Azithromycin-Nonsusceptible Shigellosis in the United States. CDCHAN-00379. Distributed via the CDC Health Alert Network, June 4, 2015. Available at: http://emergency.cdc.gov/han/han00379.asp (accessed on June 6, 2016).
- 16. Gutiérrez Garitano I, Naranjo M, Forier A, et al. Shigellosis outbreak linked to canteen-food consumption in a public institution: a matched case–control study. Epidemiol Infect 2011; 139:1956–64.
- 17. Shata MT, Stevceva L, Agwale S, et al. Recent advances with recombinant bacterial vaccine vectors. Mol Med Today 2000; 6(2):66–71.
STAPHYLOCOCCUS SPECIES
Common name for diseases: Common names for some staphylococcal illnesses include impetigo, toxic shock syndrome (TSS), scalded skin syndrome, and staphylococcal food poisoning
Occupational setting
Staphylococcal skin infections can occur in any setting where trauma to the skin occurs, including agricultural workers,1 construction workers, poultry process workers, and meat-packers.2,3 Outbreaks of skin infection outside of healthcare settings usually occur as a result of direct physical trauma, but have occurred in some unusual outdoor occupations such as river rafting guides.4 Staphylococcal infections and asymptomatic carriage of S. aureus in healthcare employees, either of which can cause outbreaks of staphylococcal infection in patients, are of particular concern in hospitals.5 Food handlers or preparers with staphylococcal skin infections have caused food-poisoning outbreaks,6 and much current food-handling regulation is directed at reducing this risk to the general public.
Exposure (route)
Humans are the reservoir for Staphylococcus species. The coagulase-negative staphylococci (S. epidermidis and S. saprophyticus) are ubiquitous inhabitants of the skin; S. aureus may colonize the skin in individuals who come into contact with it through work or other activities. Carriage of the bacteria may be either chronic or transient, and it can be transmitted from carriers to other individuals or objects. S. aureus may be cultured from 30 to 50% of healthy individuals from the nasopharynx, skin, gastrointestinal and urogenital tracts, and perineum; persistent colonization occurs in 10–20% of cases.7 Infection occurs by direct invasion of a tissue, usually through traumatic breaks in the skin or through conditions such as eczema that disrupt the skin’s integrity as a barrier.
Pathobiology
Staphylococcus is a Gram-positive, nonmotile coccus that grows in clusters. It is a facultative anaerobe that colonizes human skin. The most important human pathogen, S. aureus, is the only coagulase-positive staphylococcus species. S. epidermidis and S. saprophyticus are clinically the most important of the coagulase-negative staphylococci. They are often contaminants in culture from skin flora.
S. aureus is the most common staphylococcal pathogen in the occupational setting. Factors that predispose to infection with S. aureus include injury to normal skin, prior viral infections, immunologic compromise, indwelling foreign bodies including sutures and catheters, prior antibiotic treatment, and pre-existing illness.8S. aureus infection usually occurs through a break in the skin. The bacteria produce a wide variety of enzymes and toxins that enhance their virulence and pathogenicity. Nonspecific toxins, including hemolysins and leukocidins, and those specific to some strains, including epidermolytic toxins (which cause bullous impetigo and scalded skin syndrome), increase the invasive properties of staphylococci. Additional strains produce very specific exotoxins, which include toxic shock toxin and the enterotoxin responsible for food poisoning.9
The spectrum of disease arising from S. aureus depends on the specific organism, the location of invasion, and host characteristics. Superficial infections include folliculitis, furunculosis, skin abscesses, impetigo, mastitis (in nursing mothers), wound infections, and spreading pyodermas. Systemic infections include superficial scalded skin syndrome, a serious condition that can result in desquamation of the entire skin, and staph toxic shock syndrome, a severe illness characterized by fever, hypotension, rash with subsequent desquamation, and involvement of several organ systems.8 Toxic shock syndrome is caused by strains of S. aureus that produce a unique toxin, TSST-1 or toxic shock toxin. The disease may arise from local staphylococcal infections such as blisters10 and may be introduced from a site of colonization rather than infection. Historically toxic shock syndrome resulting from staph was associated with tampon use. However, about half of infections seen today are not related to tampon use and can occur in either sex. Organ infections caused by S. aureus include endocarditis, pericarditis, pneumonia, osteomyelitis, septic arthritis, septic bursitis, and pyomyositis. Staphylococcal bacteremia and endocarditis have occurred as a result of accidental needle sticks.
Food poisoning is caused by an S. aureus enterotoxin that produces vomiting, diarrhea, fever, and abdominal pain. The source of the organism can be direct contact of food with the skin or infectious discharge of someone harboring the organism, with subsequent incubation of the organisms and production of enterotoxin while in storage, or from an animal source, such as meat or milk that has been inadequately processed or stored. The onset of symptoms is typically only a few hours after ingestion; the enterotoxin is heat-stable and is not inactivated by subsequent cooking of food after contamination.
S. epidermidis and S. saprophyticus are not of major concern in occupational settings. S. epidermidis is typically associated with infections in patients with prosthetic or intravenous access devices. It is often a contaminant from skin flora when found in blood culture. S. saprophyticus is usually associated with urinary tract infections.
Diagnosis
Staphylococcal disease can be diagnosed by demonstrating the organism on Gram stain and culture. Specific diagnostic criteria have been established for the diagnosis of toxic shock syndrome, which must be distinguished from other diseases with similar clinical presentations, including Rocky Mountain spotted fever, leptospirosis, scarlet fever, and measles.7
Treatment
Treatment of all staphylococcal infections includes appropriate antibiotic therapy. Selection of antibiotics depends on the type of infection, the host, and the likely resistance patterns of the infecting organism, but choices include penicillins with β-lactamase inhibitors in combination (i.e., amoxicillin–clavulanate), certain cephalosporins, erythromycin and macrolide antibiotics, (clarithromycin or azithromycin) or quinolones, such as ciprofloxacin. Methicillin-resistant S. aureus (MRSA) is of concern primarily in healthcare settings because of selection pressures for bacterial antibiotic resistance in these locations. Vancomycin is indicated for the treatment of MRSA or suspected MRSA infections requiring hospitalization. Resistance to quinolone antibiotics has rapidly developed within the past decade, and their use in staphylococcal infections may be limited. Doxycycline or clindamycin administered orally is a reasonable choice for outpatient care of suspected MRSA infection.
