24
Health Aspects of Novel Hydrocolloids
Jafar M. Milani and Abdolkhalegh Golkar
Department of Food Science & Technology, Sari Agricultural Sciences and Natural Resources University (SANRU), Sari, Iran
24.1 Introduction
The term hydrocolloids includes all the polysaccharides and proteins that are widely used in industrial sectors. Hydrocolloids are important parts of our daily diet in food systems such as yogurt, mayonnaise and salad dressing, ice cream, dessert, bakery products, and so on [1,2]. The increasing occurrence of different diseases in the world has given rise to a demand for healthy foods containing natural compounds such as hydrocolloids (e.g., dietary fiber) and phytochemical compounds (e.g., antioxidants, and so on) with a high level of compounds with health benefits [3]. For many years, hydrocolloids have been used in food systems as functional ingredients for the control of microstructure, texture, flavor, and shelf life. In fact, these are a diverse group of high‐molecular‐weight polymers and may be named thickeners, gelling agents, stabilizers, bulking agents, and emulsifiers on the basis of their functionality [ 1,4]. Beside functional attributes, hydrocolloids are presently being reported to have many increasing applications in healthy foods.
The alteration of people's lifestyle has been caused by a growing awareness of the relationship between diet and health and new processing technologies. These changes have led to the production of novelty food with a high level of fiber and low‐fat content. Consequently, this has increased the demand for hydrocolloids in the food industry [5].
The term dietary fiber was first used in 1953 by Eben Hipsley in his observation publication noting that populations with diets high in fiber‐rich foods tended to also have lower rates of pregnancy toxemia [6]. Previously, the analytical term crude fiber had been used for the portion of plant foods that escaped solvent, acid, and alkali extractions [7]. The WHO (World Health Organization) and FAO (Food and Agriculture Organization) agree with the AACC (American Association of Cereal Chemists) definition but with a slight variation. They state that dietary fiber is a polysaccharide with 10 or more monomeric units which is not hydrolyzed by endogenous hormones in the small intestine [8].
Epidemiological evidence suggests that a high intake of dietary fiber is associated with numerous health benefits. The fiber hypothesis proposed by Burkitt and Trowel suggested some three decades ago that there was a link between the consumption of a diet rich in fiber and the level of protection against many of the “First World diseases” [ 7,9]. In previous research papers, the dietary fiber properties of various food hydrocolloids have been discussed. Intakes of food containing dietary fiber reduced the risk for developing different diseases such as coronary heart disease, diabetes, obesity, and so on [ 4 10–13]. In addition, it has been suggested that public awareness of these important ingredients will be required. Although several review papers have been published reporting on the health benefits of commercial food hydrocolloids [ 2, 4,14,15], none of them has addressed novel hydrocolloids in particular. So, the important points of this chapter are the investigation of the health aspects of novel hydrocolloids on the basis of recent publications.
24.2 Health Benefits of Hydrocolloids
With the spread of different disease in the world and the increasing demand for functional and healthy food products, scientists have conducted research to solve these problems. Hydrocolloids as food ingredients related to unique functional properties have been used for many years. These ingredients can be used as fibers with specific health benefits. The literature has examples of novel food hydrocolloids that exhibit important roles in foods as new dietary fiber sources in addition to their traditional applications as thickening, coating, gelling, and emulsifying agents.
Dietary fiber and whole grains are an abundant source of nutrients including vitamins, minerals, and slowly digestible carbohydrates. Also, they contain phytochemicals that are not classified as essential nutrients but may play important roles in human health [16].
First, researchers found that a diet with guar gum prolongs mouth to cecum transit time, delays gastric emptying, slows down the increase in postprandial glycemia, and aids colonic function [17–19]. In the following sections, the proposed health benefits of well‐known and novel food hydrocolloids as dietary fiber will be introduced. The health aspects of commercial hydrocolloids have been deeply studied, and some of them are summarized in Table 24.1. Numerous papers are annually published on the characterization of new hydrocolloids with health claims, and a wide range of novel hydrocolloids are reported to have health benefits in line with good functional properties in food systems. In Table 24.2, some recent studies on the possible health effects of novel hydrocolloids are presented.
Table 24.1 Health benefits of most well‐known hydrocolloids.
| Hydrocolloid | Health aspect | Reference |
| Pectin and Guar gum | Cholesterol‐lowering effect | [20] |
| Psyllium | Blood glucose and insulin lowering effect | [21] |
| Alginate | Enhancing satiety and controlled energy intake | [22] |
| Hydroxypropylmethylcellulose (HPMC) | Anti‐diabetic effect | [23] |
| Oat β‐glucan | Hypoglycemic effect, satiety effect | [24,25] |
| Gum arabic | Reduction of blood pressure, anti‐obesity effect | [26,27] |
| Chitosan | Antioxidant effect | [28] |
| Cellulose | Anti‐obesity effect, blood‐glucose‐lowering effect, Cholesterol‐lowering effect | [28–30] |
| Resistant starch | Blood glucose and insulin control | [31] |
| Barley β‐glucan | Satiety effect | [32] |
| Inulin | Prebiotic effect | [33] |
| Arabinoxylan | Anti‐diabetic effect | [34] |
| Carrageenan | Antitumoral activity | [35] |
| Alginate | Active compounds carrier | [36] |
Table 24.2 Possible health benefits of novel hydrocolloids (extracted from the published works during the years 2010–2017).
| Hydrocolloid | Health aspect | Highlight | Reference |
| Cyanobacteria Nostoc commune polysaccharides | Antioxidant activity |
|
[37] |
| Almond gum polysaccharides (Prunus amygdalus) |
Antioxidant activity Antimicrobial activity |
|
[38,39] |
| Sulfated polysaccharide from Ulva pertusa (Chlorophyta) | Antihyperlipidemic activity |
|
[40] |
| Levan and its derivative from Bacillus subtilis NRC1aza | Antitumor activity Antioxidant activity Hypolipidemic effect |
|
[41,42] |
| Levan polysaccharide | Anti‐diabetic activity |
|
[43] |
| Artemis sphaerocephala Krasch. Gum | Antioxidant activity Anti‐diabetic activity |
|
[44] |
| Extracted polysaccharides from Bryopsis plumosa | Antioxidant activity |
|
[45] |
| Polysaccharides from Sargassum thunbergii | Antitumor activity |
|
[46] |
| Acidic polysaccharide from Tuber sinoaestivum | Immunomodulator effect |
|
[47] |
| Polysaccharides from Diaphragma juglandis fructus | Antioxidant activity Antibacterial activity |
|
[48] |
| Peach‐gum‐derived polysaccharides | Anti‐diabetic effect |
|
[49] |
| Lycium barbarum L. Polysaccharides | Anticancer effect Antioxidant activity Hypoglycemic effect Neuroprotective effect |
|
[50–53] |
| Zizyphus jujuba Mill Polysaccharide | Antioxidant activity Hyperlipidemic effect Immunomodulatory activity |
|
[54–57] |
| Plantago spp. Polysaccharide | Immunomodulatory effect Antioxidant activity |
|
[58–60] |
| Morus spp. Polysaccharide | Antioxidant activity Anti‐obesity activity |
|
[61,62] |
| Rehmannia glutinosa polysaccharide | Anticancer activity |
|
[63] |
| Malva aegyptiaca polysaccharides | Antioxidant activity Antimicrobial |
|
[64] |
| Cress (Lepidium sativum) seed gum | Hypoglycemic activity Hypolipidemic activity Antimicrobial activity |
|
[65] |
24.2.1 Anti‐diabetic Effects
Diabetes mellitus is a chronic metabolic disorder characterized by a high blood glucose level (hyperglycemia) due to insulin deficiency and/or insulin resistance [66]. Diabetes is spreading worldwide, affects approximately 4% of the population, and is expected to increase by 5.4% in 2025. Diabetes is a multifactorial disease which is characterized by hyperglycemia, defects in reactive oxygen species scavenging enzymes, and high‐oxidative‐stress‐induced damage to pancreatic beta cells [67]. Generally, diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Diabetes mellitus is classified into two groups: type‐1 diabetes is characterized by the destruction of pancreatic β‐cells. Type‐2 diabetes is the major form of diabetes mellitus and is caused by insulin resistance and impaired insulin production, secretion, and function [49]. For diabetes mellitus therapy, a series of agents, including sulfonylureas, thiazolidinedione, α‐glucosidase inhibitors, and Biguanide, which are commercial products, have been used for decades. But these drugs have adverse effects depending on the amount consumed (weight gain, liver damage, bone loss, diarrhea, vomiting, and so on). As the treatment of this disease usually involves very long periods, in some cases, serious problems may occur. Although new drugs such as DPP‐4 inhibitors and GLP‐1 analogs have been introduced to the market, but their high price as well as the absence of clear safety characteristics have led to a large demand for natural drugs to treat diabetes mellitus. The new trend is for patients to use functional foods and complementary or alternative medicine. Researchers report that a variety of active compounds from natural products such as polysaccharides and dietary fibers have a high potential for application in diabetes mellitus treatment [68].
