Making And Remaking Alzheimer Disease
I … now see my reluctance to apply the term Alzheimer’s to my father as a way of protecting the specificity of Earl Franzen from the generality of a named condition. Conditions have symptoms; symptoms point to the organic basis of everything we are. They point to the brain as meat. And, where I ought to recognize that, yes, the brain is meat, I seem instead to maintain a blind spot across which I then interpolate stories that emphasize the more soul-like aspects of the self.
—Jonathan Franzen, “My Father’s Brain”1
In January 2011 I attended a lecture delivered in an engaging manner in a Montréal hospital about pathbreaking basic science research in connection with amyloid plaques and neurofibrillary tangles, long thought to be diagnostic of Alzheimer disease. The lecture was given by an invited guest from Harvard University, Bradley Hyman, and took place in a room packed with young molecular biologists from diverse countries of origin, interspersed here and there with a few clinicians. In striking contrast to the majority of basic science lecturers who launch immediately into their specialized subject making use of the obligatory PowerPoint presentation, Dr. Hyman started out by making two general statements. First, he noted the estimated number of people living with AD worldwide at the present time, and the projected number of more than 115 million by 2050, to which he added the wry comment that his audience had clearly chosen to be in the right field. Second, he referred briefly to the history of AD, noting that it was the demonstration of plaques and tangles in the brains of demented people that had allowed the condition to be definitively identified by Alois Alzheimer over 100 years ago. Hyman then reminded his audience that here they were, assembled in 2011, still struggling to understand the reasons for the formation and significance of plaques and tangles. He went on to state that his team is now able to observe the production of a single plaque or tangle in a mouse’s brain and track its growth over the ensuing hours. The audience viewed this remarkable feat on video—an innovation that may, perhaps, move us one step closer to solving part of the molecular aspects of this stubborn Alzheimer puzzle.
During his talk Bradley Hyman had not mentioned that the significance of amyloid plaques is currently being debated in the AD world, due largely to repeated failures of clinical trials designed to target the removal of amyloid plaques in the brain. Nor did he note that a second reason causing dispute is irrefutable evidence showing that among individuals whose brains exhibit plaques (whether demonstrated in vivo by means of neuroimaging, or at autopsy), a good number do not exhibit the behavioral changes associated with dementia. The difficulties posed in attempting to rigorously delineate normal from pathological aging are made starkly evident by these findings. When prodded a little by a clinician during the question period, Dr. Hyman agreed that certain people are apparently able to “maintain homeostasis” in their brains, even in the presence of amyloid plaques, acknowledging, somewhat belatedly, an entanglement of “normal” aging and dementia.
This chapter opens with a brief discussion of the “discovery” of Alzheimer disease early in the 20th century, at a time when the significance of neuropathology as causal of mental illness began to be firmly established. The history of the disease is then tracked throughout the 20th century, showing how the question of the relationship of “normal” aging to dementia has never been satisfactorily resolved. Arguments revolve around interpretations of the significance of specific neuropathological changes associated with both aging and dementia and are of immediate relevance in determining what directions to take with respect to drug development designed to limit the ravages of AD. At a more fundamental level, such debates raise questions about the ontology of AD and what exactly will count as its defining pathological signs. An obverse question then follows: what “protects” those many individuals who never become demented during life but who harbor what is believed to be definitive Alzheimer pathology in their brains? This second question, although cursorily posed quite often these days, has never been systematically examined.
Is Aging a Disease?
The evolution of senile dementia has traditionally been considered to represent an aspect of senescence which, in turn, is the normal final phase of human performance that occurs as a prelude to death. Yet there has always been vast disagreement regarding the meaning of this statement.
—Richard M. Torack, The Pathologic Physiology of Dementia2
The idea that many people become demented in old age has a very long history well documented in the major literate traditions. In As You Like It, Shakespeare’s character Jacques, a professional melancholic, tells his audience how the elderly are perceived as they pass through the last of the seven stages of human life:
Last scene of all
That ends this strange eventful history [of humankind]
Is second childishness and mere oblivion,
Sans teeth, sans eyes, sans taste, sans everything.
(Shakespeare, As You Like It, Act 2, Scene 7)
Given that the play is a comedy, it is perhaps tempting to interpret the gruesome characterization of old age as recounted by Jacques as ironic.3 But, during Shakespeare’s time, the dominant idea was one of decline and decay, not very different from our own, and irony was not in play, although it was also recognized that by no means everyone becomes demented, even when very old.4 However, until well into the 20th century, in contrast to the present day, what was characterized as senile dementia was rarely regarded as pathology, but simply as part of aging itself.
No doubt most people coped as best they could by keeping their affected relatives at home. If families simply could not or did not want to deal with dependent elderly, then some were placed in poor houses and others taken to asylums. Alternatively, they were left to wander the streets to beg and scavenge—a situation that remains all too evident in many parts of the world today. The epigraph above by the neurologist Richard Torack suggests that it was generally assumed that everyone would eventually become demented—senile dementia, one’s second childhood, was a “natural” end to life, although this might take place at a great age, by which time most people would have succumbed to some other condition.
Somatikers and Psychikers
Although senile dementia among older people was widely regarded as normal aging by the medical profession in the 18th and 19th centuries, other types of dementia that affected people of all ages were subsumed under the overarching concept of “mental derangement.” The majority of the patients housed in asylums suffered from an extreme form of dementia associated with tertiary syphilis, and epileptics too were commonly shut away in these institutions. When care was no longer provided by families, cases of senile dementia were also usually housed in these custodial asylums. Typically, these and other patients were kept in prison-like conditions, and more often than not they were constantly shackled. Alois Alzheimer worked as a psychiatrist in such an establishment at the end of the 19th century in Frankfurt, but he then moved in 1903 to commence work in Heidelberg, and then later in Munich and Bresleau, in newly founded university clinics designed for the purposes of teaching and research, where patients stayed a relatively short time before being removed to asylums once it was clear that nothing further could be done for them. A notable feature of Alzheimer’s career, then, is that it “traversed both of these psychiatric cultures.”5 Cases of senile dementia were most likely to be housed in the custodial asylums.
The numerous accounts about the “discovery” of Alzheimer disease rarely relate how Alzheimer was deeply involved in clinical care throughout his career, that he spent a great deal of time trying to converse with patients and took exceptionally comprehensive medical histories. He worked at a time when humanistic reforms were beginning to be implemented and was directly responsible for implementing nonrestraint practices together with other reforms in the large Frankfurt hospital where he was first employed, including regular bathing of patients not simply for reasons of hygiene, but also to calm and soothe them.
