4
Air and Water
Chapter Contents
Air, Hot Air, and the Power of Steam
Water Chemistry and How It Affects Your Baking
You Must Choose Your Flour, but Choose Wisely
The Science of Crispy, Chewy Cookies
Recipes
Seeded Crackers and Easy Flatbreads
Savory Baked Seitan with Spicy String Beans
Spicy Holiday Gingerbread Cookies
Patent-Violating Chocolate Chip Cookies
My Favorite Cake: Chocolate Port Cake
ONE NEEDS TO UNDERSTAND MORE THAN TIME AND TEMPERATURE TO UNDERSTAND BAKING: AIR AND WATER ARE ALSO KEY VARIABLES. While few of us would list air and water as ingredients, they’re critical to baked goods. Both breads and cakes rely on air and moisture for their texture, flavor, and appearance. Yeast adds lift and flavor to breads; baking powder and baking soda generate carbon dioxide to give cakes their rise. Air bubbles in whisked egg whites lift soufflés, lighten macaroons, and elevate angel food cakes. And what makes one chocolate chip cookie chewy and another crispy is only the difference of a few percentage points of water present in it after it’s baked.
Unlike cooking, in which the chemical composition is locked from the start—chefs can’t change the types of proteins in a fillet of salmon—baking requires a well-balanced ratio of ingredients to create gas and trap air. Achieving this balance is sometimes about precise measurements at the beginning; other times it’s about careful attention to the look and feel of a dough as it develops. If you’re an intuitive cook—winging it and adapting recipes on the fly—you’ll probably enjoy making bread. On the other hand, if you’re a methodical cook—one who enjoys precision and prefers a tidy environment—or you like to express affection though food, then baking cakes, pastries, and cookies will likely be your thing. Either way, the science behind both is fascinating.
In this chapter, we’ll start with a short examination of air, water, and flour and then cover the different ingredients used for generating air in both savory and sweet dishes: biological (yeast and bacteria), chemical (baking powder and baking soda), and mechanical (egg whites, egg yolks, and whipped cream).

Air, Hot Air, and the Power of Steam
If the ancient Greeks wrote cooking magazines, they probably would have listed fire, earth, water, and air as ingredients. Aristotle and other philosophers of his day considered these four classical elements to be fundamentally indivisible. Their proof? Adding water to fire didn’t create more of either but instead created a new “structure” they called steam.
While the ancient Greeks had a rather simplistic understanding of the science, they were on to something with their ideas about water and fire: the properties of air do change with temperature. As the temperature of air goes up, so does the potential amount of water in it. This is subtle but important: air—mostly nitrogen and oxygen, normally only 0.5 to 1% water vapor—can hold more water vapor as it heats up, if there is a source of water.
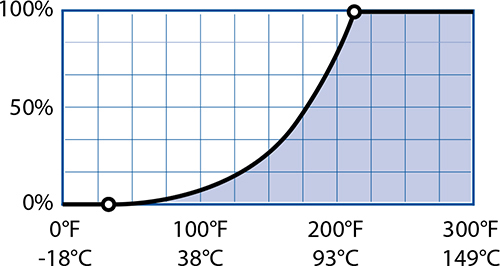
Maximum Percentage of Water Vapor by Temperature:
Hot, humid weather means more water vapor heating up your food as it bakes.
Water vapor matters in cooking because of what it does when it cools down. Technically, steam isn’t the same thing as water vapor. In science, steam refers to water droplets suspended in air while water vapor is invisible. I’ll use the science definition when talking about science. As temperature drops, the maximum percentage of water vapor in air also drops. At some point there will be too much water vapor dissolved in cooling air, causing it to condense (that point is called the dew point). You probably normally think of condensation as something that happens on a glass of iced tea on a hot summer day, but it happens in your oven too! A cold ball of cookie dough going into a hot oven will cause the air around it to cool and the water vapor in that air to condense.
Water vapor gives off an immense amount of heat when it condenses. The more water vapor there is in your oven, the stronger a thermal punch your cool cookie dough or cake batter is going to take from condensation and the quicker it’s going to heat up. A hot, dry oven will take longer to cook food than an oven at the same temperature but full of water vapor. Steam is powerful!
When you put a batch of cookies in your oven, hot air heats the cookie dough in two ways: convection and condensation (see page 143 for definitions). Convection is easy enough to imagine: hot oven air circulates over the surface of cold food, warming it up. (If your oven has a “convection” setting, that means it has a fan inside blowing air around, circulating that air faster. Using convection mode causes foods to cook faster and dry out faster, which is great for crispy pastries and crunchy breads but not so great for steamed buns or custards.)
Professional chefs often use combi steamers—ovens that control both humidity and temperature. Perhaps this will be standard for home ovens someday; until then, most of us are stuck with squirt bottles and pans full of water.
Condensation is tricky to understand because we don’t normally think about water vapor in our recipes (when’s the last time you saw a recipe that says set oven to 50% humidity?!). Changes in your kitchen’s humidity will change how foods cook from one day to the next, speeding up or slowing down how quickly they heat.
There’s no universal perfect humidity. To get a thick crunchy crust on rustic bread or crispy skin on roasted chicken, the surface needs to dry out, so you need a drier oven, at least toward the end of cooking. (Maillard reactions don’t happen when liquid water is around; see page 213.) If you’re making dinner rolls—breads with soft, lighter-colored surfaces—you’ll want a more humid oven. For steamed buns you need an even more humid cooking environment, like a steamer or rice cooker.
Adding humidity is easy enough: as your oven heats, add a baking pan of water on a lower shelf and keep it topped off. Or use a spray bottle and mist your oven before putting your dish in, taking care not to spray the light bulb (it can shatter!). Removing humidity is tougher: using an air conditioner or dehumidifier in the kitchen is your best bet.
Think about the culture and the climate in which a recipe originated. The original bakers wouldn’t be fighting against their environment; they would have adapted recipes and the desired outcome to suit their climate.
Humidity is more important for foods that involve yeast. Yeast and the enzymes it relies on are all temperature sensitive: yeast generates carbon dioxide most rapidly at around 90–95°F/32–35°C. Enzymatic reactions that the yeast relies on speed up as temperature increases, but at some point the enzymes denature and promptly stop working. (Most enzymes are proteins created by an organism and are used to break down other substances; like all proteins, they “cook” too.) Oven spring—the additional rise that dough undergoes when it first goes in the oven—depends on how quickly the surface of the bread dries out, how much sugar the enzymes produce, and how quickly the dough heats up (and thus how long the yeast survives).
The second major issue you’ll face in baking is the weather. Wintertime means lower humidity and colder indoor air temperatures, slowing down the time it takes for yeast to work (try letting the dough rise on top of your fridge or near a radiator). Summer weather brings higher humidity, leading to the chance that cakes won’t develop a strong enough “exoskeleton” and will fall (try using less water). Or it might rain one day (100% humidity, at least at room temperature), but a week later the air might drop down to 50% humidity. That’s twice the difference in the amount of water vapor and a major difference in how quickly things heat up without any change in room or oven temperatures. Careful attention to humidity, rise time, and room temperature can solve baking mysteries.
Have you checked your oven? If not, see the sidebar “The Two Things You Should Do to Your Oven RIGHT NOW” on page 35.
The other reason air is so critical in baking is the physical volume it takes up inside the food. Air expands as it heats up. Because most baked goods “set” with heat, the more air there is to expand, the more space it will take after baking, assuming egg proteins on the inside or flour starches on the outside set enough to create the necessary scaffolding to support everything after cooling.
How air gets into your batters and doughs will end up taking the rest of this chapter to explain. Recipes that use rising agents—anything that generates gas (yeast, baking soda)—rely on them to generate volume with small bubbles, almost always carbon dioxide. Anything without a rising agent, such as popovers, meringues, and soufflés, can rise only by either the expansion of already-present gas or water evaporating into gas. Regardless of the source, understanding and controlling air is an important part of the science of great baking.
Water Chemistry and How It Affects Your Baking
Water is wonderfully weird. There’s lots of trivia about water, some of it obvious (it expands in volume somewhere between 1,600 and 1,700 times when converted to gas, hence the lift it gives in some baking) and some of it brain-smashingly amazing (you can tell the rough latitude a tomato was grown at by examining its water composition).
Tap water isn’t just H2O. Among other stuff, trace amounts of minerals, additives such as chlorine, and dissolved gases can all come pouring out of the faucet and into your doughs and batters. When it comes to yeast and gluten formation (which we’ll cover in the next section), those trace minerals and anything that changes the pH of water will make a difference. You might find that a recipe that works perfectly fine in one location will need tweaking when made elsewhere, due to differences in the water alone!
First, let’s talk about trace minerals. Trace minerals in water—primarily calcium (Ca2+) and magnesium (Mg2+)—occur naturally in water, being absorbed as the water passes through calcium- and magnesium-containing rock such as limestone and dolomite. Our bodies need these minerals; they’ve been present in water since time immemorial. The water supplies in different regions vary, with different ratios and different amounts of dissolved trace minerals, and those changes impact food. (There’s some thought that the different types of teas in the UK evolved from how differences in water supplies changed their flavor. For example, Scotland gets most of its water from surface sources such as rainwater while Southeast England gets most of its water from aquifers, leading to different levels of trace minerals that will interact with compounds in the tea.)
The term water hardness refers to the concentrations of dissolved trace minerals in water, soft water being a low concentration and hard water being high. There’s no exact scale for water hardness because temperature, combinations of minerals, and pH all change how these minerals interact with other things (especially gluten). Researchers generally use parts per million (ppm) of calcium as a measure, so we’ll go with that. As the quantity of calcium increases, water is said to be harder, presumably because the minerals literally “harden” things.