Medical surveillance
Reporting to local health authorities is required for cases or outbreaks of staphylococcal food poisoning, for community outbreaks (especially in schools or camps) of other staphylococcal infections, for epidemics in hospitals, and for cases of toxic shock syndrome in most of the Unites States and in other countries. Patient screening, with nasal swab for staphylococcal carriage, may be performed in medical facilities where nosocomial transmission of S. aureus is suspected. Screening of medical personal is controversial and there is no proven reduction in MRSA transmission in a hospital setting.11
Prevention
Food handlers should follow strict hygienic practices, including proper handwashing technique and use of gloves. Additional precautions, including temporary work removal, should be taken if they have purulent lesions of the hands, nose, or face. Food itself should be appropriately cooked, processed, and rapidly refrigerated when not used. Meat-packers should be provided with appropriate tools and personal protective equipment to minimize the risk of exposure and skin trauma, such as cut-resistant gloves. Equipment should also be cleaned regularly.
Healthcare workers are more likely to be asymptomatic carriers of S. aureus than the general population12,13 and must be scrupulous in the use of handwashing techniques and personal protective equipment to prevent transmission to susceptible individuals. The optimal management, including surveillance guidelines decolonization therapy and work restrictions, has not been clearly determined.14 It is important to reduce the opportunity for needlesticks and other trauma through the use of work practices, equipment redesign, and personal protective equipment, and to sterilize equipment and other fomites such as microscopes and ocular eyepieces that may be contaminated to eliminate potential reservoirs for infection.
References
- 1. Pardo-Castello V. Common dermatoses in agricultural workers in the Caribbean area. Indust Med Surg 1962; 31:305–7.
- 2. Barnham M and Kerby J. A profile of skin sepsis in meat handlers. J Infect 1984; 9:43–50.
- 3. Fehrs LJ, Flanagan K, Kline S, et al. Group A beta-hemolytic streptococcal skin infections in a US meat-packing plant. JAMA 1987; 258:3131–4.
- 4. Decker MD, Lybarger JA, Vaughn WK, et al. An outbreak of staphylococcal skin infections among river rafting guides. Am J Epidemiol 1986; 124:969–76.
- 5. Patterson WB, Craven DE, Schwartz DA, et al. Occupational hazards to hospital personnel. Ann Intern Med 1985; 102:658–80.
- 6. Eisenberg MS, Gaarslev K, Brown W, et al. Staphylococcal food poisoning aboard a commercial aircraft. Lancet 1975; ii:595–9.
- 7. Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339:520–32.
- 8. Noble WC. Skin bacteriology and the role of Staphylococcus aureus in infection. Br J Dermatol 1998; 139(Suppl 53):9–12.
- 9. Berkeley SF, McNeil JG, Hightower AW, et al. A cluster of blister-associated toxic shock syndrome in male military trainees and a study of staphylococcal carriage patterns. Military Med 1989; 154:496–9.
- 10. Godfrey ME and Smith IM. Hospital hazards of staphylococcal sepsis. JAMA 1958; 166:1197–201.
- 11. Hawkins G, Stewart S, Blatchford O, et al. Should healthcare workers be screened routinely for methicillin-resistant Staphylococcus aureus? A review of the evidence. J Hosp Infect 2011; 77:285–9.
- 12. Rongpharpi SR, Hazarika NK, and Kalita H. The prevalence of nasal carriage of Staphylococcus aureus among healthcare workers at a tertiary care hospital in Assam with special reference to MRSA. J Clin Diagn Res 2013; 7:257–60.
- 13. Dulon M, Peters C, Schablon A, et al. MRSA carriage among healthcare workers in non-outbreak settings in Europe and the United States: a systematic review. BMC Infect Dis 2014; 14:363.
- 14. Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35 Suppl 2:S108–32.
STREPTOCOCCUS SPECIES
Common names for diseases: Impetigo, erysipelas, strep throat, scarlet fever, rheumatic fever
Occupational setting
Like staphylococcal infections, the most common occupational streptococcal infections are skin infections, which are prominent among workers with frequently traumatized or abraded skin, including construction workers, foresters, and farmers. Group A β-hemolytic streptococcal and S. pyogenes infections have been reported in slaughterhouse workers after pig bites.
Streptococcal septicemia from S. pyogenes has also been reported in a mortuary technician who punctured himself while conducting a post-mortem examination.1 Healthcare workers can be asymptomatic carriers of streptococci (group A or B) and transmit nosocomial infection to patients.2 There are several other species of Streptococcus, especially those found in several animal species, that rarely cause skin infections or systemic disease in humans. S. agalactiae (group B), S. milleri (α-hemolytic), and S. equisimilis (group C) have been associated with local and systemic infections in persons with exposure to pigs and meat-packers and poultry handlers.3-6
An excess of pneumococcal pneumonia has been noted in welders, although these workers were considered to have an increased susceptibility to infection from unspecified welding fumes rather than direct exposure to S. pneumoniae.7
Streptococcus suis Type II (group R β-hemolytic streptococci) was first noted in 1968 as the cause of a syndrome of meningitis and sepsis in both pigs and humans.8 Groups at risk include pig farmers and handlers of raw pork, such as meat-packers and butchers.9 A 2014 meta-analysis reported that S. suis was most prevalent in Asia and that occupation along with risky food consumption was the main risk factor. They found a case fatality rate of 12%.10 Meningitis, endocarditis, and arthritis are seen in S. suis infection.
Exposure (route)
Streptococci are ubiquitous human pathogens. Skin infection probably occurs through breaks in the skin arising from laceration, trauma, surgery, or skin breakdown, although in some cases the breaks may be unnoticed. Respiratory exposure occurs through inhalation of droplets. S. suis colonizes the snout and pharynx of healthy pigs; diseased pigs may exhibit a bacteremia. Transmission to humans occurs from exposure to work with pigs or raw pork products, most likely through minor skin breaks, although transmission may also take place through respiratory exposure.
Pathobiology
Streptococci are Gram-positive, non-spore-forming bacteria that typically grow in pairs or chains of spherical cells. They are classified as β-hemolytic if a clear zone of hemolysis surrounding bacterial colonies is seen on sheep blood agar medium. If the zone is only partly clear (usually noted as a greenish tint, giving rise to the term “viridans” streptococci), they are considered α-hemolytic. Another classification scheme, developed by Lancefield, designates groups (A–D, G, and R) based on antigenic differences.