However, Alzheimer and his close colleague Bielschowsky declared that their stated mission was to move psychiatry forward with “the assistance of the microscope,”6 and Alzheimer had always shown a predilection from the time he did research as a medical student for drawing remarkable pathohistological diagrams.7 But, at heart, he was clearly also a caring, dedicated clinician. As his biographers state, “Alzheimer was an obsessed doctor and scientist.”8 His purpose was not to “reduce” the condition of dementia entirely to neuropathology, but to establish irrefutable links between clinical changes and pathology seen at autopsy.
Above all, Alzheimer wanted the medical world to recognize that mental illnesses have an undeniable material component. There was an obvious political reason for taking such a position because it could then be established that dementia-like conditions are not part of the spiritual/theological domain, but undeniably biological in origin and therefore not attributable with moral implications. A related reason, linked to the first, is that Alzheimer was an early adherent of the idea of “cortical localization,” and hence was classed by his contemporaries as a “somaticizer.” On the other hand, given the attention that he paid to his patients, and his predilection for improving their care, it is not unreasonable to surmise that Alzheimer was also seeking to reduce the stigma so often associated with the mentally ill and the inhumane treatment that was their lot. Common thinking of the day drew on the concept of “degeneracy” in which it was assumed that certain people, notably the poor, were predisposed to hereditary degenerative disorders, including mental derangement, exacerbated by alcoholism, sexual excess, venereal disease, and other “immoral” behavior, and it seems that Alzheimer questioned this demeaning thesis.
In order to better understand the “discovery” of Alzheimer disease, it is necessary to briefly touch on theories about causation in the early days of psychiatry. A tension has been evident throughout the history of Western medicine between accounts that favor somatic origins of mental illness and those that privilege social and individual behavioral causes including, on occasion, narratives about retribution by otherworldly entities. By the turn of the 19th century, when the earliest signs of the reform movement in the care of the mentally ill began to take shape, a “moral therapy” movement emerged. One of its pioneers was Philippe Pinel, who directed attention for the first time to the patient’s “story” in an effort to discern what might have taken place in the life of the patient to precipitate her or his illness. Pinel asserted that because no organic lesions could be seen in the brain, the belief that mental illness has material origins must be a false premise. His ideas were extraordinarily influential among physicians in both Europe and North America, but it was not long before the somaticists “struck back.”9 Phrenologists of the late 18th century postulated the idea of localization of “faculties” in different parts of the cortex of the human brain, and over the ensuing decades, debate moved toward the somatic end of the spectrum. This move was bolstered by experimentation carried out by Broca, Ferrier, and others. In 19th-century Germany tensions between so-called localists and holists were most apparent, culminating in an attempt at a synthesis by Wilhelm Griesinger, whose arguments were well known to Alzheimer. Griesinger’s position, described as “multifactorial,” postulated predisposing factors including “psychical causes” that bring about a state of “intense irritation of the brain.”10
By the late 19th century, the subspecialties of “organic neuropsychiatry” and “brain pathology” took form as new, hardened articulations for somaticism. As Michel Foucault was to note when commenting on this shift, “Disease is an autopsy in the darkness of the body,”11 and findings from numerous autopsies conducted by Jean-Martin Charcot, known today as the “father” of modern neurology, and his colleagues at the Paris asylums of Saltpêtrière and Bicêtre anchored these new specialties. These institutions housed 3,000 to 4,000 patients and hence were a rich source of material for brain dissections. In 1899, Charles Hughes, the editor of The Alienist and Neurologist and a follower of organic neuropsychiatry, wrote,
There is no such thing as insanity without disease … involving the brain. … There is no expression of mental derangement without a substratum of cortex disease, either in the neuron, in the enveloping membranes of the brain, in the nourishing blood supply, in the behavior of the vaso-motor system mechanism.12
But when dealing with elderly patients, a pathologically oriented position was more difficult to sustain because of the continuing widespread acceptance of an inevitable, “natural” decline among the aged, bringing about the onset of a second childhood, as Jacques in As You Like It reminds us.
Senility in Old Age
From classical times in Europe until early in the 19th century, influenced by ideas that originally emanated from the Middle East, the life cycle was conceptualized as rather clearly defined epochs based on age. Certain illnesses and conditions were associated with specific epochs in the life cycle thought to bring about instability in the nervous system, notably at pubescence and the climacteric (believed to be common to both men and women). The term “dementia” was assigned to people of any age and was used to indicate “any state of psychological dilapidation associated with chronic brain disease.”13 However, among the elderly it was assumed that an inevitable depletion of the vitality necessary for life was the primary cause of dementia, and from the beginning of the 19th century “senile dementia” was used almost exclusively when referring to the elderly—a condition of normal aging, in which, as some medical practitioners noted, memory loss rather than the florid symptoms associated with “derangement” was usually the primary symptom. Clearly, delineating normal from pathological has never been easy; “they implode,” as Cohen puts it.14
It was in this milieu, in which neuropathology was being recognized as the key to understanding the class of conditions known as dementia and, paradoxically, senile dementia continued to be widely recognized as “normal,” that Alzheimer began in 1901 to write a remarkably extensive clinical history of the 51-year-old woman whose case fascinated him, and who came to be known in the medical literature as Auguste D. When Alzheimer first met her in the Asylum for the Insane and Epileptic in Frankfurt am Main, where he was employed as a senior physician, Auguste Deter had just had a lunch of cauliflower and pork. Alzheimer asked her,
“What are you eating?”
“Spinach.”
She chewed the meat.
“What are you eating now?”
“First I eat the potatoes and then the horseradish.”
Two days later Alzheimer noted that Auguste D was “constantly fearful and at a loss. She said, over and over, ‘I won’t let myself be cut,’ acted as if she were blind, and when walking about groped the faces of other patients, and was often struck by them in return. When asked what she was doing she said: ‘I have to tidy up.’ ”15 For months, until he left the hospital in Frankfurt, Alzheimer saw his patient virtually every day, making extensive notes of his attempted conversations with her and of her moods and behavior. Three months after he first met Auguste D, she was neither able to converse with Alzheimer nor answer his questions. Her behavior, Alzheimer wrote, was now hostile, and she lashed out when he tried to examine her. He noted that she often screamed spontaneously for hours on end and wandered about aimlessly, sometimes having paroxysmal fits that lasted for several hours. After he left Frankfurt, Alzheimer continued to inquire about his former patient, and when she died in April 1906, five years after first being admitted to hospital, Alzheimer immediately requested that he be given her brain for autopsy. His biographers note, “Alzheimer believed that behind the clinical symptoms, marked by forgetfulness and jealous fantasies, a new ‘peculiar disease,’ as he put it, could be found in Auguste D.”16
Seduced by Plaques and Tangles
The innovation that many agree brought about the “discovery” of Alzheimer disease was a silver precipitation technique first developed by the Italian scientist Camillo Golgi in 1873, and shortly thereafter modified by the Spanish neurologist and photographer Santiago Ramón y Cajal. This novel technique radically transformed the emerging discipline of the neurosciences, and was regarded as such an important breakthrough that these two scientists were jointly awarded the Nobel Prize for medicine. Prior to the development of effective staining techniques it had already been postulated by the somaticists that psychiatric disorder must be intimately associated with specific demonstrable changes in the gross anatomy of the brain, but this could not be proven beyond noting anatomical changes detectable by eye, the significance of which was speculative. Nevertheless, efforts were made as early as the 1850s to create subcategories of mental illness based on gross neuroanatomical lesions found at autopsy. Commencing in the 1880s, microscopic examinations (figure 1.1) were carried out in earnest and, with improved preservation and fixing and the development of new stains, it was possible to discern in closer detail the material changes assumed to be causal of psychiatric disorder and senility.17 As the neurologist Peter Whitehouse has noted,

Figure 1.1.
