7
Balangu (Lallemantia royleana) Seed Gum
Asad Mohammad Amini
Department of Food Science and Technology, Faculty of Agriculture, University of Kurdistan (UOK), Sanandaj, Iran
7.1 Introduction
Lallemantia royleana (Benth. in Walla) is an annual herb belonging to the Labiatae (or Lamiaceae) family (Figure 7.1a). With about 220 genera and almost 4000 species, the Labiatae family is considered as one of the largest and most distinctive flowering plants worldwide. Lallemantia royleana is widely grown in different regions of Europe and the Middle East, such as Turkey, India, Pakistan, and Afghanistan, especially in Iran, with about 410 species and subspecies. The common names of L. royleana are Balangu‐Shirazi or briefly Balangu (in Persian) and Tukhum‐malanga (in Urdu) [1–3].

Figure 7.1 (a) The Lallemantia royleana plant, (b) the seeds of Balangu, and (c) the seeds soaked in water.
For centuries, the seeds of Balangu (Figure 7.1b) have been considered as a folk medicinal herb by traditional Persian practitioners and prescribed for the treatment of gastrointestinal disease, respiratory ailments, kidney and urinary disorders, and have also been applied as a liniment for skin complications. Some recent studies have showed antioxidant, antibacterial, anesthetic, and hypocholesterolemic properties [2–9]. Also, it has been used in a range of products such as beverages (namely, Tokhm‐e‐Sharbati) and bread in Iran and Turkey [ 1,4].
The average values of the length, diameter, geometric mean diameter, sphericity, and surface area of Balangu seeds are reported as 3.148 mm, 0.720 mm, 1.176 mm, 37.4%, and 4.362 mm2, respectively. Also, the average unit mass, thousand mass, true and apparent volumes, true density, bulk density, porosity, terminal velocity, and filling and emptying angles of repose were determined as 0.0016 g, 1.67 g, 1.51 mm3 and 2.467 mm3, 1046.68 kg m−3, 739.50 kg m−3, 29.19%, 4.05 m s−1, and 27.24° and 17.23°, respectively. The static coefficient of friction was 0.221 on glass and 0.432 on rubber. On the basis of the texture analysis results, the average rupture force, hardness, and toughness are reported to be 1482.06 g, 3280.76 g, and 12902.83 g s, respectively [10].
The seeds of Balangu have been shown to mainly consist of carbohydrates (45.25%), crude fiber (30.67%), ash (3.63%), oil (18.27%), and protein (25.60%). Fatty acid including linoleic, oleic, palmitic, and stearic acid in addition to beta‐sitosterol are the main constituents of seed oil [ 3, 10,11]. The most valuable characteristic of Balangu seeds is that they adsorb water very quickly and form a turbid, sticky, and tasteless mucilaginous layer around the outer surface (Figure 7.1c).
7.2 Extraction and Purification
Although high amounts of mucilage form around the seeds in the presence of sufficient water, it adheres to the seed and cannot be separated easily. In this regard, there is a need for mechanical separation of mucilage from the hydrated seeds. Hereinafter, the separated mucilage is called Balangu seed gum (BSG).
The process parameters influencing BSG extraction were investigated for the first time by Mohammad Amini [11], and an optimized method of extraction was proposed which has been extensively utilized by others. The parameters including temperature (25–85 °C), the water‐to‐seed ratio (30:1 to 70:1), pH (5–9), and NaCl concentration (0–0.6 M) were studied with respect to gum yield, apparent viscosity, and absorbance of gum solutions, and specific optical rotation of the hydrolyzed gum. The results revealed that temperature and the water‐to‐seed ratio had a significant influence on the responses (dependent variables) of the extracted gum, while pH and salt concentration had minor effects. With respect to the gum yield and solution viscosity, the optimal conditions for extraction were found to be 85 °C, 59:1, and 7.5 for temperature, the water‐to‐seed ratio, and pH, respectively. The salt concentration parameter was found to be eliminated, that is, zero molar salt solution. The reported characteristics of optimized gum were 35%, 670 mPa s, 13.2°, and 0.63 for the yield, apparent viscosity (25 °C, 46.16 s−1, 1%), specific optical rotation, and absorbance, respectively [ 11–13].
The influence of different temperatures (20–80 °C), pH (5–9), and the water‐to‐seed ratio (20:1 and 30:1) on gum extraction kinetics has been investigated. It has been reported that raising the extraction temperature enhanced the gum yield by about 34%, while the highest yield (22.6%) was achieved under neutral conditions. Also, the results revealed that the mass transfer coefficient increased by about 3.6% as a response to increasing the water‐to‐seed ratio [14].