Viscous forms of dietary fiber have been shown to improve blood glucose control by trapping ingested carbohydrates inside the viscous gel formed after digestion. As a result, sugars are absorbed into the bloodstream more slowly, limiting the rise in blood glucose seen after a meal. High‐viscosity fibers usually reduce the palatability of products, and this property is a major problem for practical application, although they are necessary for maximizing the beneficial effect on blood glucose [69]. In addition, some suggest that insoluble fiber increases the passage rate of foodstuff through the gastrointestinal tract, decreasing the absorption of nutrients (such as simple carbohydrates). Insoluble fiber can cause reduced appetite and food intake, and short‐chain fatty acids (via fermentation) have been shown to reduce the postprandial glucose response [8]. Recently, an improvement in glycemic control was observed with psyllium in patients with type‐2 diabetes mellitus [70].
Jia et al. [71] investigated the hypoglycemic and hypolipidemic activities of Laminaria japonica polysaccharides (LJPs) by alloxan injection and found that LJP administration prevented body weight loss, decreased fasting blood glucose levels, and increased serum insulin levels in diabetic mice. Furthermore, it decreased total cholesterol, total triglyceride, and LDL‐C levels, and increased HDL‐C levels in these mice [71]. The same results were also seen in the studies of Li et al. [72].
Huang et al. [73] isolated a fucose‐containing exopolysaccharide from a culture broth of Enterobacter cloacae Z0206 with molecular weight 1.1 × 106 Da and composed of fucose, glucose, galactose, glucuronic acid, and pyruvic acid in the approximate molar ratio 2:1:3:1:1, and found that exopolysaccharide exhibited hypoglycemic and hypolipidemic effects, possibly through regulating AMP‐activated protein kinase and SirT1‐mediated effects on carbohydrate and lipid metabolism. Selenium‐ECZ‐EPS (exopolysaccharide) is a water‐soluble selenium‐enriched exopolysaccharide which is isolated from the submerged culture broth of E. cloacae Z0206. Se‐ECZ‐EPS significantly reduces fasting blood glucose, glycated serum proteins (GSPs), total cholesterol, and total triglyceride contents in the liver [73].
Cunha et al. [66] found that galactomannan from Caesalpinia ferrea seeds lowered hyperglycemia in diabetic rats and significantly decreased serum TAG (mediated effects on carbohydrate and lipid metabolism). The anti‐diabetic benefits of these hydrocolloids are associated with smooth glucose uptake and slow starch digestion [74]. As seaweed contains a large number of soluble polysaccharides, they therefore have potential functions as dietary fiber. Thus, they might be considered to have beneficial effects on cardiovascular diseases risk factors [75].
24.2.2 Antioxidant Activity and Cancer Prevention
There has been increasing interest in researching natural antioxidants since they can protect the human body from free radicals and retard the progress of many chronic diseases and cancer [76]. Accordingly, there is a growing interest in applying new natural antioxidant compounds to prevent metabolic disorders of oxidative stress origin [64]. A number of natural polysaccharides and their derivatives have been demonstrated to possess potent antioxidant activities and potential applications as antioxidants [41].
In the last decade, it has been reported that some seaweed sulfated polysaccharides (such as carrageenan and fucoidan) showed antioxidant activities [77–79]. The antioxidant capacity of commercial carrageenan from Gigartina skottsbergii and Schizymenia binderi, and fucoidan from Lessonia vadosa was also evaluated by the oxygen radical absorbance capacity method [80]. In addition, peach‐gum‐derived oligosaccharides showed high hydroxyl radical scavenging activity (86.12%) and 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH) radical scavenging activity (91.70%) at a concentration of 100 µg ml−1 as well as high reducing capacity at a concentration of 50 µg ml−1 [81].
Bouaziz et al. [38] reported that almond gum oligosaccharide (by enzymatic hydrolysis) had significant antioxidant and antimicrobial activity. This oligosaccharide has been tested in beef meat preservation, and microbial growth and lipid oxidation were monitored for nine days at 4 °C. They found significant inhibitions (p < 0.05) of lipid oxidation and microbial growth in ground beef meat containing almond gum oligosaccharide [38].
Cancer treatment strategies are actually focused on improving three main strategies: (1) prevention, based on promoting lifestyles associated with low tumorigenesis risks; (2) surgery, consisting often in the ablation of the tumor, ideally before the epithelial‐mesenchymal transition, which leads to metastasis; and (3) by inducing tumoral cell death via targeted radio‐ or chemotherapy [75]. Epidemiological studies have suggested a reverse association between intake of dietary fiber, the ingested parts of plant materials, and risk of colon cancer. Dietary fibers influence colon carcinogenesis by increased fecal bulk, reduced colonic transit time, and diluted fecal toxin contents, which consequently reduce the exposure of colonic mucosa to the luminal carcinogens. In addition, the interaction between dietary fiber and colonic microbiota and bile acids, and the production of short‐chain fatty acids resulting from fermentation are believed to protect against colon cancer development [82].
Dahech et al. [43] investigated the antitumor and anti‐cytotoxic effect of Levan polysaccharide produced by Bacillus licheniformis. In the in vitro antitumor activity test of Levan against some tumor cell lines, relatively significantly high activity was observed against hepatocellular carcinoma, human (HepG2). The strongest inhibiting activity appeared at the highest dosage of Levan (12.5 mg ml−1) [43].