If you’ve ever encountered scale buildup on faucets—the bane of household cleaning—it may be calcium carbonate or calcium stearate. Calcium from hard water can combine with carbon dioxide in the air or with stearic acid from soap; vinegar, being ~5% acetic acid, will dissolve it.
Because hard water has more calcium (and generally more magnesium), it makes gluten tougher, less elastic (elasticity is the ability to spring back into shape), and less able to stretch, all three of which will lead to denser baked goods. Depending upon how hard your water is, you may need to adjust recipes to compensate accordingly.
Water treated with sodium carbonate? Your water will have more dissolved sodium in it and you may need to use less salt to compensate for flavor and texture problems.
Water treated with chlorine? Leave a pitcher of it out overnight for the chlorine to dissipate, lest it interfere with yeast.
If your water is too hard—you’ll know because yeast-based goods won’t ferment as well, breads will come out denser, and vegetables and beans will cook “tough”—try using filtered water as a first attempt. No water filter? Try boiling your water, which will remove any dissolved carbon dioxide and in turn cause calcium carbonate to precipitate out. If neither option works, and your recipe allows for it, see if cutting down on the salt or adding an acid—a squirt of lemon juice (citric acid), a tiny pinch of vitamin C powder (ascorbic acid), or some vinegar (acetic acid)—fixes it.
Range (calcium parts per million) |
Problems |
Fixes |
<60 ppm: Soft water |
Soft, sticky doughs; |
Increase salt |
60–120 ppm: Moderately hard water |
Potentially tough |
Filter water |
>120 ppm: Hard water |
Doughs not rising; toughness |
Increase yeast; add acid; decrease salt; filter water |
Water that’s too soft can produce sticky doughs and present problems for yeast, which, like us, needs minerals to grow and reproduce. If you know you’re adding the right amount of water based on ratios, try adding a modest amount of salt. Too much salt, though, and you’ll land on the “too tough” side of hardness, plus your bread will end up tasting salty!
What about the pH of your water?
If you have alkaline water (pH above 7—also usually hard, but not necessarily) and are baking with yeast, you’ll need to add an acidic ingredient to compensate. Baked goods that rely on yeast need water with a pH below 7, because yeast uses sugar as an energy source and sugar is created from starch by pH-sensitive enzymes (e.g., amylase in flour). Likewise, if your recipe is generating bubbles of carbon dioxide by using baking soda as a base and you have alkaline water, you may need to cut back on the baking soda; otherwise, you might have unreacted baking soda in your final baked goods along with its unpleasant, soapy taste.
You shouldn’t have to deal with water that’s too acidic: the United States EPA (Environmental Protection Agency) recommends a pH of tap water between 6.5 and 8.5. For most of us, the pH of our water isn’t an issue in baking, but it can be for those with especially hard water, which is usually basic.
(PS: Debates about how much salt you should cook beans with often overlook water differences: some ~15% of cooks have too-soft water; then there’s the pH of the water. More salt makes beans cook quicker; more acidic water slows down their cooking. Mushy beans are related to too-long cooking time; flatulence occurs with some beans that are not presoaked and are cooked too briefly. Speaking of boiling salty water, it’s true that salt raises the boiling point, but by so little that that’s not why it can change cooking times. It’s the chemical changes, not the physical changes, that can do that.)

