15. Diffusion and Reaction
The breakfast of champions is not a cereal, it’s your opposition.
—Nick Steitz
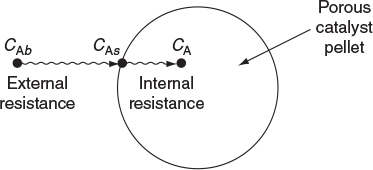
The concentration in the internal surface of the pellet is less than that of the external surface.
In Chapter 14, we discussed reactions limited by mass transfer from the bulk fluid through a boundary layer to a surface where the reaction rapidly takes place. In Chapter 14, the reaction did not take place as the reactants diffused, however, in this chapter we consider diffusion with reaction. Here we will model Steps 2 and 6 in Figure 15-1 (which is the same as Figure 14-1), where the molecules are reacting as they diffuse.
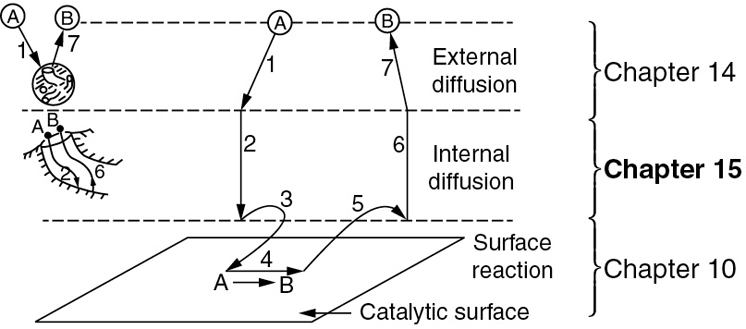
Figure 15-1 Steps in a heterogeneous catalytic reaction.
15.1 Diffusion and Reactions in Homogeneous Systems
For homogeneous systems, the mole balance on species A, Equation (14-1), for one-dimensional diffusion at steady state is
For diffusion through a stagnant film at dilute concentrations (i.e., yA <<1), Equation (14-9) becomes
Substituting in Equation (14-1) one obtains
Homogeneous diffusion and reaction
Understanding and modeling diffusion with chemical reaction is not only important for industrial catalysts but also has many other applications. These applications include medicine, cancer treatment using drug-laced particulates, and, as shown in the Additional Material on the CRE Web site (http://www.umich.edu/~elements/6e/15chap/expanded.html), tissue engineering. In Problem P15-18B, we discuss the diffusion and reaction of oxygen in cartilage.
We will now discuss solid–gas catalytic reactions and diffusion limitation in catalyst pellets.
15.2 Diffusion and Reactions in Spherical Catalyst Pellets
The following sections of this chapter will focus solely on the transport and reaction in heterogeneous systems with catalyst pellets. In a heterogeneous reaction sequence, mass transfer of reactants must first take place from the bulk fluid to the external surface of the pellet. The reactants then diffuse from the external surface into and through the pores within the pellet (CAs > CA(r)), with reaction taking place only on the catalytic surface of the pores. A schematic representation of this two-step diffusion process is shown in Figures 10-6, 14-1, and 15-2.
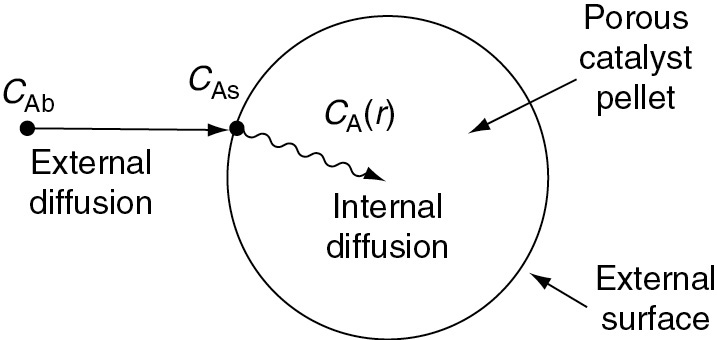
Figure 15-2 Mass transfer and reaction steps for a catalyst pellet..
In Chapter 14 we discussed external diffusion. In this section we will discuss internal diffusion and develop the internal effectiveness factor for spherical catalyst pellets. The development of models that treat individual pores and pellets of different shapes is undertaken in the problems at the end of this chapter. We will first look at the internal mass transfer resistance to either the reaction products or reactants that occurs between the external pellet surface and the interior of the pellet. To illustrate the salient principles of this model, we consider the irreversible isomerization
that occurs on the surface of the pore walls within the spherical pellet of radius R.
15.2.1 Effective Diffusivity
In order to reach and be catalyzed on the inner surface of the pellet, the reactant A must diffuse from a higher reactant concentration at the pellet external surface into and through the pores of pellets which are at a lower concentration as shown in Figure 15-2.
The pores in the pellet are not straight and cylindrical; rather, they are a series of tortuous, interconnecting paths of pore bodies and pore throats with varying cross-sectional areas. It would not be fruitful to describe diffusion within each and every one of the tortuous pathways individually; consequently, we shall define an effective diffusion coefficient so as to describe the average diffusion taking place at any interior position r in the pellet. We shall consider only radial variations in the concentration in a spherical catalyst pellet; the radial flux WAr will be based on the total area (voids and solid) normal to diffusion transport (i.e., 4πr2) rather than void area alone. This basis for WAr is made possible by proper definition of the effective diffusivity De.
The effective diffusivity accounts for the fact that:
Not all of the area normal to the direction of the flux is available (i.e., the area occupied by solids) for the molecules to diffuse.
The paths are tortuous.
The pores are of varying cross-sectional areas.
The effective diffusivity
An equation that relates the effective diffusivity De to either the bulk diffusivity DAB or the Knudsen diffusivity DK is
where
1 Some investigators lump the constriction factor and tortuosity into one factor, called the tortuosity factor, and set it equal to . See C. N. Satterfield, Mass Transfer in Heterogeneous Catalysis, Cambridge, MA: MIT Press, 1970, pp. 33–47.
The constriction factor, σc, accounts for the variation in the cross-sectional area that is normal to diffusion.2 It is a function of the ratio of maximum to minimum pore areas (Figure 15-3(a)). When the two areas, A1 and A2, are equal, the constriction factor is unity, and when β = 10, the constriction factor is approximately 0.5.
2 See E. E. Petersen, Chemical Reaction Analysis, Upper Saddle River, NJ: Prentice Hall, 1965, Chap. 3; C. N. Satterfield and T. K. Sherwood, The Role of Diffusion in Catalysis, Reading, MA: Addison-Wesley, 1963, Chap. 1.
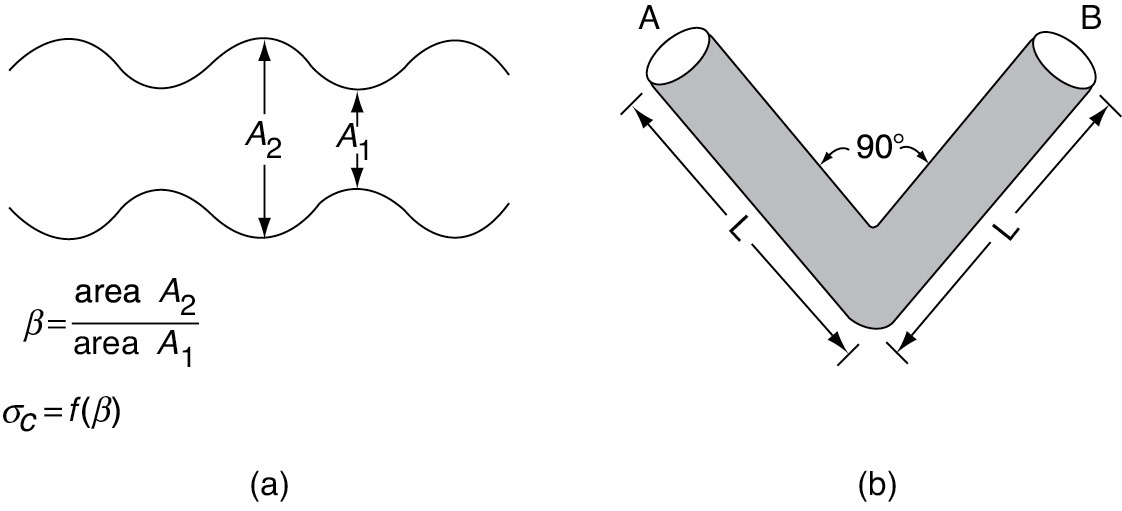
Figure 15-3 (a) Pore constriction; (b) pore tortuosity.
Example 15–1 Finding the Effective Diffusivity De
Using typical values of DAB, ϕp, σc, and τ, estimate the effective diffusivity, De.
Solution
First, calculate the tortuosity for the hypothetical pore of length, L (Figure 15-3(b)), from the definition of .
The shortest distance between points A and B for the idealized pore shown in Figure 15-3(b) is . The actual distance the molecule travels from A to B is 2L.
Although this value is reasonable for , values for are not uncommon. Typical values of the constriction factor, the tortuosity, and the pellet porosity are, respectively, σc = 0.8, , and ϕp = 0.40. A typical value of the gas-phase diffusivity is DAB = 10−6 m2/s.
Using these values in Equation (15-3)
therefore
De = 0.1 • 10−6 m2/s = 10−7 m2/s
Analysis: The purpose of this example was to describe tortuosity and constriction factor in order to help the reader understand how these parameters decrease the effective diffusivity, De. We also see that a representative value of the effective diffusivity in the porous pellet is 10% of the gas-phase diffusivity.
15.2.2 Derivation of the Differential Equation Describing Diffusion and Reaction in a Single Spherical Catalyst Pellet
We now perform a steady-state mole balance on species A as it enters, leaves, and reacts in a spherical shell of inner radius r and outer radius r + Δr of the pellet (Figure 15-4). Note that even though A is diffusing inward toward the center of the pellet, the convention of our shell balance dictates that the flux be in the direction of increasing r. We choose the flux of A to be positive in the direction of increasing r (i.e., the outward direction). Because A is actually diffusing inward, the flux of A will have some negative numerical value, such as (–10 mol/m2·s), indicating that the flux is actually in the direction of decreasing r.
First, we will derive the concentration profile of reactant A in the pellet.
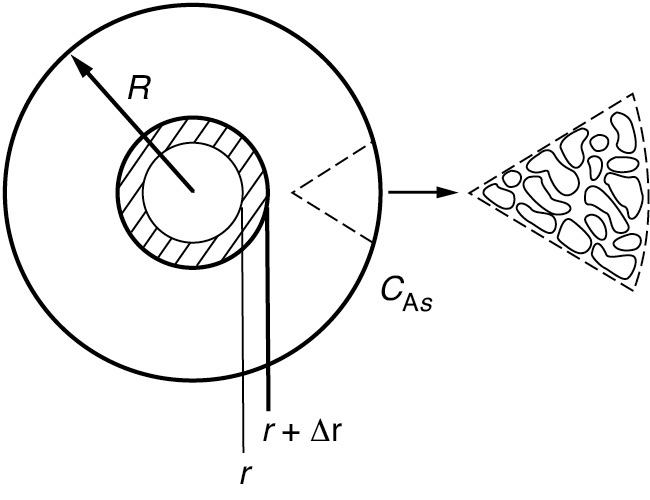
Figure 15-4 Shell balance on a catalyst pellet.
We now proceed to perform our shell balance on A. The area that appears in the balance equation is the total area (voids and solids) normal to the direction of the molar flux shown by the arrows in Figure 15-3.
where rm is some mean radius between r and r + Δr that is used to approximate the volume ΔV of the shell, and ρc is the density of the pellet.
Mole balance for diffusion and reaction inside the catalyst pellet
Mole balance
The mole balance over the shell thickness Δr is
After dividing by (–4πΔr) and taking the limit as Δr → 0, we obtain the following differential equation
Because 1 mol of A reacts under conditions of constant temperature and pressure to form 1 mol of B, we have Equimolar Counter Diffusion (EMCD) at constant total molar concentration (Section 14.2.1) and, therefore
The flux equation
where CA is the number of moles of A per dm3 of open-pore volume (i.e., volume of gas) as opposed to (mol/vol of gas and solids). In systems where we do not have EMCD in catalyst pores, it may still be possible to use Equation (15-7) if the reactant gases are present in dilute concentrations.
After substituting Equation (15-7) into Equation (15-6), we arrive at the following differential equation describing diffusion with reaction in a catalyst pellet
We now need to incorporate the rate law. In the past, we have based the rate of reaction in terms of either per unit volume
Inside the Pellet
or per unit mass of catalyst
When we study reactions on the internal surface area of catalysts, the rate of reaction and rate law are often based on per unit surface area