Alois Alzheimer, 1911. Neuritic plaque, in Javier DeFelipe, Cajal’s Butterflies of the Soul: Science and Art (2010). Reproduced with the permission of Oxford University Press p.278
The staining process is a centrally important one that, literally, brings the lesion into the medical gaze. As part of a process that made a thing visible, it thereby made it (appear) real. The staining process asserted a commonality of the pathologic lesions.18
The silver precipitation technique was refined in 1902 by the German scientist Max Bielschowsky and shortly thereafter made use of by Alois Alzheimer to stain sections of the brain tissue he had procured after the death of Auguste D together with that from several other patients diagnosed with the disease soon to become his eponym. The histological slides allowed Alzheimer to see with the aid of a microscope what had not before been evident, namely the clumped structures he labeled as neurofibrillary tangles that would thereafter be recognized as one of the key autopsy signifiers of Alzheimer disease.19
Neurofibrils are present in normal cells and had been observed well before Alzheimer’s time, but the stain clearly showed the extent to which they had accumulated excessively to form abnormally dense twisted fibers inside the nerve cells. A second signifier of Alzheimer disease, amyloid plaques, found between the nerve cells (neurons), were also visible when he applied a different stain, methyl blue-eosin, but plaques had already been definitively described by several other medical researchers working at the end of the 19th century.
Both Alzheimer and Bielschowsky had spent a great deal of time dealing with patients with the dementia-like condition associated with tertiary syphilis; they had dissected the brains of many patients who had died of this disease and of epilepsy—the condition that more than any other would lead to an acceptance of localization theory.20 Alzheimer had also studied anatomical changes in the brains of older demented patients where syphilis was not implicated. He and his colleagues were already convinced that lesions visible to the naked eye in the autopsied brains of demented patients, even without the aid of a microscope, were important signifiers of mental illness; what they saw when looking down the microscope at preparations of brain tissue only confirmed their beliefs.
Alzheimer gave a report at a meeting of the South West German Alienists on November 4, 1906, about the case of Auguste D. He informed his audience that she had “presented” with progressive cognitive impairment, hallucinations, delusions, and “marked psychosocial incompetence.” Alzheimer added that on postmortem he had found brain atrophy, arteriosclerotic changes, senile plaques, and neurofibrillary tangles.21 He concluded his presentation with the following comment:
Taken in all, we clearly have a distinct disease process before us. Such processes have been discovered in great numbers in recent years. This observation suggests to us that we should not be content to locate any clinically unclear cases of illness in one of the familiar categories of disease known to us to save ourselves the effort of understanding them. There are undoubtedly far more mental illnesses than are listed in our textbooks. In many such cases, a later histologic examination will allow us to elucidate the case.22
Alzheimer’s report of Auguste D’s case received a poor reception at the meeting. No one present answered the chair’s call for questions, and the paper was rebuffed by the so-called anti-Kraepelinians present, who argued that “there can be no talk of nosological specificity.” They were adamantly opposed to the possibility that specific pathological anatomy accounted for named mental illnesses.23 Even though Alzheimer’s reputation was well established, and the leading psychiatrist of the day with whom Alzheimer worked, Emil Kraepelin, ably defended him, the assembled group moved on to discuss a paper delivered by a disciple of Sigmund Freud.
While Alzheimer was working in the Frankfurt asylum he was dealing with a large number of cases of senile dementia, but in the ensuing years, after he moved to the teaching hospitals, he was no longer responsible for such patients on an ongoing basis because they were relatively quickly transferred to asylums. In the years following his presentation of the case of Auguste D, Alzheimer and his colleagues reported only eight similar cases; at least two of these had already been reported elsewhere, and, furthermore, their authenticity was questionable. These patients were not elderly and therefore not “typically” senile, and there were serious doubts as to whether the cases represented anything significantly new that warranted revising the current taxonomy. What is more, the second key case written up by one of Alzheimer’s colleagues, that of a 56-year-old laborer, Johann F, showed no signs of neurofibrillary tangles at postmortem.24
But a move toward recognition of localization theory was very much in the air despite the existence of vocal anti-Kraepelinians. L. W. Weber, a senior physician practicing in Göttingen, noted in 1905, “It is a fact now scarcely contested, that all mental disorders depend on pathological processes in the brain … in this sense every mental disorder may be termed a brain disease.” And in 1910, Emil Kraepelin, in the eighth edition of his extraordinarily influential revised textbook on psychiatry, made a cautious but nevertheless clear distinction between conditions he described as presenile and senile dementia. The former, presenile dementia, he named Alzheimer disease, but he noted, “The clinical interpretation of Alzheimer disease is still unclear at the moment.”25 The question remains as to why Kraepelin took this position on the basis of what appears to be fragile evidence. The historian German Berrios argues that the re-reporting of several of the cases suggests that there may have been great pressure in the laboratory where Alzheimer worked alongside Kraepelin to find evidence for a “new” disease, but it has never been satisfactorily established whether or not Alzheimer in the end agreed with Kraepelin’s decision to name this disease, tagged or not, with his name. The comments of one of Alzheimer’s close colleagues, Gaetano Perusini, strongly suggest that Alzheimer continued to believe that he had documented nothing but a few atypical forms of senile dementia in which age was the sole distinguishing feature, but vacillation on his part is also evident.26 It seems undeniable that the institutional constraints imposed by teaching and research hospitals in which Alzheimer worked for most of his life, in which long-term care was not provided, ensured that he could not observe his patients in a manner that he had carried out so successfully while caring for Auguste D in an asylum.27
Among the reasons put forward by historians for Kraepelin’s apparently hasty move to name Alzheimer disease are the following: Kraepelin did so for scientific reasons because he himself was convinced that the cases being reported demanded taxonomical revisions. But evidence for the veracity of this reason remains slim. The existence of a rival neurological department in Prague headed up by Arnold Pick, who would shortly have another form of dementia named after him, has been given as a second reason. But the most commonly offered reason is that Kraepelin was feeling threatened by Sigmund Freud and the increasing interest being shown in a psychoanalytic approach to the interpretation and management of mental illness. Hence, it is argued, Kraepelin was experiencing a sense of urgency to document the pathological substrates of mental conditions in order to put them on a sound footing. However, Berrios argues strongly against this position and reminds us that in principle Freud, himself a neurologist, had no antipathy to recognition of organic, localized foundations of dementia.28
It is also pertinent to note that Kraepelin was not a hard-nosed somaticist. He had traveled to many parts of the world, and strongly believed that it was important to establish a comparative psychiatry. In common with many like-minded thinkers of his day, and profoundly influenced by his trip to Southeast Asia in 1904, Kraepelin postulated the “psychic character” of peoples who live in similar environments, and following in the footsteps of his teacher Wilhelm Wundt, he called for the formation of a discipline of comparative ethnopsychology.29 In short, a satisfactory explanation for Kraepelin’s apparent rush to name a new disease is still wanting, but clearly the idea that localized changes in the brain cause mental illness was sufficiently well established for Kraepelin to feel justified in principle to make his move, even though the supporting evidence was rather slim.