Where you live determines how much gluten will form in your bread dough.
MODIFIED VERSION OF MAP BY US GEOLOGICAL SURVEY, DEPARTMENT OF THE INTERIOR/USGS.
You Must Choose Your Flour, but Choose Wisely
Light, fluffy foods like bread need two things: air and something to trap that air. This might seem obvious, but without some way of holding on to air while cooking, croissants would be as flat as pie crust. This is where your choice of flour comes in.
Flour is, most generically speaking, ground-up “stuff”: usually grain, usually wheat. Flours made from other grains—rice, buckwheat, corn/maize—are also commonly used, and flours made from seeds and nuts give us even more choices, like almond flour, chickpea flour, and amaranth flour.
Wheat flour, as an ingredient, has many properties that professional and industrial bakers need to consider, but the selection of flours available to the home baker is generally limited to a handful based on commodity crops, with the main differences being how much gluten can be formed. Hopefully soon we’ll see a renaissance in wheat flour, just as we have with other ingredients such as apples and coffee beans. (Although, seriously, how many varieties of apples do we need?) Until then, most of us are stuck with just a handful of choices, so here is some wisdom that can aid you in choosing and working with flour, lest your breads turn to dust and blow away (“he chose...poorly,” as the line goes).
Most wheat flour sold in the United States is AP (short for all-purpose) flour, named such because it’s generally suited for most baking tasks. AP flour is made from the endosperm of the wheat grain and creates about 10–12% gluten (by weight) when worked. When you read “flour” in a recipe, this is what you should use. In parts of Europe, flour is classified by ash content, a measure of mineral content. Ash content is determined by which parts and ratios of the kernel are used. Using only the endosperm yields a lower ash content and whiter flour—e.g. Italian “00” (“doppio zero”)—and being more refined is generally ground finer. While there’s no guarantee that a “00” flour will be lower in protein or more finely ground than a higher ash one, most “00” flours are similar to finely ground AP flour.
Wheat allergies and gluten sensitivity are different things—someone can be allergic to proteins in wheat but have no issue with gluten in other flours, and vice versa. If you’re cooking for someone with a wheat allergy, see page 450. For gluten sensitivities, use ingredients that don’t form gluten, such as rice, buckwheat, corn, or quinoa.
You’ll sometimes see recipes call for whole wheat flour or cake or pastry flour instead. With whole wheat flour, the wheat grain’s bran and germ are milled along with the endosperm, so the flour has more fiber (bran!) and creates less gluten (the proteins for gluten come primarily from the endosperm). Cake and pastry flours are similar to AP flour but form less gluten, either because they use softer wheats that have less protein or because of chemical processing (bleaching) that changes the flour.
Gluten gets a lot of attention in baking because it’s what creates structure in baked goods. Gluten is created when two proteins—glutenin and gliadin in the case of wheat—come into contact with each other to form what chemists call crosslinks: bonds between molecules that hold them together. In the kitchen, bakers create this crosslinking by adding water and then mixing, but instead of talking about crosslinks, they speak of “developing the gluten.” During mixing, the two proteins bind together with water, and the resulting gluten molecules in turn stick together to form an elastic, stretchy membrane. That membrane traps air bubbles from ingredients like yeast, baking soda, and even water to give baked goods their height and springy texture.
Understanding how to control gluten formation will vastly improve your baked goods. Do you want a chewy texture? Do you want something with lift and rebound when it’s pressed? Then you’ll need to develop enough gluten to provide the necessary texture and elasticity. If you’re trying to create a fluffy pancake, crumbly cake, or crispy cookie, you’ll want to decrease the amount of gluten, either by reducing the amount of gluten-forming proteins or by adding ingredients that disrupt that gluten, such as butter, egg yolks, and sugar.
Let’s start with the easy part: controlling the amount of gluten by changing the amount of protein. Wheat is the most common source of gluten and creates the highest percentage of it. Different strains of wheat have different concentrations of glutenin and gliadin proteins, based on the growing climate, so varying the source of wheat will vary the amount of protein in its flour. Other grains, like rye and barley, have the necessary proteins but in smaller quantities. Flours made from corn, rice, buckwheat, and quinoa won’t form any gluten.
Phyllo dough—also spelled filo dough—is an unleavened dough used in pastries like baklava. It’s made by mixing flour and water and repeatedly folding and rolling to develop gluten. It’s also paper-thin: the sheets I checked were 0.0065” / 0.175 mm thick. Phyllo dough remains flexible while moist, but becomes brittle when dry. Take care to not let it dry out when working with it and use a spray bottle of water to moisten it if necessary.

Changing the cultivar of wheat, changing the way the flour is milled, or blending in nonwheat flours will change how much gluten exists that will trap air. If you’re used to working with AP flour, substituting whole wheat flour or flours from other grains will reduce the amount of gluten and give you a flatter (possibly still tasty!) loaf. Switching to bread flour (start with 50% by weight and add a little more water) will increase the amount of gluten, resulting in a higher loaf.
What if you want the flavor of a certain type of flour (say, whole wheat flour or buckwheat) but need more gluten? You can add wheat gluten, wheat flour that has had bran and starch removed, yielding a 70%+ gluten content. If you want to swap out AP flour for whole wheat flour, replace 10% of the flour (by weight) with wheat gluten to add back the right quantity of gluten. (If substituting whole wheat flour for regular flour, you’ll also want to use extra water—the bran and germ will absorb it—or decrease the amount of flour; either way, let the dough rest twice as long.)
Choosing the right types of flours is the easy way of controlling how much gluten exists in your baked goods. Use wheat flours higher in the necessary proteins to create more; use softer wheat flours or other types to reduce it. The other way is more complicated but sometimes necessary: prevent the glutenin and gliadin from forming crosslinks, or break those crosslinks after they form.

Gluten levels of various grains and common flours. Besides wheat, both barley and rye form noticeable amounts of gluten, although rye also contains substances that interfere with its ability to form gluten.
Why biscuits are Southern food and Wonder Bread came from the Midwest
Colder climates favor flour cultivars with more glutenin and gliadin proteins. Flour in, say, France won’t be identical to that grown in the US, and different regions will differ, too. Where your flour comes from will change its properties. Since different mills use different flours, try baking with a couple of different brands.

Consider the following tips for managing gluten levels:
Use fats and sugar to reduce gluten formation.
Cookies crumble and cakes are tender because of fat and sugar, both of which prevent gluten from forming. Oil, butter, and egg yolks all add fat to doughs, preventing crosslinking, while sugar is hygroscopic and snaps up the water before gluten does. If your baked goods aren’t coming out with a desirable crumbly texture, one possible fix is to increase the fats (hence “one egg plus one egg yolk”) or sugar (if not too sweet).
Use mechanical agitation and rest time to develop gluten.
Mechanical agitation (a.k.a. kneading) physically rams proteins together, increasing the odds that they’ll form gluten. Letting dough sit also develops gluten by giving wheat’s glutenin and gliadin proteins time to combine as the dough subtly moves. This is why the no-knead bread recipe on page 261 works.
Don’t overmix.
Too much kneading weakens gluten. Mixing a batter or dough initially develops gluten by bringing the necessary proteins together, but after a few minutes, enzymes in the flour will cause the gluten to break down.
Ever wonder why some recipes tell you to mix “just until incorporated” (muffins) and others say “mix for a few minutes” (breads and dinner rolls)? Researchers use Farinograph charts to check dough viscosity over time as it’s mixed, and one look at such a chart explains it all. It takes about a minute of mixing for a flour-and-water dough to have formed enough gluten to give a chewy, breadlike texture. Mixing less than that will avoid that texture—good in muffins, not so good in breads. On the other extreme, mixing for more than a few minutes will cause enzymes in the flour to break down the gluten, deteriorating below the magic “500 Brabender Unit” threshold. (Brabender Units are an arbitrary measure of viscosity.) These one-minute and five-minute rules will vary depending upon your dough and ingredients, but they’re good rules of thumb.