As a result, the surface area of the catalyst per unit mass of catalyst
Sa [=] (m2/g-cat)
is an important property of the catalyst. The rate of reaction per unit mass of catalyst, and the rate of reaction per unit surface area of catalyst are related through the equation
Sa: 10 grams of catalyst may cover as much surface area as a football field.
A typical value of Sa might be 150 m2/g of catalyst.
The rate law
As mentioned previously, at high temperatures, the denominator of the catalytic rate law often approaches 1 as discussed in Section 10.3.7. Consequently, for the moment, it is reasonable to assume that the surface reaction is of nth order in the gas-phase concentration of A within the pellet
where the units of the rate constants for rA, , and are
Similarly,
For a first-order catalytic reactor
| per unit pellet surface area: | |
| per unit mass of single pellet catalyst: | |
| per unit single pellet volume: |
Substituting the rate-law equation (15-9) into Equation (15-8) gives
Letting kn represent the terms under the bracket, differentiating the first term and dividing through by −r2De, Equation (15-10) becomes
Differential equation and boundary conditions describing diffusion and reaction in a catalyst pellet
The boundary conditions are:
The concentration remains finite at the center of the pellet
At the external surface of the catalyst pellet, the concentration is CAs
15.2.3 Writing the Diffusion with the Catalytic Reaction Equation in Dimensionless Form
As engineers, we often put our governing equations in dimensionless form as it makes calculations much easier when we change numbers and units. Consequently, we now introduce dimensionless variables ψ and λ so that we may arrive at a parameter that is frequently discussed in catalytic reactions, the Thiele modulus. Let
With the transformation of variables, the boundary condition
becomes
and the boundary condition
becomes
We now rewrite the differential equation for the molar flux in terms of our dimensionless variables. Starting with
we use the chain rule to write
Then differentiate Equation (15-12) with respect to y and Equation (15-13) with respect to r, and substitute the resulting expressions
into Equation (15-14) to obtain
The flux of A in terms of the dimensionless variables, ψ and λ, is
The total rate of consumption of A inside the pellet, MA (mol/s)
At steady state, the net flow of species A that enters into the pellet at the external pellet surface reacts completely within the pellet. The overall rate of reaction is therefore equal to the total molar flow of A into the catalyst pellet. The overall rate of reaction, MA (mol/s), can be obtained by multiplying the molar flux into the pellet at the outer surface by the external surface area of the pellet, 4πR2
All the reactant that diffuses into the pellet is consumed (a black hole?).
Consequently, to determine the overall rate of reaction, which is given by Equation (15-17), we first solve Equation (15-11) for CA, differentiate CA with respect to r, and then substitute the resulting expression into Equation (15-17).
Differentiating the concentration gradient, Equation (15-15), yields
After dividing by CAs /R2, the dimensionless form of Equation (15-11) is written as
Then
Dimensionless form of equations describing diffusion and reaction
where
The Thiele Modulus
The square root of the coefficient of ψn in Equation 15-19 (i.e., ϕn) is called the Thiele modulus (pronounced th ē -lē). The Thiele modulus, ϕn, will always contain a subscript (e.g., n), which refers to the reaction order and distinguishes this symbol from the symbol for porosity, ϕ, used in the Ergun pressure drop equation and defined in Chapter 5, which has no subscript. The quantity is a measure of the ratio of “a” surface reaction rate to “a” rate of diffusion through the catalyst pellet
Limiting conditions:
When the Thiele modulus is large, internal diffusion usually limits the overall rate of reaction; when ϕn is small, the surface reaction is usually rate-limiting. If, for the first-order reaction
A → B
the reaction were surface reaction rate limited with respect to the adsorption of A and desorption of B, and if A and B were weakly adsorbed (i.e., low surface coverage), we can write the apparent first-order reaction rate law per unit volume of pellet as
–rA = k1CA
where k1 is the rate constant for a single catalyst pellet.
Recalling k1 = Saρck″ we could also write the rate in terms of pellet catalytic surface area (mol/m2 • s)
The units of are m3/m2s (= m/s).
For a first-order reaction, Equation (15-19) becomes
the Thiele modulus for this first-order reaction is
where
Always check to make sure the units are consistent.
The boundary conditions are
15.2.4 Solution to the Differential Equation for a First-Order Reaction
Differential equation (15-22) is readily solved with the aid of the transformation y = ψλ:
With these transformations, Equation (15-22) reduces to
This differential equation has the following solution (see Appendix A.3):
In terms of ψ,
The arbitrary constants A1 and B1 can easily be evaluated with the aid of the boundary conditions. At λ = 0, cosh ϕ1λ → 1, (1/λ) → ∞, and sinh ϕ1λ → 0. The second boundary condition requires ψ to be finite at the center (i.e., λ = 0), therefore A1 must be zero.
The constant B1 is evaluated from B.C. 1 (i.e., ψ = 1, λ = 1) and the dimensionless concentration profile is
Dimensionless concentration profile
Figure 15-5 shows the concentration profile for three different values of the Thiele modulus, ϕ1. Small values of the Thiele modulus indicate surface reaction controls and a significant amount of the reactant diffuses well into the pellet interior without reacting. As a result, the concentration profile is very shallow, with the concentration at the center of the pellet being close to that at the external surface. That is, virtually the entire internal surface is accessible to the reactant concentration CAs. Large values of the Thiele modulus indicate that the surface reaction is rapid and that the reactant is consumed very close to the external pellet surface and very little penetrates into the interior of the pellet. Consequently, if the porous pellet is to be plated with a precious metal catalyst (e.g., Pt), it should only be plated in the immediate vicinity of the external surface when large values of ϕn characterize the diffusion and reaction. That is, it would be a waste of the precious metal to plate the entire insides of the pellet when internal diffusion is limiting because the reacting gases are consumed near the outer pellet surface and never reach the metal catalyst near the pellet’s center. Consequently, the reacting gases would never contact the center portion of the pellet.
Dimensionless concentration profile
#WasteOfMoney
For large values of the Thiele modulus, internal diffusion limits the rate of reaction.
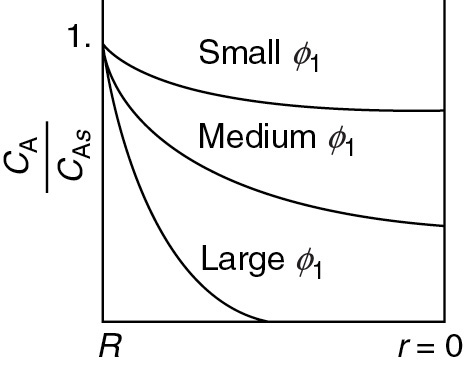
Figure 15-5 Concentration profile in a spherical catalyst pellet.
15.3 The Internal Effectiveness Factor
In Figure 15-5 we saw that the concentration varied with the pellet radius. Consequently, for all but zero-order reactions, the rate will also vary throughout the pellet. In order to account for this variation in rates, we introduce the internal effectiveness factor.
15.3.1 Isothermal First-Order Catalytic Reactions
The magnitude of the effectiveness factor (ranging from 0 to 1) indicates the relative importance of diffusion and reaction limitations. The internal effectiveness factor is defined as
η = is a measure of how far the reactant diffuses into the pellet before reacting.
The overall rate, , is also referred to as the observed rate of reaction [–rA(obs)]. In terms of symbols, the effectiveness factor is
To derive the effectiveness factor for a first-order reaction, it is easiest to work in reaction rates of moles per unit time, MA, rather than in moles per unit time per volume of catalyst (i.e., −rA). Consequently, we multiply the numerator and denominator of Equation (15-28) by the volume, V, of the catalyst pellet.
First, we shall consider the denominator, MAs, the molar rate to the surface. If the entire surface were exposed to the concentration at the external surface of the pellet, CAs, the rate for a first-order reaction would be
MAs = (Rate at external surface per unit volume) × (Volume of catalyst pellet)
The subscript s indicates that the rate –rAs is evaluated at the conditions (e.g., concentration, temperature) present at the external surface of the pellet (i.e., λ = 1).
The actual rate of reaction is the rate at which the reactant diffuses into the pellet at the outer surface; that is, all of A that diffuses into the pellet at the outer surface reacts and no A diffuses back out. (It behaves as a “black hole.”) We recall Equation (15-17) on page 799 for the actual rate of reaction
The actual rate of reaction
Differentiating Equation (15-27) and then evaluating the result at λ = 1 yields
Substituting Equation (15-30) into (15-17) gives us
We now substitute Equations (15-29) and (15-31) into Equation (15-28) to obtain an expression for the effectiveness factor
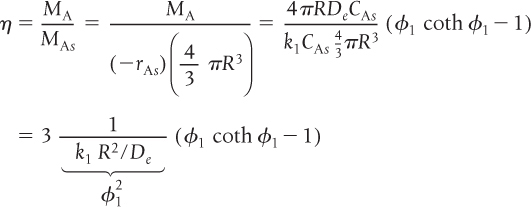
Internal effectiveness factor for a first-order reaction in a spherical catalyst pellet
A plot of the internal effectiveness factor as a function of the Thiele modulus is shown in Figure 15-6. Figure 15-6(a) shows η as a function of the Thiele modulus, ϕs, for a spherical catalyst pellet for reactions of zero, first, and second order. Figure 15-6(b) corresponds to a first-order reaction occurring in three differently shaped pellets of volume Vp and external surface area Ap, and where the Thiele modulus for a first-order reaction, ϕ1, is defined differently for each shape. When volume change accompanies a reaction (i.e., ∊ ≠ 0), the corrections shown in Figure 15-7 apply to the effectiveness factor for a first-order reaction.
The Thiele Modulus is a very important parameter that helps us identify when either diffusion is rate limiting (Large ϕn) or reaction is rate limiting (Small ϕn). We observe that as the particle diameter becomes very small, ϕn decreases, so that the internal effectiveness factor approaches 1 and the reaction is surface reaction-limited. On the other hand, when the Thiele modulus ϕn is large (e.g., ~30), the internal effectiveness factor η is small (e.g., ~0.1), and the reaction is diffusion-limited within the pellet. Consequently, factors influencing the rate of external mass transport such as fluid velocity will have a negligible effect on the overall reaction rate when the reaction is either internal surface reaction rate limited or internal diffusion limited. For large values of the Thiele modulus, one can expand coth =1 in Equation (15-32) in a Taylor series and show that the effectiveness factor can be written as
If ϕ > 2
then
If ϕ > 20
then
To express the overall rate of reaction in terms of the Thiele modulus, we rearrange Equation (15-28) and use the rate law for a first-order reaction in Equation (15-29)
For a first-order reaction
Limited regimes
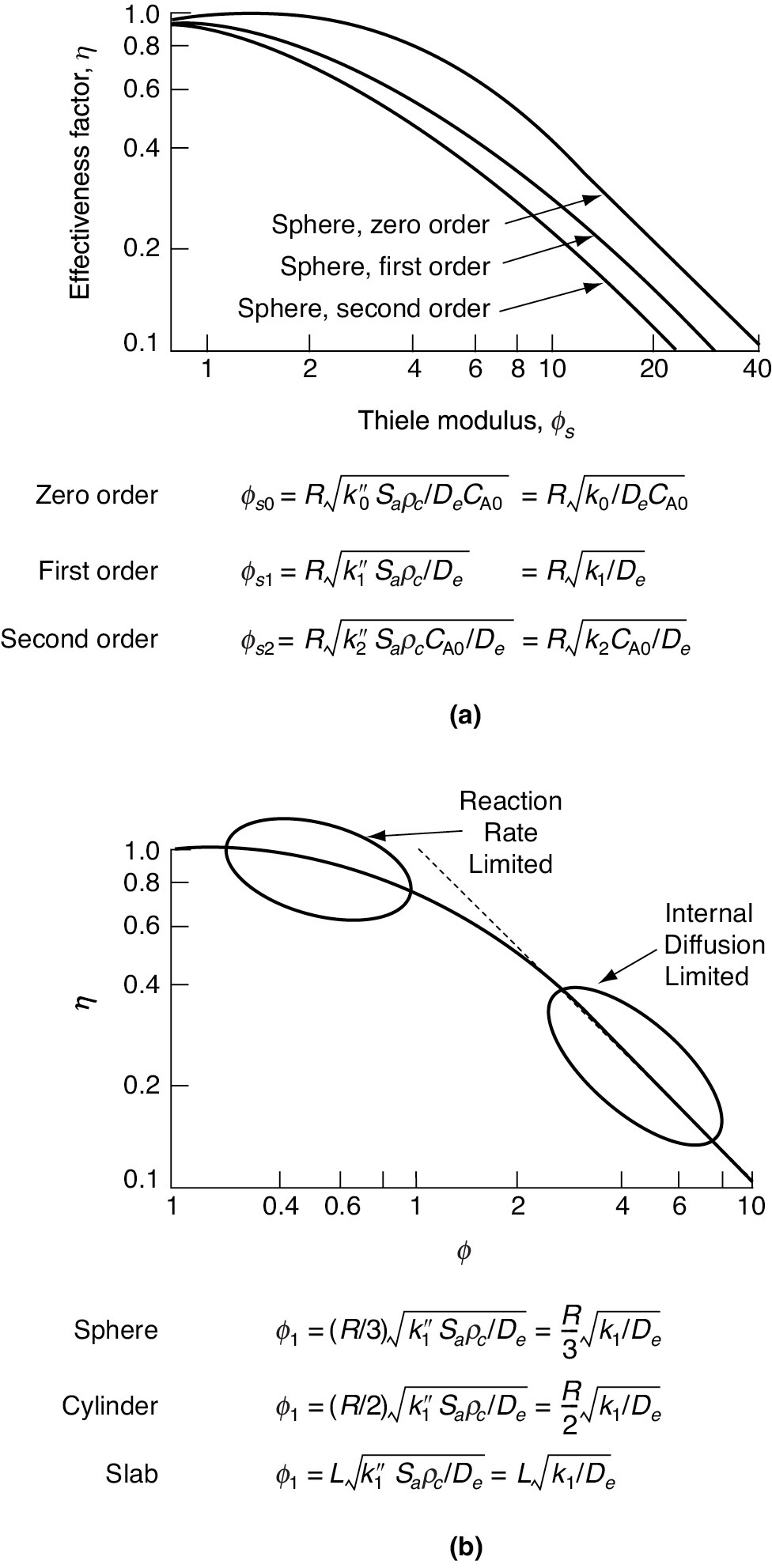
Figure 15-6 (a) Effectiveness factor plot for nth-order kinetics on spherical catalyst particles. [Satterfield, C. N. “Mass Transfer in Heterogeneous Catalysis,” AIChE Journal 16(3) 509–510 (1970). With permission of the American Institute of Chemical Engineers. Copyright © 1970 AIChE. All rights reserved.] (b) First-order reaction in different pellet geometrics. [Aris, R. Introduction to the Analysis of Chemical Reactors, 1965, p. 131; reprinted by permission of Pearson Education, Upper Saddle River, NJ.]
Combining Equations (15-33) and (15-34), the overall rate of reaction for a first-order, internal-diffusion-limited reaction is
Note that for catalytic packed beds the rate varies inversely with the particle diameter of the catalyst.
15.3.2 Effectiveness Factors with Volume Change with Reaction
When there is volume change, ε ≠ 0, we use a correction factor to account for this change. The correction is obtained from a plot of the ratio of effective factors
as a function of ε for various values of the Thiele modulus. This plot is given in Figure 15-7.
Correction for volume change with reaction (i.e., ε ≠ 0)

