Introduction
The man who has ceased to learn ought not to be allowed
to wander around loose in these dangerous days.
—M. M. Coady
A. Who Is the Intended Audience?
This book was written with today’s students in mind. It provides instantaneous access to information; does not waste time on extraneous details; cuts right to the point; uses more bullets to make information easier to access; and includes new, novel problems on chemical reaction engineering (e.g., solar energy).1 The interaction between the text and Web site (http://www.umich.edu/~elements/6e/) breaks new ground and provides one of the most comprehensive active learning resources available. With the advent of sliders in both Wolfram and Python, students can explore the reactions and the reactor in which they occur, by carrying out simulation experiments and then writing a set of conclusions to describe what they found.
1 This Introduction is a condensed version of the full Preface/Introduction found on the Web site (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf).
This book and interactive Web site are intended for use as both an undergraduate-level and a graduate-level text in chemical reaction engineering. The undergraduate course/courses usually focus on Chapters 1–13; the graduate course material includes topics such as diffusion limitations, effectiveness factors (discussed in Chapters 14 and 15), nonideal reactors, and residence time distribution (discussed in Chapters 16–18) along with the additional material and Professional Reference Shelf (PRS) on the Web site.
This edition emphasizes chemical reactor safety by ending each chapter with a safety lesson called And Now… A Word From Our Sponsor-Safety (AWFOS–S). These lessons can also be found on the Web site at http://umich.edu/~safeche/.
B. What Are the Goals of This Book?
B.1 To Have Fun Learning Chemical Reaction Engineering (CRE)
Chemical reaction engineering (CRE) is a great subject that is fun to learn and is the heart of chemical engineering. I have tried to provide a little Michigan humor as we go. Take a look at the humorous YouTube videos (e.g., “Black Widow” or “Chemical Engineering Gone Wrong”) that illustrate certain principles in the text. These videos were made by chemical engineering students at the universities of Alabama and Michigan. In addition, I have found that students enjoy the Interactive Computer Games (ICGs) that, along with the videos, are linked from the CRE homepage (http://www.umich.edu/~elements/6e/index.html).
B.2 To Develop a Fundamental Understanding of Reaction Engineering
The second goal of this book is to help the reader clearly understand the fundamentals of CRE. This goal is achieved by presenting a structure that allows the reader to solve reaction engineering problems through reasoning rather than through memorization and recall of numerous equations and the restrictions and conditions under which each equation applies (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf.
B.3 To Enhance Thinking Skills
A third goal of this text is to enhance critical thinking skills and creative thinking skills. How does the book help enhance your critical and creative thinking skills? We discuss ways to achieve this enhancement in Table P-2, Critical Thinking Questions; Table P-3, Critical Thinking Actions; and Table P-4, Practicing Creative Thinking, in the complete preface on the CRE Web site (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf) and also from the Problem Solving Web site (http://umich.edu/~scps/).
C. What Is the Structure of CRE?
C.1 What Are the Concepts That Form the Foundation of CRE?
The strategy behind the presentation of material is to build continually on a few basic ideas in CRE to solve a wide variety of problems. The building blocks of CRE and the primary algorithm allow us to solve isothermal CRE problems through logic rather than memorization. We start with the Mole Balance Building Block (Chapter 1) and then place the other blocks one at a time on top of the others until we reach the Evaluate Block (Chapter 5), by which time we can solve a multitude of isothermal CRE problems. As we study each block, we need to make sure we understand everything in that block and be sure not to cut corners by leaving anything out so we don’t wind up with a stack of cylindrical blocks. An animation of what happens to such a stack is shown at the end of Lecture 1 notes (http://www.umich.edu/%7Eelements/6e/lectures/umich.html).
For nonisothermal reactions, we replace the “Combine” building block in Figure I-1 with the “Energy Balance” building block because nonisothermal reactions almost always require a computer-generated solution. Consequently, we don’t need the “Combine” block because the computer combines everything for us. From these pillars and building blocks, we construct our CRE algorithm:
Mole Balance + Rate Laws + Stoichiometry + Energy Balance + Combine → Solution
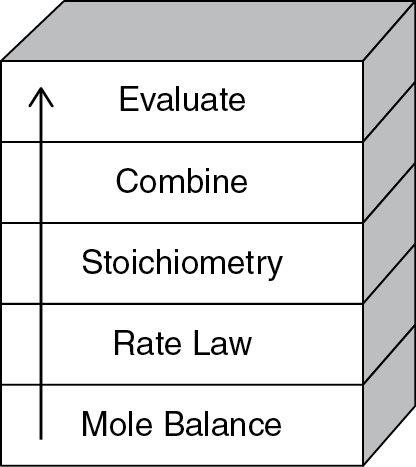
Figure I-1 Building blocks.