Brabender Units versus time (in minutes) shows the viscosity of a dough as it’s mixed.
Pay attention to water.
Quantity matters: you need enough water for gluten to form, but add too much and the proteins won’t bump into each other. In bread dough, aim for about a 0.60:0.65 ratio of water to flour (about 30–35% water by weight); more than that, and you’ll get large, irregular holes, which can be nice in rustic bread, but not sandwich bread. Flours with more gluten will absorb a little more water, so adjust the amount of water accordingly. Due to evaporative cooling, batters with too much water will end up with surface issues and stall out, leading to cakes that fall after baking; if you see that, cut back on wet ingredients. You’ll face similar issues if the humidity is too high, so reduce wet ingredients in this case too.
Ingredients like sugar, flour, and salt all absorb atmospheric moisture, so changes in humidity will change the amount of water they bring to the recipe. Ideally, buy and store them in airtight containers; otherwise, on humid days, reserve a fifth or so of your liquid ingredients and add in what’s necessary to achieve consistency.
Pay attention to minerals and salt.
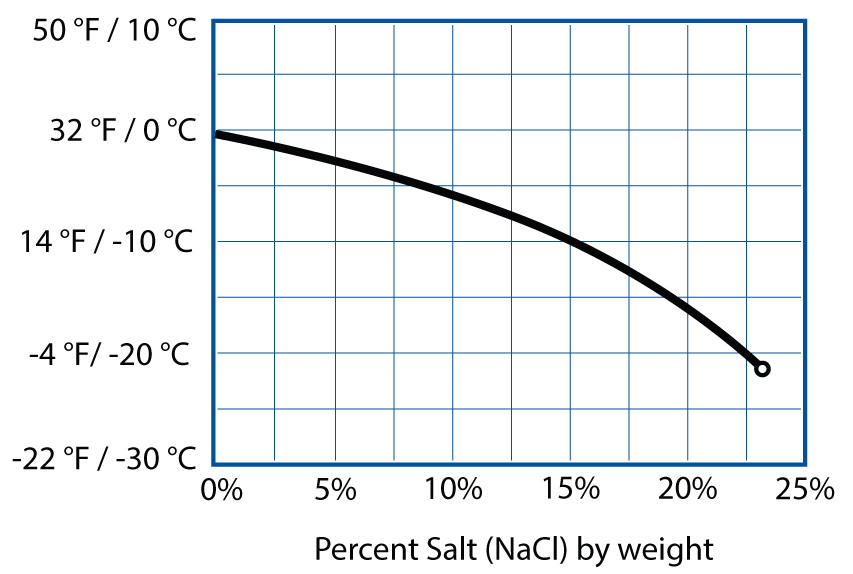
Gluten also needs some amount of calcium or magnesium from dissolved minerals in water; you can counterbalance too much or too little by adjusting the amount of salt in your dough. As for salt, there’s some wiggle room, but in breads, try to keep salt at between 1% and 2% of the total weight for optimal lift. Finally, be mindful of high pH levels: if your water is alkaline, add an acid (vitamin C, lemon juice, vinegar). (See page 240 for more on how water impacts your baking.)

Loaf volume (cc) by percent salt (NaCl).
Error Tolerances in Baking
In pastries and cakes, the error tolerance in measurement—the amount you can be off by and still have good results—is much tighter than in many breads and savory dishes. Even small changes in the ratios between flour, water, sugar, and fat will cause large changes in how some baked goods turn out.
Without enough water, glutenin and gliadin won’t properly form gluten, which is good for scones, biscuits, and pie shells but bad for higher-gluten goods like bread. But too much water also creates problems: bread will end up with large air pockets and cakes won’t set correctly and will collapse in on themselves.
Likewise, if you add less shortening than intended for something like a cookie or pie crust, more gluten can form and give you a tougher pie shell. If you use too much shortening, though, doughs won’t rise as much and will turn out short; that’s how shortbread got its name.
Consider the ingredients for the following two double-crust pie dough recipes.
Joy of Cooking |
Martha Stewart’s Pies & Tarts |
||||||
Baker's % |
Weight |
Volume |
Ingredient |
Baker's % |
Weight |
Volume |
Ingredient |
100% |
240g |
1 3/4 cups |
flour |
100% |
300g |
2 1/8 cups |
flour |
60% |
145g |
2/3 cup |
shortening |
– |
– |
– |
(no shortening) |
11.25% |
27g |
2 tablespoons |
butter |
76% |
227g |
1 cup |
butter |
25% |
59g |
1/4 cup |
water |
19.7% |
59g |
1/4 cup |
water |
0.8% |
2g |
1/2 teaspoon |
salt |
2% |
6g |
1/2 tablespoon |
salt |
– |
– |
– |
(no sugar) |
2% |
6g |
1/2 tablespoon |
sugar |
The numbers in the first and fifth columns are “baker’s percentages,” which normalize the quantities relative to the quantity of flour. Among other things, this makes it easy to compare differences between recipes.
Comparing these two recipes, you can see that the ratio of flour to fats ranges from 1:0.71 to 1:0.76, and the Joy of Cooking version calls for a higher percentage of water.
However, butter isn’t the same thing as shortening. Butter is about 13–19% water and ~1% milk solids; shortening is only fat. With this in mind, look at the recipes again. The Martha Stewart version has 76g of butter (per 100g of flour), for about 64g of fat. Joy of Cooking’s version, with shortening and butter, has 69g of fat per 100g of flour. The quantity of water is also roughly equal between the two once the water present in the butter is factored in.
If you’re following a recipe that doesn’t give a weight for flour, you’ll need to guess how many grams of flour per cup the writer intended. If the recipe came from the US, try using 140 grams as a starting guess; if it’s of European origin, try 125 grams.
When following recipes with tight error tolerances—usually pastries, rarely breads—always use a digital weight scale. This one change in your approach to baking will have the biggest impact on how things turn out.
Yeast
We’ve talked about how flour and water create gluten, and how wonderful gluten is for trapping air, but how do we actually get the air in there to begin with? Biologically based leaveners—primarily yeast, but also bacteria for salt-rising breads—are surely the oldest method for generating air. Presumably, a prehistoric baker first discovered that a bowl of flour and water left out overnight (much to the annoyance of whoever was washing the dishes) would ferment. Bread was so critical in the Roman Empire that a representative of the baker’s guild had a seat in the senate. Agriculture has been involved in politics for a long, long time. Using yeast in baking goes back even further.
There’s nothing magical about the strains of yeast we use, other than someone taking notice of their flavor and thinking, “Hey, this one tastes pretty good; I think I’ll hang on to it!” Friendship bread—the “chain letter” of yeast—has been passed around for decades.
Yeast is a single-celled fungus that consumes sugar and other sources of carbon and creates carbon dioxide, ethanol, and other byproduct compounds. All three of these make yeast useful: carbon dioxide gives lift, ethanol sterilizes and preserves beverages, and the byproducts give sourdough breads their distinctive flavors. Over the years we’ve “domesticated” many strains of yeast by selective breeding: Saccharomyces cerevisiae—more simply called baker’s yeast—is used in baking; other strains are useful in beer production (usually Saccharomyces pastorianus, named after Louis Pasteur—lucky guy).
Before domestication of yeast, bread makers would have relied on any ambient yeasts present in their environment, saving and sharing successful strains. Not that the “roulette gambling method” of picking your yeast is recommended when you’re working in your kitchen—leaving a bowl of unseeded dough out has a decent chance of ending up poorly, with a foul strain of yeast generating unpleasant-tasting sulfur and phenol compounds, or possibly worse. This is why you should add a starter strain: providing a particular strain ensures that it will dominate any other yeasts that might be present in the environment. (If your breads rise too quickly—you’ll know by loaves failing to rise and being porous—cut back on the amount of starter you use.)
Like any living critter, yeast prefers to live in a particular temperature zone, with different strains preferring different temperatures. Baker’s yeast works best at room temperature (55–75°F / 13–24°C). Other strains used in cooking, primarily for brewing (lagers and steam beers), prefer cellarlike environments of around 32–55° F / 0–13°C. Regardless of where your culinary adventures take you, keep in mind the temperature range that the yeast you’re using likes. Too cold, and the yeast will slumber and hardly rise; too hot, and it’ll die.
The Quest for Great Pizza
How can a book called Cooking for Geeks not have a spread on pizza? Regardless of whether or not you identify as a geek, pizza is as much fun to make as it is to eat. You should learn to make it. Really. The stuff you can get delivered to your door is far, far inferior to what you can make at home. There’s a time for a tomatoey, cheesy slice of pizza—usually around 2 a.m. on a weekend. The rest of the time, though? A well-lived life includes relishing the nuances of great, homemade pizza.
First, the pizza dough. While you can buy pizza dough at your grocery store, I get better results when I make dough from scratch, and this is the way my dad taught me. I’m so keen on making your own pizza dough that I’ve included two different recipes: a simple no-knead version on page 271, and the yeast-free recipe on page 286 for the impatient (trust me, I understand).