In 2014, Salehi and Kashaninejad investigated the influence of different drying methods, including oven drying (40–80 °C for 48 h), freeze drying, and vacuum drying (100 mbar, 50 °C) on the physicochemical characteristics of BSG. It should be noted that the BSG extraction conditions used by the authors were far from the optimum, that is, 25 °C and 20:1 water‐to‐seed ratio. They reported that the apparent viscosity of gums dried at higher temperatures was lower in the air drying method, 56 mPa s for gum dried at 80 °C versus 161 mPa s at 40 °C. Also, the highest apparent viscosity was exhibited by freeze‐dried BSG (203 mPa s). On the basis of the results of large‐deformation texture analysis, the highest hardness, stickiness, consistency, and adhesiveness (46.9 g, 14.6 g, 487.8 g s, and 130.8 g s, respectively) were measured for freeze‐dried gums. Referring to the color characteristics, the highest values have been observed in the case of air‐dried gums in comparison to vacuum‐ or freeze‐dried gums, so that the higher the drying temperature, the darker the color of BSG [15].
More recently, a two‐step extraction procedure was utilized by Ali et al. [16]. In the first step, they heated the seeds in boiling water for 4 to 5 h with continuous stirring, followed by concentrating (50% volume), filtering, and cooling to ambient temperature. Subsequently, they added acetone to precipitate the mucilage with continuous stirring, followed by collecting, filtering, and oven drying at 45 °C. Compared to the gum extracted by the method of Mohammad Amini (2007), which is highly soluble in cold water, the gum extracted by this method has been reported to be soluble in warm water, slightly soluble in cold water, and insoluble in benzene, chloroform, ethanol, and acetone. The BSG extracted by this method was reported to be free of alkaloids and tannins, as well as protein or amino acids, unlike the gum extracted by the optimized method, which contains 0.87% protein [16].
Farhadi [18] extracted BSG gum according to the optimized method of Mohammad Amini [11] and then performed purification using a three‐step procedure introduced by Piazza et al. [17]. The method consists of a hexane‐chloroform (1:1 ratio) extraction of nonpolar substances for 24 h, subsequent ethanol reflux to bleach and eliminate free sugars, and the final step of centrifugation (3500 rpm for 10 min), vacuum filtration, and dialysis (3500 Da MWCO cellulose membrane) to eliminate any insoluble constituents [18].
7.3 Physicochemical and Structural Properties
The average chemical composition of BSG has been reported to be as follows: 61.74% carbohydrates, 0.87% (Folin phenol method) or 1.66% (Kjeldahl method) proteins, 29.66% crude fiber, and 8.33% ash based on wet‐weight basis [ 10–12]. The results of chemical composition analysis have been also reported to be as follows: 75.87% carbohydrates, 8.24% ash, and 2.71% protein on the dry‐weight basis [4]. The purified BSG treated with hexane‐chloroform has also been analyzed, the results revealing that the major constituent is carbohydrates with 77.1%, followed by ash (13.1%) and protein (1.6%). In addition, the author reported on the basis of elemental (CHNO) analysis that the highly purified form of BSG had 33.39%, 4.05%, 35.45%, and 0.31% C, H, O, and N, respectively [18].
The high amounts of uronic acid (20.33%) in BSG, comparable to that of commercial hydrocolloids, reflects a polyelectrolyte nature, and therefore a higher degree of solubility will be observed [4], a characteristic which was mentioned previously.
On the basis of the analysis of monosaccharides by high‐performance anion exchange chromatography (HPAEC), it has been reported that the constituent monosaccharides of BSG (Table Table 7.1) are arabinose, galactose, rhamnose, xylose, and glucose [4]. Similarly, Farhadi [18] reported on the basis of GC–MS analysis that the monosaccharide composition of BSG is mainly composed of rhamnose, galactose, arabinose, and galacturonic acid, and the gum contains both neutral and acidic (uronic acid) sugars (Table Table 7.1) [18].
Table 7.1 Monosaccharide composition of BSG.