24.2.3 Immunostimulant Activity
Immunomodulation is considered an important biological function of natural polysaccharides, which act as immunomodulators or biological response modifiers. Various reports have suggested that polysaccharides and proteoglycans with high arabinose and galactose content exhibit immunomodulatory activities, including complement fixing activities and/or modulation of macrophage function. The results from in vitro tests of cashew nut tree gum exudate using murine peritoneal macrophages showed that this gum can be used as an anti‐inflammatory [83]. Also, a recent study suggested that water‐soluble polysaccharide from Erythronium sibiricum bulb is a potential immunostimulator. An in vitro assay showed that this polysaccharide significantly promoted the proliferation and neutral red phagocytosis of RAW 264.7 macrophage cells. Moreover, it stimulated the production of secretory molecules (nitric oxide, TNF‐α, and IL‐1β) of RAW 264.7 macrophage cell in a dose‐dependent manner [84]. Ji et al. [85] found that Ziziphus jujuba polysaccharide fractions (RQP1d and RQP2d) induced significant increases in nitric oxide formation in RAW 264.7 cells, and both extracts stimulate the innate immune response. However, RQP1d and RQP2d are dissimilar in their chemical compositions and molecular weights. Low concentrations of RQP2d had a synergistic effect with lipopolysaccharide on splenocyte proliferation. The immunomodulatory actions of polysaccharides are associated with their molecular weights, chemical compositions, glycosidic linkages, and so on [85].
24.2.4 Antimicrobial Activity
Various researchers have attempted to find new antimicrobials to inhibit food spoilage and food poisoning, which are important problems in the food industry [64]. Nowadays, it is possible to control pathogenic microorganisms in foods by synthetic antimicrobials. But the increase of bacterial resistance to conventional antimicrobial agents and consumer awareness of the side effects of these compounds has motivated the search for novel substrates of natural origin. According to Kubo et al. and Campos et al., cashew tree gum has antimicrobial activity against several microorganisms, which is attributed to the presence of anacardic acid [86,87].
Peach‐gum‐derived oligosaccharides demonstrated antimicrobial activity against Bacillus subtilis, Staphylococcus aureus, and Escherichia coli at a concentration of 100 µg ml−1 [81]. On the basis of recent researches, almond gum polysaccharides (Prunus amygdalus) have potent antimicrobial activities against the pathogenic strains S. aureus, Pseudomonas aeruginosa, Salmonella typhimurium, Enterococcus faecalis, and E. coli [ 38, 39].
Alginate, fucoidans, and laminaran extracts were tested for their antimicrobial activity against bacteria (E. coli, Staphylococcus, Salmonella, and Listeria). Sodium alginate has been established as a strong antibacterial agent [88]. In addition, alginates can be used as bioactive coatings against Listeria monocytogenes for fish products during refrigerated storage such as cold‐smoked salmon slices and fillets [89].
24.2.5 Anti‐obesity
Obesity has become a worldwide epidemic affecting people on every continent. More than just obesity itself, the major drawback of being obese is the high incidence of the associated health risks, like metabolic syndrome, type‐2 diabetes, and cardiovascular problems. It is generally accepted that a disruption in the balance between energy intake and energy expenditure is an extremely important factor in the incidence of obesity [90]. Its rate is increasing dramatically, and it has been estimated that 58% of the world population will become obese by 2030 [91]. So, one of the interesting approaches of the food industry for the prevention of weight gain is to provide products with high satiating capacities and low‐energy densities. Dietary fibers seem to be ideal candidates for the achievement of this function [74]. Increasing dietary fiber consumption may decrease energy absorption by diluting a diet's energy availability while maintaining other important nutrients [8]. Calame et al. found that blends of gum arabic (EmulGold® and PreVitae®) are able to satiety enhancement and decrease the caloric intake significantly after consumption [90].
Several studies have been performed that suggest a link between seafood consumption and obesity‐related disorders [92,93]. Several actions have been proposed for the mechanism linking seaweed's polysaccharides and obesity disorders. One of them is the action of fiber in the seaweed's biomass, and another is attributed to antioxidants, minerals, and omega 3 fatty acids that interfere in obesity prevention [75].
Generally, the capability of dietary fiber to decrease body weight could be related to the following:
- (A) Fermentation of soluble fiber resulting in the production of GLP‐1 (glucagon‐like peptide) and peptide YY (PYY), which play a satiety role.
- (B) Dietary fiber also may significantly decrease energy intake.
- (C) Dietary fiber may decrease the dietary metabolizable energy, which is the gross energy minus the energy lost in the feces, urine, and combustible gases [8].
In Figure 24.1, the effect of hydrocolloids on the passage rate and enzyme digestion processes of foods during small intestine transfer is shown. Hydrocolloids can play a key role in modulating small intestinal behavior [94]. As seen in Figure 24.1, enzymes and bile are secreted into the first section (duodenum), digestion/absorption and water/salt uptake occur in parallel throughout the rest of the small intestine, and bile is re‐absorbed at the end of the jejunum and in the ileum. The concentration of non‐digested hydrocolloids increases along the small intestine due to the uptake of both water and nutrients [12].
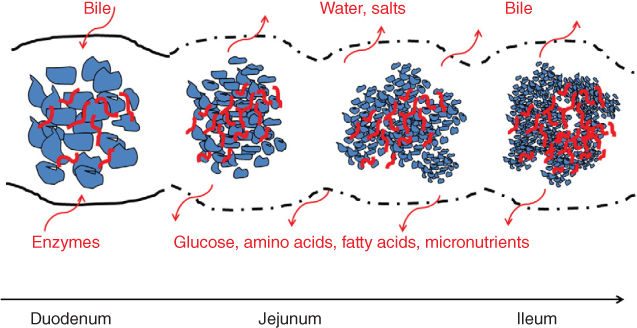
Figure 24.1 Schematic illustration of particulate food remnants and non‐digested hydrocolloids (red symbols) in the small intestine.
Source: Adapted from Gidley [12] with permission from Elsevier.
The presence of hydrocolloids in either viscous soluble form or as an encapsulating matrix is likely to have a major effect on the rate of small intestinal enzymatic digestion of starch, proteins, and lipids. This could be due to one or more of (1) slow transport or restricted access of enzyme to its substrate, (2) direct inhibition of enzyme activity through site‐specific binding, and (3) slow or restricted transport of products from the enzyme to the site of absorption. Recent modeling and experimental data suggest that factor (3) may be important in viscous solutions [95].
24.2.6 Blood Pressure and Cholesterol‐Lowering Effects (Cardiovascular Health)
Despite significant development in its prevention, cardiovascular disease remains the leading cause of death in the United States and most Western countries. Saturated fat, cholesterol intake, and increasing cis‐saturated fat intake are the major parameters in the risk of cardiovascular diseases [20]. High consumption of whole grains is associated with a significant reduction in cardiovascular diseases. Psyllium gum and oat β‐glucan are the most widely used sources of soluble fiber and have been approved for health benefits related to protection from cardiovascular diseases by the FDA. The mechanisms for an association between fiber linkage and cardiovascular disease are unclear, but it is suggested that fiber can reduce blood cholesterol levels by altering cholesterol and bile acid absorption and by its effects on hepatic lipoprotein production and cholesterol synthesis [ 74, 10]. The relationship between water‐soluble fibers and decreasing serum LDL cholesterol concentrations are summarized in Figure 24.2. The viscous water‐soluble fibers form a thick unstirred water layer in the intestinal lumen, thereby decreasing the (re)absorption of cholesterol and bile acids. This leads to an increased fecal output of these two components. As a result, hepatic conversion of cholesterol into bile acids increases, hepatic pools of free cholesterol decrease, and – to reach a new steady state – endogenous cholesterol synthesis will increase. This leads to increased activities of 7‐α‐hydroxylase and HMG‐CoA reductase to compensate for the losses of bile acids and cholesterol from the liver stores. In addition, hepatic LDL cholesterol receptors are upregulated to re‐establish hepatic free cholesterol stores. These processes will ultimately lead to decreased serum LDL cholesterol concentrations [20].