The slides on which Alzheimer’s initial observations were made were lost for many years. Remarkably, they came to light in 1998. Upon reexamination it was agreed that Alzheimer’s conclusions had been entirely accurate by present-day standards. Atrophied cells and plaques were clearly present in the preparations made of brain tissue from both Auguste D and Johann F, but a massive number of neurofibrillary tangles appeared only on the slides of tissue taken from Auguste.
The Partial Eclipse of Alzheimer Disease
Despite its baptism by Kraepelin, Alzheimer disease lost its way for four decades. Numerous scientists and clinicians of the day disagreed with Kraepelin’s designation of a new disease, and no systematic follow-up was undertaken to consolidate its recognition. It was already well known at the time, largely on the basis of the autopsied brains of syphilitics and epileptics, that amyloid plaques and neurofibrillary tangles occur in individuals other than those diagnosed with Alzheimer’s. This effectively weakened any argument made for a relationship between these specific neuropathological findings and the behavioral changes seen in patients diagnosed with presenile dementia.30 A second reason is that the outbreak of the First World War ensured that resources were diverted away from basic laboratory work to the war effort. And a further reason was that the energies of the drug companies of the day were directed toward what was termed arteriosclerotic or vascular dementia, on the assumption that pharmacological agents would soon be found to combat this problem. Senile dementia continued to be associated primarily with aging itself, and the matter of early age of onset alone did not prove to be sufficiently convincing evidence for presenile dementia to be accepted as a distinct disease. This was the case even though Alzheimer’s close colleagues of the day started to use the label, and Alzheimer himself continued to insist, as he had done since his presentation of Auguste Deter’s case, “We must reach a stage in which the vast well-known disease groups must be subdivided into many smaller groups, each with its own clinical and anatomical characteristics.”31
Although Alzheimer spoke up for recognition of links between localized neuroanatomy and behavioral changes, scholars who know the literature of the time well and have facility with German have made comments such as the following: “Alzheimer himself could be counted among the ‘doubters’ who did not necessarily believe that AD represented anything but a precocious form of senile dementia.”32 Even so, for Alzheimer, both presenile dementia and senile dementia were not “normal” aging but rather irreversible conditions, the material reality of which could be located in the brain. When plaques were relabeled in 1910 as “senile plaques,” this confounded the matter further—plaques were now specifically associated with aging, but they were also understood as the prime signifier of dementia as a disease.33
During the 1920s more cases of presenile dementia were documented, but hesitation about its validity as an isolable disease persisted, leaving the problem of Alzheimer disease and its relationship to aging unresolved. Another line of thought further muddied the waters: a good number of experts of the day believed that cerebral arteriosclerosis was the primary cause of dementia. Meantime it was reported in the 1930s by a German researcher, on the basis of autopsy findings, that “84% of persons dying over the age of 65 had ‘senile plaques’ in their brains,” suggesting that plaques are, in effect, a normal part of aging.34 The upshot of the uncertainty was that the dementias were “seriously neglected” for many years, in part because they fell into a no-man’s land, neither fish nor fowl, neither neurological nor psychiatric disorder, and geriatrics did not as yet exist as a specialty.35 This situation was to persist until the 1970s.36
Medicalization of Aging
During the latter part of the 19th century medical concern about the plight of the elderly increased, resulting in the formation of what would become the specialty of geriatric medicine and hence the beginnings of the medicalization of old age. In his Lectures on Senile and Chronic Diseases, published in 1867, Charcot noted that the importance of studying diseases of old age was no longer contested.37 France, Germany, and Great Britain were at the center of this move that then spread to North America. Early on, certain of the key figures in this emerging field started to describe old age itself as a disease-like condition.38 Emphasis was given to the slowing of body activity, loss of sociability, becoming bedridden, and “senile degeneration.”39 As the historian Martha Holstein has noted, “[I]n what may seem contradictory to modern readers, turn of the century investigators often described ‘normal’ aging somewhat quixotically as pathological or at least as ‘a quasi-pathological process of cell and tissue degeneration.’ ”40 Thus dementia could be simultaneously “normal” and “pathological.” Ignatz Nascher, the founder of gerontology in the United States, argued that “senile changes” were “deviations in degree … usually permanent, progressive, and uncontrollable; rarely remissive or changeable.”41 But the historian Jesse Ballenger points out that despite a widespread negative stereotype about the ravages of senility there was a large popular literature available at the time informing its readers how to avoid senility by paying “careful attention to hygiene, exercising, seeking out the companionship of the young, and many other stratagems.”42
Ballenger insists that what is known as the “dark ages” of dementia research, from about the 1920s until 1970, is not an accurate depiction. He points out that between the mid-1930s and the 1950s a surge of interest in senile dementia took place, notably among American psychiatrists. This was in part stimulated by what was described as a “demographic avalanche of aging”43 under way, with the result that state mental institutions in the United States were becoming clogged with elderly senile patients, thus detracting from the professional authority of psychiatry. At the same time, the profession of gerontology argued forcefully for recognition of and provision for diseases that affect older patients in disproportionate numbers. Among the U.S. psychiatrists of the day who turned to a consideration of senile dementia, David Rothschild is perhaps the best known.
Rothschild argued that because plaques and tangles are found in several medical conditions and not simply in senile dementia, these formations should be understood as a generalized tissue reaction in response to a number of biological factors. But Rothschild was not content with this observation alone; together with his colleagues he began to examine the relationship of emotional and personality disturbances to Alzheimer’s. Rothschild argued explicitly that the usual approach to senile dementia is reductionistic, noting that there is “too exclusive a preoccupation with cerebral pathology.” He went on, “The changes occur in living, mentally functioning persons who may react to a given situation, including an organic one, in various ways.”44 Rothschild argued that people have differing abilities to compensate for organic lesions and that this should be investigated. To reinforce his position, Rothschild emphasized the contradictory findings shown repeatedly since Alzheimer’s time, namely that the presence and degree of dementia in a living patient often show discrepancies with the presence and degree of pathological structures at autopsy. Ballenger suggests that Rothschild’s publications were so influential that the therapeutic nihilism usual among psychiatrists when confronted by senile patients began to break down.