Group A streptococcus (Streptococcus pyogenes) is the most important human pathogen, causing both streptococcal skin infections and pharyngitis. Streptococcal skin infections include erysipelas, a rapidly progressive skin infection accompanied by fever and, in some cases, bacteremia; pyoderma or impetigo, a localized purulent skin infection; and cellulitis.11 Lymphangitis and lymphadenitis may also occur. The most severe form of group A streptococcal infection is necrotizing fasciitis, an infection of subcutaneous tissue with relative sparing of overlying skin and underlying muscle. Symptoms include severe local pain and tenderness, fever, and systemic toxicity. There is rapid progression to tissue gangrene and death unless the infection is quickly treated.11,12 Elaboration of pyrogenic exotoxins in strains of S. pyogenes is responsible for the streptococcal toxic shock syndrome, characterized by tachycardia, tachypnea, fever, chills, and diarrhea, progressing to septic shock and organ failure. Approximately half the cases are seen in association with necrotizing fasciitis. Skin or vaginal mucosa appears to be the portal of entry in 60% of cases, with the rest probably arising from bacteremia originating in the pharynx.11
Streptococcal pharyngitis, along with skin infection, is one of the most common streptococcal illnesses; its importance lies in its potential sequelae. Although nasopharyngeal carriage of group A streptococci declines somewhat from childhood to adulthood, it is not uncommon to see pharyngitis in adults, and outbreaks in crowded conditions (i.e., military barracks) can occur. Outbreaks may also arise from foodborne or waterborne transmission. Clinically, an incubation period of several days is followed by fever and sore throat. The posterior pharynx is red and edematous, the tonsils are enlarged and frequently have a patchy white exudate, and the cervical lymph nodes are swollen and tender. Although very early treatment may shorten the duration of symptoms and the period of communicability, the primary purpose and importance of treatment is to prevent complications of the infection.
Complications and sequelae of streptococcal pharyngitis include local head and neck infections, including otitis media, sinusitis, peritonsillar cellulitis or abscess, suppurative lymphadenitis, and bacteremia. Scarlet fever, which is caused by a toxin (erythrogenic toxin) produced by some strains, is characterized by a distinctive rash and, in more severe cases, systemic toxicity. Acute rheumatic fever and acute post-streptococcal glomerulonephritis are two delayed complications of streptococcal infection. Both are inflammatory diseases that develop after the streptococcal infection itself has resolved. Acute rheumatic fever is a systemic illness that involves connective tissue, primarily in the joints, manifesting as acute arthritis. Other organs may be affected, including the heart, skin, and blood vessels, leading to carditis, erythema marginatum and subcutaneous nodules, and chorea. Acute post-streptococcal glomerulonephritis can follow either pharyngitis or pyoderma and consists of a proliferative glomerular disease that is manifested clinically by edema, proteinuria, hematuria, and hypertension.
S. suis infection is usually manifested by a flu-like prodromal illness, followed by fever and meningismus. Hearing loss and vestibular dysfunction with ataxia occur in approximately 50% of cases. Other manifestations of infection may include endocarditis, arthritis, septicemia with shock and disseminated intravascular coagulation, and rhabdomyolysis.13
Diagnosis
Diagnosis in most cases of infection is made by Gram stain and culture of the organism. Kits are available to detect group A antigen for rapid diagnosis of streptococcal pharyngitis. Throat culture is the preferred method of diagnosis; however, to distinguish streptococcal pharyngitis from other causes of exudative pharyngitis such as C. diphtheriae, C. haemolyticum, N. gonorrhoea, M. pneumoniae, Yersinia enterocolitica, and several species of viruses. Mononucleosis should be suspected in cases of exudative pharyngitis, particularly in adolescents and younger adults. Detection of specific streptococcal antibodies such as antistreptolysin O is not useful in the diagnosis of acute infections.
S. suis infection should be considered in cases of systemic illness where a history of exposure to pigs or raw pork products is obtained or suspected. Signs of meningitis or septicemia with characteristic findings of hearing loss and vestibular dysfunction are nonspecific but may lead to a higher index of suspicion for the infection. The bacteria can be cultured from both blood and cerebrospinal fluid.
Treatment
Treatment of streptococcal infections requires antibiotic therapy. Penicillin is the antibiotic of choice, macrolides like erythromycin and β-lactam antibiotics as well as clindamycin are also effective; addition of the latter may be advisable in cases of streptococcal toxic shock or toxin-related illness. Steptococci are usually resistant to sulfonamides, flouroquinolones, and tetracyclines. Pharyngitis should be treated for 10 days to prevent post-streptococcal complications, unless a single dose of a long-acting penicillin is used intramuscularly. Most strains of S. suis are penicillin-sensitive, though consideration should be given to addition of a second antibiotic until culture and sensitivity results are obtained. Aggressive treatment and surgical debridement is essential in necrotizing fasciitis to reduce the mortality from this condition. Additional supportive measures, including fluid resuscitation and mechanical ventilation, may be necessary for serious infections and streptococcal toxic shock.
Medical surveillance
There are no recommended medical screening activities for streptococcal diseases. Community or school screening programs for identifying group A streptococcal carriers have not been shown to be effective. Epidemics must be reported to local health authorities in the United States.
Prevention
Prevention of streptococcal disease requires good hygiene practices. Food handlers should use strict personal hygiene, and food handlers with active streptococcal respiratory infections should be considered for job reassignment. Food itself should be appropriately cooked, processed, and refrigerated. Meat-packers and meat-handlers should be provided with appropriate tools and personal protective equipment to minimize the risk of exposure and skin trauma, such as cut-resistant gloves. Equipment should also be cleaned regularly. Antibiotic prophylaxis of asymptomatic swine herds and changes in breeding conditions have not been well studied.9Pneumococcal vaccine (the polyvalent vaccine for S. pneumoniae) is indicated for those at risk of pneumococcal disease on the basis of underlying health status and age, not occupational exposure.14
References
- 1. Hawky PM, Pedler SJ, and Southall PJ. Streptococcus pyogenes: a forgotten occupational hazard in the mortuary. Br Med J 1980; 281:1058.
- 2. Patterson WB, Craven DE, Schwartz DA, et al. Occupational hazards to hospital personnel. Ann Intern Med 1985; 102:658–80.
- 3. Fehrs LJ, Flanagan K, Kline S, et al. Group A beta-hemolytic streptococcal skin infections in a US meat-packing plant. JAMA 1987; 258:3131–4.
- 4. Phillips G, Efstratiou A, Tanna A, et al. An outbreak of skin sepsis in abattoir workers caused by an “unusual” strain of Streptococcus pyogenes. J Med Microbiol 2000; 49:371–4.
- 5. Barnham M, Kerby J, Skillin J. An outbreak of streptococcal infection in a chicken factory. J Hyg 1980; 84:71–5.
- 6. Barnham M. Pig bite injuries and infection: report of seven human cases. Epidemiol Infect 1988; 101:641–5.
- 7. Coggon D, Inskip H, Winter P, et al. Lobar pneumonia: an occupational disease in welders. Lancet 1994; 344:41–3.