Second, the oven. Oven temperature determines how the pizza’s crust will set up. Place your baking stone on a middle rack, and then preheat your oven to 375°F / 190°C for a doughier crust or at least 450°F / 230°C for a crispier one. (See page 370 for high-heat pizzas.)
Par-bake your dough. Par-baking isn’t part of most pizza recipes, but I’m a fan. By cooking the dough first, you avoid the risks of soggy dough and burned toppings.
1 Sprinkle a large cutting board with flour.
2 Take 1 pound (450g) of the dough and form it into a ball using your hands, kneading and folding it. The dough should be just slightly sticky, but not so much that it actually remains stuck to your hands. If it’s too sticky, dredge the dough in flour.
3 Continue to work the dough until it reaches a firm consistency and has good elasticity when stretched.
4 Work the dough into a flat, round disc, and then roll it into a circle or rectangle.
5 Transfer the pizza dough to the oven, carefully picking it up and laying it onto the baking stone (use a pizza peel or sturdy sheet of clean cardboard if needed).
6 Let the pizza bake for 3–5 minutes, until the dough has set. If the dough puffs up in one place, use a chef’s knife to poke a small hole in the bubble and then use the flat side of the knife blade to push the puffed portion back down.
7 Remove the par-baked pizza from the oven and set it back onto your cutting board.
Toppings. Add sauce and toppings. Choosing these is the art of pizza: it is a blank canvas upon which you can paint whatever flavors you’re craving. Some general thoughts:
• You can’t go wrong with a thin layer of tomato sauce and some slices of good mozzarella, topping it with basil leaves after baking.
• If you’re out of tomato sauce, anything from a thin coating of olive oil to a white cheese sauce will work (see Béchamel Sauce on page 105).
• For toppings such as onions and sausage, sauté them before placing them on the pizza. Cooking the dough and toppings separately removes all the headache of trying to get everything to cook at the same time, leaving just three goals: melting the cheese (assuming you’re using some) to fuse the ingredients together, browning the edge of the crust, and browning the top surface of the toppings.
Cooking. Finish cooking by transferring the dressed pizza into the oven and baking it until the pizza has begun to turn golden brown, about 8–12 minutes. When in doubt, overcook it: a beautifully browned crust (I didn’t say blackened) looks and tastes great.
Bacteria
Bacteria are used in all sorts of food—yogurt, kimchi, cheese, chocolate—and bacteria can generate gas, so it’s not that far of a leap to wonder how to create bacteria-leavened foods. Alas, using bacteria as a leavening agent is downright rare.
The only recipe I’m aware of is salt-rising bread, possibly named from the use of a mound of warm salt to keep a bowl warm overnight in cooler climates. Salt-rising bread used to be popular in some Midwestern communities and was literally prized: the Iowa State Agricultural Society awarded one Mrs. M. L. Harding of Des Moines $5 for her salt-rising bread in 1889. (The last state fair I went to featured deep-fried Twinkies slathered in strawberry jam for $5—how times have changed!)
Leavening relies on the bacteria Clostridium perfringens to generate hydrogen for lift. While the idea of flammable bread is oddly appealing, the problem for me is C. perfringens: it’s the same bacteria that causes millions of cases of foodborne illnesses annually. To be fair, there are multiple strains of C. perfringens, and no illnesses have been linked to salt-rising bread. Researchers checked a few samples for relevant toxins and found nothing, attributing the lack of toxins to the particular strain, but also noted “the very real possibility” that other batches could contain the wrong strain. If you do want to give it a try, search online for Harold McGee’s article “The Disquieting Delights of Salt-Rising Bread.”
Of course, bacteria show up elsewhere in baking bread all the time: lactobacillus is what gives sourdough bread its distinctive flavor, with different species creating different flavors based on the byproducts they generate during fermentation. Lactobacillus has other benefits, too: it reduces the potential for mold growth in the baked loaf and improves the nutritional aspects.
Making sourdough bread is easy enough: add sourdough starter and water to whatever flour you like, and knead away. Making the sourdough starter, though, takes longer. Sourdough starter—sometimes called mother dough—is commonly created by gambling on the wild bacteria and yeast in your environment. Mix equal parts by weight of water and flour in an open container, cover with cheesecloth or a towel to keep flies out while allowing air to flow, stir twice daily, start feeding a few tablespoons of flour and water after a few days. After a week, you should have something that smells like sourdough; if not, try again. This “wild fermentation” method usually works, and I greatly respect the tradition and culture (pardon the pun) that it comes from and the practitioners who use it.
Sourdough Starter
There is a small chance that the strain of bacteria that settles into a wild starter won’t be safe—it needs to produce enough acetic acid to drive the pH low enough to prevent other bacteria from cohabitating. Using good strains of Lactobacillus and commercial yeast removes that risk.
Mix together 2 cups (500 mL) lukewarm water, 1 teaspoon (5g) yeast, 1 tablespoon (12g) sugar, and ¼ cup (60g) plain yogurt (with live cultures) and then knead in 2 cups (280g) of bread flour. Stir a few times daily, following the wild fermentation method.
Baking Soda
While yeast allows for the creation of many delicious foods, it has two potential drawbacks: time and flavor. Commercial bakers with high volumes and those of us with limited time to play in the kitchen can’t always afford to wait for yeast to do its thing. Then there are the flavors and aromas generated by yeast, which clash with the flavors in something like a chocolate cake. The simplest answer to these problems is baking soda:
A bicarbonate (HCO3–) that’s bound with a sodium atom (related compounds use potassium or ammonium to similar effect). When added to water, the bicarbonate dissolves and is able to react with acids to generate CO2.
Anyone who’s done the science fair project of using vinegar and baking soda to make a volcano can tell you that the mixture generates a whole lot of gas really quickly (along with a whole lot of mess). But in the kitchen, baking soda remains one of the bigger mysteries for bakers. How is it different from baking powder? How do you know which one to use?
The standard answer goes something like: “Baking soda reacts with acids, so use it only when your ingredients are acidic.” As simple explanations go, this covers most of the cases in cooking. But baking soda is more complicated—it also reacts with itself under heat—and worthy of a brief digression into the chemistry. I promise this’ll be short.
The baking soda you buy in the store is a specific chemical: sodium bicarbonate, NaHCO3. Without something for sodium bicarbonate to dissolve into, it’s an inert white powder. Upon getting wet—any moisture from any ingredient will do—the sodium bicarbonate dissolves, meaning that the sodium ions are free to run around separately from the bicarbonate ions.
The sodium in sodium bicarbonate is just there to transport the bicarbonate to your food. It does make the food slightly saltier, incidentally, which is why industrial food manufacturers will sometimes use things like potassium bicarbonate: potassium is good for you, and this avoids the sodium for people on a low-sodium diet.
This is where the alkaline and basic nature of things comes in. Most of us are familiar with the pH scale (the H stands for hydrogen; it’s unclear what the p stands for; “power” and “potential” are the best guesses). The pH scale is a measure of the amount of available hydrogen ions in a solution. Chemicals that affect the number of hydrogen ions can be classified in one of two ways:
Acids (pH below 7)
Proton donors—chemicals that increase the number of hydronium ions (H3O+; the hydrogen binds with a water molecule) in the solution
Bases (pH above 7)
Proton receivers—chemicals that bind with hydronium ions, reducing their available concentration in a solution
Baking soda’s bicarbonate ion has an interesting property called amphotericity: it can react with either an acid or a base. In the kitchen, very few things have a basic pH—egg whites, maybe your tap water in some situations, and that’s pretty much it—so you can safely ignore baking soda’s ability to react with bases and just think of it as something that reacts with acids.
In a glass of pure water with a spoonful or two of baking soda, there’s not much for the bicarbonate ions to interact with, so they just float around and taste generally nasty. But if you were to add a spoonful of vinegar—which contains acetic acid—to that glass, the bicarbonate ions would react with the acetic acid and generate carbon dioxide. Depending upon the amount of bicarbonate you started with, after you added the vinegar to the glass it would be in one of three states (none of which involves being half full or half empty):
• Bicarbonate ions still available; no acetic acid ions available
• No bicarbonate ions available; acetic acid ions still available
• Neither bicarbonate nor acetic acid ions freely available
Baking soda doesn’t need an acid to generate carbon dioxide; heat will do it, too. Boil some water and toss in a spoonful of baking soda. The sodium bicarbonate will break down and foam up.
In baking, it’s this last state—a neutral balance—that you want to reach. Too much baking soda can leave food with a soapy, yucky taste. Not enough baking soda will leave food slightly acidic (which is okay) but miss out on possible lift (which is probably not okay—your food will be flat). To get the “just right” state, I’ll repeat one of my favorite quotes: “Dosage matters!”
Obviously, we don’t bake “baking soda and water”; take a sip of baking soda in water sometime if you wonder why. Baking soda is usually called for in recipes that also use more acidic ingredients: fruit juices, buttermilk, molasses. Sugar and flour are slightly acidic, but not enough to normally merit much baking soda. (We’ll get to baking powder in the next section.) If you make substitutions in a recipe—say, you don’t have buttermilk so you use regular milk in its place—pay attention to the corresponding change in pH. In the case of buttermilk, you’d need to add one tablespoon (15 mL) of white vinegar or lemon juice for every cup (240 mL) of milk (and reduce the amount of milk by a tablespoon); this will provide the acids necessary for the baking soda to react with. Of course, you’ll be missing the pleasantly tangy flavor of the buttermilk, but at least you won’t be eating flat waffles!
How much baking soda to use depends on the pH of the ingredients in your dish. Short of testing the pH (see http://cookingforgeeks.com/book/ph-tester/), experimentation is the easiest way to work out the ideal ratio: increase the amount of baking soda in your recipe until you get the desired lift or can taste the baking soda. If you’re still not getting enough lift at this point, switch to adding baking powder. This balancing act between acids and baking soda isn’t a problem with baking powder; the ratio of acids to bicarbonate in the powder is preset by the manufacturer, as we’ll see in the next section.