C.2 What Is the Sequence of Topics in Which This Book Can Be Used?
The selection and order of topics and chapters are shown in Figure P-3 in the Complete Preface/Introduction on the Web site (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf). There are notes in the margins, which are meant to serve two purposes. First, they act as guides or commentary as one reads through the material. Second, they identify key equations and relationships that are used to solve CRE problems.
Margin Notes
D. What Are the Components of the CRE Web Site?
The interactive companion Web site material has been significantly updated and is a novel, and integral part of this book. The main purposes of the Web site are to serve as an interactive part of the text with enrichment resources. The home page for the CRE Web site (http://www.umich.edu/~elements/6e/index.html) is shown in Figure I-2. For discussion of how to use the Web site and text interactively, see Appendix I.

Figure I-2 Screen shot of the book’s companion Web site
(http://www.umich.edu/~elements/6e/index.html).
D.1 How to Use the Web Site
I would like to expand a bit on a couple of things that we use extensively, namely the useful links. These items can be accessed by clicking on the Chapter number on the Home Page. After clicking on Chapter 1 shown in Figure I-3, one will arrive at
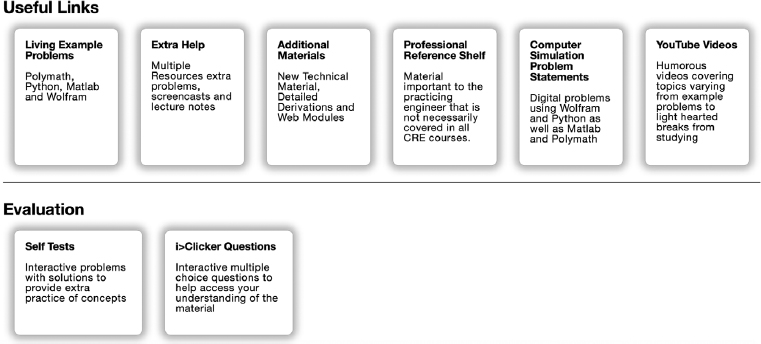
Figure I-3 Access to useful links
(http://www.umich.edu/~elements/6e/01chap/obj.html#/).
The important point I want to make here is the list of all resources shown in Figures I-3 and I-4. In addition to listing the objectives for this chapter, you will find all the major hot buttons, such as

Figure I-4 Useful links.
The Living Example Problems (LEPs), including COMSOL, have all numerical Example Problems programmed and read for use with the click of a button. The Extra Help includes interactive notes, screen casts, and techniques that facilitate learning and studying. The Additional Material and Professional Reference Shelf provide expanded derivations and material that is relevant to CRE, but did not make the final cut owing to limitations of the thickness of the book; that is, students can’t concentrate about CRE if their backpacks are so heavy they are suffering from carrying them. The Self Tests and i>Clicker Questions help readers gauge their level of understanding.
D.2 Living Example Problems (LEPs)
What are LEPs? LEPs are Living Example Problems that are really simulations that can be used to carry out experiments on the reactor and the reactions occurring inside the reactor. Here, rather than being stuck with the parameter values the author gives, the LEPs allow you to change the value of a parameter and see its effect on the reactor’s operation. LEPs have been unique to this book since their invention and inclusion in the Third Edition of this title, published in 1999. However, Wolfram and Python have allowed us to take LEPs to a new level, resulting in a minor paradigm shift. The LEPs use simulation software, which can be downloaded directly onto one’s own computer in order to “play with” the key variables and assumptions. Using the LEPs to explore the problem and asking “What if…?” questions provide students with the opportunity to practice critical and creative thinking skills. To guide students in using these simulations, questions for each chapter are given on the Web site (e.g., http://www.umich.edu/~elements/6e/12chap/obj.html).2 In this edition, there are more than 80 interactive simulations (LEPs) provided on the Web site. It is the author’s strong belief that using the LEP sliders will develop an intuitive feel for Chemical Reaction Engineering (CRE).