- 8. Zanen HC and Engel HW. Porcine streptococci causing meningitis and septicaemia in man. Lancet 1975; 1(7919):1286–8.
- 9. Dupas D, Vignon M, and Geraut C. Streptococcus suis meningitis: a severe noncompensated occupational disease. J Occup Med 1992; 34:1102–5.
- 10. Huong VT, Ha N, Huy NT, et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis 2014; 20:1105–14.
- 11. Bison AL and Stevens DL. Streptococcal infections of skin and soft tissue. N Engl J Med 1996; 334:240–5.
- 12. Green RJ, Dafoe DC, and Raffin TA. Necrotizing fasciitis. Chest 1996; 110:219–29.
- 13. Tambyah PA, Kumarasinghe G, Chan HL, et al. Streptococcus suis infection complicated by purpura fulminans and rhabdomyolysis: case report and review. Clin Infect Dis 1997; 24:710–2.
- 14. Centers for Disease Control. Update on adult immunization: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR 1991; 40(RR-12):43–4.
TREPONEMA PALLIDUM
Common name for disease: Syphilis
Occupational setting
Treponema pallidum is the causative agent of syphilis, a sexually transmitted disease. The incidence of primary and secondary syphilis is increasing in the United States. According to CDC, reported cases increased by 10% between 2012 and 2013.1 Occupational groups at increased risk of syphilis are those who have direct, primarily genital, contact with infectious lesions, such as sex workers.
In the early part of the century, infection was reported among some laboratory workers who were handling animals infected with strains of T. pallidum. Transmission may have occurred through scratches, bites, or self-inoculation.2
Exposure (Route)
Transmission of the organism among adults takes place through direct contact of mucous membrane or skin with an infectious lesion and almost always involves sexual contact. Infectious lesions include chancres, skin rashes, mucous patches, or condylomata lata. Syphilis can also be transmitted vertically, resulting in congenital syphilis, and by contact with infected human blood through transfusion or autoinoculation.
Pathobiology
After an incubation period of 9–90 days (median of 21 days), a primary lesion develops at the site of infection. The chancre of primary syphilis is typically painless and indurated with a well-defined border. It usually heals within 2 months. Secondary syphilis, a systemic disease, typically occurs 6–8 weeks after the primary lesion is healed. A wide variety of clinical manifestations may be present, including involvement of the skin, mucous membranes, and lymphatic, renal, gastrointestinal, and skeletal systems. The most characteristic lesion at this stage is the punctate or pox-like rash, which appears most often on the palms and soles.3 Late or tertiary syphilis occurs years after primary infection. Consequences of syphilis at this stage include neurosyphilis, with numerous clinical manifestations including ataxia and ocular lesions, cardiovascular syphilis, which may be seen as aortitis and valvular disease, and a “benign” form in which the characteristic syphilitic gumma may be found in organs including liver and bone systems.
Diagnosis
As T. pallidum is extremely difficult to grow in culture, diagnosis is made either by direct examination or serologic testing. Identification of the organism is made by dark-field microscopic examination of material (scrapings or exudate) from an active lesion or lymph node. Serologic tests fall into two categories. Those that detect antigenic indicators of host tissue damage (the Venereal Disease Research Laboratory (VDRL), rapid plasma reagin or RPR tests) are nonspecific but inexpensive and can be used for initial testing of individuals suspected of having disease or for population screening. Specific treponemal antigen testing is used to confirm the diagnosis in individual patients. These tests include microhemagglutinin assays for T. pallidum antibody (MHA-TP) and the fluorescent treponemal antibody absorption (FTA-ABS) test.3, 4
Treatment
All stages of syphilis can be treated with one 2.4-million-unit dose of intramuscular benzathine penicillin.5 It is notable however that there are no clear guidelines about duration of treatment and so RPR or VDRL titers must be followed to ensure they are decreasing. Penicillin desensitization is recommended for those that are allergic to penicillin. Those with suspected neurosyphilis should undergo a lumbar puncture to assess T. pallidum infection and titer, and will need an extended course of intravenous penicillin. As with treatment of other spirochetes, there is a risk of Jarisch–Herxheimer reaction during therapy.
Medical surveillance
There are no recommended occupational screening activities for syphilis. Syphilis is a reportable disease in the United States and many other countries.
Prevention
Infection with T. pallidum is prevented by treatment of infected individuals and the use of condoms and antiseptic prophylactic agents. In addition, healthcare workers should use appropriate personal protective equipment in cases where the organism is being handled when examining patients in whom clinical syphilis is suspected.
References
- 1. Centers for Disease Control and Prevention, Division of STD Prevention. Sexually Transmitted Disease Surveillance 2013. Available at: http://www.cdc.gov/std/stats13/surv2013-print.pdf (accessed on June 6, 2016).
- 2. Collins CH and Kennedy DA. Microbiological hazards of occupational needlestick and “sharps” injuries. J Appl Bacteriol 1987; 62:385–402.
- 3. Brown TJ, Yen-Moore A, and Tyring SK. An overview of sexually transmitted diseases. J Am Acad Dermatol 1999; 41:511–32.
- 4. Larsen SA, Steiner BM, and Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev 1995; 8:1–21.
- 5. Clement ME, Okeke NL, and Hicks CB. Treatment of syphilis: a systematic review. JAMA 2014; 312:1905–17.
VIBRIO CHOLERAE
Common name for disease: Cholera
Occupational setting
Persons at risk of cholera include travelers to areas where cholera is endemic1 and those in occupations where exposure to contaminated seawater or food is possible.2 Those who handle or consume undercooked shellfish may also be at risk.3 Healthy commercial divers have been found to become colonized following dives at contaminated sites.4 Outbreaks have occurred in hospitals and affected laboratory workers.5, 6 In 2012, six states reported cases of cholera serogroup O1 to CDC; most were international travelers returning from endemic areas, while one was laboratory-acquired.7
Exposure (route)
Exposure is through ingestion of food or water containing live organisms, or through direct contact with water bearing the organisms.
Pathobiology
Vibrios are curved, flagellated, Gram-negative rods. The organism is found in surface waters (both fresh and salt water) all over the world. V. cholerae is a diverse species, consisting of numerous different strains. Strains are primarily grouped according to the type of cell wall O antigen present. Most cases of epidemic cholera are due to serogroup O1. Within this serogroup, there are also subdivisions into three serotypes: Ogawa, Inaba, and Hikojima, as well as two biotypes: classical and El Tor. However, not all O1 strains are pathogenic. While some members of serogroups O2–O138 have the potential to cause isolated cases of cholera,8 they are not thought to result in epidemics. V. cholerae O139 (Bengal strain) has been isolated from a large proportion of cases in the recent cholera epidemic in Asia.9 New serotypes are likely to be implicated in future epidemics.10
When ingested with water or food, V. cholerae passes through the stomach into the small bowel, where it adheres to the mucosal lining. There, it secretes an enterotoxin that acts on intestinal cell receptors, mediated by cyclic adenosine monophosphate, to cause the active secretion of sodium chloride into the gut lumen. This, in turn, causes a voluminous watery diarrhea, which may occur at a rate of 1 L/h.