Knowing the pH of your ingredients will help you understand when to use baking soda.
Why do some recipes call for sifting ingredients?
Sifting used to be necessary to remove husks, bugs, and whatever else ended up in flour, but those days are long gone. And weighing ingredients removes the need to deal with density differences. Sifting does aerate flour and quickly mixes in other dry ingredients, both of which can be done more easily by whisking. If you do need to sift—say, to really mix cocoa powder and flour—you can use a strainer over a bowl.

The Science of Crispy, Chewy Cookies
One of the biggest advantages that home bakers have is time. Commercial products are made at least half a day in advance, usually longer, so manufacturers have to come up with clever tricks to mimic what happens in your kitchen. What if we could learn those manufacturing tricks and try them ourselves?
A freshly baked cookie—“just like Mom used to make!”—is crispy on the outside and chewy in the middle. Some enterprising researchers at UC Davis proved this by building an oven inside of their MRI machine and then baking cookies in it, using the MRI to scan what happened to the water inside the dough as it baked. (I’d love to see the grant application for that one.)
A dozen cookies and MRIs later, the researchers had proof: the edge of the cookie definitely dries out—and to a remarkable extent—during baking. After a day or two, however, the moisture evens back out and the cookies revert to having a uniform ductile, soft texture, losing that fresh-baked quality. (And a week later, the sugars recrystallize—that’s how the cookie crumbles!)
Crispy-chewy chocolate chip cookies are incredibly hard to make, at least commercially. But good luck calling up and asking the elves making cookies at any of the large commercial manufacturers for tips: these sorts of things are trade secrets, with stories of industrial espionage that spy novelists and Jason Bourne would appreciate. Luckily for us there is one place where industry has to spill its secrets: patents. And in this case, US Patent #4,455,333 (http://cookingforgeeks.com/book/cookie-patent/) has the answers.