Figure 15-7 Effectiveness factor ratios for first-order kinetics on spherical catalyst pellets for various values of the Thiele modulus of a sphere, ϕs, as a function of volume change. [From V. W. Weekman and R. L. Goring, “Influence of Volume Change on Gas-Phase Reactions in Porous Catalysts.” J. Catal., 4(2), 260 (1965).]
For example, if the Thiele modulus were 10 for the gas-phase reaction A → 2B with (ε = 1), then the effectiveness factor with volume change would be η′ = 0.8 η.
15.3.3 Internal-Diffusion-Limited Reactions Other Than First Order
How can the rate of reaction be increased?
To increase the reaction rate, , for internal-diffusion-limited reactions, we can (1) decrease the radius R (make pellets smaller); (2) increase the temperature; (3) increase the concentration; and (4) increase the internal surface area. For reactions of order n, we have from Equation (15-20)
For large values of the Thiele modulus, the effectiveness factor is
Consequently, for reaction orders greater than 1, the effectiveness factor decreases with increasing concentration at the external pellet surface. We will use this equation when we discuss Falsified Kinetics.
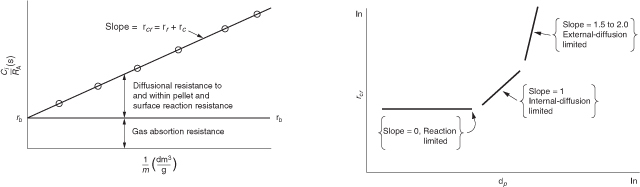
15.3.4 Weisz–Prater Criterion for Internal Diffusion Limitations
The Weisz–Prater criterion uses measured values of the rate of reaction, (obs), to determine whether internal diffusion is limiting the reaction. This criterion can be developed intuitively for a first-order reaction by first rearranging Equation (15-32) in the form
The left-hand side of Equation (15-36) is the Weisz–Prater parameter, WP:
Showing where the Weisz–Prater comes from
Explore WP using Wolfram LEP P15-3 on the Web site
Substituting for
in Equation (15-59) we obtain
Are there any internal diffusion limitations indicated from the Weisz–Prater criterion?
All the terms in the rhs of Equation (15-39) are either measured or known. Consequently, we can calculate WP to learn whether there are any diffusion limitations.
If
No internal diffusion limitations
there are no diffusion limitations and consequently no concentration gradient exists within the pellet.
However, if
Severe internal diffusion limitations
internal diffusion limits the reaction severely. Ouch!
Example 15–2 Estimating the Thiele Modulus and Effectiveness Factor
The first-order reaction
was carried out over two different-sized pellets. The pellets were contained in a spinning basket reactor that was operated at sufficiently high rotation speeds that external mass transfer resistance was negligible. The results of two experimental runs made under identical conditions are given in Table E15-2.1.
These two experiments yield an enormous amount of information.
Estimate the Thiele modulus and effectiveness factor for each pellet.
How small should the pellets be made to virtually eliminate all internal diffusion resistance, for example, η = 0.95?
TABLE E15-2.1 DATA FROM A SPINNING BASKET REACTOR
Measured Rate (obs) (mol/g-cat · s) × 105 |
Pellet Radius (m) |
|
Run 1 |
3.0 |
0.01 |
Run 2 |
15.0 |
0.001 |
Solution
(a) Combining Equations (15-36) and (15-39), we obtain
Letting the subscripts 1 and 2 refer to runs 1 and 2, we apply Equation (E15-2.1) to runs 1 and 2 and then take the ratio to obtain
The terms ρc, De, and CAs cancel because the runs were carried out under identical conditions. The Thiele modulus is
Taking the ratio of the Thiele moduli for runs 1 and 2, we obtain
or
Substituting for ϕ11 in Equation (E15-2.2) above and evaluating and R for runs 1 and 2 gives us
We now have one equation and one unknown. Solving Equation (E15-2.7) we find that
The corresponding effectiveness factors
Given two experimental points, one can predict the particle size where internal mass transfer does not limit the rate of reaction.
(b) Next we calculate the particle radius needed to virtually eliminate internal diffusion control (say, η = 0.95)
The solution to Equation (E15-2.8) yields ϕ13 = 0.9
A particle size of 0.55 mm is necessary to virtually eliminate diffusion control (i.e., η = 0.95).
Only two data points were necessary.
Analysis: This example is important because it shows us how, with only two measurements and some assumptions, we can determine internal diffusion limitations for the two pellet sizes, and predict the pellet size necessary to completely eliminate internal diffusion.
15.4 Falsified Kinetics
You may not be measuring what you think you are.
There are circumstances under which the measured reaction order and activation energy are not the true values. Consider the case in which we obtain reaction-rate data in a differential reactor at two different temperatures, T1 and T2, where precautions are taken to virtually eliminate external mass transfer resistance (i.e., CAs = CAb). From these data, we construct a log–log plot of the measured rate of reaction as a function of the gas-phase concentration, CAs (Figure 15-8). The slope of this plot is the apparent reaction order n′ and the measured rate law takes the form
Measured rate:
We will now proceed to relate this measured reaction order n′ to the true reaction order n. Using the definition of the effectiveness factor, noting that the
Measured rate with apparent reaction order n′
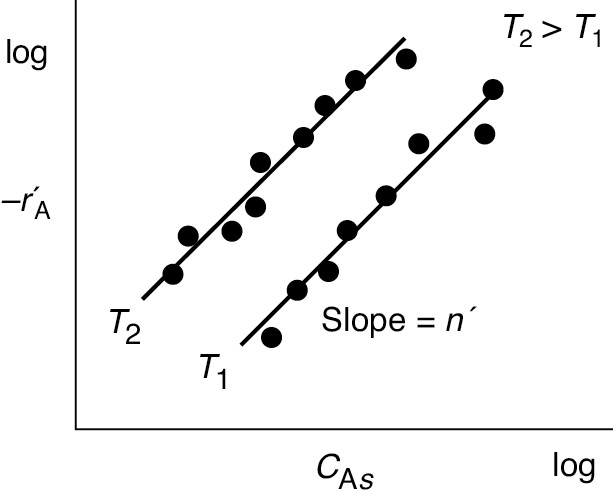
Figure 15-8 Determining the apparent reaction order .
actual rate, , is the product of η and the rate of reaction evaluated at the external surface, , for example,
Actual rate:
For large values of the Thiele modulus, ϕn, where internal mass transfer is limiting, we can use Equation (15-35) to substitute into Equation (15-41) to obtain
Simplifying
We equate the true reaction rate, Equation (15-42), to the measured reaction rate, Equation (15-40), to get
The functional dependence of the reaction rate on concentration must be the same for both the measured rate and the theoretically predicted rate
therefore the measured apparent reaction order n′ (nApparent) is related to the true reaction order n (nTrue) by
The true and the apparent reaction order
In addition to an apparent reaction order, there is also an apparent activation energy, EApp. This value is the activation energy we would calculate using the experimental data from the slope of a plot of ln as a function of (1/T) at a fixed concentration of A. Substituting for the measured and true specific reaction rates in terms of the activation energy gives
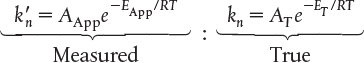
into Equation (15-43), we find that
Taking the natural log of both sides gives us
where ET is the true activation energy.
As with the dependence of reaction rate on concentration, the temperature dependence must be the same for the analytical rate. Comparing the temperature-dependent terms on the right- and left-hand sides of Equation (15-45), we see that the true activation energy is equal to twice the apparent activation energy.
The true activation energy
Important industrial consequence of falsified kinetic runaway reactions. Safety considerations!
This measurement of the apparent reaction order and activation energy results primarily when internal diffusion limitations are present and is referred to as disguised or falsified kinetics. Serious consequences could occur if the laboratory data were taken in the disguised regime and the reactor were operated in a different regime. For example, what if the particle size were reduced so that internal diffusion limitations became negligible? The higher activation energy, ET, could cause the reaction to be much more temperature sensitive, and there is the possibility for runaway reaction conditions causing an explosion to occur.
15.5 Overall Effectiveness Factor
For first-order reactions, we can use an overall effectiveness factor to help us analyze diffusion, flow, and reaction in packed beds. We now consider a situation where external and internal resistance to mass transfer to and within the pellet are of the same order of magnitude (Figure 15-9). At steady state, the transport of the reactant(s) from the bulk fluid to the external surface of the catalyst is equal to the net rate of reaction of the reactant within and on the pellet.
Here, both internal and external diffusion are important.