The incubation period after exposure ranges from 6 hours to 5 days. Prodromal symptoms consist of abdominal discomfort and anorexia. This is followed by diarrhea that progresses from brown to a “rice water” appearance (due to mucus secretion). The complications of cholera all derive from the rapid loss of fluids and electrolytes and include hypotension, hypoglycemia, electrolyte imbalance, renal failure, and acidosis. Rarely, an ileus may occur. Death is most often due to the loss of glucose, electrolytes, and fluid. It should be noted that in most studies of institutional outbreaks, over 75% of those infected are asymptomatic.5
Diagnosis
Diagnosis of cholera can be made by dark-field examination of stool or by culture. A history of travel to areas with endemic cholera is also helpful.
Treatment
The cornerstone of cholera treatment is fluid and electrolyte replacement, either orally or by intravenous rehydration. With adequate rehydration, the case fatality rate is low, 2% in a case series from the United States.10 Oral rehydration solutions can be made from salt and sugar (5 g sodium chloride and either 20 g glucose or 40 g sucrose per liter of water) or according to the World Health Organization formulation (3.5 g sodium chloride, 2.5 g sodium bicarbonate, 1.5 g potassium chloride, and 20 g glucose per liter of water). Since this formulation does not decrease (and may even increase) the duration and volume of diarrhea, formulations that add starch from a variety of sources have been advocated.11 Most cases can be successfully treated with oral rehydration alone; however, more severe diarrhea may require intravenous rehydration. Antibiotics are useful in shortening the duration of infection in moderate to severe cases. Tetracycline, doxycyline, erythromycin, and trimethoprim–sulfamethoxazole have all been used successfully. However, multiple antibiotic resistance has emerged among strains of V. cholerae. Currently, the quinolones, such as ciprofloxacin and norfloxacin, have shown excellent results and offer the advantages of efficacy with a short course or even a single dose.12
Medical surveillance
Reporting of cholera cases and epidemics to local health authorities is mandated in virtually all countries. There are no recommended medical screening activities for this disease.
Prevention
Prevention of cholera requires avoiding contaminated food and water. The absence of municipal water chlorination has been identified as a major contributor to the reemergence of cholera in South America.13
An inactivated bacteria cholera vaccine is available, but it is not normally recommended. The vaccine is about 50% effective in preventing illness for 3–6 months, does not prevent transmission of infection, and may not offer cross-protection against diarrhea caused by V. cholerae O139.14 Newer oral live attenuated vaccines have been developed which appear to offer protection for up to 2 years.15
Persons with cholera infections should not return to work until clearance of the organism is complete as documented by negative stool cultures. In outbreaks, asymptomatic carriers should be identified and treated to eradicate the organism. Healthcare workers caring for patients with cholera should apply appropriate enteric precautions.
References
- 1. Cooper G, Hadler JL, Barth S, et al. Cholera associated with international travel, 1992. MMWR 1992; 41:664–7.
- 2. Hunt MD, Woodward WE, Keswick BH, et al. Seroepidemiology of cholera in Gulf coastal Texas. Appl Environ Microbiol 1988; 54(7):1673–7.
- 3. Weber JT, Mintz ED, Canizares R, et al. Epidemic cholera in Ecuador: multidrug-resistance and transmission by water and seafood. Epidemiol Infect 1994; 112(1):1–11.
- 4. Huq A, Hasan JA, Losonsky G, et al. Colonization of professional divers by toxigenic Vibrio cholerae O1 and V. cholerae non-O1 at dive sites in the United States, Ukraine and Russia. FEMS Microbiol Lett 1994; 120(1–2):137–42.
- 5. Goh KT, Teo SH, Lam S, et al. Person-to-person transmission of cholera in a psychiatric hospital. J Infect 1990; 20(3):193–200.
- 6. Huhulescu S, Leitner E, Feierl G, et al. Laboratory-acquired Vibrio cholerae O1 infection in Austria, 2008. Clin Microbiol Infect 2010; 16:1303–4.
- 7. Newton A, Kendall M, Vugia DJ, et al. Increasing rates of vibriosis in the United States, 1996–2010: review of surveillance data from 2 systems. Clin Infect Dis 2012; 54 Suppl 5:S391–5.
- 8. Dalsgaard A, Forslund A, Bodhidatta L, et al. A high proportion of Vibrio cholerae strains isolated from children with diarrhoea in Bangkok, Thailand are multiple antibiotic resistant and belong to heterogenous non-O1, non-O139 serotypes. Epidemiol Infect 1999; 122(2):217–26.
- 9. Bhattacharya SK, Bhattacharya MK, Nair GB, et al. Clinical profile of acute diarrhoea cases infected with the new epidemic strain of Vibrio cholerae O139: designation of the disease as cholera. J Infect 1993; 27:11–15.
- 10. Weber JT, Levine WC, Hopkins DP, et al. Cholera in the United States, 1965–1991. Risks at home and abroad. Arch Intern Med 1994; 14:551–6.
- 11. Rabbani GH. The search for a better oral rehydration solution for cholera. N Engl J Med 2000; 342(5):345–7.
- 12. Usubutun S, Agalar C, Diri C, et al. Single dose ciprofloxacin in cholera. Eur J Emerg Med 1997; 4(3):145–9.
- 13. Ries AA, Vugia DJ, Beingolea L, et al. Cholera in Piura, Peru: a modern urban epidemic. J Infect Dis 1992; 166(6):1429–33.
- 14. Albert MJ, Alam K, Ansaruzzaman M, et al. Lack of cross-protection against diarrhea due to Vibrio cholerae O139 (Bengal strain) after oral immunization of rabbits with V. cholerae O1 vaccine strain CVD103-HgR. J Infect Dis 1994; 169:230–1.
- 15. Graves P, Deeks J, Demicheli V, et al. Vaccines for preventing cholera. Cochrane Database Syst Rev 2000; 2:CD000974.