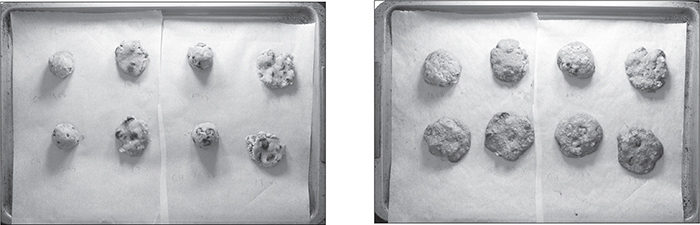
Each patent includes a background description written to set the stage for the invention, and those descriptions can be a great source for a clear summary of “how things work.” From reading a few cookie-related patents, you’ll quickly learn that soft cookies have a water concentration of 6% and higher, while crispy cookies are drier. This makes sense—moisture is a key variable in texture. So how can you control the moisture in your cookies?
Check out the ingredients listed on packaged crispy cookies as compared to the same brand’s packaged chewy cookies. In the brand I checked, cornstarch and molasses show up only in the chewy ones.
Crispy cookies are actually the easier of the two to make: create a dough that holds less water, or bake your dough longer, and the final product will be drier. To make chewy cookies, you have to formulate the dough so that it holds on to more water as it bakes, but you can’t just add more water into cookie dough (that’s what causes cookies to flatten out and results in burnt, feathered edges). Here are the common ways of making cookies chewier:
Substitute glucose/fructose-based sugars for sucrose. In baking, sugars dissolve in water from eggs and butter. As the dough heats up, the sugar water forms a syrup, but—this is the key!—different types of sugars will absorb different amounts of water (the solutions saturate at different points). Sucrose molecules, being roughly twice the size of fructose and glucose molecules, don’t create a solution with as much water, cup for cup. This means a dough that uses simpler sugars will hold on to more water. Lots of white sugar (sucrose)? You’ll get crispy cookies. More brown sugar (sucrose, glucose, and fructose)? You’ll have chewier cookies. Corn syrup? You’ll get even chewier cookies (it’s 100% glucose—high-fructose corn syrup is different from what you buy at the store). Glucose and fructose sugars are monosaccharides—the simplest form of sugar—and will keep more moisture in the cookie, so any source of those will work.
Everyone has an opinion about how gooey, chewy, or crispy a cookie should be. I’ve had one person insist on eating almost-raw “6-minute cookies”—baked at 350°F / 180°C for 6 minutes—while serious milk-dunkers wouldn’t consider anything less than a 15-minute cookie acceptable.
As a rough rule of thumb, for a ½-ounce (14g) cookie baked at 350°F / 180°C:
• 7–9 minutes: gooey
• 10–12 minutes: chewy
• 13–15+ minutes: crispy
If your cookies aren’t coming out the way you like, in terms of gooey-chewy-crispy, change how long you’re baking them. Using the same dough, crispy cookies will take about 25–30% longer to bake than chewy cookies.
If you want really crispy, thoroughly golden brown cookies, drop the temperature to 275°F / 140°C and bake for about 30 minutes.
Add cornstarch. Cornstarch doesn’t dissolve in cold water, but as it heats up it will gelatinize, absorbing water, and prevent that water from leaving the cookie as it bakes. (Speaking of patents, there’s one that adds a ground-up gel, something sort of like Jell-O, into the dough—yet another clever trick for chewy cookies.)
Use bread flour. Gluten, too, will increase chewiness, as its elastic nature means that the baked good won’t fracture and break. Using a higher-gluten flour will modestly aid you, although it’s not common in chewy dough recipes; there’s a lot of sugar and fat in the dough to get in the way. Melting butter affects this variable: the water from the butter, when melted, will help with gluten formation (see page 249 for more on controlling gluten).
Bake them for less time. In addition to making dough that holds on to water better, there’s another obvious trick for making chewier cookies: don’t bake the cookies as long! (Chilling the dough is a related tactic, but you could just bake them for less time.) I looked at the baking times listed for the first six recipes I found online for “chewy chocolate chip cookie recipe”; the average bake time was 12 minutes, 20 seconds. “Crispy chocolate chip cookie recipe”? 14 minutes, 55 seconds—a full 2½ minutes longer! (The average temperatures were only a few degrees off, essentially equivalent.)
In reality, chewy versus crispy cookies ends up being a balancing act of all of these tricks, along with subtler tactics, such as tweaking the dough’s pH or, depending upon the type of cookie, including humectants such as raisins, which hold on to water.
Baking Powder
Baking powder solves the “balancing act” problem I wrote about when describing baking soda. By including acids alongside baking soda, baking powder eliminates the need to balance the ratio of acidic ingredients:
A self-contained leavening system that generates carbon dioxide in the presence of water, baking powders by definition contain a baking soda and acids that react with the baking soda.
Because the acids are mixed into baking powder, the types and quantity can be optimized. Baking powder, at its simplest, can be made with just one type of bicarbonate and one type of acid. Baking powders are typically fancier than this, though. Different acids have different rates of reaction and different reaction temperatures, so using multiple types of acid creates a baking powder that’s essentially time-released. This isn’t just clever marketing: in baked goods, if the CO2-generating reaction occurs too slowly, you’ll end up with a dense, flat product. And if those reactions happen too quickly, the food won’t have time to properly set to hold on to the gas, resulting in outcomes like collapsed cakes.
Baking Powder Substitute
Mix 2 parts cream of tartar to 1 part baking soda. Cream of tartar—potassium hydrogen tartrate—will dissolve in water, freeing tartaric acid (C4H6O6) to react with the sodium bicarbonate.
Double-acting baking powder—the stuff you’ll find at the grocery store—uses both slow- and fast-acting acids to help prevent these types of problems. Fast-acting acids, such as tartaric acid (in cream of tartar) and monocalcium phosphate monohydrate, can work at room temperature; slow-acting acids, such as sodium aluminum sulfate, need heat and time to release CO2.
As long as the ratio of ingredients in your baked products is roughly correct and you’re baking within an acceptable temperature range, baking powder is unlikely to be the culprit in failed baking experiments. The different acids used can impart a taste—some people find that baking powder made with sodium aluminum sulfate tastes more bitter—so if you’re experiencing an “off” taste, check the ingredients list and choose another product accordingly. If you’re seeing unexpected results with a commercial baking powder, check whether your ingredients are highly acidic. Acidity impacts baking powder; more acidic ingredients in a recipe will require less baking powder. If that doesn’t turn up any suspects, check how long it has been since the baking powder was opened. Even though commercial baking powders contain cornstarch, which absorbs moisture to extend the shelf life, the chemicals in baking powder will eventually react with each other. Standard shelf life is about six months after opening.
Yeast-Free Pizza Dough
This quick-rising pizza dough is especially handy if someone has a yeast allergy or if you’re craving pizza in the next hour. Whisk 3–4 cups (420–560g) of flour with 1 teaspoon (6g) of salt and 2 teaspoons (10g) of baking powder. Add 1 cup (240g) of water and knead to create a dough that has roughly a 66–75% hydration level. Let rest for 15 minutes before using.
Egg Whites
Whisked egg whites are the Styrofoam of the culinary world: besides acting as space fillers in cakes, waffles, and soufflés and as insulators in desserts like lemon meringue pie, when overcooked, they taste kind of like Styrofoam, too. All metaphors aside, egg whites are much more forgiving than many cooks realize. With a little attention spent on understanding the chemistry and a bit of experimentation, you can easily master egg white foams.
Whisked egg whites work by trapping air within a liquid, creating a foam: a mixture of a solid or liquid surrounding a dispersion of gas; that is, the gas (usually air) is dispersed through the liquid or solid, not in a single big cavity. Bread is a solid foam; whipped egg whites are a liquid foam.
Unlike yeast, baking soda, or baking powder, all of which rely on the chemical makeup of the food, egg whites hold on to air based on their physical properties. You can’t just add mechanical leaveners—typically whisked egg whites, but as we’ll see later, also egg yolks and whipped cream—to a dish without considering the impact of the moisture or fat that they also add. Adding these types of ingredients can throw off the ratios between flour and water or between sugar and fats.
The key to understanding egg whites is to understand how foams themselves work. Whisking egg whites turns them into a light, airy foam by trapping air bubbles in a mesh of denatured proteins. Since regions of the proteins that make up egg whites are hydrophobic—literally, “water fearing”—they normally curl up and form tight little balls to avoid interacting with the water. But when whisked, those regions of the proteins are slammed against air bubbles and unfold, and as more and more proteins are knocked against an air bubble, they form a layer around the bubble and essentially trap it in the liquid, creating a foam that’s stable.
There are a few things that can go wrong in whisking egg whites: fats interfering with their formation; overwhisking leading to their breakdown; or leaving them to sit too long, which allows water in the foam to drag proteins away as it drains out. These issues won’t impact some uses of egg whites. If you’re adding whites into waffle batter, for example, any water weeping out from whisked whites will be absorbed by the batter. But in meringues, that water will form a puddle around the cookie as it bakes—not good.
Oils, especially from egg yolks or any trace oils in the whisking bowl, prevent egg whites from being whisked into as stable a foam because they’re also able to interact with the hydrophobic sections of the proteins. While many recipes admonish you to not get a drop of yolk in your whites, a very small amount won’t ruin egg whites’ ability to foam—but it can change how stable they are before being set by baking. (There’s an old paper that says one drop of yolk decreases the potential volume of a foam made from a single large egg white from 135 mL to 40 mL—perhaps true in some industrial applications, but when I tried it in my kitchen, nothing close to that decrease happened.)
When whisking, take care to not overwhisk. Doing so will cause the formation of smaller and smaller air bubbles, which will decrease the flexibility and elasticity of the foam and cause it to become more unstable. Egg whites whisked to dry peaks—billowy, almost cloudlike shapes on a whisk—won’t expand as much in baked goods; taken too far, they’ll become brittle.
There are some culinary tricks for increasing the stability of egg white foams. Some recipes call for adding sugar or cream of tartar early in the whisking process. These ingredients don’t interfere with the formation of protein-based foams because they don’t interact with the hydrophobic sections. Small amounts of acids will even help stabilize the foam and aid in baking by increasing the temperature at which the egg white proteins set, allowing for more air expansion.
When it comes to working whisked egg whites into other ingredients, like a batter, fold them in using a flat spatula, putting part of the foamed egg white on top of the batter and then cutting through the mixture and turning up the heavier batter on top of the whisked whites. Once the egg whites are foamed up, it takes quite a bit of effort to get them to break down. Exposing the whites to fats before whisking can be a problem, but once the eggs are whisked, they’re much more resilient. Try whisking an egg white to soft peak stage, then adding ½ teaspoon (3g) olive oil and continuing to whisk. It might surprise you how long it takes before the oil starts to noticeably interact with the foam, and even then, the foam remains mostly stable.
Making the Most of Whisked Egg Whites
Whisked egg whites trap air bubbles inside a tangled net of egg white proteins to create an egg white foam, but the way those proteins are tangled and how the whites are used in cooking changes how much volume the whisked egg whites can provide.
The physics of egg white foams is fascinating. Foams are colloids, mixtures of different substances. We’ll cover these more later (see page 379), but for now, just know there are two types: liquid-air foams and solid-air foams. Bread is a solid-air foam; whisked egg white is a liquid-air one. It’s the liquid in egg white foams that presents the challenges of great whisked egg whites.
Egg white foams have two variables: capacity (how much air the foam can hold) and stability (how much the volume decreases over time). Capacity and stability are primarily determined by the size of the air bubbles, the viscosity of the liquid, and how thick or thin the walls between adjacent air bubbles are. How you control these things is another matter.
Acids and cream of tartar
Just as the pH of egg whites changes how easily boiled eggs peel (see page 193), it also changes the volume of egg white foams. Older eggs won’t foam up as much. Adding an acid fixes this, but decreases the foam’s stability. Cream of tartar is commonly used because of its mild taste (try licking a finger lightly dusted with some; it’ll taste only mildly sour after a few seconds); other acids, like citric acid via lemon juice, also work but can impart too strong of a taste. Any time a recipe depends on whisked egg whites for volume and you’re stuck with older eggs, add a pinch of cream of tartar—1/8 teaspoon (0.5g) per egg white.
Sugar
The liquid in the foam structure slowly drains down due to gravity, so anything that slows that drainage increases the stability. Adding sugar makes liquids more viscous but also increases how long it takes to whisk whites to optimal volume. If you’re using whisked whites in a recipe that calls for sugar, try splitting the sugar between the components.
Water
Adding more water to egg whites decreases viscosity, so it’s no surprise that adding it will decrease stability. However, adding water—up to ~40% by weight—will increase capacity, which can be useful in quickly cooked recipes.
Choice of bowl
Fats interfere with the development of egg white foams, leading to a smaller capacity to hold air. Because different materials retain fats differently, what you whisk your whites in can change the results.
Avoid plastic bowls. Plastic is chemically similar enough to lipids that they stick to it and are impossible to completely wash away. Whisking egg whites in a plastic bowl reduces their volume because of this oil lingering in the bowl. (Of course, it’s fine to whip cream in a plastic bowl; more fat isn’t going to interfere with its fat-based foam structure.)
Stainless steel and glass bowls are fine to use. They won’t hold on to problematic fats, assuming you’ve washed them well. Some metals, like copper, will react with proteins in the egg white—in a good way!—leading to a more stable foam. (The same chemistry that makes stainless steel stainless also means it won’t give off any metal ions.) It’s not a subtle effect: when I whisk egg whites in a copper bowl, they’re definitely easier to work with. It’s not copper specifically: zinc and iron can have a similar effect, although reportedly lead to a red tint. In theory any noble metal, even very unreactive ones like silver and gold, should react with the sulfur in the egg whites. (Harold McGee investigated this with silver and found good results; I have yet to find someone who’s tried it in a gold or rhodium bowl.) Copper bowls are expensive, but if you find you’re whipping up egg whites a lot, it’s probably worth breaking down and spending the money on one. (If you have a gold bowl, my mailing address is...)
Egg Yolks
If Eskimos have N words for describing snow, the French and Italians have N+1 words for describing dishes involving egg yolks. Egg yolks are used in almost all cultures for many purposes, from sticking bread crumbs to fish to adding a shiny gloss to baked goods. What might not be evident is that egg yolks, just like egg whites, can also be used to create airy foams by trapping air bubbles.
Egg yolks are much more complex than egg whites: they’re ~51% water, ~16% protein, ~32% fat, and ~1% carbohydrates, while egg whites are only protein (~11%) and water. In their natural state, egg yolks are an emulsion: a mixture of two liquids that are immiscible—that is, unable to mix (think oil and water). Mayonnaise is the classical culinary example. In egg yolks, the fats and water are held in suspension by some of the proteins, which act as emulsifiers—compounds that can hold immiscible liquids in suspension. For more on the chemistry of emulsions, see page 429.
Like egg white foams, egg yolk foams trap air with denatured proteins that form a mesh around air bubbles. Whisking yolks won’t form a foam, though; to denature the proteins in the yolk you’ll need to use heat. The optimal temperature for egg yolk foam creation is 162°F / 72°C. Temperatures hotter than that cause the proteins to coagulate, leading to a loss of air and affecting the texture.