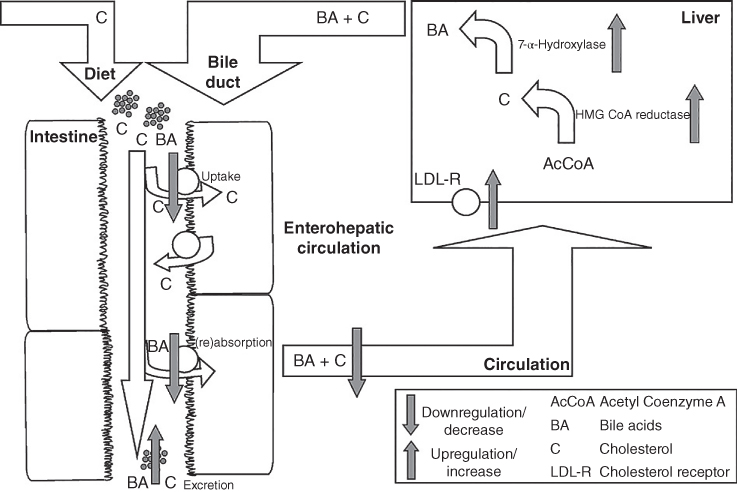
Figure 24.2 Postulated hypocholesterolemic mechanism of water‐soluble fibers.
Source: Adapted from Theuwissen and Mensink [20] with permission from Elsevier.
Panlasigui et al. found that carrageenan‐enriched diets are beneficial for lipid balance in regard to lowering the risk of the cardiovascular disease [96]. In addition, Paxman et al. proposed that alginate has indirect cardiovascular beneficial effects by modulating glucose and cholesterol uptake from the small intestine [97]. Researchers reported that Acacia (sen) SUPERGUM™ is suitable for diabetes mellitus patients and could assist in the control of the systolic blood pressure to reduce the risk of renal impairment [98].
Oat β‐glucan influences the blood cholesterol levels, and LDL cholesterol‐lowering effects depend on viscosity, which is controlled by the molecular weight and amount of oat β‐glucan solubilized in the intestine [99].
24.2.7 Mineral Absorption Effect
Previous animal and human studies have reported increased rates of calcium absorption and associated improvements in bone mineral density with ingestion of prebiotics [100]. In addition to encapsulation within hydrocolloid gels, plant tissues, or other food structures, it is possible that nutrients bind directly to food structures. This phenomenon is now recognized as being important for a range of phytonutrients, particularly phenolic compounds, which appear to be bound sufficiently strongly to plant cell walls that they escape solubilization and uptake in the stomach and small intestine [12].
The dietary fiber fermentation in the large intestine can influence the intestinal absorption of elements. Short‐chain fatty acids are fermentation products that are responsible for lowering the pH of cecal content, which in turn increases mineral solubility, leading to improved mineral absorption. Animal studies have revealed enhanced absorption of calcium, magnesium, and iron with gluco‐oligofructose, fructo‐oligofructose, and inulin in the colon, and five out of eight studies in humans also show a benefit, most importantly in adolescents [101]. In addition, inulin influences the intestinal absorption of calcium and magnesium in rats [102].
24.2.8 Prebiotic Effects
A prebiotic is a food ingredient that is not hydrolyzed by human digestive enzymes in the upper gastrointestinal tract and beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria (Bifidobacteria or Lactobacilli) in the colon that can improve host health [74]. Dietary ingredients such as indigestible carbohydrates modify the gut microflora in favor of probiotics and hence potentially reduce the risk of colorectal diseases [103]. Typical of prebiotics are inulin and oligofructose, both naturally present in a number of fruits and vegetables, and another resistant oligosaccharide such as inline‐type fructans [10]. Although all prebiotic is fiber, not all fiber is prebiotic. Classification of a food ingredient as a prebiotic requires a scientific demonstration that the ingredient [104]:
- Resists gastric acidity, hydrolysis by mammalian enzymes, and absorption in the upper gastrointestinal tract
- Is fermented by the intestinal microflora
- Selectively stimulates the growth and/or activity of intestinal bacteria potentially associated with health and well‐being
The ability to favorably alter the intestinal microflora has been demonstrated by a number of other fiber and plant food sources. A specific role for resistant starch in stimulation of bacteria able to produce butyric acid has been reported [105].
Acacia gum was shown to produce a greater increase in bifidobacteria and lactobacilli than an equal dose of inulin and resulted in fewer gastrointestinal side effects, such as gas and bloating [90]. Polydextrose consumption resulted in a dose‐dependent decrease in Bacteroides, as well as an increase in lactobacilli and bifidobacteria [106]. Wheat dextrin has also been shown to increase lactobacilli and reduce Clostridium perfringens and increase bifidobacteria [107]. Psyllium was found to have a prebiotic effect in healthy women [108]. Some reports exist about the role of prebiotics in cancer prevention [101], benefits in atopic disease [109], and reduction of blood cholesterol and triglycerides [101].
24.2.9 Biomedical Applications
Other applications of hydrocolloids are tissue engineering (including biological signaling, cell adhesion, cell proliferation, cell differentiation, cell responsive degradation, and remodeling), wound dressing/healing, and drug delivery systems; the above three major topics are grouped under biomedical applications. Hydrocolloids have some advantages over synthetic polymers in that they are nontoxic, biodegradable, biocompatible, and less expensive [14].
Alginate, chitin, chitosan, hyaluronic acid, cellulose, chondroitin sulfate, starch, and their derivatives have been studied as biomaterials for tissue engineering applications [110]. Moreover, chitin and chitosan as scaffolds for tissue engineering [111], composites of chitosan with hydroxyapatite, and grafted chitosan with carbon nanotubes have been developed for artificial bone and bone regeneration [112]. Application of other polysaccharides for bone, cartilage, and/or skin tissue engineering applications have also been explored [113].
Hydrocolloids have been widely used to prepare wound healing materials. Collagen sponge dressing was used to treat skin wounds in the rat [114]. Hooper et al. studied the antimicrobial activity of a RESTORE silver alginate dressing with a silver‐free control dressing using a combination of in vitro culture and imaging techniques. The data highlighted the rapid speed of kill and antimicrobial suitability of this RESTORE silver alginate dressing on wound isolates and its overwhelming ability to manage a microbial wound bioburden in the management of infected wounds [115]. Moreover, sodium alginate films containing natural essential oils have been reported that may be applied as disposable wound dressings [116].
Various hydrocolloid‐based drug delivery systems have been developed for specific targeted delivery or controlled release. The release of entrapped drugs or certain molecules can be triggered by the changes of conditions such as pH, ions, temperature, certain molecules, and so on [117]. Several hydrocolloids such as pectin, chitin, chitosan, guar gum, xanthan gum, gellan gum, dextran, and chondroitin have also been developed for drug delivery or controlled drug release [118,119].
24.2.10 Other Benefits
Some hydrocolloids can be applied to prevent the human enamel from erosion (dissolution and softening). For examples, Beyer et al. [120] reported that pectin, alginate, and gum arabic have potential to reduce citric acid erosion in soft drinks. The suggested interactions of such biopolymers with the enamel surface and between biopolymers are graphically shown in Figure 24.3. In the case of enamel erosion, two interactions are proposed: (1) interaction between negatively charged carboxyl groups of polymers and Ca2+‐ions of the enamel surface and (2) interaction between negatively charged carboxyl groups of different polymer molecules in the presence of positively charged Ca2+‐ions by forming a chelate complex [120].