VIBRIO SPECIES OTHER THAN V. CHOLERAE (V. PARAHEMOLYTICUS, V. VULNIFICUS)
Common name for disease: None
Occupational setting
Individuals at risk for non-cholera Vibrio infection are those in close contact with both aquatic environments such as commercial divers,1 fishermen,2 fish farmers,3 and workers who handle seafood or shellfish.4 Outbreaks have also occurred aboard cruise ships.5 Bacteria in this group have been described as “occupational pathogens,” since asymptomatic carriage rates approaching 4% have been documented in high-risk worker groups, which result in sporadic outbreaks.6 In some coastal areas of the United States, non-cholera Vibrio infections are increasing, possibly due to warming ocean temperatures.7
Exposure (route)
Exposure is through ingestion or direct contact with marine organisms, or with water containing live organisms.
Pathobiology
Vibrios are curved, flagellated, Gram-negative rods. These organisms are found in surface waters (fresh and salt water) around the world. A number of Vibrio species have been identified which may cause disease in humans. Most, like V. cholerae, cause toxic gastrointestinal disease. Several species, however, also have varying degrees of predilection for causing soft tissue infections, usually at the site of pre-existing skin breaks or wounds. Sepsis and death are well-known complications of either presentation, but typically only in those with chronic disease.8 In cases complicated by sepsis, there may be lower extremity edema and bullae.9
The most important species to consider are V. parahaemolyticus and V. vulnificus. V. parahaemolyticus is a common cause of diarrheal disease throughout the world. The most frequent route of exposure is consumption of raw shellfish, usually oysters.9 Four such outbreaks have occurred in the United States since 1997.9 Other Vibrio species primarily associated with gastroenteritis include V. mimicus,10V. hollisae,11 and V. fluvialis.12
V. vulnificus causes local wound infections that may follow an aggressive course with rapid spread and necrosis of surrounding tissue.13 Other conditions associated with this organism include corneal ulcers4 and a fulminant systemic illness characterized by a hemorrhagic rash, fever, gastroenteritis, and hypotension.14 Other vibrios that cause local wound infections are V. alginolyticus,15V. damsela,16 and V. metschnikovii.17 The infections occur frequently in pre-existing wounds or skin breaks, or they develop as an acute otitis.1 The most common route of exposure is direct contact with open seawater.13
Diagnosis
All Vibrio infections are diagnosed by culturing the organism from stool, blood, or wound samples. A history of exposure to seawater or raw shellfish, or ingestion of raw or under-cooked shellfish, is helpful in the diagnosis. Development of a severe cellulitis of the extremities after exposure to seawater, especially if the cellulitis does not respond to aminoglycosides, should raise the suspicion of infection with Vibrio species.
Treatment
Most of the gastrointestinal diseases caused by non-cholera vibrios are self-limited and do not require specific therapy other than rehydration.
Local wound infections generally respond to antibiotics such as tetracycline, with chloramphenicol or penicillin as a second choice. Surgical debridement is frequently required when soft-tissue necrosis is present.13 Systemic infection requires parenteral antibiotic therapy.
Medical surveillance
There are no recommended medical screening or surveillance activities for non-cholera Vibrio infections.
Prevention
There is no effective vaccine for any of the non-cholera Vibrio infections. Prevention of foodborne disease consists primarily of proper handling, cooking, and refrigeration. Immunocompromised individuals, particularly those with chronic liver disease, should avoid ingestion of uncooked shellfish.13 The concentration of Vibrio species in seawater increases with increasing water temperature, increasing the risk of contamination for seafood harvested during the summer.18
Individuals with pre-existing wounds should take precautions to avoid exposure to sea water or other potentially contaminated material.
References
- 1. Tsakris A, Psifidis A, and Douboyas J. Complicated suppurative otitis media in a Greek diver due to a marine halophilic Vibrio sp. J Laryngol Otol 1995; 109(11):1082–4.
- 2. Hoi L, Dalsgaard A, Larsen JL, et al. Comparison of ribotyping and randomly amplified polymorphic DNA PCR for characterization of Vibrio vulnificus. Appl Environ Microbiol 1997; 63(5):1674–8.
- 3. Bisharat N, Agmon V, Finkelstein R, et al. Clinical, epidemiological, and microbiological features of Vibrio vulnificus biogroup 3 causing outbreaks of wound infection and bacteraemia in Israel. Israel Vibrio Study Group. Lancet 1999; 354(9188):1421–4.
- 4. Massey EL and Weston BC. Vibrio vulnificus corneal ulcer: rapid resolution of a virulent pathogen. Cornea 2000; 19(1):108–9.
- 5. Centers for Disease Control. Gastroenteritis caused by Vibrio parahaemolyticus aboard a cruise ship. MMWR 1978; 27:65–6.
- 6. Morris JG Jr. Non-O group 1 Vibrio cholerae: a look at the epidemiology of an occasional pathogen. Epidemiol Rev 1990; 12:179–91.
- 7. Jones EH, Feldman KA, Palmer A, et al. Vibrio infections and surveillance in Maryland, 2002–2008. Public Health Rep 2013; 128:537–45.
- 8. Klontz KC. Fatalities associated with Vibrio parahaemolyticus and Vibrio cholerae non-O1 infections in Florida (1981 to 1988). South Med J 1990; 83(5):500–2.
- 9. Centers for Disease Control. Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from Long Island Sound—Connecticut, New Jersey, and New York, 1998. MMWR 1999; 48(3):48–51.
- 10. Campos E, Bolanos H, Acuna MT, et al. Vibrio mimicus diarrhea following ingestion of raw turtle eggs. Appl Environ Microbiol 1996; 62(4):1141–4.
- 11. Carnahan AM, Harding J, Watsky D, et al. Identification of Vibrio hollisae associated with severe gastroenteritis after consumption of raw oysters. J Clin Microbiol 1994; 32(7):1805–6.
- 12. Klontz KC, Cover DE, Hyman FN, et al. Fatal gastroenteritis due to Vibrio fluvialis and nonfatal bacteremia due to Vibrio mimicus: unusual vibrio infections in two patients. Clin Infect Dis 1994; 19(3):541–2.
- 13. Howard RJ and Bennett NT. Infections caused by halophilic marine Vibrio bacteria. Ann Surg 1993; 217(5):525–30.
- 14. Serrano-Jaen L and Vega-Lopez F. Fulminating septicaemia caused by Vibrio vulnificus. Br J Dermatol 2000; 142(2):386–7.
- 15. Mukherji A, Schroeder S, Deyling C, et al. An unusual source of Vibrio alginolyticus-associated otitis: prolonged colonization or freshwater exposure? Arch Otolaryngol Head Neck Surg 2000; 126(6):790–1.
- 16. Tang WM and Wong JW. Necrotizing fasciitis caused by Vibrio damsela. Orthopedics 1999; 22:443–4.
- 17. Hansen W, Freney J, Benyagoub H, et al. Severe human infections caused by Vibrio metschnikovii. J Clin Microbiol 1993; 31(9):2529–30.