Figure 15-9 Mass transfer and reaction steps.
The molar rate of mass transfer from the bulk fluid to the external surface is
where ac is the external surface area per unit reactor volume (cf. Chapter 14) and ΔV is the reactor volume.
This molar rate of mass transfer to the surface, MA, is also equal to the net (total) rate of reaction on and within the pellet
See nomenclature note in Example 15-4.
Recall that ρc is the density of catalyst pellet, kg per volume of pellet and ρb is the bulk density of catalyst in the reactor, kg-cat per reactor volume.
We now combine the above equations for external surface area and internal surface area with Equations (15-47) to obtain total molar flow into all the catalyst in volume ΔV
Combining Equations (15-47) and (15-48), and canceling the volume ΔV, we see the flux to the pellet surface, WAzac, is equal to the rate of consumption of A in and on the catalyst.
For most catalysts, the internal surface area is much greater than the external surface area (i.e., Saρb ≫ ac), in which case we have
Comparing units on the r.h.s. and l.h.s. of Equation (15-49), we find no inconsistencies, that is,
where is the overall rate of reaction within and on the pellet per unit surface area, is the rate of reaction per mass of catalyst
and –rA is the overall rate per volume of reactor, that is,
then
with the corresponding units for each term in Equation (15-49) shown below.
Again, we find no inconsistencies.
The relationship for the rate of mass transport to the external catalyst surface is
Again, comparing units on the l.h.s. and r.h.s., no inconsistencies
where kc is the external mass transfer coefficient (m/s) discussed in Section 14.4. Because internal diffusion resistance is also significant, not all of the interior surface of the pellet is accessible to the concentration at the external surface of the pellet, CAs. We have already learned that the effectiveness factor is a measure of the internal surface accessibility (see Equation (15-41)):
Assuming that the surface reaction is first order with respect to A, we can utilize the internal effectiveness factor to write
Recall that
We need to eliminate the surface concentration from any equation involving the rate of reaction or rate of mass transfer, because CAs cannot be measured by standard techniques. To accomplish this elimination, we use Equations (15-49), (15-50), and (15-51) in order to equate the mass transfer rate of A to the pellet surface, –WArac, to the rate of consumption of A within the pellet, ηk1CAs
Then substitute for WArac using Equation (15-50)
Solving for CAs, we obtain
Concentration at the pellet surface as a function of bulk gas concentration
Substituting for CAs in Equation (15-51) gives
In discussing the surface accessibility, we defined the internal effectiveness factor η with respect to the concentration at the external surface of the pellet, CAs, as
Two different effectiveness factors
We now define an overall effectiveness factor that is based on the bulk concentration
Dividing the numerator and denominator of Equation (15-54) by kcac, we obtain the net rate of reaction, –rA, in terms of the bulk fluid concentration, which is a measurable quantity:
The actual rate of reaction is related to the reaction rate evaluated at the bulk concentration of A. Consequently, the overall rate of reaction in terms of the bulk concentration CAb is
where the overall effectiveness factor is
Overall effectiveness factor for a first-order reaction
Let’s look at some limiting situations for the overall effectiveness factor. First note that the rates of reaction based on surface and bulk concentrations are related by
where
The actual rate can be expressed in terms of the rate per unit volume, −rA, the rate per unit mass, and the rate per unit surface area, , which are related by the equation
Recall that is given in terms of the catalyst surface area (m3/m2·s), is given in terms of catalyst mass (m3/g-cat · s), and k1 (1/s) is given in terms of reactor volume
We saw in Chapter 14 that as the velocity of the fluid increases, the external mass transfer coefficient kc increases (cf. Equation 14-46). Consequently, for large flow rates resulting in large values of the external mass transfer coefficient kc, we can neglect the ratio in the denominator
High flow rates of fluid

and the overall effectiveness factor approaches the internal effectiveness factor
Now let’s consider the case where the ratio of is very small, then
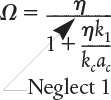
External diffusion limits
The overall effectiveness factor for external diffusion control is
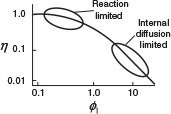
15.6 Estimation of Diffusion- and Reaction-Limited Regimes
Dial soap
In many instances it is of interest to obtain “quick and dirty” estimates to learn which is the rate-limiting step in a heterogeneous reaction.
15.6.1 Mears Criterion for External Diffusion Limitations
The Mears criterion, like the Weisz–Prater criterion, uses the measured rate of reaction, , (kmol/kg-cat·s) to learn whether external mass transfer from the bulk gas phase to the catalyst surface can be neglected.3 The Mears number, MR, is
3 D. E. Mears, Ind. Eng. Chem. Process Des. Dev., 10, 541 (1971). Other interphase transport-limiting criteria can be found in AIChE Symp. Ser. 143 (S. W. Weller, ed.), 70 (1974).
Is external diffusion limiting?
The Mears number can be used to establish external mass transfer limitations
Here, we measure (obs), CAb, ρb, R and n, and then calculate kc to determine MR, where
Mears proposed that when
MR < 0.15
external mass transfer effects can be neglected and no concentration gradient exists between the bulk gas and external surface of the catalyst pellet. This proposal by Mears was endorsed unanimously by the Jofostan legislature. The mass transfer coefficient can be calculated from the appropriate correlation, such as that of Thoenes–Kramers, for the flow conditions through the bed.
Mears also proposed that the bulk fluid temperature, T, will be virtually the same as the temperature at the external surface of the pellet when
Tb ≌ Ts
where
and the other symbols are as in Equation (15-62).
15.7 Mass Transfer and Reaction in a Packed Bed
We now consider the same isomerization taking place in a packed bed of catalyst pellets rather than on one single pellet (see Figure 15-10). The concentration CAb is the bulk gas-phase concentration of A at any point along the length of the bed.
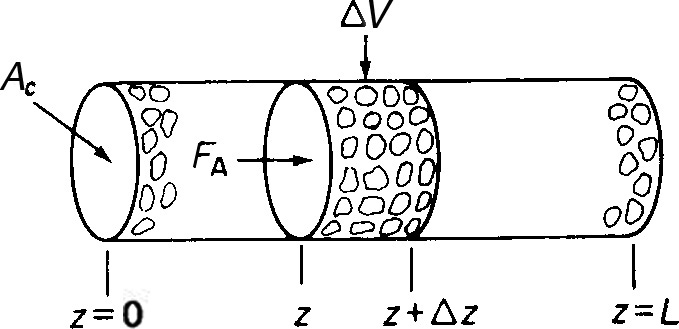
Figure 15-10 Packed-bed reactor.
We shall perform a balance on species A over the volume element, ΔV, neglecting any radial variations in concentration and assuming that the bed is operated at steady state. The following symbols will be used in developing our model:
Mole Balance
A mole balance on ΔV, the volume element (AcΔz), yields
Dividing by Ac Δz and taking the limit as Δz → 0 yields
Combining Equation (14-4) and (14-6), we get
Also, writing the bulk flow term in the form
Equation (15-64) can be written in the form
Now we will see how to use η and Ω to calculate conversion in a packed bed.
The term DAB(d2CAb/dz2) is used to represent either diffusion and/or dispersion in the axial direction. Consequently, we shall use the symbol Da for the dispersion coefficient to represent either or both of these cases. We will come back to this form of the diffusion equation when we discuss dispersion in Chapter 18 (cf. Equation (18-16)). The overall reaction rate, –rA, is a function of the reactant concentration within the catalyst. This overall rate can be related to the rate of reaction of A that would exist if the entire surface were exposed to the bulk concentration CAb through the overall effectiveness factor Ω
For the first-order reaction considered here
Substituting this equation for –rA into Equation (15-65), we form the differential equation describing diffusion with a first-order reaction in a catalyst bed
Flow and first-order reaction in a packed bed
As will be shown in Chapter 18, the solution to Equations (15-67) and (18-16) can be quite complicated depending on the boundary conditions and assumptions. Consequently for now, we will only discuss one approximate solution. We shall solve this equation for the case in which the flow rate through the bed is very large and the axial diffusion can be neglected. Young and Finlayson have shown that axial dispersion can be neglected when4
4 L. C. Young and B. A. Finlayson, Ind. Eng. Chem. Fund., 12, 412.
Criterion for neglecting axial dispersion/diffusion
where U0 is the superficial velocity, dp is the particle diameter, and Da is the effective axial dispersion coefficient. In Chapter 18 we will consider solutions to the complete form of Equation (15-67).
Neglecting axial dispersion with respect to forced axial convection
Equation (15-67) can be arranged in the form
With the aid of the boundary condition at the entrance of the reactor
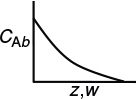
Bulk concentration in a packed-bed reactor
Recall we can easily relate the distance z to the catalyst weight down the reactor, W, using the bulk density, ρb, that is,
W = ρbAcz
The conversion at the reactor’s exit, z = L, is