Moreover, Rothschild’s arguments were drawn on to create a highly influential literature of the day in which normative societal attitudes toward aging and management of the elderly were extensively criticized. The locus of senile mental deterioration should no longer be located in the aging brain, it was now argued; rather, society strips the older person of a reason for living.45 The psychodynamic variation on this position also aired at this time was that the forgetfulness associated with senility is a form of repression, a protection against the fear and frustration associated with growing old in the mid-20th century. By the 1970s discussion highlighting systematic discrimination against the elderly was common, but at the same time it was acknowledged that a minority do indeed suffer from severe organic problems that require investigation.
The Politicization of Alzheimer Disease
Ballenger points out that during these decades a limited organic approach to senility never entirely disappeared and that, commencing from the 1960s, five innovative changes took place that once again put Alzheimer disease firmly on the map. First, in the 1960s, a large series of brain autopsies of patients diagnosed with dementia were carried out in England. It was asserted that the vast majority of these brains showed relatively few signs of arteriosclerotic changes (the pathology that had occupied both drug companies and neuroscientists for some time), but that they exhibited numerous plaques and tangles as described decades earlier by Alzheimer.46 These clinicians argued explicitly that the amount of damage caused by the plaques and tangles corresponded closely to the severity of the behavioral changes in patients. However, the researchers were also obliged to acknowledge that in a considerable proportion of their nondemented control group, plaques, tangles, and brain shrinkage were markedly evident at autopsy.
A second significant change was the development of the electron microscope, permitting important refinements in classification of neuropathologies. For the first time, specific biochemical changes were associated with structural changes made visible by this powerful new instrument. It was possible to distinguish clearly between the composition of plaques and tangles, and arguments began to take place about the primacy of plaques or tangles in disease causation. A psychological approach to dementia was pushed to one side, to be replaced by a “new agenda for pathological research.”47
Third, in the 1970s, the influential neurologist Robert Katzman publicly insisted that received wisdom of the time, namely that aging inevitably results in senility, be abandoned. Several commentators pointed out that the word “senile” had in effect become a term of abuse and, furthermore, that its use perpetuated discrimination against the elderly.48 Katzman, following a suggestion first made in 1948 by R. D. Newton on the basis of 150 autopsies carried out at Middlesex Hospital in London, declared that both “presenile dementia” and all cases of senility should be recognized as pathology, be labeled as Alzheimer disease, and understood as entirely distinct from normal aging.49 Katzman’s position has been described as a “neo-Kraepelin” concept of Alzheimer disease,50 and it culminated in what has been characterized as the “rediscovery” of this condition.51 As Ballenger notes, “[B]y the end of the 1970s, if senility had not been eradicated, as an earlier generation of gerontologic activists had dreamed, it had at least been thoroughly disciplined—relegated by biomedical scientists to various discrete, well-defined disease entities that, at least in theory, no longer contaminated the entire experience of aging.”52
It was at this time that Alzheimer disease began to be billed by Robert Katzman and others as the fourth or fifth leading cause of death in the United States. Writing Katzman’s obituary in the New York Times in 2008, Roger Segelken argued that a major transformation had come about in the view of the medical community after the publication of an editorial by Katzman in 1976 in the Archives of Neurology, followed in 1977 by a conference that he organized. In the editorial Katzman described AD as a “major killer” and discussed its “malignancy.” Prior to its publication, fewer than 150 articles in all had been published on Alzheimer disease. From the publication of the editorial until the time of the publication of Katzman’s obituary in 2008, over 45,000 articles had been published,53 a number that continues to rise exponentially.
The fourth innovation in the 1970s was the formation in the United States of the National Institute on Aging (NIA), founded specifically to foster a comprehensive research program on aging. And fifth was the emergence of an incipient Alzheimer’s movement, and its consolidation commencing from 1977 as the Alzheimer’s Disease and Related Disorders Association (ADRDA), with strong support from the first director of the NIA, the gerontologist and psychiatrist Robert Butler. Activities of the ADRDA in turn increased the legitimacy of the NIA, in large part because it lobbied government and also set about raising money for research into Alzheimer disease.54 At this time the language associated with an epidemic, “a ticking time bomb,” began to be evident, together with concerns about looming skyrocketing medical and social costs.55 However, Whitehouse and colleagues have pointed out that emphasis was given from the outset to finding effective treatments and ultimately a means of prevention of AD, rather than paying attention to the desperate needs of caregivers.
It was argued by advocacy groups that widespread recognition of Alzheimer’s as a disease was first essential and that, when making a case to the NIA, an emphasis on curative medicine would be the best way of addressing not only the search for a cure, but also the social and personal burdens imposed by the disease. Not surprisingly, little money was set aside to specifically deal with caregiving.56 Similarly, in Great Britain, the Medical Research Council denoted research into dementia and Alzheimer disease as a priority area.57 The sociologist Patrick Fox points out that although clearly efforts to treat and cure AD are admirable, it must be kept in mind that this endeavor is a business that involves powerful economic interests centered on “the marketplace of disease diagnosis and treatment.”58 It is also fostered by a “discourse of hope” sustained by involved families.59 This characterization of AD continues to be relevant and accounts in part for the consolidation of what came to be the dominant AD paradigm known as the “amyloid cascade hypothesis,” believed to be causal of a buildup of plaques in the brain (see the following chapter).