2See Introduction section D and Appendix I for ideas on how to use the LEPs.
LEP Sliders
The simulations labeled Stop and Smell the Roses are comprehensive-interactive simulations that will provide significant insight and an intuitive feel for the reactor and the reaction when you take the time to explore the parameters using the Wolfram or Python sliders. #wellworthyourtime

Figure I-5 shows a screen shot of the LEPs for Chapter 5. One simply clicks on the hot button of the desired programming language (Wolfram, Python) and the program loads, then uses the sliders to explore the reactors operating variables and the property parameters.
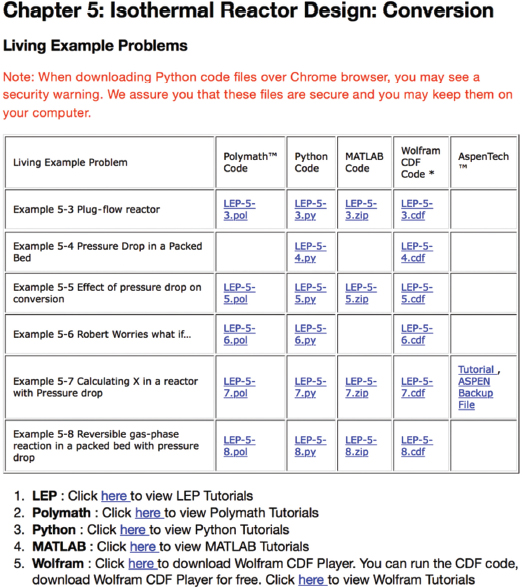
Figure I-5 Living Example Problems (LEPs).
It has been shown that students using inquiry-based learning (IBL) have a much greater understanding of information than students educated by traditional methods (Univers. J. Educ. Res., 2(1), 37–41 (2014)).3,4 The learning was most definitely enhanced when it came to questions that required interpretation such as, “Why did the temperature profile go through a minimum?” Each chapter has a section on Computer Simulations and Experiments that will guide students in practicing IBL. Students have commented that the Wolfram slider LEPs are a very efficient way to study the operation of a chemical reactor. For example, one can carry out a simulation experiment on the reactor (e.g., LEP 13-2) to investigate what conditions would lead to unsafe operation.
3 Ibid, Adbi, A.
4 Documentation of the advantages of IBL can be found at Studies in Higher Education, 38(9), 1239–1258 (2013), https://www.tandfonline.com/doi/abs/10.1080/03075079.2011.616584
You will note the tutorials listed just below the screen shot of the Living Example Problems page. There are 11 Polymath tutorials, and one LEP tutorial for each Polymath, Wolfram, Python, and MATLAB in later chapters. There are also six COMSOL tutorials. To access the LEP software you want to use, that is, Polymath, Wolfram, Python, or MATLAB, just click on the appropriate hot button, and then load and run the LEPs in the software you have chosen. Homework problems using the LEPs have been added to each chapter that requires the use of Wolfram, Python, and Polymath. The use of the LEP sliders will allow students to vary the reaction and reactor parameters to get a thorough understanding of the Computer Simulation Problems.
D.3 Extra Help
The components of Extra Help are shown in Figure I-6.
Figure I-6 Screen shot of Extra Help.
The Learning Resources give an overview of the material in each chapter through the Interactive Summary Notes. These notes include on-demand derivations of key equations, audio explanations, additional resources such as Interactive Computer Games (ICGs), computer simulations and experiments, Web modules of novel applications of CRE, solved problems, study aids, Frequently Asked Questions (FAQs), Microsoft PowerPoint lecture slides, and links to LearnChemE videos. The Web modules consist of a number of examples that apply key CRE concepts to both standard and nonstandard reaction engineering problems (e.g., glow sticks, the use of wetlands to degrade toxic chemicals, and pharmacokinetics of death from a cobra bite). The Web modules can be loaded directly from the CRE Web site (http://www.umich.edu/~elements/6e/web_mod/index.html). These resources are described in Appendix I.
D.4 Additional Material
The additional material shown in Figure I-7 includes derivations, examples, and novel applications of CRE principles that build on the CRE algorithm in the text.

Figure I-7 Screen shot of Additional Materials.
D.5 Professional Reference Shelf
This material is important to the practicing engineer, such as details of the industrial reactor design for the oxidation of SO2 and design of spherical reactors and other material that is typically not included in the majority of chemical reaction engineering courses.