- 18. Shapiro RL, Altekruse S, Hutwagner L, et al. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States, 1988–1996. Vibrio Working Group. J Infect Dis 1998; 178(3):752–9.
YERSINIA PESTIS
Common names for disease: Plague
Occupational setting
Human plague arises from infection with Yersinia pestis, a bacterium that is maintained in a natural reservoir of small rodents and the fleas that infest them. Since 1925, plague in the United States has been associated with exposures to wild rodents only in the southwestern states, primarily around the Four Corners region where New Mexico, Arizona, Utah, and Colorado join, and in California.1 The disease is also endemic in many areas of South America, Africa, and Southeast Asia. Historically, throughout the world, urban and domestic rats have been the most important reservoirs for epidemic plague. As control measures have reduced the proximity of rats to humans in urban areas, foci of the sporadic illness have shifted to rural areas where burrowing mammals make their habitat. The oriental rat flea (Xenopsylla cheopis) is the most important vector. In the United States, plague is maintained in well-established enzootic foci among wild rodents, including rock squirrels, the California ground squirrel, prairie dogs, chipmunks, and woodrats. Although they are not part of the enzootic cycle, are only incidentally infected, and rarely develop overt illness, rabbits and hares, deer, antelope, gray fox, badger, bobcat, and coyote have been occasionally associated with human plague in hunters and trappers. Plague associated with rock squirrels is the most important cause of human disease in North America, as housing developments have been introduced into habitats where plague was enzootic. There were 13 cases of human plague in four states in the United States in 2006, the highest number in recent years (Figure 22.10).2 Hunters, trappers, foresters, rangers, and others working in remote locations where contact with rodent habitats might be expected are at risk for contracting plague, as are veterinarians in enzootic areas. In 2009, a wildlife biologist in the Grand Canyon died of plague after conducting a necropsy. Military personnel and individuals who might be stationed in areas where rats or other animal reservoirs are present would also be at increased risk.
FIGURE 22.10 Plague – reported cases among humans, United States, 1970–2012.
Source: http://www.cdc.gov/plague/maps.
Exposure (route)
Plague is transmitted from infected animals to humans by several species of rodent fleas, including in the United States the ground squirrel fleas Diamana montana and Thrassis bacchi. Domestic cats can also transmit the infection after consuming plague-infected animals, or after bites from infected rodent fleas, to humans by bites or scratches.3 Human-to-human transmission can occur in pneumonic plague, and such transmission constitutes a public health emergency. Human-to-human pneumonic plague transmission has not been reported in the United States since a 1925 epidemic in Los Angeles. Human pneumonic plague can be acquired from domestic cats with secondary pneumonic plague; this mode of transmission has been associated with disease in veterinarians. Y. pestis can also be acquired through infectious fluids or tissues entering through cuts or abrasions in the skin, a mode of transmission most commonly seen in hunters and trappers who skin infected animals.
Pathobiology
Y. pestis is a Gram-negative, nonmotile coccobacillus. Human infection is associated with four common clinical presentations: bubonic plague, septicemic plague, pneumonic plague, and meningitis. From 2 to 6 days after the bite of an infected flea, the patient develops a febrile illness, followed by the development of very painful suppurating lymphadenopathy (buboes) proximal to the bite. These are seen most commonly in the groin, axillae, or cervical region.1 Septicemic plague is usually secondary to untreated bubonic plague and is usually rapidly fatal without treatment. Hematogenous dissemination can affect any organ system, but most commonly involves lungs, eyes, meninges, joints, and skin. Occlusion of small cutaneous blood vessels can result in necrosis and gangrene of the fingers and skin (which gave rise to the term “Black Death” in the fourteenth century). Meningitis is a rarer complication that follows inadequately treated bubonic plague and is more commonly associated with axillary buboes. Case fatality rates for untreated bubonic and septicemic plague exceed 50% in most reports.4
Pneumonic plague can be secondary to septicemia or arise as a primary infection after the inhalation of infectious droplet nuclei. Primary pneumonic plague has a short incubation period and can spread rapidly in close contacts; its presence is therefore a public health emergency, as the infected case will generate infective aerosols. In the United States, the few reported human cases of primary pneumonic plague have been acquired from domestic cats that developed secondary pneumonic infection. Survival is unlikely unless treatment is initiated within 18 hours after onset of respiratory symptoms.4
Diagnosis
Infectious clinical material can be examined with light microscopy after appropriate staining. Buboes and blood can also provide specimens for culture. Large quantities of bacteria in lesions and blood typically make bacteriologic diagnosis relatively easy when the etiology is suspected. However, the use of automated systems for laboratory detection has resulted in diagnostic errors and delayed diagnosis.5 In patients with symptoms associated with plague, identification of unusual organisms (e.g., P. luteola, Y. pseudotuberculosis, or A. lwoffii) in the blood using automated systems should trigger further clinical and laboratory evaluation.5 If plague is suspected, treatment should be started and samples should be sent to public health laboratories for confirmation.5 A rapid fluorescent antibody test is available at certain reference laboratories.
Treatment
Streptomycin has been the traditional treatment of choice, but due to its toxicity and limited availability, gentamicin has become a more accepted initial treatment. Tetracycline can be used if gentamicin is contraindicated. Chloramphenicol is the preferred treatment for meningitis and endophthalmitis.
Medical surveillance
There are no recommended human medical screening activities for plague. Suspected and confirmed cases of plague are required to be immediately reported to local health authorities worldwide. Monitoring of rodent populations for increased die-offs and evidence of epizootics should be performed in endemic areas.
Prevention
Discussion of the prevention of human plague can be grouped into three categories: (i) very high-risk populations such as laboratory workers or medically underserved endemic or epidemic areas; (ii) exposure to human plague cases or epizootic foci; and (iii) environmental management of hyperendemic residential or recreational foci.1 A 2013 survey of wildlife workers’ use of personal protective equipment, found there was significant room for improvement and that equipment was rarely used and seldom accessible to workers.6 An inactivated plague vaccine is available in the United States, consisting of a primary immunization series of two injections and boosters every 6 months, and is recommended for laboratory workers with frequent exposure to Y. pestis and persons such as mammalogists, ecologists, and other field workers who have regular contact with wild rodents or their fleas in areas in which plague is enzootic or epizootic.4
Management of contacts of human plague cases and of exposures to epizootic plague are potential public health emergencies. Contacts should be carefully identified, and appropriate follow-up, including consideration of prophylactic antibiotic therapy, should be ensured. Available environmental control measures for management of epizootic plague include application of pesticides to control fleas; closure of recreational areas to humans; and judicious use of rodenticides. Control may also be exercised through management of the environment near the interface of human and rodent activities, such as appropriate handling of trash, and removal of rock piles, and dilapidated buildings that provide habitats for rodents. Catastrophic events, such as war or natural disasters, which disrupt normal sanitary activities, may lead to spread of plague from rural foci into urban centers; control measures should be reinstituted as rapidly as possible to prevent this scenario.