Fox notes that interest in the newly formed organization began to explode in 1980 after a letter from a family member of an Alzheimer disease victim was published in the nationally syndicated column “Dear Abby.” Following this publication, the ADRDA received more than 30,000 letters, precipitating interest among the public in AD as nothing else before it had done.60 It rapidly became clear that many families desperately hoped for assistance with caregiving, but, of equal importance, they wanted senility recognized as a disease of the brain for which a cure should be sought out, thus challenging the stigma associated with dementia, and at the same time shifting the moral burden for the occurrence of the disease away from themselves. These families were adamant that they had little time for psychosocial or psychodynamic models of senility. Fox and colleagues have recently shown how this two-edged sword—the tension between cure and care—has been exacerbated over time, particularly following the Reagan years, so that support for care of AD patients has been reduced today to little more than tax credits for most affected families in the United States.61
When I talked with the neurogeneticist John Hardy in 2008, he stressed that consolidation of the concept of Alzheimer disease as a singular condition “was just a political maneuver to get funding, and some people then actually came to believe that this is the case.” Writing in the early 1990s about the rise of a medical model to tackle the problem of dementia, the eminent psychiatrist William Lishman, associated with the Institute of Psychiatry at the Maudsley Hospital in London, sounds a cautionary note: “Certain observations remain obstinately to remind us that aging and Alzheimer’s dementia are interlinked closely.”62 Lishman notes that many of the clinical features of Alzheimer’s, notably memory loss, are strongly associated simply with aging. And so too are changes within the brain, above all, the presence of plaques and tangles in the hippocampus. He points out other biological changes in common between the aging process and Alzheimer’s, and concludes with the important observation that, as individuals move into old age, it becomes increasingly difficult to distinguish Alzheimer’s and old age because of their common features. Lishman insists that the possibility must be entertained that Alzheimer disease is, after all, “simply brain aging.” He argues that if “a marker is found that is indisputably associated with the condition labeled as Alzheimer’s alone, a marker never seen in healthy aging individuals,” then the idea that Alzheimer’s and neural aging are indistinguishable must be dismissed.63 Lishman concludes that the aging process itself may be under strong genetic and environmental influences so that in certain circumstances it will be “accelerated and intensified,” in effect becoming a form of “precocious aging.”64 And he adds, “In all persons the shift from so-called ‘normality’ to becoming ‘a case of Alzheimer’s dementia’ will occur when the process has passed a certain threshold in its development.”65
The recently deceased British psychiatrist Martin Roth argued in the 1990s for research into what he described as “reserve capacity,” by which he meant the likelihood that certain individuals have an abundance of neurons that permit them to stay above a crucial threshold in which mental activity is preserved throughout life. In contrast to that of Lishman, the position that Roth took is one of a marked discontinuity between aging and dementia; he argued that when a crucial threshold is passed, the involved molecular pathways diverge, even though some overlap may be possible. As far as Roth was concerned, “an aging person either does or does not have AD,” and those without AD may have some memory loss, but they do not lose their sense of identity, ability to retain any information, or capacity for reasoning. Nor do they die as a result of being demented. Roth was adamant: “AD cannot be accounted for in terms of a continuous and predictable extension of normal mental aging.”66 But he nevertheless acknowledged that genetics and environment no doubt play a role in accounting for why some individuals are more vulnerable to AD than are others.
A Brief History of Normal
Michel Foucault’s work on the “archaeology of medical perception” illustrates how the rise of anatomical pathology in the 19th century helped create the standardized body of modern, scientific medicine.67 He argued that representations of the body in premodern medicine were in effect “mute,” a blank slate on which the timeless truth of disease was to be deciphered through a parsing of signs. Gradually, the “truth” of disease was displaced into the body: “[O]pen up a few cadavers,” exhorted the great Parisian anatomist Bichat, “and you will see disappear the obscurity that observation alone could not dissipate.”68 The body could now “speak,” but only once the differences there observed could be understood as pathological deviations from a healthy norm. This marked a shift from the earlier notion that diseases are pure “essences” that mark the body from within.
Foucault extended his argument to note that it became possible to “see” differences among abnormalities made visible when bodies were dissected. This variation was interpreted either as different diseases or as different stages in the unfolding of the same disease, that could then be linked back to the signs and symptoms experienced and spoken about by patients that were at times visible on the surface of their bodies. The anatomized body became an invariant, a standardized measure of disease mechanism. Extrapolating from the cadaver to ailing patients made it possible to imagine the symptoms that afflicted them as signifiers of invisible processes deep within the body; thus was “localization theory” solidified.
Until well into the 19th century use of the term “normal” was virtually limited to the fields of mathematics and physics. It was not until an internalizing approach to the body based on anatomy took hold that arguments about the relationship between normal and abnormal biological states were seriously debated for the first time. Auguste Comte, writing in 1851, noted a major shift in conceptualization that had taken place when the physician Broussais argued in the 1820s that the phenomenon of disease is of essentially the same kind as that of health and, thus, health and disease differ from each other only in “intensity.”69 Application of epidemiological methods to the study of populations allows this continuum to be mapped, and the results can then be made use of in clinical settings, with the assumption that all human bodies are biologically equivalent. Conversely, findings about individual bodily conditions such as those observed in clinical studies may be extrapolated to entire human populations, although, given that such findings are not representative of any given population, they often introduce major problems in connection with interpretation as to their significance.70 Broussais postulated not only that normality should be understood as being on a continuum with pathology but also, furthermore, that deviation must be understood with reference to a “normal” state.71 This theme was taken up and expanded upon by several influential thinkers during the course of the 19th century, among them Auguste Comte and Claude Bernard.
In the 1960s, the philosopher and physician Georges Canguilhem, Foucault’s teacher, in writing a synthesis of the work of the previous century in connection with normality, noted that “strictly speaking … there is no biological science of the normal. There is a science of biological situations and conditions called normal.” Canguilhem concluded that normality can be understood only in context, “as situated in action,” and moreover, diversity does not infer sickness, nor does “objective” pathology exist outside of the laboratories, clinics, and operating theatres where it is made visible.72 Canguilhem’s argument, contra Broussais, was that the “normal” and the “pathological” are two fundamentally different states that cannot logically be placed on the same continuum. Their reconciliation along a biological continuum, Canguilhem argued, was in fact an artifact of decontextualized clinical and laboratory methods used in biomedical research, and “normalization” can lead to the mistaken assumption that what is statistically “abnormal” is inevitably pathological or, alternatively, that no pathology lies in what is statistically “normal.” Discussion in contemporary Alzheimer circles about “exceeding a threshold,” “maintaining homeostasis,” “reserve capacity,” and continuities and discontinuities between normal aging and dementia reflect this much older debate about disease, and the relationship of normal and pathological. The confusion about this relationship is most often expressed in current scientific articles about Alzheimer disease in terms of the oxymoron “normal subjects exhibit neuropathology,” or some close equivalent.
When Is Pathology Normal?
The brain, to a greater extent than other major organs, has long presented a formidable barrier for medical diagnosticians. Until very recently no technologies existed that allowed one to “see” into the living brain, and for this reason, from Alzheimer’s time on, two diagnoses have been made use of: the first is a clinical diagnosis based on an apparent decline over time in cognitive function, determined by means of psychological testing and clinical judgment, and the second is a postmortem diagnosis based on autopsy findings. Clinicians I have talked to in specialty clinics usually insist that when a diagnosis is made by an experienced physician, one who has tracked a patient for a good number of months or even years, the diagnosis is accurate in the great majority of cases; the Alzheimer’s is “real.” During this time, extended conversations have taken place at each meeting with the patient and a caregiver who has been requested to accompany the patient to the clinic, and various psychological tests have also been repeatedly administered. It is clinicians, of course, who must deal with the complaints, concerns, and the assessment of “functional deficits” associated with dementia. But they are required to write a diagnosis of “possible” or “probable” AD on patients’ charts, on the assumption that only at autopsy will the truth be revealed.