D.6 Computer Simulations, Experiments, and Problems
As discussed in section D.2, these problems help guide students to understand how the parameters and operating conditions affect the reaction and the reactors. These problems are in the printed version of the second edition of Essentials of Chemical Reaction Engineering, and the sixth edition of Elements of Chemical Reaction Engineering, but not in the printed version of the fifth edition of Elements of Chemical Reaction Engineering.
D.7 YouTube Videos
Here, you will find links to humorous YouTube videos made by students in Professor Alan Lane’s 2008 chemical reaction engineering class at the University of Alabama, as well as videos from the University of Michigan’s 2011 CRE class, which includes the ever-popular chemical engineering classic, “Reaction Engineering Gone Wrong.” If you have a humorous YouTube video on CRE, I would be happy to consider linking to it.
D.8 COMSOL
The COMSOL Multiphysics software is a partial differential equation solver that is used with Chapters 13 and 18 to view both axial and radial temperature and concentration profiles. For users of this text, COMSOL has provided a special Web site that includes a step-by-step tutorial, along with examples. See Figure 18-15 on page 964 and also (https://www.comsol.com/books/elements-of-chemical-reaction-engineering-5th/models). Further details are given in the Living Example Problems on the Web site.
E. Why Do We Assign Homework Problems?
The working of homework problems facilitates a true understanding of CRE. After reading a chapter, the student may feel they have an understanding of the material. However, when attempting a new or slightly different application of CRE in a homework problem, students sometimes need to go back and reread different parts of the chapter to get the level of understanding needed to eventually solve the homework problem. Polymath is a most user-friendly software and is recommended to solve these end-of-chapter problems.
I would like to point out research has shown (J. Exp. Psychol. Learn. Mem. Cogn., 40, 106–114 (2014)) that if you ask a question of the material before reading the material you will have greater retention. Consequently, the first question of every chapter will have such a question on that chapter’s material. For Chapter 1, the question is, “Is the generation term, G, the only term in the mole balance that varies for each type of reactor?” The questions that follow are qualitative in Q1-2A and Q2-3A, and so on.
It is recommended that students first work through Computer Simulation Problems that use MATLAB, Python, and Wolfram before going on to other problems. These example problems are a key resource. The subscript letter (A, B, C, or D) after each problem number denotes the difficulty of the problem (i.e., A = easy; D = difficult). The A- and B-level problems should be worked before tackling the more challenging homework problems in a given chapter.
F. Are There Other Web Site Resources?
CRE Web Site (http://www.umich.edu/~elements/6e/index.html). A complete description of all the educational resources and ways to use them can be found in Appendix I.
Safety Web Site. During the past two years, a safety Web site has been developed for all core chemical engineering courses (http://umich.edu/~safeche/). A section at the end of each chapter called And Now… A Word From Our Sponsor-Safety (AWFOS-S) has taken the tutorials and distributed them in chapters throughout the text. A safety module for both the T2 Laboratory incident (http://umich.edu/~safeche/assets/pdf/courses/Problems/CRE/344ReactionEngrModule(1)PS-T2.pdf) and the Monsanto incident (http://umich.edu/~safeche/assets/pdf/courses/Problems/CRE/344ReactionEngrModule(2)PS-Monsanto.pdf) can be found on the safety Web site. A safety algorithm is included in both of these modules.
What Entertainment Is on the Web Site?
A. YouTube Videos. The humorous videos are discussed in Section D, what are the components of the CRE Web site, above.
B. Interactive Computer Games (ICGs). Students have found the Interactive Computer Games to be both fun and extremely useful for reviewing the important chapter concepts and then applying them to real problems in a unique and entertaining fashion. The following ICGs are available on the Web site:
Quiz Show I (Ch. 1)
Reactor Staging (Ch. 2)
Quiz Show II (Ch. 4)
Murder Mystery (Ch. 5)
Tic Tac (Ch. 5)
Ecology (Ch. 7)
The Great Race (Ch. 8)
Enzyme Man (Ch. 9)
Catalysis (Ch. 10)
Heat Effects I (Ch. 12)
Heat Effects II (Ch. 12)
As you play these interactive games, you will be asked a number of questions related to the corresponding material in the textbook. The ICG keeps track of all the correct answers and at the end of the game displays a coded performance number that reflects how well you mastered the material in the text. Instructors have a manual to decode the performance number.