References
- 1. Craven RB and Barnes AM. Plague and tularemia. Infect Dis Clin North Am 1991; 5:165–75.
- 2. Centers for Disease Control and Prevention. Human plague—four states, 2006. MMWR Morb Mortal Wkly Rep 2006; 55:940–3.
- 3. Doll JM, Zeitz PS, Ettestad P, et al. Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am J Trop Med Hyg 1994; 51:109–14.
- 4. Centers for Disease Control and Prevention. Prevention of plague: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1996; 45(RR-14):1–15.
- 5. Tourdjman M, Ibraheem M, Brett M, et al. Misidentification of Yersinia pestis by automated systems, resulting in delayed diagnoses of human plague infections—Oregon and New Mexico, 2010–2011. Clin Infect Dis 2012; 55(7):e58–60.
- 6. Bosch SA, Musgrave K, and Wong D. Zoonotic disease risk and prevention practices among biologists and other wildlife workers—results from a national survey, US National Park Service, 2009. J Wildl Dis 2013; 49:475–585.
YERSINIA PSEUDOTUBERCULOSIS AND ENTEROCOLITICA
Common names for disease: None
Occupational setting
Yersinia enterocolitica and Y. pseudotuberculosis are widespread in natural settings and have been isolated from wild and domestic animals, foods, water, and soil. Y. enterocolitica is a frequent cause of diarrhea and gastroenteritis in European countries and North America. Swine and pigs are common asymptomatic carriers of the bacteria, with Yersiniae frequently isolated from pigs’ tongues and tonsils, and abattoir workers have been found to have an elevated risk of infection.1 Other animal reservoirs of the infection include rodents, rabbits, sheep, cattle, horses, dogs, and cats. Workers who come in contact with refrigerated meat after slaughter including butchers, meat-handlers, and meat-packers, are also at risk, as Yersiniae can propagate at low temperatures. Y. pseudotuberculosis is a zoonosis of the aforementioned animals and also several species of birds (turkeys, ducks, geese, pigeons). It is an uncommon disease, more frequent in children and during the winter months and is associated with exposure to animals or common-source outbreaks from contaminated food or water.
Exposure (route)
Transmission occurs via ingestion of contaminated food or water and by direct contact with infected animals, possibly by a fecal–oral route. Fecal–oral transmission between humans has not been documented.
Pathobiology
These Yersiniae are facultatively anaerobic Gram-negative bacilli that are motile when grown at 25°C. Asymptomatic infection with either Yersinia species is common. The prevalence of antibodies to Yersiniae is up to 10% in the general population and up to 40% in slaughterhouse workers. The incubation period for Y. enterocolitica enterocolitis ranges from 1 to 11 days.2 Symptomatic infection most commonly is a diarrheal illness with fever and severe abdominal pain that can mimic acute appendicitis. Septicemia is less common, has an untreated case fatality ratio of 50%, and is usually associated with moderate to severe underlying medical problems such as diabetes or cancer. Interestingly, in one study, abattoir workers were reported to have almost a fourfold increased risk, and pig farmers a twofold increased risk of appendectomy compared to grain or berry farmers. The authors hypothesized that the severe abdominal pain associated with Yersinia infections in these workers could have accounted for the increased risk.3 Postinfection complications include reactive polyarthritis, erythema nodosum, and eye inflammation (e.g., iridocyclitis).1 Person-to-person transmission of Y. enterocolitica has also been reported as a cause of septicemia arising from blood transfusions.4 Patients developed the abrupt onset of fever and hypotension within 50 minutes after transfusion had begun; four of six patients reported between 1989 and 1991 died from the infection. Transient bacteremia in donors with proliferation of the bacterium under cold storage conditions was considered responsible for the contamination of blood products. Y. pseudotuberculosis most commonly causes mesenteric adenitis in adults, mimicking acute appendicitis, which is often self-limiting.
Diagnosis
Culture of appropriate clinical specimens (usually stool samples) often yields Yersiniae. Cold enrichment increases the yield of cultures by selectively favoring the growth of Eurasian, although, because of asymptomatic colonization, care must be taken with culture results that become positive only after prolonged culture. Serologic tests using adsorption methods (to remove cross-reacting antibodies) are also useful in diagnosis. If transfusion-associated bacteremia is suspected, the residual blood in the bag should be examined by Wright–Giemsa or other hematologic stain, and cultured.4
Treatment
Enterocolitis and mesenteric adenitis are usually self-limited, and the need for antibiotic therapy in these conditions is unclear. Doxycycline and trimethoprim–sulfamethoxazole are effective in complicated gastrointestinal infection or focal extraintestinal infection.2 There is resistance to quinolones.5 Septicemia should be treated with a combination of doxycycline and an aminoglycoside. Laparotomy for suspected appendicitis should be avoided if Yersinia infection is a likely diagnosis.
Medical surveillance
There are no recommended medical screening activities. Reporting of cases is mandatory in many areas in the United States and in many other countries.
Prevention
Prevention should focus on the animal reservoirs of the infection. Institution of work practices to minimize contamination of meat, such as altered methods of slaughter of pigs and avoidance of prolonged refrigeration of meat before consumption, is advised. Careful handwashing and cleaning of surfaces after food preparation is essential to prevent bacterial spread to other foods. Personal protective equipment use may also afford some protection from infection, but its effectiveness has not been evaluated.
References
- 1. Merilahti-Palo R, Lahesmaa R, Granfors K, et al. Risk of Yersinia infection among butchers. Scand J Infect Dis 1991; 23:55–61.
- 2. Cover TL, Aber RC. Yersinia enterocolitica. N Engl J Med 1989; 321:16–22.
- 3. Seuri M. Risk of appendicectomy in occupations entailing contact with pigs. Br Med J 1991; 301:345–6.
- 4. Centers for Disease Control and Prevention. Epidemiologic Notes and Reports Update: Yersinia enterocolitica bacteremia and endotoxin shock associated with red blood cell transfusions—United States, 1991. MMWR 1991; 40:176–8.
- 5. Capilla S, Ruiz J, Goñi P, et al. Characterization of the molecular mechanisms of quinolone resistance in Yersinia enterocolitica O:3 clinical isolates. J Antimicrob Chemother 2004; 53:1068–71.