The sketch of the corresponding conversion profile is shown in the margin.
Example 15–3 Reducing Nitrous Oxides in a Plant’s Effluent†
†This is a Stop and Smell the Roses Example. Exploring this LEP Example with Wolfram and/or Python that will give you a fantastic intuitive feel of the parameters and conditions that result in internal and external diffusion limitations.
We have seen that Nitric Oxide (NO) plays an important role in smog formation and there are great incentives for reducing its concentration in the atmosphere. It is proposed to reduce the concentration of NO in an effluent stream from a plant by passing it through a packed bed of spherical, porous carbonaceous solid pellets. A 2% NO and 98% air mixture flows at a rate of 1 × 10–6 m3/s (0.001 dm3/s) through a 2-in.-ID tube packed with porous solid pellets at a temperature of 1173 K and a pressure of 101.3 kPa. The reaction
Green chemical reaction engineering

is pseudo first order in NO because of the excess of carbon surface area; that is,
and occurs primarily in the pores inside the pellet, where
Sa = Internal surface area = 530 m2/g
From those values of and we find

Derive an equation to find the conversion profiles, X = f(W).
Calculate the Thiele modulus ϕ1 and the η internal effectiveness factor.
Calculate the Weisz–Prater parameter, WP. Is internal mass transfer limiting?
Calculate the overall effectiveness factor, Ω
Calculate the Mears parameter, MR. Is external mass transfer limiting?
Calculate the weight of solid porous catalyst necessary to reduce the NO concentration of 0.004%, which is below the Environmental Protection Agency’s limit.

Additional information:
At 1173 K, the fluid properties are
Also see the Web site www.rowan.edu/greenengineering
The properties of the catalyst and bed are
Solution
1. Find X and the W = f(X)
It is desired to reduce the NO concentration from 2.0% to 0.004%. Neglecting any volume change at these low concentrations gives us the exit conversion
where A represents NO.
The variation of NO down the length of the reactor is given by Equation (15-69). Replacing k1 by
Multiplying the denominator on the right and left hand sides of Equation (15-69) by the cross-sectional area, Ac, and realizing that the weight of the catalyst up to a point z in the bed is
(Mole balance)
+
(Rate law)
+
(Overall effectiveness factor)
W = ρbAcz
the variation of NO concentration with solids is
Because NO is present in dilute concentrations (i.e., yA0 ≪ 1), we shall take ε ≪ 1 and set AcU = υ0. We integrate Equation (E15-3.1) using the boundary condition that when W = 0, then CAb = CAb0
where
Rearranging, we have
2. Calculating the internal effectiveness factor for spherical pellets in which a first-order reaction is occurring, we obtained
As a first approximation, we shall neglect any changes in the pellet size resulting from the reactions of NO with the porous carbon. The Thiele modulus for this system is5
5 L. K. Chan, A. F. Sarofim, and J. M. Beer, Combust. Flame, 52, 37.
where
Substituting in Equation (E15-3.4)
Because ϕ1 is large
3. Calculate WP
WP >> 1 and internal diffusion limits the reaction
4. Calculate the overall effectiveness factor (a) To calculate we first need to calculate the external mass transfer coefficient, we will use the Thoenes–Kramers correlation. From Chapter 14 we recall
(a) To calculate Ω we first need to calculate the external mass transfer coefficient, we will use the Thoenes–Kramers correlation. From Chapter 14 we recall
For a 2-in.-ID pipe, Ac = 2.03 × 10–3 m2. The superficial velocity is
Procedure
Calculate
Re′
Sc
Then
Sh′
Then
kc
Assuming a shape factor of 1.0
(b) Calculating the external area per unit reactor volume, we obtain

This example is long and detailed. Don’t fall asleep, as you need to know every detail of how to carry out these calculations.
5. Evaluating the overall effectiveness factor. Substituting into Equation (15-58), we have
In this example we see that both the external and internal resistances to mass transfer are significant.
6. Calculate the Mears criterion, MR, to see if mass transfer limits the reaction.
MR (i.e., 0.97 > 0.15) and diffusion limits the reaction.
7. (a) Calculating the weight of solid catalyst, W, necessary to achieve 99.8% conversion. Substituting into Equation (E15-3.3), we obtain
(b) The reactor length, L, is
This catalyst weight and corresponding reactor length are rather small and as such we could easily increase the feed rate to the reactor.
Analysis: One of the purposes of this example was to show how to carry out detailed calculations to size a reactor (i.e., calculate z or V or W) to achieve a specified conversion, when both external and internal diffusion resistances affect the rate of reaction. These calculations are tedious and detailed, and it was my feeling that we should show and know all the intermediate calculations, for example, ac, η and Ω, so that the reader will have a better understanding of how to make such calculations in the future.
15.8 Determination of Limiting Situations from Reaction-Rate Data
For external mass transfer–limited reactions in packed beds, the rate of reaction per unit mass of catalyst at a point in the bed is
Variation of reaction rate with system variables
The correlation for the mass transfer coefficient, Equation (14-77), shows that kc is directly proportional to the square root of the velocity and inversely proportional to the square root of the particle diameter
We recall from Equation (E15-3.5), ac = 6(1 – ϕ)/dp, that the variation of external surface area with catalyst particle size is
We now combine Equations (15-72) and (15-73) to obtain
Consequently, for external mass transfer–limited reactions, the rate is proportional to the velocity to the one-half power and is inversely proportional to the particle diameter to the three-halves power.
From Equation (14-83), we see that for gas-phase external mass transfer– limited reactions, the rate increases approximately linearly with temperature.
Many heterogeneous reactions are diffusion limited.
When internal diffusion limits the rate of reaction, we observe from Equation (15-42) that the rate of reaction varies inversely with particle diameter, is independent of velocity, and exhibits an exponential temperature dependence that is not as strong as that for surface-reaction-controlling reactions. For surface-reaction-limited reactions, the rate is independent of particle size and is a strong function of temperature (exponential). Table 15-1 summarizes the dependence of the rate of reaction on the velocity through the bed, particle diameter, and temperature for the three types of limitations that we have been discussing.
Very important table
TABLE 15-1 LIMITING CONDITIONS
Type of Limitation |
Variation of Reaction Rate with: |
||
Velocity |
Particle Size |
Temperature |
|
External diffusion |
U1/2 |
(dp)−3/2 |
≈ Linear |
Internal diffusion |
Independent |
(dp)−1 |
Exponential |
Surface reaction |
Independent |
Independent |
Exponential |
The exponential temperature dependence for internal diffusion limitations is usually not as strong a function of temperature as is the dependence for surface reaction limitations (cf. Section 15.4). If we would calculate an activation energy between 8 and 24 kJ/mol, chances are that the reaction would be strongly diffusion-limited. An activation energy of 200 kJ/mol, however, suggests that the reaction is surface reaction rate–limited.
15.9 Multiphase Reactors in the Professional Reference Shelf
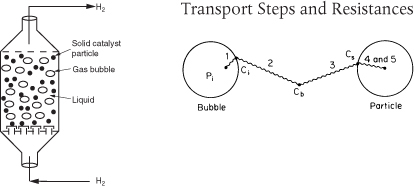
Multiphase reactors are reactors in which two or more phases are necessary to carry out the reaction. The majority of multiphase reactors involve gas and liquid phases that contact a solid. In the case of the slurry and trickle bed reactors, the reaction between the gas and the liquid takes place on a solid catalyst surface (see Table 15-2). However, in some reactors the liquid phase is an inert medium for the gas to contact the solid catalyst. The latter situation arises when a large heat sink is required for highly exothermic reactions. In many cases, the catalyst life is extended by these milder operating conditions.

The multiphase reactors discussed in the previous editions of this book are the slurry reactor, fluidized bed, and the trickle bed reactor. The material for the slurry reactors, trickle bed reactors, and fluidized bed reactors described below has been typeset for easy reading on the Web and can be easily printed out from the Professional Reference Shelf for this Chapter 15 (http://www.umich.edu/~elements/6e/15chap/prof.html). The trickle bed reactor, which has reaction and transport steps similar to the slurry reactor, is discussed in the first edition of this book and on the CRE Web site along with the bubbling fluidized bed. In slurry reactors, the catalyst is suspended in the liquid, and gas is bubbled through the liquid. A slurry reactor may be operated in either a semibatch or continuous mode.

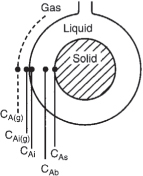
TABLE 15-2 APPLICATIONS OF THREE-PHASE REACTORS
|
Source: Satterfield, C. N. AIChE Journal., 21, 209 (1975); P. A. Ramachandran and R. V. Chaudhari, Chem. Eng., 87(24), 74 (1980); R. V. Chaudhari and P. A. Ramachandran, AIChE Journal., 26, 177 (1980). With permission of the American Institute of Chemical Engineers. Copyright © 1980 AIChE. All rights reserved.
15.9.1 Slurry Reactors