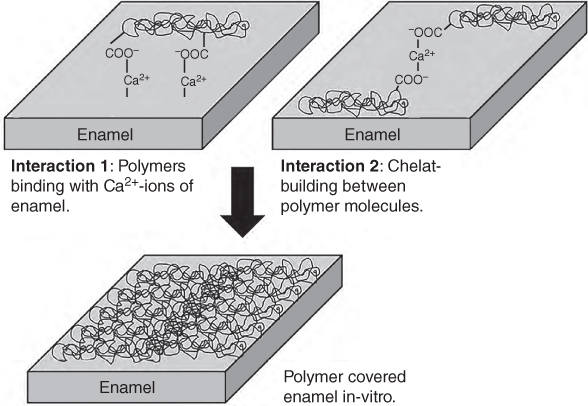
Figure 24.3 Two models of erosion inhibiting polymers in citric acid solutions.
Source: Adapted from Beyer et al. [120] with permission from Elsevier.
In addition, some fiber‐containing foods may need to be avoided in certain allergies. In fact, dietary fiber is fermented in the colon by anaerobic bacteria into short‐chain fatty acids, mainly acetate, butyrate, and propionate. These short‐chain fatty acids bind metabolite‐sensing G‐protein‐coupled receptors. These receptors are expressed on epithelial cells as well as on immune cells [121].
Moreover, seaweeds contain a variety of polysaccharides and fibers that can be used in the prevention and treatment of various diseases in humans [75]. For examples, fucoidans are fucose‐containing sulfated heteropolysaccharides [122]; fucoxanthin is a metabolite [123]; carrageenans [35] are extracted from red seaweeds, where this substance plays a structural function; and laminarin is an active component from the brown seaweeds [124]. They have been documented in various biological activities including antiviral, anti‐inflammatory, anticoagulant, antiangiogenic, immunomodulatory, and anti‐adhesive activity. Moreover, the anti‐constipation effect of some hydrocolloids such as psyllium, gellan gum, karaya gum, and xanthan have been reported [2].
24.3 Conclusions and Recommendations
There has been an extremely alarming growth in chronic diseases such as cardiovascular disease, diabetes mellitus, and cancer, which has been connected to the overconsumption of high‐fat as well as high‐calorie foods. Also, the immoderate consumption of food carbohydrates has been a source of concern, and a joint FAO/WHO report has required people to decrease the consumption of sugars and to correspondingly increase dietary fiber consumption [125]. Hydrocolloids are the most important groups of food components that, in addition to functionality, have beneficial characteristics in the digestive tract and subsequent nutritional and health outcomes. Food industries are changing the production approach in response to people's awareness and increasing demand for healthy food products. Nowadays, hydrocolloids (particularly dietary fiber) as one of the health components have been considered. As mentioned above and on the basis of published data, it can be suggested that usually, bioactive polysaccharides of natural origin such as algae have several biological effects in both in vitro and in vivo experiments. The information reviewed here may be helpful to design product formulations with novel hydrocolloids in view of their potential for therapeutic use or for use as ingredients in functional foods. In fact, the application of novel hydrocolloids as dietary fibers and functional ingredients is vital for the future studies and development of food industries.
References
- 1 Dickinson, E. (2003). Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocolloids 17 (1): 25–39.
- 2 Li, J.‐M. and Nie, S.‐P. (2016). The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocolloids 53: 46–61.
- 3 Devi, P.B., Vijayabharathi, R., Sathyabama, S. et al. (2014). Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. Journal of Food Science and Technology 51 (6): 1021–1040.
- 4 Viebke, C., Al‐Assaf, S., and Phillips, G.O. (2014). Food hydrocolloids and health claims. Bioactive Carbohydrates and Dietary Fibre 4 (2): 101–114.
- 5 Phillips, G.O. and Williams, P.A. (2009). Introduction to food hydrocolloids. In: Handbook of Hydrocolloids, 1–22. New York: CRC Press.
- 6 Hipsley, E.H. (1953). Dietary “fibre” and pregnancy toxemia. British Medical Journal 2 (4833): 420.
- 7 Burkitt, D. and Trowell, H. (1977). Dietary fibre and western diseases. Irish Medical Journal 70 (9): 272.
- 8 Lattimer, J.M. and Haub, M.D. (2010). Effects of dietary fiber and its components on metabolic health. Nutrients 2 (12): 1266–1289.
- 9 Burkitt, D. and Trowell, H. (1979). Nutritional intake, adiposity, and diabetes. British medical journal 1 (6170): 1083.
- 10 Buttriss, J. and Stokes, C. (2008). Dietary fibre and health: an overview. Nutrition Bulletin 33 (3): 186–200.
- 11 Kendall, C.W., Esfahani, A., and Jenkins, D.J. (2010). The link between dietary fibre and human health. Food Hydrocolloids 24 (1): 42–48.
- 12 Gidley, M.J. (2013). Hydrocolloids in the digestive tract and related health implications. Current Opinion in Colloid & Interface Science 18 (4): 371–378.
- 13 Varela, P. and Fiszman, S. (2013). Exploring consumers' knowledge and perceptions of hydrocolloids used as food additives and ingredients. Food Hydrocolloids 30 (1): 477–484.
- 14 Liu, J., Willför, S., and Xu, C. (2015). A review of bioactive plant polysaccharides: biological activities, functionalization, and biomedical applications. Bioactive Carbohydrates and Dietary Fibre 5 (1): 31–61.
- 15 Fuller, S., Beck, E., Salman, H. et al. (2016). New horizons for the study of dietary fiber and health: a review. Plant Foods for Human Nutrition 71 (1): 1–12.
- 16 Liu, R.H. (2003). Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. The American Journal of Clinical Nutrition 78 (3): 517S–520S.
- 17 Jenkins, D.A., Newton, C., Leeds, A. et al. (1975). Effect of pectin, guar gum, and wheat fibre on serum‐cholesterol. The Lancet 305 (7916): 1116–1117.
- 18 Jenkins, D., Leeds, A.R., Slavin, B. et al. (1979). Dietary fiber and blood lipids: reduction of serum cholesterol in type II hyperlipidemia by guar gum. The American Journal of Clinical Nutrition 32 (1): 16–18.
- 19 Cummings, J., Branch, W., Jenkins, D. et al. (1978). Colonic response to dietary fibre from carrot, cabbage, apple, bran, and guar gum. The Lancet 311 (8054): 5–9.
- 20 Theuwissen, E. and Mensink, R.P. (2008). Water‐soluble dietary fibers and cardiovascular disease. Physiology & Behavior 94 (2): 285–292.
- 21 Moreaux, S.J.J., Nichols, J.L., Bowman, J.G. et al. (2011). Psyllium lowers blood glucose and insulin concentrations in horses. Journal of Equine Veterinary Science 31 (4): 160–165.
- 22 Jensen, M.G., Knudsen, J.C., Viereck, N. et al. (2012). Functionality of alginate based supplements for application in human appetite regulation. Food Chemistry 132 (2): 823–829.
- 23 Maki, K.C., Carson, M.L., Miller, M.P. et al. (2007). High‐viscosity hydroxypropylmethylcellulose blunts postprandial glucose and insulin responses. Diabetes Care 30 (5): 1039–1043.