G. How Can One’s Critical Thinking and Creative Thinking Skills Be Enhanced?
(http://umich.edu/~scps/html/probsolv/strategy/crit-n-creat.htm)
A third goal of this book is to enhance critical and creative thinking skills. How does one enhance their critical thinking skills? Answer: By learning how to ask critical thinking questions and taking critical thinking actions of the type given on the Web site in Tables P-2 and P-3. Further discussion is found in the Complete Preface-Introduction on the Web site (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf).
The goal to enhance creative thinking skills is achieved by using a number of problems that are open-ended to various degrees. With these, students can practice their creative skills by exploring the example problems, as outlined at the beginning of the homework problems of each chapter, and by making up and solving an original problem using the suggestions in Table P-4 on the Web site (http://www.umich.edu/~elements/6e/toc/Preface-Complete.pdf).
One of the major goals at the undergraduate level is to bring students to the point where they can solve complex reaction problems, such as multiple reactions with heat effects, and then ask “What if . . . ?” questions and look for optimum operating conditions and unsafe operating conditions. The solution to one problem exemplifies this goal: the Manufacture of Styrene (Chapter 12, Problem P12-26C). This problem is particularly interesting because two reactions are endothermic and one is exothermic.
Ethylbenzene → Styrene + Hydrogen: Endothermic
Ethylbenzene → Benzene + Ethylene: Endothermic
Ethylbenzene + Hydrogen → Toluene + Methane: Exothermic
The student could get further practice in critical and creative thinking skills by adding any of the following exercises (x), (y), and (z) to any of the end-of-chapter homework problems.
(x) How could you make this problem easier? More difficult?
(y) Critique your answer by writing a critical thinking question.
(z) Describe two ways you could work this problem incorrectly.
H. What’s New in This Edition?
This textbook and Web site interaction is a mini paradigm shift in active learning. There is a symbiotic relationship between the Web site and the textbook that allows the student to get an intuitive feel of the reactions and reactors. Here the students use the software packages of Wolfram, Python, MATLAB, and Polymath to explore the reactions and the reactors. In addition, this edition maintains all the strengths of the previous editions of Elements of Chemical Reaction Engineering by using algorithms that allow students to learn chemical reaction engineering through logic rather than memorization. Figure I-8 shows the Extra Help associated with the Chapter 1 material.
Figure I-8 Screen shot of Extra Help.
The Web site has been greatly expanded to address the Felder/Solomon Inventory of Different Learning Styles5 through interactive Summary Notes, i>clicker questions and Interactive Computer Games (ICGs). For example, as discussed in Appendix I the Global Learner can get an overview of the chapter material from the Summary Notes; the Sequential Learner can use all the i>clicker questions and ![]() hot buttons; and the active learner can interact with the ICGs and use the
hot buttons; and the active learner can interact with the ICGs and use the ![]() hot buttons in the Summary Notes.
hot buttons in the Summary Notes.
5https://www.engr.ncsu.edu/stem-resources/legacy-site/
The Web site for this new edition provides thorough interactive example problems using Polymath, Wolfram, Python, and MATLAB. These software packages are used to perform experiments on the reactor and the reactions and to then write a set of conclusions describing what the experiments revealed. In addition, there is a Safety Section at the end of each chapter that is linked to the safety Web site (http://umich.edu/~safeche/).
As with the past edition, an Aspen tech tutorial is provided for four example problems on the CRE Web site (http://www.umich.edu/~elements/6e/software/aspen.html).
And most importantly we have to always remember that:
Hopefully all intensive laws tend often to have exceptions. Very important concepts take orderly, responsible statements. Virtually all laws intrinsically are natural thoughts. General observations become laws under experimentation.
I. How Do I Say Thank You?
There are so many colleagues and students who contributed to this book that it would require another chapter to thank them all in an appropriate manner. I again acknowledge all my friends, students, and colleagues for their contributions to the sixth edition of Elements of Chemical Reaction Engineering. I would like to give special recognition as follows.