In turn, neuropathologists I have interviewed insist that pathological findings represent the “true” diagnosis of AD, even though they acknowledge that their findings do not always correspond precisely with those of other pathologists. Clinicians and pathologists agree that clinical and neuropathological diagnoses are, for the most part, in concurrence, but acknowledge that this is not always the case. These discrepancies have been evident since Alzheimer’s time, as we have seen, and were highlighted yet again when findings from the so-called Nun study were first published. This research commenced in 1986 and involved 678 Catholic sisters who belonged to an order called the School Sisters of Notre Dame located in seven regions of the United States. Statements written by these nuns when they were young women about why they wanted to enter the order, carefully stored for decades, were matched with neuropsychological test results administered throughout the latter part of their lives from age 75 on, and then subsequently linked to autopsy findings after death (every nun had agreed when she entered the project to donate her brain for autopsy).
It is argued on the basis of this study that those individuals who showed imagination and complexity in their thinking while young (that is, exhibited “high idea density,” in the language of the researchers) were less likely to succumb to Alzheimer disease when they grew older.73 This finding was not related to number of years of formal education, and was borne out as the autopsy results gradually accumulated: 90% of those nuns whose brains exhibited extensive neuropathology had shown “low idea density” as 20-year-olds. This research gave an enormous boost to what came to be known as the “cerebral reserve” hypothesis—such reserve being laid down commencing in utero.
Of even greater interest for the present discussion was the finding that a small proportion of the nuns who coped very ably with the neuropsychological battery of tests turned out at autopsy to have extensive signs of plaques and tangles, once again confirming observations made repeatedly over the past 80 years. Conversely, it was also clear that a few individuals whose autopsied brains revealed a relatively small number of anatomical changes exhibited all the behavioral signs of dementia while alive.74
David Snowdon, the principal investigator in this study, made the following comment about one of the nuns whom he came to know well:
Sister Mary, the gold standard for the Nun study, was a remarkable woman who had high cognitive test scores before her death at 101 years of age. What is more remarkable is that she maintained this high status despite having abundant neurofibrillary tangles and senile plaques, the classic lesions of Alzheimer disease.75
When asked to comment on the findings of the Nun’s study, a Montréal neuropathologist had this to say:
Well, if I find little neuropathology and the clinician says there’s “probable AD,” then I have to find a reason why. And if I don’t think the quantitative changes of what I’m looking at in the brain is Alzheimer disease, then I have to find another reason. Is it vascular dementia? Is it another neurodegenerative disease? So, I mean, when the patient is clinically demented, there’s got to be something in the brain to explain that. The difficult thing is, we know that there’s no direct correlation between the density of plaques and tangles, and the clinical presentation. So we can have a brain that’s full of neuropathology, but clinically the patient was not very demented. We can’t categorize and say this is “moderate” Alzheimer’s, this is “severe” Alzheimer’s just by histological findings. That means nothing. All we can say is that there are sufficient tangles to confirm the clinical diagnosis, but there’s no direct relation so as you can say that if you have more than 20 plaques in this area, or so many tangles, that’s a severe case of the disease. So there’s no correlation between clinical severity and histological severity.
Of course, neuropathology is the expression par excellence of localization theory in practice. Pathologists often know relatively little about the patient whose brain tissue they examine under the microscope, and all they have in front of them may be the clinician’s final diagnostic report of “probable dementia.” Their task is one of verification or falsification and, if the latter, to account for the death using a different diagnosis. We begin to get a sense here of just how uncertain it is as to what should count as an AD “case”—making the creation of robust figures for use in public health arenas and in creating population databases highly problematic. Moreover, the way in which the person is, in effect, “disappeared,” to be transformed into a thoroughly decontextualized AD case, one reduced entirely to neuropathology, is strikingly evident. Thus, in contrast to autopsy findings in connection with many other conditions, the pathological report does not provide definitive causal information—rather, it confirms that Alzheimer’s is present in the brain. The situation cannot be compared with a laboratory demonstration of the syphilis spirochete or the exposure of a brain tumor at autopsy. The intricacies involved were highlighted for me when an experienced neuropathologist remarked, without irony, “I’ve never seen two human brains in which the pathological signs of dementia are the same.”
Another epidemiological project, one in which the population was carefully delineated, assessed the neuropathological status at death of 456 individuals who had agreed to donate their brains for research and had previously been tested clinically for AD. This study showed, as expected, that the neuropathological features assumed to be diagnostic of Alzheimer disease and dementia, including plaques, tangles, and cell loss (figure 1.2), are more evident in older than in younger subjects. However, although the correlation between postmortem neuropathology and clinically diagnosed AD and dementia was strong in the group aged 60 to 75, this finding was shown to be less and less strong with increasing age. The researchers concluded that a considerable overlap exists in the so-called neuropathological features present at postmortem of individuals aged 75 and older who were clinically diagnosed with dementia while alive, and those without dementia.76
It was argued that additional factors other than the simple presence of Alzheimer neuropathology must determine the clinical expression of dementia, especially in those aged 75 years and older. The researchers insist, “[T]he pathological basis of dementia should be considered as an interaction among pathological changes, compensatory mechanisms, and underlying synaptic dysfunction” and, “cognitive dysfunction in later life is a life-span issue and is affected by genetic, developmental, and lifestyle factors, accumulated neural insults, innate and acquired cerebral reserve and compensatory mechanisms, and age-related decline.”77
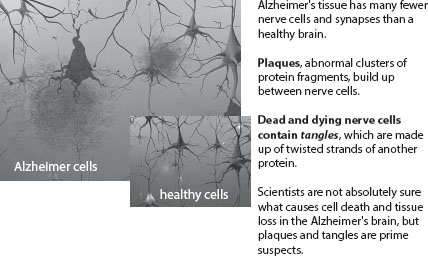
Figure 1.2.
Under the microscope: Plaques and tangles. Reproduced with the permission of the Alzheimer’s Association, from Inside the Brain: An Interactive Tour, Alzheimer’s Association, http://www.alz.org/alzheimers_disease_4719.asp. This tour is available in 14 different languages.