Whipped Cream
Unlike eggs, in which proteins provide the structure for foam, cream relies on fats to provide the structure for a foam when whipped. During whisking, fat globules in the cream lose their outer membranes, exposing hydrophobic portions of the molecules—you’re literally stripping off part of the surface of each microscopic bubble of fat. These exposed parts of the fat globules can then either bind with other fat globules (butter!) or pack around an air bubble by aligning the stripped region with the air, creating a dense air-filled foam once enough of them have been aggregated together.
Another technique for “whipping” cream is to pressurize it with gas and then spray it. If you’ve ever used a can of whipped cream from the grocery store, you’re “making” whipped cream this way. The gas dissolves into the liquid and then, upon spraying, rapidly bubbles out of saturation, foaming up the cream. From a structural point of view, whipped cream created this way is entirely different from foams created by whisking. Instead of a 3D mesh of surfactants holding on to the air bubbles—the “stripped regions” of the fat globules—the air bubbles from canned whipped cream are essentially just in suspension. Whipped cream from a can will fill about twice the volume, per weight, as whisked whipped cream, but it’s also less stable and will collapse—the fat globules from the pressurized can presumably are intact. We’ll cover other uses of pressure-created foams using cream whippers on page 313.
When working with whipped cream, keep in mind that the fats provide the structure. If the cream gets too warm, the fats will melt, so be sure to chill your bowl and the cream before whisking.

Whipped cream from a can foams up to twice the volume (15 mL expands to 67 mL) as hand-whisked cream (15 mL expands to 34 mL), but also collapses over time.
Whipping high-quality cream increases its volume by about 80%, while whipped egg whites can expand by over 600%!

Percentage of fat in dairy products. If the cream doesn’t have enough fat, there won’t be enough fat globules to create a stable foam.