- 24 Dong, J., Cai, F., Shen, R. et al. (2011). Hypoglycaemic effects and inhibitory effect on intestinal disaccharidases of oat beta‐glucan in streptozotocin‐induced diabetic mice. Food Chemistry 129 (3): 1066–1071.
- 25 Pentikäinen, S., Karhunen, L., Flander, L. et al. (2014). Enrichment of biscuits and juice with oat β‐glucan enhances postprandial satiety. Appetite 75: 150–156.
- 26 Glover, D.A., Ushida, K., Phillips, A.O. et al. (2009). Acacia (sen) SUPERGUM™ (Gum Arabic): an evaluation of potential health benefits in human subjects. Food Hydrocolloids 23 (8): 2410–2415.
- 27 Musa, H., Fedail, J., Ahmed, A. et al. (2016). Effect of gum arabic on oxidative stress markers in the liver of high fat diet induced obesity in mice. Gums and Stabilisers for the Food Industry 18: 256–263: Royal Society of Chemistry.
- 28 Delorme, C.B. and Wojcik, J. (1982). Interaction of dietary protein with cellulose in the adaptation of caloric dilution by weaning rats. The Journal of Nutrition 112 (1): 21–28.
- 29 Takahashi, T., Karita, S., Ogawa, N. et al. (2005). Crystalline cellulose reduces plasma glucose concentrations and stimulates water absorption by increasing the digesta viscosity in rats. The Journal of Nutrition 135 (10): 2405–2410.
- 30 Maki, K.C., Davidson, M.H., Torri, S. et al. (2000). High‐molecular‐weight hydroxypropylmethylcellulose taken with or between meals is hypocholesterolemic in adult men. The Journal of Nutrition 130 (7): 1705–1710.
- 31 Reader, D.M., O, Connell, B.S., Johnson, M.L. et al. (2002). Glycemic and insulinemic response of subjects with type 2 diabetes after consumption of three energy bars. Journal of the American Dietetic Association 102 (8): 1139–1142.
- 32 Vitaglione, P., Lumaga, R.B., Montagnese, C. et al. (2010). Satiating effect of a barley beta‐glucan–enriched snack. Journal of the American College of nutrition 29 (2): 113–121.
- 33 Gibson, G.R., Beatty, E.R., Wang, X. et al. (1995). Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108 (4): 975–982.
- 34 Lu, Z.X., Walker, K.Z., Muir, J.G. et al. (2000). Arabinoxylan fiber, a byproduct of wheat flour processing, reduces the postprandial glucose response in normoglycemic subjects. The American Journal of Clinical Nutrition 71 (5): 1123–1128.
- 35 Zhou, G., Xin, H., Sheng, W. et al. (2005). In vivo growth‐inhibition of S180 tumor by mixture of 5‐Fu and low molecular λ‐carrageenan from Chondrus ocellatus. Pharmacological Research 51 (2): 153–157.
- 36 Ching, S.H., Bansal, N., and Bhandari, B. (2017). Alginate gel particles–a review of production techniques and physical properties. Critical Reviews in Food Science and Nutrition 57 (6): 1133–1152.
- 37 Wang, H.‐B., Wu, S.‐J., and Liu, D. (2014). Preparation of polysaccharides from cyanobacteria Nostoc commune and their antioxidant activities. Carbohydrate Polymers 99: 553–555.
- 38 Bouaziz, F., Helbert, C.B., Romdhane, M.B. et al. (2015). Structural data and biological properties of almond gum oligosaccharide: application to beef meat preservation. International Journal of Biological Macromolecules 72: 472–479.
- 39 Bouaziz, F., Koubaa, M., Helbert, C.B. et al. (2015). Purification, structural data and biological properties of polysaccharide from Prunus amygdalus gum. International Journal of Food Science & Technology 50 (3): 578–584.
- 40 Qi, H., Huang, L., Liu, X. et al. (2012). Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohydrate Polymers 87 (2): 1637–1640.
- 41 Abdel‐Fattah, A.M., Gamal‐Eldeen, A.M., Helmy, W.A. et al. (2012). Antitumor and antioxidant activities of levan and its derivative from the isolate Bacillus subtilis NRC1aza. Carbohydrate Polymers 89 (2): 314–322.
- 42 Belghith, K.S., Dahech, I., Hamden, K. et al. (2012). Hypolipidemic effect of diet supplementation with bacterial levan in cholesterol‐fed rats. International Journal of Biological Macromolecules 50 (4): 1070–1074.
- 43 Dahech, I., Belghith, K.S., Belghith, H. et al. (2012). Partial purification of a Bacillus licheniformis levansucrase producing levan with antitumor activity. International Journal of Biological Macromolecules 51 (3): 329–335.
- 44 Hu, X.‐Z., Xing, X.‐H., Zhang, Z.‐M. et al. (2011). Antioxidant effects of Artemis sphaerocephala Krasch gum, on streptozotocin‐induced type 2 diabetic rats. Food Hydrocolloids 25 (2): 207–213.
- 45 Song, H., Zhang, Q., Zhang, Z. et al. (2010). In vitro antioxidant activity of polysaccharides extracted from Bryopsis plumosa. Carbohydrate Polymers 80 (4): 1057–1061.
- 46 Jin, W., Liu, G., Zhong, W. et al. (2017). Polysaccharides from Sargassum thunbergii: monthly variations and anti‐complement and anti‐tumor activities. International Journal of Biological Macromolecules 105 (2): 1526–1531.
- 47 Li, X., Zhang, B., Li, J. et al. (2017). Purification, characterization, and complement fixation activity of acidic polysaccharides from Tuber sinoaestivum. LWT‐Food Science and Technology 85: 82–88.
- 48 Meng, Q., Li, Y., Xiao, T. et al. (2017). Antioxidant and antibacterial activities of polysaccharides isolated and purified from Diaphragma juglandis fructus. International Journal of Biological Macromolecules 105 (1): 431–437.
- 49 Wang, L., Liu, H.‐M., Xie, A.‐J. et al. (2018). Chinese quince (Chaenomeles sinensis) seed gum: structural characterization. Food Hydrocolloids 75: 237–245.
- 50 Mao, F., Xiao, B., Jiang, Z. et al. (2011). Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Medical Oncology 28 (1): 121–126.
- 51 Liang, B., Jin, M., and Liu, H. (2011). Water‐soluble polysaccharide from dried Lycium barbarum fruits: isolation, structural features and antioxidant activity. Carbohydrate Polymers 83 (4): 1947–1951.
- 52 Zhu, J., Liu, W., Yu, J. et al. (2013). Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydrate Polymers 98 (1): 8–16.
- 53 Jin, M., Huang, Q., Zhao, K. et al. (2013). Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. International Journal of Biological Macromolecules 54: 16–23.
- 54 Chang, S., Hsu, B., and Chen, B. (2010). Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. International Journal of Biological Macromolecules 47 (4): 445–453.
- 55 Yue, Y., Wu, S., Zhang, H. et al. (2014). Characterization and hepatoprotective effect of polysaccharides from Ziziphus jujuba Mill. Var. spinosa (Bunge) Hu ex HF Chou sarcocarp. Food and Chemical Toxicology 74: 76–84.
- 56 Ji, X., Liu, F., Peng, Q. et al. (2018). Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from Ziziphus Jujuba cv. Muzao. Food Chemistry 245: 1124–1130.
- 57 Zhang, L., Liu, X., Wang, Y. et al. (2017). In vitro antioxidative and immunological activities of polysaccharides from Zizyphus Jujuba cv. Muzao. International Journal of Biological Macromolecules 95: 1119–1125.