First of all, I am indebted to Ame and Catherine Vennema family, whose gift of an endowed chair greatly facilitated the completion of this project. My colleague Dr. Nihat Gürmen coauthored the original Web site during the writing of the fourth edition of Elements of Chemical Reaction Engineering. He has been a wonderful colleague to work with. I also would like to thank University of Michigan undergraduate ChE students who served early on as webmasters for the CRE Web site namely Arthur Shih, Maria Quigley, Brendan Kirchner, and Ben Griessmann. More recently CSE students, Jun Kyungjun Kim, Elsa Wang, Wen He, Kiran Thwardas, Tony Hanchi Zhang, Arav Agarwal, and Lisa Ju Young Kim worked on both the CRE Web site and the Safety Web site.
Michael B. Cutlip, coauthor of Polymath, not only gave suggestions and a critical reading of the first edition, but also, most importantly, provided continuous support and encouragement throughout the course of this project. Professor Chau-Chyun Chen provided two AspenTech examples. Ed Fontes at COMSOL Multiphysics not only provided encouragement, but also provided a COMSOL Web site containing a tutorial with CRE examples. Julie Nahil, senior content producer at Pearson for all of my book projects, has been fantastic throughout. She provided encouragement, attention to detail, and a great sense of humor, which were greatly appreciated. Indian Institute of Technology (IIT)–Guwahati chemical engineering graduate Mayur Tikmani was amazing in helping to get this text to the compositor in time. He provided all of the Wolfram coding for the LEP examples; when necessary, checked and corrected all the Polymath, Wolfram, Python, and MATLAB tutorials on the CRE Web site; and also helped proofread all the chapters. A number of summer interns have helped with preparation of the additional material for the book, especially the Safety Web site, as well as related material. Kaushik Nagaraj developed and provided the MATLAB coding for the simulations in Section 3.5 while Jakub Wlodarczyk (Warsaw University of Technology, Poland) checked all of the i>clicker questions and solutions. Students from Indian Institute of Technology, Bombay, who contributed to AWFOS-S at the end of each chapter include Kaushik Nagaraj, Triesha Singh, Reshma Kalyan Sundaram, Kshitiz Parihar, Manan Agarwal, Kushal Mittal, and Sahil Kulkarni. Vaibav Jain from IIT Delhi worked on the Solutions Manual. From the University of Michigan, Kara Steshetz, Alec Driesenga, Maeve Gillis, and Lydia Peters also worked on the Safety material.
I would like to thank the following people for various different reasons: Waheed Al-Masry, David Bogle, Lee Brown, Hank Browning, Thorwald Brun, John Chen, Stu Churchill, Dave Clough, Jim Duderstadt, Tom Edgar, John Falconer, Claudio Vilas Boas Favero, Rich Felder, Asterios Gavriilidis, Sharon Glotzer, Joe Goddard, Robert Hesketh, Mark Hoefner, Jay Jorgenson, Lloyd Kemp, Kartic Khilar, Costas Kravaris, Steve LeBlanc, Charlie Little, Kasper Lund, the Magnuson family, Joe Martin, Susan Montgomery, our parents, Guiseppe Parravano, Max Peters, Sid Sapakie, Phil Savage, Jerry Schultz, Johannes Schwank, Mordechai Shacham, Nirala Singh who class-tested this edition, Michael Stamatakis, Klaus Timmerhaus, my good friend Jim Wilkes, June Wispelwey, my grandchildren Max and Joe (aka “Jofo”) Fogler, Sophia and Nicolas Bellini, my children, Peter, Rob, and Kristi, my parents, the Emeritus Faculty Friday Lunch Group, and the Starbucks staff at Plymouth Road Mall, where most of my final editing of this book was accomplished.
Laura Bracken is very much a part of this book. I appreciate her excellent deciphering of equations and scribbles, her organization, her discovery of mistakes and inconsistencies, and her attention to detail in working with the galleys and page proofs. Through all this was her ever-present wonderful disposition. Thanks, Radar!!
Finally, to my wife Janet, love and thanks. Not only did she type the first edition of this book–can you believe on a Royal Select typewriter!–she also was a sounding board for so many things in this edition. She was always willing to help with the wording and sentence structure. For example, I often asked her, “Is this the correct phrase or word to use here?” or “Should I mention Jofostan here?” Jan also helped me learn that creativity involves knowing what to leave out. Without her enormous help and support the project would never have been possible.
HSF
Ann Arbor, Michigan
May 2020
Updates, FAQs, Web Modules, LEPs, exciting new applications, and typographical errors can all be accessed from the Home page on the companion Web site:
www.umich.edu/~elements/6e/index.html