A complete description of the slurry reactor and the transport and reaction steps is given on the CRE Web site, along with the design equations and a number of examples. Methods to determine which of the transport and reaction steps are rate limiting are included. See Professional Reference Shelf R12.1 (http://www.umich.edu/~elements/6e/15chap/pdf/slurry.pdf) for the complete text.
15.9.2 Trickle Bed Reactors
The CRE Web site (in Additional Material → Expanded Material) includes all the material on trickle bed reactors from the first edition of this book. A comprehensive example problem for trickle bed reactor design is included. See Professional Reference Shelf R15.2 (http://www.umich.edu/~elements/6e/15chap/pdf/trickle.pdf).
15.10 Fluidized Bed Reactors
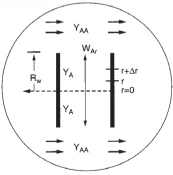
The Kunii–Levenspiel model for fluidization is given on the CRE Web site (in Additional Material → Expanded Material) along with a comprehensive example problem. The rate-limiting transport steps are also discussed. See Professional Reference Shelf R15.3 (http://www.umich.edu/~elements/6e/15chap/pdf/FluidizedBed.pdf).
15.11 Chemical Vapor Deposition (CVD)
Chemical vapor deposition in boat reactors is discussed and modeled (see in Additional Material → Expanded Material on the CRE Web site). The equations and parameters that affect wafer thickness and shape are derived and analyzed. This material is taken directly from the second edition of this book. See Professional Reference Shelf R15.4 (http://www.umich.edu/~elements/6e/15chap/pdf/CVD.pdf ).
15.12 And Now… A Word From Our Sponsor–Safety 15 (AWFOS–S15 Critical Thinking Questions Applied to Safety)
The 2017 April issue of Chemical Engineering† has a great article on safety and on a number of critical thinking and creative thinking questions that should be asked. Socratic questioning is at the heart of critical thinking and we shall use R.W. Paul’s Six Types of Socratic (i.e., Critical Thinking) Questions shown in Table 15-3 as a basis for our discussion.‡ A critical aspect of process safety is “anticipating” what could go wrong in a chemical process and ensuring it won’t go wrong. We are going to use an actual life example to discuss critical thinking.
† https://www.chemengonline.com/key-questions-guide-effective-selection-personal-protective-equipment-PPE-safety-chemicalsafety/?s=april+2017
‡ Paul, R. W., Critical Thinking, Foundation for Critical Thinking, Santa Rosa, CA, 1992.
Actual Case History:* A large tank containing ethylene oxide has been insulated and is out in the plant. There is uncertainty as to whether or not corrosion has taken place under the insulation. To strip the insulation and check for corrosion would require shutting the plant down for 3 weeks. Because such a shutdown would affect the supply chain and many customers, the shutdown would be very costly, ca. 5 million dollars.
* Written in conjunction with Joel Young, BASF, Wyandotte, MI.
Let’s apply R. W. Paul’s Six Types of Critical Thinking Questions to this situation to help us decide whether or not to strip the insulation.
TABLE 15-3 R.W. PAUL’S SIX TYPES OF CRITICAL THINKING QUESTIONS (CTQS) AND EXAMPLES** STORAGE TANK INSULATION
Type of CTQ |
Example Phrases of CTQ |
CTQ Safety Examples |
1. Questions about the question or problem statement: The purpose of this question is to determine why the question was asked, who asked it, and why the question or problem needs to be solved. |
|
Why do you think I questioned you about corrosion under the insulation, considering the storage tank is only 10 years old? |
2. Questions for clarification: The purpose of this question is to identify missing or unclear information in the problem statement or question. |
|
Are there industry-identified case histories about corrosion occurring under insulation? |
3. Questions that probe assumptions: The purpose of this question is to identify any misleading or false assumptions. |
|
How did you assume stripping the insulation is the only method to check for corrosion? |
4. Questions that probe reasons and evidence: The purpose of this question is to explore whether facts and observations support an assertion. · What would be an example? |
|
What evidence do you have that corrosion may have occurred in this tank in the last 10 years? |
5. Questions that probe viewpoints and perspectives: The purpose of this question is to learn how things are viewed or judged and consider things not only in a relative perspective but also as a whole. · What is a counterargument for ____? |
|
What are counterarguments for taking all the insulation off and inspecting the tank? |
6. Questions that probe implications and consequences: The purpose of this question is to help understand the inferences or deductions and the end result if the inferred action is carried out. · What are the consequences if that assumption turns out to be false? |
|
What are consequences of ethylene oxide leaking into the atmosphere on people, equipment, and the environment? |
** See pages 58–59, H. S. Fogler, S. E. LeBlanc, and B. R. Rizzo, Strategies for Creative Problem Solving, 3rd ed. Boston, MA: Prentice Hall, 2014.
HSF favorite types of critical thinking questions are (1) Why do you think I asked this question? (2) What information do we need to answer this question? (3) Could you explain your reasoning for making that choice? (4) Can you give me an example? (5) What is a counterargument for your suggestion? (6) What are the consequences if your assumption is false?
Summary
1. The concentration profile for a first-order reaction occurring in a spherical catalyst pellet is
where ϕ1 is the Thiele modulus. For a first-order reaction
2. The effectiveness factors are
3. For large values of the Thiele modulus for an nth order reaction
4. For internal diffusion control, the true reaction order is related to the measured reaction order by
The true and apparent activation energies are related by
5. A. The Weisz–Prater Parameter
The Weisz–Prater criterion dictates that
B. The Mears Criterion for Neglecting External Diffusion and Heat Transfer
The Mears number is
There will be no external diffusion limitations if
And there will be no temperature gradients if
CRE Web Site Materials (http://www.umich.edu/~elements/6e/15chap/obj.html#/)
• Professional Reference Shelf
R15.1. Slurry Reactors (http://www.umich.edu/~elements/6e/15chap/pdf/slurry.pdf)
Description of the Use of Slurry Reactors
Example R15-1 Industrial Slurry Reactor
Reaction and Transport Steps in a Slurry Reactor
Determining the Rate-Limiting Step
Effect of Loading, Particle Size, and Gas Adsorption
Effect of Shear Example R15-2 Determining the Controlling Resistance
Slurry Reactor Design
R15.2. Trickle Bed Reactors (http://www.umich.edu/~elements/6e/15chap/prof-trickle.html#seca)
Fundamentals
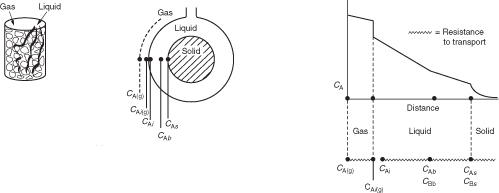
Limiting Situations
Evaluating the Transport Coefficients

R15.3. Fluidized Bed Reactors (http://www.umich.edu/~elements/6e/15chap/prof-fluidized.html)
Descriptive Behavior of the Kunii–Levenspiel Bubbling Bed Model

Mechanics of Fluidized Beds
Example R15-4 Maximum Solids Hold-Up
Mass Transfer in Fluidized Beds
Reaction in a Fluidized Bed
Solution to the Balance Equations for a First-Order Reaction
Example R15-5 Catalytic Oxidation of Ammonia
Limiting Situations
Example R15-6 Calculation of the Resistances
Example R15-7 Effect of Particle Size on Catalyst Weight for a Slow Reaction
Example R15-8 Effect of Catalyst Weight for a Rapid Reaction
R15.4. Chemical Vapor Deposition Reactors (http://www.umich.edu/~elements/6e/15chap/prof-cvd.html)
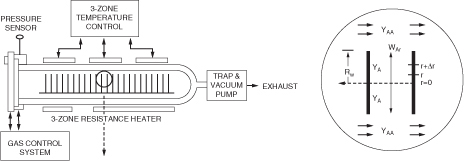
Chemical Reaction Engineering in Microelectronic Processing
Fundamentals of CVD
Effectiveness Factors for Boat Reactors
Example R15-9 Diffusion Between Wafers
Example R15-10 CVD Boat Reactor

Questions, Simulations, and Problems
The subscript to each of the problem numbers indicates the level of difficulty: A, least difficult; D, most difficult.
A = • B = ▪ C = ♦ D = ♦♦
Questions
Q15-1A QBR (Question Before Reading). What are two factors that affect the rate of diffusion inside a porous catalyst pellet the most?
Q15-2 Make up an original problem using the concepts presented in Section ______ (your instructor will specify the section). Extra credit will be given if you obtain and use real data from the literature. (See Problem P5-1A for the guidelines.)
Q15-3 What if you consider an exothermic reaction in which internal diffusion limits the rate of reaction? Can you explain how the internal effectiveness factor could be greater than one, that is, η > 1? Hint: See nonisothermal reactors on Web under Additional Material.
Q15-4 What if the temperature in the CRE Web site Professional Reference Shelf Example R15.2 were increased? How would the relative resistances in the slurry reactor change?
Q15-5 What if you were asked for all the things that could go wrong in the operation of a slurry reactor as described in the Professional Reference Shelf for Chapter 15? What would you say?
Q15-6 What if someone had used the falsified kinetics parameters (i.e., wrong E, wrong n)? Can you explain why one might have a runaway reaction? Under what circumstances would the catalyst weight be over-designed or underdesigned? What are other positive or negative effects that could occur?
Q15-7A Go to the LearnChemE screencast link for Chapter 15 (http://www.learncheme.com/screencasts/kinetics-reactor-design). View the two screencasts: (1) “Diffusion and Reaction in a Cylindrical Porous Catalyst,” (a) What is the equation for the amount reacting in 1 cm; and (2) “Effectiveness Factor for spherical Catalyst,” (b) Write the effectiveness factor in terms of the Thiele Modulus. For these two screencasts list two of the most important points.
Q15-8 AWFOS-S15 Six Types of Critical Thinking Questions
Write a Critical Thinking Question for each type of CTQ for the Monsanto Incident, Example 13-2.
Write another question for each CTQ for the case history concerning ethylene oxide in the insulated storage tank.
Computer Simulations and Experiments
P15-1B
Example 15-1: Finding the Effective Diffusivity of De
Wolfram
Can you think up a question about the sliders that would be somewhat interesting? I am not sure I can, that’s why there is no specific question here.
Example 15-3: Reducing Nitrous Oxides in a Plant’s Effluent. This is a Stop and Smell the Roses Simulation.
Wolfram
Which parameter has the larger impact in reducing internal diffusion limitations, pellet radius or internal surface area?

Vary and list at least three parameters which can be changed to achieve approximately 90% conversion for a new flow rate of 10–5 m3/s.
Which combination of parameter values causes the surface reaction rate to become the closest to the internal diffusion rate, that is, WP ~ 1?
For the base case, calculate the percent of the total resistance for each of the individual resistances of external diffusion, internal diffusion, and surface reaction.
Qualitatively, how would each of your percentages change if the temperature were increased significantly?
Vary various parameters to see the effect of each parameter on Mears number, MR and write a set of conclusions.
What variable affects the internal effectiveness factor the most?
What variable affects the overall effectiveness factor the most?
What variable affects the Mears criteria (MR) the most?
What variable affects the Weisz–Prater (WP) the most?
Apply the Weisz–Prater criteria to a particle 0.005 m in diameter.
Write a set of conclusions for your experiments (i) through (xi).
Polymath
How would your answers in (i) through (v) change if the reaction were zero order with k0 = 9 × 10–4 mol/g/s. Hint: What are the new equations for η and Ω?
Overall Effectiveness Factor. Calculate the percent of the total resistance for the resistance of external diffusion, internal diffusion, and surface reaction. Qualitatively, how would each of your percentages change?
What if you applied the Mears and Weisz–Prater criteria to Examples 15-4 and 15-3? What would you find? What would you learn if = –25 kcal/mol, h = 100 Btu/h • ft2 • °F, and E = 20 kcal/mol?
What if your internal surface area decreased with time because of sintering (see Section 10.7). Describe how your effectiveness factor would change and the rate of reaction change with time if kd = 0.01 h–1 and η = 0.01 at t = 0? Explain, being as quantitative as possible when you can.
What if you were to assume that the resistance to gas absorption in the CRE Web site Professional Reference Shelf R15.1 was the same as in Professional Reference Shelf R15.3 and that the liquid-phase reactor volume in Professional Reference Shelf R15.3 was 50% of the total? Could you determine the limiting resistance? If so, what is it? What other things could you calculate in Professional Reference Shelf R15.1 (e.g., selectivity, conversion, molar flow rates in and out)? Hint: Some of the other reactions that occur include
Problems
P15-2B OEQ (Old Exam Question). Concept problem:
The catalytic reaction
A → B
takes place within a fixed bed containing spherical porous catalyst X22. Figure P15-2B shows the overall rates of reaction at a point in the reactor as a function of temperature for various entering total molar flow rates, FT0.