One member of this research team, the British epidemiologist Carol Brayne, has been arguing for years that research into dementia should move away from a model that poses as its basic question “has he got it?” to one of “how much of it has he got?” and “why?”78 And cumulative research in recent years based on population research has confirmed that so-called mixed dementias—usually of vascular dementia and Alzheimer disease—are the most common; some findings suggest that the vascular changes may initiate the dementia.79 Another study found that “dementia of unknown etiology” accounted for 5% of all cases of dementia among patients dying in their 70s, 21% of patients dying in their 80s, and 48% of patients dying in their 90s. It was concluded that a significant percentage of demented patients older than 80 years do not meet pathological criteria for either AD or a second relatively common kind of dementia (Lewy body dementia).80
Almost as an afterthought, one of the most highly recognized and experienced of AD experts, John Morris, suggests along with his coauthor in the concluding sentence of a 2006 article, “An additional research focus, of course, should determine why many older adults with neuropathologic evidence of AD manage to remain cognitively normal despite numerous AD lesions littering their brains.”81
A Diffuse Clinical Syndrome
Since the beginning of the 20th century two major ontological matters have persisted in the AD world. The first, as a recent article title in the British Medical Journal put it, is, “What do we mean by Alzheimer disease?”82 The second, linked to the first, is the question of whether or not AD is an inevitable part of “normal” aging, or is it a bona fide neuropathological disease, entirely different from aging? Throughout the 20th century repeated efforts were made to have AD recognized as a disease, not merely to aid in the search for a cure but also, as we have seen, for powerful social and political reasons. However, in the first decade of the 21st century emerging research, largely resulting from epidemiological population-based data, has shown repeatedly that the question of what exactly constitutes AD, and who might be at risk for it, remains unanswered. These findings tip the scales toward an argument for the inextricable entanglement of dementia with aging, but, even so, an approach grounded in localization theory remains dominant, in large part because pharmaceutical companies support it.
Two researchers have recently called for the condition glossed as Alzheimer disease to be recognized as a “diffuse clinical syndrome,” one that reflects the gradual accumulation of “multiple pathologies, arising from multiple interlocking risk factors over the life course.”83 Alzheimer’s is not a “yes-no clinical diagnostic category,” they argue, nor can one tell when a symptom gradient associated with AD-like symptoms commences, because it is increasingly becoming clear that events that happen in utero and in early life may well be implicated. Richards and Brayne insist that if indeed Alzheimer’s is a diffuse clinical syndrome, as they argue, then a therapeutic “silver bullet” is unlikely to be forthcoming, and the focus should instead be on better management of the numerous factors throughout the life cycle linked to increased risk for becoming demented. These authors conclude,
No straightforward correspondence exists between higher mental function and the burden of lesions in the aging brain. If this shifts the focus away from detailed diagnostic classification made on the basis of assumed clinical-pathological correlation and towards a global pragmatic approach to the needs of patients and carers, and to modifiable lifetime risk factors, then the apparent loss of scientific precision is a gain to clinical practice.84
This article highlights inherent, incommensurable tensions among the four principal sets of expert actors in the Alzheimer world: basic science researchers, often with drug company support; academically based clinicians; physicians whose specialties are gerontology or family medicine and general practitioners working in primary care; and those researchers in epidemiology and public health. Basic scientists and academic clinicians (very many of whom work in specialist memory clinics) constitute the heart of the biomedical endeavor that favors localization theory.
The sociologists Cambrosio and Keating characterize contemporary biomedicine as a “bio-clinical” hybrid in which tight working links are established between what is termed in the medical world “bench and bedside.” The bodies of patients in tertiary care settings (whether hospitalized or ambulatory) are a source of endless research material, in the form of specimens, imaging data, medical records, and so on, deposited in computerized repositories that may be drawn on in order to both refine extant knowledge and generate new knowledge that will then, in turn, be standardized and made available for use in clinical practice. Clinical trials using human subjects also take place in tertiary care settings, most of which are designed with the purpose of developing new drugs. In effect, patients in such settings become hybrids—patient/research subjects—the moment they enter the hospital. In laboratory investigations, the disease or condition takes priority over the actual condition of human research subjects. But this is not to suggest that the clinical care given to hospitalized tertiary care patients who are also research subjects is wanting in any way, although on occasion this may the case, depending on the location. John Le Carré’s novel The Constant Gardener depicts an appalling exploitative research situation that is not complete fiction in certain poor countries where drug company interests are at work.
Aging as a Continuum
In an illuminating article, the sociologist/historian team of Tiago Moreira and Paolo Palladino has written about the role that gerontologists have played in recent years in both the United States and the United Kingdom in bringing about a substantial transformation in how diseases of aging should be managed. This transformation is described as an era moving toward “health promotion and disease prevention in the 21st century.”85 They cite one of the last articles written by Robert Butler together with colleagues, in which it is argued that to persist with the customary anatomical divisions of the body characteristic of 19th- and 20th-century medicine on which medical departments and clinical care are based is inadequate to address the numerous chronic, long-term illnesses of the late 20th and early 21st centuries. Criticism from several sources, including that of the U.K. House of Lords, is summarized by Moreira and Palladino as follows:
The protracted temporal unfolding of these illnesses is so nearly coterminous with ageing that it unsettles the epistemic pairing of the “normal” and the “pathological” that underpins the clinical perspective on ageing. Furthermore, this pairing assumes that the two states can be situated proximally and intervened upon directly, but this obscures the understanding of the diverse and complex processes involved.86
The argument made by the Science and Technology Committee of the House of Lords called for research into the “generic” process of aging.87 This committee in effect charged that the current organization of biomedicine no doubt serves clinicians and researchers well, but fails with respect to the elderly themselves.88 It is also the case, since the time in the 1970s when Alzheimer’s was fully recognized as a disease, that the U.S. government approach to aging has been similarly grounded in a disease-specific program that, paradoxically, was initially headed up by Robert Butler.
Today, gerontologists, whose numbers continue to be relatively small, especially in North America, together with certain primary care physicians, are arguing for a new approach, one that sets out from a theoretical stance similar to that taken by the evolutionary biologist Tom Kirkwood: “aging is a continuum, affecting all of us all the time.” Kirkwood elaborates by pointing out that “there are scientific connections between birth, early years, childhood and adolescence that have major impacts on health and quality of life in middle and old age.”89 His position, in common with several of the medical researchers cited above, is that aging is inextricably entangled with conditions such as dementia that become manifest in later life, and recognition of this entanglement should become the cornerstone in a move toward their prevention.
Nor surprisingly, many basic science researchers such as Dr. Hyman, whose work tracking the formation of plaques in mice was mentioned at the beginning of this chapter, continue to put their energies into unraveling the molecular pathways thought to be uniquely associated with the onset of Alzheimer disease. Such research receives the bulk of available corporate and government funding because if a cure for this condition is to be found, then it will be based on results obtained from this kind of research. Basic science research is also strongly encouraged by advocacy groups, notably the AD societies and individuals working for them, whose fund-raising activities depend to a great extent on promotion of the idea that a cure for this devastating condition is just over the horizon. The tension between localization and entanglement stances has, if anything, become more aggravated than ever in an aging world where no cure for AD is in sight.
In the following chapter I turn to a discussion of repeated efforts to standardize an Alzheimer diagnosis—a task that continues to be extraordinarily difficult to achieve, in turn suggesting that the AD phenomenon is indeed heterogeneous, and simultaneously putting into question claims about the numbers of AD cases.