- 58 Zhao, H., Wang, Q., Sun, Y. et al. (2014). Purification, characterization and immunomodulatory effects of Plantago depressa polysaccharides. Carbohydrate Polymers 112: 63–72.
- 59 Gonçalves, S., Grevenstuk, T., Martins, N. et al. (2015). Antioxidant activity and verbascoside content in extracts from two uninvestigated endemic Plantago spp. Industrial Crops and Products 65: 198–202.
- 60 Zubair, M., Nybom, H., Lindholm, C. et al. (2016). Promotion of wound healing by Plantago major L. leaf extracts–ex‐vivo experiments confirm experiences from traditional medicine. Natural Product Research 30 (5): 622–624.
- 61 Yuan, Q., Xie, Y., Wang, W. et al. (2015). Extraction optimization, characterization and antioxidant activity in vitro of polysaccharides from mulberry (Morus alba L.) leaves. Carbohydrate Polymers 128: 52–62.
- 62 Choi, J.W., Synytsya, A., Capek, P. et al. (2016). Structural analysis and anti‐obesity effect of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Carbohydrate Polymers 146: 187–196.
- 63 Xu, L., Zhang, W., Zeng, L. et al. (2017). Rehmannia glutinosa polysaccharide induced an anti‐cancer effect by activating natural killer cells. International Journal of Biological Macromolecules 105 (1): 680–685.
- 64 Fakhfakh, N., Abdelhedi, O., Jdir, H. et al. (2017). Isolation of polysaccharides from Malva aegyptiaca and evaluation of their antioxidant and antibacterial properties. International Journal of Biological Macromolecules 105 (2): 1519–1525.
- 65 Behrouzian, F., Razavi, S.M., and Phillips, G.O. (2014). Cress seed (Lepidium sativum) mucilage, an overview. Bioactive Carbohydrates and Dietary Fibre 3 (1): 17–28.
- 66 Cunha, A.P., Ribeiro, A.C., Ricardo, N.M. et al. (2017). Polysaccharides from Caesalpinia ferrea seeds–chemical characterization and anti‐diabetic effects in Wistar rats. Food Hydrocolloids 65: 68–76.
- 67 Dahech, I., Belghith, K.S., Hamden, K. et al. (2011). Antidiabetic activity of Levan polysaccharide in alloxan‐induced diabetic rats. International Journal of Biological Macromolecules 49 (4): 742–746.
- 68 Wang, P.‐C., Zhao, S., Yang, B.‐Y. et al. (2016). Anti‐diabetic polysaccharides from natural sources: a review. Carbohydrate Polymers 148: 86–97.
- 69 Flammang, A.M., Kendall, D.M., Baumgartner, C.J. et al. (2006). Effect of a viscous fiber bar on postprandial glycemia in subjects with type 2 diabetes. Journal of the American College of Nutrition 25 (5): 409–414.
- 70 Feinglos, M.N., Gibb, R.D., Ramsey, D.L. et al. (2013). Psyllium improves glycemic control in patients with type‐2 diabetes mellitus. Bioactive Carbohydrates and Dietary Fibre 1 (2): 156–161.
- 71 Jia, X., Yang, J., Wang, Z. et al. (2014). Polysaccharides from Laminaria japonica show hypoglycemic and hypolipidemic activities in mice with experimentally induced diabetes. Experimental Biology and Medicine 239 (12): 1663–1670.
- 72 Li, X., Yu, Z., Long, S. et al. (2012). Hypoglycemic effect of Laminaria japonica polysaccharide in a type 2 diabetes mellitus mouse model. ISRN Endocrinology 2012: 507462.
- 73 Huang, M., Wang, F., Zhou, X. et al. (2015). Hypoglycemic and hypolipidemic properties of polysaccharides from Enterobacter cloacae Z0206 in KKAy mice. Carbohydrate Polymers 117: 91–98.
- 74 Redgwell, R.J. and Fischer, M. (2005). Dietary fiber as a versatile food component: an industrial perspective. Molecular Nutrition & Food Research 49 (6): 521–535.
- 75 Déléris, P., Nazih, H., and Bard, J. (2016). Seaweeds in human health. In: Seaweed in Health and Disease Prevention, 319–367. Amsterdam: Academic Press.
- 76 Xing, R., Liu, S., Guo, Z. et al. (2005). Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorganic & Medicinal Chemistry 13 (5): 1573–1577.
- 77 Berteau, O. and Mulloy, B. (2003). Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 13 (6): 29R–40R.
- 78 Zhang, Q., Yu, P., Li, Z. et al. (2003). Antioxidant activities of sulfated polysaccharide fractions from Porphyra haitanesis. Journal of Applied Phycology 15 (4): 305–310.
- 79 Campo, V.L., Kawano, D.F., da Silva, D.B. et al. (2009). Carrageenans: biological properties, chemical modifications and structural analysis–a review. Carbohydrate Polymers 77 (2): 167–180.
- 80 Barahona, T., Chandía, N.P., Encinas, M.V. et al. (2011). Antioxidant capacity of sulfated polysaccharides from seaweeds. A kinetic approach. Food Hydrocolloids 25 (3): 529–535.
- 81 Yao, X.‐C., Cao, Y., and Wu, S.‐J. (2013). Antioxidant activity and antibacterial activity of peach gum derived oligosaccharides. International Journal of Biological Macromolecules 62: 1–3.
- 82 Wu, W.‐T., Yang, L.‐C., and Chen, H.‐L. (2014). Effects of konjac glucomannan, inulin and cellulose on acute colonic responses to genotoxic azoxymethane. Food Chemistry 155: 304–310.
- 83 Yamassaki, F., Lenzi, R., Campestrini, L. et al. (2015). Effect of the native polysaccharide of cashew‐nut tree gum exudate on murine peritoneal macrophage modulatory activities. Carbohydrate Polymers 125: 241–248.
- 84 Kasimu, R., Chen, C., Xie, X. et al. (2017). Water‐soluble polysaccharide from Erythronium sibiricum bulb: structural characterisation and immunomodulating activity. International Journal of Biological Macromolecules 105 (1): 452–462.
- 85 Ji, X., Peng, Q., Yuan, Y. et al. (2017). Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): a review. Food Chemistry 227: 349–357.
- 86 Kubo, I., Muroi, H., and Kubo, A. (1995). Structural functions of antimicrobial long‐chain alcohols and phenols. Bioorganic & Medicinal Chemistry 3 (7): 873–880.
- 87 Campos, D.A., Ribeiro, A.C., Costa, E.M. et al. (2012). Study of antimicrobial activity and atomic force microscopy imaging of the action mechanism of cashew tree gum. Carbohydrate Polymers 90 (1): 270–274.
- 88 Holdt, S.L. and Kraan, S. (2011). Bioactive compounds in seaweed: functional food applications and legislation. Journal of Applied Phycology 23 (3): 543–597.
- 89 Neetoo, H., Ye, M., and Chen, H. (2010). Bioactive alginate coatings to control Listeria monocytogenes on cold‐smoked salmon slices and fillets. International Journal of Food Microbiology 136 (3): 326–331.
- 90 Calame, W., Thomassen, F., Hull, S. et al. (2011). Evaluation of satiety enhancement, including compensation, by blends of gum Arabic. A methodological approach. Appetite 57 (2): 358–364.
- 91 Kelly, T., Yang, W., Chen, C.‐S. et al. (2008). Global burden of obesity in 2005 and projections to 2030. International Journal of Obesity 32 (9): 1431–1438.