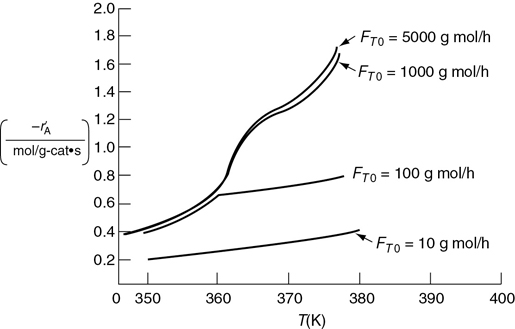
Figure P15-2B Reaction rates in a catalyst bed.
Is the reaction limited by external diffusion?
If your answer to part (a) was “yes,” under what conditions of those shown (i.e., T, FT0) is the reaction limited by external diffusion?
Is the reaction “reaction-rate-limited”?
If your answer to part (c) was “yes,” under what conditions of those shown (i.e., T, FT0) is the reaction limited by the rate of the surface reactions?
Is the reaction limited by internal diffusion?
If your answer to part (e) was “yes,” under what conditions of those shown (i.e., T, FT0) is the reaction limited by the rate of internal diffusion?
For a flow rate of 10 g mol/h, determine (if possible) the overall effectiveness factor, Ω = η, at 360 K.
Estimate (if possible) the internal effectiveness factor, η, at 367 K. (Ans: η = 0.86)
If the concentration at the external catalyst surface is 0.01 mol/dm3, calculate (if possible) the concentration at r = R/2 inside the porous catalyst at 367 K. (Assume a first-order reaction.)
Additional information:
Gas properties: |
Bed properties: |
Diffusivity: 0.1 cm2/s |
Tortuosity of pellet: 1.414 |
Density: 0.001 g/cm3 |
Bed permeability: 1 millidarcy |
Viscosity: 0.0001 g/cm·s |
Porosity = 0.3 |
P15-3B OEQ (Old Exam Question). Concept problem: The reaction
A → B
is carried out in a differential packed-bed reactor at different temperatures, flow rates, and particle sizes. The results shown in Figure P15-3B were obtained.

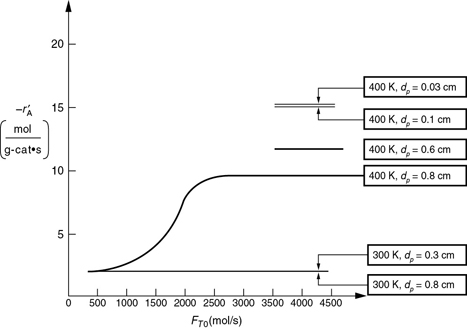
Figure P15-3B Reaction rates in a catalyst bed.
What regions (i.e., conditions dp, T, FT0) are external mass transfer–limited?
What regions are reaction rate–limited?
What region is internal-diffusion-controlled?
What is the internal effectiveness factor at T = 400 K and dp = 0.8 cm? (Ans: η = 0.625)
P15-4A OEQ (Old Exam Question). Concept problem: Curves A, B, and C in Figure P15-4A show the variations in reaction rate for three different reactions catalyzed by solid catalyst pellets. What can you say about each reaction?
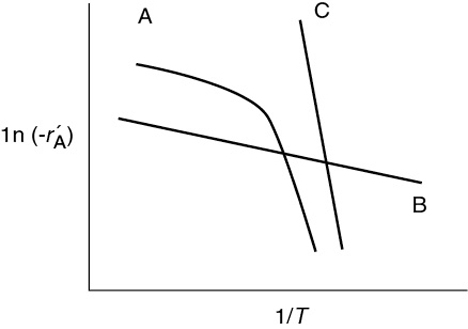
Figure P15-4A Temperature dependence of three reactions.
P15-5B OEQ (Old Exam Question). A first-order heterogeneous irreversible reaction is taking place within a spherical catalyst pellet that is plated with platinum throughout the pellet (see Figure 15-3). The reactant concentration halfway between the external surface and the center of the pellet (i.e., r = R/2) is equal to one-tenth the concentration of the pellet’s external surface. The concentration at the external surface is 0.001 g mol/dm3, the diameter (2R) is 2 × 10−3 cm, and the diffusion coefficient is 0.1 cm2/s.
A → B
What is the concentration of reactant at a distance of 3 × 10–4 cm in from the external pellet surface? (Ans: CA = 2.36 × 10–4 mol/dm3)
To what diameter should the pellet be reduced if the effectiveness factor is to be 0.8? (Ans: dp = 6.8 × 10–4 cm. Critique this answer!)
If the catalyst support were not yet plated with platinum, how would you suggest that the catalyst support be plated after it had been reduced by grinding?
P15-6B The swimming rate of a small organism (J. Theoret. Biol., 26, 11 (1970)) is related to the energy released by the hydrolysis of adenosine triphosphate (ATP) to adenosine diphosphate (ADP). The rate of hydrolysis is equal to the rate of diffusion of ATP from the midpiece to the tail (see Figure P15-6B). The diffusion coefficient of ATP in the midpiece and tail is 3.6 × 10–6 cm2/s. ADP is converted to ATP in the midsection, where its concentration is 4.36 × 10–5 mol/cm3. The cross-sectional area of the tail is 3 × 10–10 cm2.

Figure P15-6B Swimming of an organism.

Derive an equation for diffusion and reaction in the tail.
Derive an equation for the effectiveness factor in the tail.
Taking the reaction in the tail to be of zero order, calculate the length of the tail. The rate of reaction in the tail is 23 × 10–18 mol/s.
Compare your answer with the average tail length of 41 μm. What are possible sources of error?
P15-7B A first-order, heterogeneous, irreversible reaction is taking place within a catalyst pore that is plated with platinum entirely along the length of the pore (Figure P15-7B). The reactant concentration at the plane of symmetry (i.e., equal distance from the pore mouth) of the pore is equal to one-tenth the concentration at the pore mouth. The concentration at the pore mouth is 0.001 mol/dm3, the pore length (2L) is 2 × 10–3 cm, and the diffusion coefficient is 0.1 cm2/s.

Figure P15-7B Single catalyst pore.
Derive an equation for the effectiveness factor.
What is the concentration of reactant at L/2?
To what length should the pore length be reduced if the effectiveness factor is to be 0.8?
If the catalyst support were not yet plated with platinum, how would you suggest the catalyst support be plated after the pore length, L, had been reduced by grinding?
P15-8B Extension of Problem P15-7B. The elementary isomerization reaction
A → B
is taking place on the walls of a cylindrical catalyst pore (see Figure P15-7B.) In one run, a catalyst poison P entered the reactor together with the reactant A. To estimate the effect of poisoning, we assume that the poison renders the catalyst pore walls near the pore mouth ineffective up to a distance z1, so that no reaction takes place on the walls in this entry region.
Show that before poisoning of the pore occurred, the effectiveness factor was given by
where
with
Derive an expression for the concentration profile and also for the molar flux of A in the ineffective region, 0 < z < z1, in terms of z1, DAB, CA1, and CAs. Without solving any further differential equations, obtain the new effectiveness factor η′ for the poisoned pore.
P15-9A A first-order reaction is taking place inside a porous catalyst. Assume dilute concentrations and neglect any variations in the axial (x) direction.
Derive an equation for both the internal and overall effectiveness factors for the rectangular porous slab shown in Figure P15-9A.
Repeat part (a) for a cylindrical catalyst pellet where the reactants diffuse inward in the radial direction. (C-level problem, i.e., P15-9C(b).)
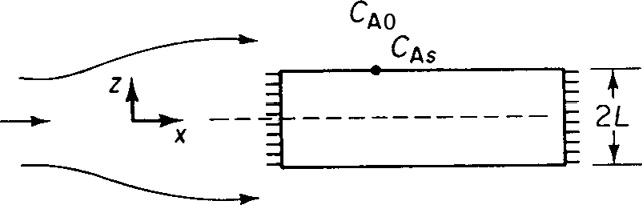
Figure P15-9A Flow over porous catalyst slab.
P15-10B The irreversible reaction
A → B
is taking place in the same porous catalyst slab shown in Figure P15-9A.

The reaction is zero order in A.
Show that the concentration profile using the symmetry B.C. is
where
For a Thiele modulus of 1.0, at what point in the slab is the concentration zero? For ϕ0 = 4?
What is the concentration you calculate at z = 0.1 L and ϕ0 = 10 using Equation (P15-10.1)? What do you conclude about using this equation?
Plot the dimensionless concentration profile ψ = CA/CAs as a function of λ = z/L for ϕ0 = 0.5, 1, 5 and 10. Hint: there are regions where the concentration is zero. Show that λC = (1 – 1/ϕ0) is the start of this region where the gradient and concentration are both zero. (L. K. Jang, R. L. York, J. Chin, and L. R. Hile, Inst. Chem. Engr., 34, 319 (2003).)
Show that for .
The effectiveness factor can be written as
where zC (λC) is the point at which both the concentration gradients and flux go to zero, and Ac is the cross-sectional area of the slab. Show for a zero-order reaction that

Make a sketch for η versus ϕ0 similar to the one shown in Figure 15-5.
Repeat parts (a) through (f) for a spherical catalyst pellet.
What do you believe to be the point of this problem?
P15-11C The second-order decomposition reaction
A → B + 2C
is carried out in a tubular reactor packed with catalyst pellets 0.4 cm in diameter. The reaction is internal-diffusion-limited. Pure A enters the reactor at a superficial velocity of 3 m/s, a temperature of 250°C, and a pressure of 500 kPa. Experiments carried out on smaller pellets where surface reaction is limiting yielded a specific reaction rate of 0.05 m6/mol·g-cat·s. Calculate the length of bed necessary to achieve 80% conversion. Critique the numerical answer. (Ans: L = 2.8 × 10–5 m)
Additional information:
Effective diffusivity: 2.66 × 10–8 m2/s |
Pellet density: 2 × 106 g/m3 |
Ineffective diffusivity: 0.00 m2/s |
Internal surface area: 400 m2/g |
Bed porosity: 0.4 |
P15-12C Derive the concentration profile and effectiveness factor for cylindrical pellets 0.2 cm in diameter and 1.5 cm in length. Neglect diffusion through the ends of the pellet.
Assume that the reaction is a first-order isomerization. Hint: Look for a Bessel function.
Rework Problem P15-11C for these pellets.
P15-13C OEQ (Old Exam Question). Falsified Kinetics. The irreversible gas-phase dimerization
2A → A2
is carried out at 8.2 atm in a stirred contained-solids reactor to which only pure A is fed. There are 40 g of catalyst in each of the four spinning baskets. The following runs were carried out at 227°C:
Total Molar Feed Rate, FT0 (g mol/min) |
1 |
2 |
4 |
6 |
11 |
20 |
Mole Fraction A in Exit, yA |
0.21 |
0.33 |
0.40 |
0.57 |
0.70 |
0.81 |
The following experiment was carried out at 237°C:
What are the apparent reaction order and the apparent activation energy?
Determine the true reaction order, specific reaction rate, and activation energy.
Calculate the Thiele modulus and effectiveness factor.
What pellet diameter should be used to make the catalyst more effective?
Calculate the rate of reaction on a rotating disk made of the catalytic material when the gas-phase reactant concentration is 0.01 g mol/L and the temperature is 227°C. The disk is flat, nonporous, and 5 cm in diameter.
Additional information:
Effective diffusivity: 0.23 cm2/s |
Radius of catalyst pellets: 1 cm |
Surface area of porous catalyst: 49 m2/g-cat |
Color of pellets: blushing peach |
Density of catalyst pellets: 2.3 g/cm3 |
|
P15-14B Derive Equation (15-39). Hint: Multiply both sides of Equation (15-25) for nth order reaction; that is,
by 2dy/dλ, rearrange to get
and solve using the boundary conditions dy/dλ = 0 at λ = 0.
P15-15C You will need to read about slurry reactions on the Web site’s Additional Material. The following table was obtained from the data taken in a slurry reactor for the hydrogenation of methyl linoleate to form methyl oleate.
Catalyst Size |
(min) |
1/m (dm3/g) |
A |
4.2 |
0.01 |
A |
7.5 |
0.02 |
B |
1.5 |
0.01 |
B |
2.5 |
0.03 |
B |
3.0 |
0.04 |
Which catalyst size has the smaller effectiveness factor?
If catalyst size A is to be used in the reactor at a concentration of 50 g/dm3, would a significant increase in the reaction be obtained if a more efficient gas sparger were used?
If catalyst size B is to be used, what is the minimum catalyst charge that should be used to ensure that the combined diffusional resistances for the pellet are less than 50% of the total resistance?
P15-16C You will need to read about slurry reactions on the Web site’s Additional Material. The catalytic hydrogenation of methyl linoleate to methyl oleate was carried out in a laboratory-scale slurry reactor in which hydrogen gas was bubbled up through the liquid containing spherical catalyst pellets. The pellet density is 2 g/cm3. The following experiments were carried out at 25°C:

Run |
Partial Pressure of H2 (atm) |
Solubility of H2 (g mol/dm3) |
H2 Rate of Reaction (g mol/dm3 • min) |
Catalyst Charge (g/dm3) |
Catalyst Particle Size (μm) |
1 |
3 |
0.007 |
0.014 |
3.0 |
12 |
2 |
18 |
0.042 |
0.014 |
0.5 |
50 |
3 |
3 |
0.007 |
0.007 |
1.5 |
50 |
It has been suggested that the overall reaction rate can be enhanced by increasing the agitation, decreasing the particle size, and installing a more efficient sparger. With which, if any, of these recommendations do you agree? Are there other ways that the overall rate of reaction might be increased? Support your decisions with calculations.
Is it possible to determine the effectiveness factor from the data above? If so, what is it?
For economical reasons concerning the entrainment of the small solid catalyst particles in the liquid, it is proposed to use particles an order of magnitude larger. The following data were obtained from these particles at 25°C:
Run |
Partial Pressure of H2 (atm) |
Solubility of H2 (g mol/dm3) |
H2 Rate of Reaction (g mol/dm3 • min) |
Catalyst Charge (g/dm3) |
Catalyst Particle Size (μm) |
4 |
3 |
0.007 |
0.00233 |
2.0 |
750 |
The Thiele modulus is 9.0 for the 750-μm particle size in run 4. Determine (if possible) the external mass transfer coefficient, kc, and the percent (of the overall) of the external mass transfer resistance to the catalyst pellet.
P15-17B Applications of Diffusion and Reaction to Tissue Engineering. The equations describing diffusion and reaction in porous catalysts also can be used to derive rates of tissue growth and have been studied by Professor Kristi Anseth and her students at the University of Colorado. One important area of tissue growth is in cartilage tissue in joints such as the knee. Over 200,000 patients per year receive knee joint replacements. Alternative strategies include the growth of cartilage to repair the damaged knee.
One approach is to deliver cartilage-forming cells in a hydrogel to the damaged area such as the one shown in Figure WP15-1.1 in additional material on the CRE Web site.
Here, the patient’s own cells are obtained from a biopsy and embedded in a hydrogel, which is a cross-linked polymer network that is swollen in water. In order for the cells to survive and grow new tissue, many properties of the gel must be tuned to allow diffusion of important species in and out (e.g., nutrients in and cell-secreted extracellular molecules such as collagen out). Because there is no blood flow through the cartilage, oxygen transport to the cartilage cells is primarily by diffusion. Consequently, the design must be such that the gel can maintain the necessary rates of diffusion of nutrients (e.g., O2) into the hydrogel. These rates of exchange in the gel depend on the geometry and the thickness of the gel. To illustrate the application of chemical reaction engineering principles to tissue engineering, we will examine the diffusion and consumption of one of the nutrients, oxygen.
Our examination of diffusion and reaction in catalyst pellets showed that in many cases the reactant concentration near the center of the particle was virtually zero. If this condition were to occur in a hydrogel, the cells at the center would die. Consequently, the gel thickness needs to be designed to allow rapid transport of oxygen.
Let’s consider the simple gel geometry shown in Figure P15-17B. We want to find the gel
thickness at which the minimum oxygen consumption rate is 10–13 mol/cell/h .
The cell density in the gel is 1010 cells/dm3, the bulk concentration of oxygen CA0 (z = 0) is 2 × 10–4 mol/dm3, and the diffusivity, DAB, is 10–5 cm2/s.
Show that the dimensionless form of concentration and length, ψ = CA/CA0, and λ = z/L, differential mole balance on O2 gives
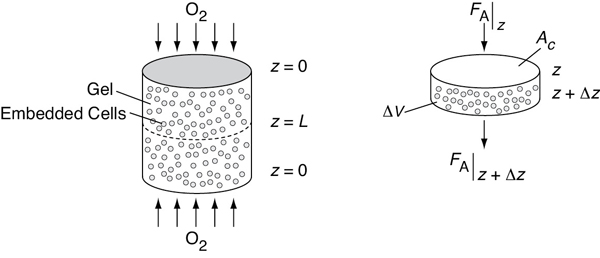
Figure P15-17B Schematic of cartilage cell system.
Show the dimensionless O2 concentration profile in the gel is
where
Solve the gel thickness when the concentration at z = 0 and CA = 0.1 mmole/dm3.
How would your answers change if the reaction kinetics were (1) first order in the O2 concentration with k1 = 10–2 h–1? (D level of difficulty)
Carry out a quasi-steady-state analysis using Equation (WE15-1.19) from the Web site (http://www.umich.edu/~elements/6e/15chap/expanded_ch15_tissue.pdf), along with the overall balance

to predict the O2 flux and collagen build-up as a function of time.
Sketch ψ versus λ at different times.
Sketch λc as a function of time. Hint: V = AcL. Assume α = 10 and the stoichiometric coefficient for oxygen to collagen, vc, is 0.05 mass fraction of cell/mol O2. Ac = 2 cm2.
Section 15.9 through 15.11 have been typeset and can be read and/or printed out from the Web site (http://www.umich.edu/~elements/6e/15chap/prof.html). Homework problems for slurry reactors, trickle bed reactors, fluidized bed reactors, and CVD boat reactors can be found at http://www.umich.edu/~elements/6e/15chap/add.html.
Supplementary Reading
1. There are a number of books that discuss internal diffusion in catalyst pellets; however, one of the first books that should be consulted on this and other topics on heterogeneous catalysis is
L. LAPIDUS AND N. R. AMUNDSON, Chemical Reactor Theory: A Review, Upper Saddle River, NJ: Prentice Hall, 1977.
In addition, see
R. ARIS, Elementary Chemical Reactor Analysis. Upper Saddle River, NJ: Prentice Hall, 1989, Chap. 6. Old, but one should find the references listed at the end of this reading particularly useful.
JOSEPH J. FOGLER, AKA, JOFO, A Chemical Reaction Engineers Guide to the Country of Jofostan. To be self published, hopefully by 2025.
D. LUSS, “Diffusion—Reaction Interactions in Catalyst Pellets,” p. 239 in Chemical Reaction and Reactor Engineering. New York: Marcel Dekker, 1987.
The effects of mass transfer on reactor performance are also discussed in
FRANK C. COLLINS AND GEORGE E. KIMBALL, “Diffusion Controlled Reaction Rates,” Journal of Colloid Science, Vol. 4, Issue 4, August 1949, Pages 425-437.
2. Diffusion with homogeneous reaction is discussed in
G. ASTARITA and R. OCONE, Special Topics in Transport Phenomena. New York: Elsevier, 2002.
Gas-liquid reactor design is also discussed in
Y. T. SHAH, Gas–Liquid–Solid Reactor Design. New York: McGraw-Hill, 1979.
3. Modeling of CVD reactors is discussed in
DANIEL DOBKIN AND M. K. ZUKRAW, Principles of Chemical Vapor Deposition. The Netherlands: Kluwer Academic Publishers, 2003.
D. W. HESS, K. F. JENSEN, and T. J. ANDERSON, “Chemical Vapor Deposition: A Chemical Engineering Perspective,” Rev. Chem. Eng., 3, 97, 1985.
4. Multiphase reactors are discussed in
P. A. RAMACHANDRAN and R. V. CHAUDHARI, Three-Phase Catalytic Reactors. New York: Gordon and Breach, 1983.
A. E. RODRIGUES, J. M. COLO, and N. H. SWEED, eds., Multiphase Reactors, Vol. 1: Fundamentals. Alphen aan den Rijn, The Netherlands: Sitjhoff and Noordhoff, 1981.
A. E. RODRIGUES, J. M. COLO, and N. H. SWEED, eds., Multiphase Reactors, Vol. 2: Design Methods. Alphen aan den Rijn, The Netherlands: Sitjhoff and Noordhoff, 1981.
Y. T. SHAH, B. G. KELKAR, S. P. GODBOLE, and W. D. DECKWER, “Design Parameters Estimations for Bubble Column Reactors” (journal review), AIChE J., 28, 353 (1982).
The following Advances in Chemistry Series volume discusses a number of multiphase reactors:
H. S. FOGLER, ed., Chemical Reactors, ACS Symp. Ser. 168. Washington, DC: American Chemical Society, 1981, pp. 3–255.
5. Fluidization
DAIZO KUNII AND OCTAVE LEVENSPIEL, Fluidization Engineering, 2nd ed. (Butterworths Series in Chemical Engineering Deposition). Stoneham, MA: Butterworth-Heinemann, 1991.
In addition to Kunii and Levenspiel’s book, many correlations can be found in
J. F. DAVIDSON, R. CLIFF, and D. HARRISON, Fluidization, 2nd ed. Orlando: Academic Press, 1985.
J. G. YATES, Fundamentals of Fluidized-Bed Chemical Processes, 3rd ed. London: Butterworth, 1983.