- 92 Ramirez‐Higuera, A., Quevedo‐Corona, L., Paniagua‐Castro, N. et al. (2014). Antioxidant enzymes gene expression and antihypertensive effects of seaweeds Ulva linza and Lessonia trabeculata in rats fed a high‐fat and high‐sucrose diet. Journal of Applied Phycology 26 (1): 597–605.
- 93 Kumar, S.A., Magnusson, M., Ward, L.C. et al. (2015). Seaweed supplements normalise metabolic, cardiovascular and liver responses in high‐carbohydrate, high‐fat fed rats. Marine Drugs 13 (2): 788–805.
- 94 Ryan, A.T., Feinle‐Bisset, C., Kallas, A. et al. (2012). Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. The American Journal of Clinical Nutrition 96 (3): 474–482.
- 95 Tharakan, A., Norton, I., Fryer, P. et al. (2010). Mass transfer and nutrient absorption in a simulated model of small intestine. Journal of Food Science 75 (6): E339–E346.
- 96 Panlasigui, L.N., Baello, O.Q., Dimatangal, J.M. et al. (2003). Blood cholesterol and lipid‐lowering effects of carrageenan on human volunteers. Asia Pacific Journal of Clinical Nutrition 12 (2): 209–214.
- 97 Paxman, J., Richardson, J., Dettmar, P. et al. (2008). Daily ingestion of alginate reduces energy intake in free‐living subjects. Appetite 51 (3): 713–719.
- 98 Phillips, A.O. and Phillips, G.O. (2011). Biofunctional behaviour and health benefits of a specific gum Arabic. Food Hydrocolloids 25 (2): 165–169.
- 99 Wolever, T.M., Tosh, S.M., Gibbs, A.L. et al. (2010). Physicochemical properties of oat β‐glucan influence its ability to reduce serum LDL cholesterol in humans: a randomized clinical trial. The American Journal of Clinical Nutrition 92 (4): 723–732.
- 100 Alexiou, H. and Franck, A. (2008). Prebiotic inulin‐type fructans: nutritional benefits beyond dietary fibre source. Nutrition Bulletin 33 (3): 227–233.
- 101 Elia, M. and Cummings, J. (2007). Physiological aspects of energy metabolism and gastrointestinal effects of carbohydrates. European Journal of Clinical Nutrition 61: S40–S74.
- 102 Coudray, C., Rambeau, M., Feillet‐Coudray, C. et al. (2005). Dietary inulin intake and age can significantly affect intestinal absorption of calcium and magnesium in rats: a stable isotope approach. Nutrition Journal 4 (1): 29.
- 103 Adebola, O., Corcoran, O., and Morgan, W.A. (2013). Protective effects of prebiotics inulin and lactulose from cytotoxicity and genotoxicity in human colon adenocarcinoma cells. Food Research International 52 (1): 269–274.
- 104 Slavin, J. (2013). Fiber and prebiotics: mechanisms and health benefits. Nutrients 5 (4): 1417–1435.
- 105 Champ, M., Langkilde, A.‐M., Brouns, F. et al. (2003). Advances in dietary fibre characterisation. 2. Consumption, chemistry, physiology and measurement of resistant starch; implications for health and food labelling. Nutrition Research Reviews 16 (2): 143–161.
- 106 Hengst, C., Ptok, S., Roessler, A. et al. (2009). Effects of polydextrose supplementation on different faecal parameters in healthy volunteers. International Journal of Food Sciences and Nutrition 60 (sup 5): 96–105.
- 107 Lefranc‐Millot, C., Guérin‐Deremaux, L., Wils, D. et al. (2012). Impact of a resistant dextrin on intestinal ecology: how altering the digestive ecosystem with NUTRIOSE®, a soluble fibre with prebiotic properties, may be beneficial for health. Journal of International Medical Research 40 (1): 211–224.
- 108 Elli, M., Cattivelli, D., Soldi, S. et al. (2008). Evaluation of prebiotic potential of refined psyllium (Plantago ovata) fiber in healthy women. Journal of Clinical Gastroenterology 42: S174–S176.
- 109 Fanaro, S., Boehm, G., Garssen, J. et al. (2005). Galacto‐oligosaccharides and long‐chain fructo‐oligosaccharides as prebiotics in infant formulas: a review. Acta Paediatrica 94 (s449): 22–26.
- 110 Oliveira, J.T. and Reis, R. (2011). Polysaccharide‐based materials for cartilage tissue engineering applications. Journal of Tissue Engineering and Regenerative Medicine 5 (6): 421–436.
- 111 Wan, A.C. and Tai, B.C. (2013). CHITIN—a promising biomaterial for tissue engineering and stem cell technologies. Biotechnology Advances 31 (8): 1776–1785.
- 112 Venkatesan, J. and Kim, S.‐K. (2010). Chitosan composites for bone tissue engineering—an overview. Marine Drugs 8 (8): 2252–2266.
- 113 Rinaudo, M. (2008). Main properties and current applications of some polysaccharides as biomaterials. Polymer International 57 (3): 397–430.
- 114 Chvapil, M., Chvapil, T.A., and Owen, J.A. (1986). Reaction of various skin wounds in the rat to collagen sponge dressing. Journal of Surgical Research 41 (4): 410–418.
- 115 Hooper, S.J., Percival, S.L., Hill, K.E. et al. (2012). The visualisation and speed of kill of wound isolates on a silver alginate dressing. International Wound Journal 9 (6): 633–642.
- 116 Liakos, I., Rizzello, L., Scurr, D.J. et al. (2014). All‐natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. International Journal of Pharmaceutics 463 (2): 137–145.
- 117 Alvarez‐Lorenzo, C., Blanco‐Fernandez, B., Puga, A.M. et al. (2013). Crosslinked ionic polysaccharides for stimuli‐sensitive drug delivery. Advanced Drug Delivery Reviews 65 (9): 1148–1171.
- 118 Liu, Z., Jiao, Y., Wang, Y. et al. (2008). Polysaccharides‐based nanoparticles as drug delivery systems. Advanced Drug Delivery Reviews 60 (15): 1650–1662.
- 119 Pachuau, L. and Mazumder, B. (2013). Colonic drug delivery systems based on natural polysaccharides and their evaluation. Mini Reviews in Medicinal Chemistry 13 (13): 1982–1991.
- 120 Beyer, M., Reichert, J., Heurich, E. et al. (2010). Pectin, alginate and gum Arabic polymers reduce citric acid erosion effects on human enamel. Dental Materials 26 (9): 831–839.
- 121 Tan, J., McKenzie, C., Vuillermin, P.J. et al. (2016). Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Reports 15 (12): 2809–2824.
- 122 Cumashi, A., Ushakova, N.A., Preobrazhenskaya, M.E. et al. (2007). A comparative study of the anti‐inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 17 (5): 541–552.
- 123 Beppu, F., Niwano, Y., Tsukui, T. et al. (2009). Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. The Journal of Toxicological Sciences 34 (5): 501–510.
- 124 Menshova, R.V., Ermakova, S.P., Anastyuk, S.D. et al. (2014). Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis. Carbohydrate Polymers 99: 101–109.
- 125 Brennan, C.S. and Tudorica, C.M. (2008). Evaluation of potential mechanisms by which dietary fibre additions reduce the predicted glycaemic index of fresh pasta. International Journal of Food Science & Technology 43 (12): 2151–2162.