Source: Reproduced from Razavi et al. [4] and Farhadi [18] with permissions from Elsevier.
| Sugar (%) | Razavi et al. (2016) | Farhadi (2017) |
| Arabinose | 37.88 | 7.55 |
| Galactose | 33.54 | 30.03 |
| Rhamnose | 18.44 | 5.45 |
| Xylose | 6.02 | — |
| Glucose | 4.11 |
2.96
a
5.88 b |
| Mannose | — | 6.47 |
| Fructose | — | 19.10 |
| Glucuronic acid | — | 4.70 |
| Galacturonic acid | — | 10.18 |
a Determined as α‐D‐glucose.
b Determined as β‐D‐glucose.
The presence of peptides or amino‐substituted of monosaccharides (such as glucosamine) along with carboxyl groups in BSG composition or structure has been reported. On the other hand, the presence of the pyranose form of monosaccharides, either in the form of α‐ and β‐isomers, has been revealed by FT‐IR analysis. The NMR analysis of BSG has led to the elucidation that sugar constituents and their linkages are as follows: D‐Galp linked by α‐(1 → 4), β‐(1 → 3), and β‐(1 → 6) linkages; β‐(1 → 3) linked L‐Arap, and L‐Rhap linked by α‐(1 → 2) linkages [ 4, 18].
Using high‐performance size exclusion chromatography (HPSEC) and static light scattering (SLS), some molecular parameters of BSG have been measured (Table Table 7.2). The molecular weight of BSG has been reported by Mohammad Amini and Razavi [19] to be 3.65 Da × 106 Da (determined using dynamic light scattering), which was higher than the values measured using HPSEC and SLS (Table Table 7.2), and is comparable to the values reported for some commercial hydrocolloids [4].
Table 7.2 Molecular parameters of BSG.
Source: Reproduced from Razavi et al. [4] with permission from Elsevier.
| Parameter | Quantity |
| Refractive index increment (dn/dc) | 0.176 mL g−1 |
| Weight average molecular weight (M w) | 1.294 × 106 Da
a
0.639 × 106 Da b |
| Polydispersity index (PDI) | 1.186 |
| Apparent hydrodynamic radius (R h) | 74.964 nm |
| Radius of gyration (R g) | 104.837 nm a
60.8 nm b |
| R g/R h | 1.378 |
a Measured by HPSEC at 40 °C.
b Measured by SLS at 25 °C.
The surface tension of BSG dispersions with 0.1%–1% (w/v) has been measured at 20 °C by Razavi et al. [4]. It was observed that the surface tension decreases in the concentration range 0.1%–0.75%, while it has increased at higher concentrations. The data provided by the authors showed that the reduction in surface tension by BSG is concentration dependent, and there is a limiting concentration of 0.75%; therefore, lower concentrations of the gum can be satisfactorily used in oil‐in‐water emulsions. The authors related the moderate surface activity of BSG to the scarce amounts of proteins in the chemical composition of the gum. The surface activity of BSG is comparable to some polysaccharide gums such as fenugreek and ghatti [4].
Other investigated the physicochemical attributes of BSG, which have been reported to be as follows: angle of repose 27.89° and swelling index 108.31 [16].
From the results of the different physicochemical and structural characterizations, it can be concluded that BSG could be categorized as an arabinogalactan with rhamnose and galacturonic acid distributed in its molecular structure, similar to gum arabic and gum ghatti [18].
In conclusion, on the basis of the data on BSG structural and chemical properties in the literature, it can be considered as a promising hydrocolloid with potential applications as stabilizing, thickening, gelling, emulsifying, suspending, and super‐disintegrating agent in food and pharmaceutical industries. Although some invaluable studies have been performed on BSG structure and chemical composition, still more comprehensive work has to be done to elucidate the complete structure, the position of linkages, and substitutions of this novel hydrocolloid.
7.4 Rheological Properties
7.4.1 Dilute Solution Properties
The intrinsic viscosity, which is a valuable molecular parameter of hydrocolloids in the dilute solution domain, was rather high for BSG compared to commercial gums: 72.36 dL g−1 determined by viscometry [19] and 23.06 dL g−1 determined using HPSEC [4].
On the basis of the shape parameter and hydrodynamic radius (Table Table 7.3), it has been revealed that BSG molecules adopt a conformation between the flexible chain and random coil in good solvents, which implies a semi‐rigid and relative compact conformation, which may contribute to its high intrinsic viscosity [ 4, 19].
The influence of different ions (Na+ and Ca2+) and disaccharides (sucrose and lactose) on intrinsic viscosity of BSG has been studied. It was found that contrary to water, which is a good solvent for BSG based on the Huggins constant (0.33) and second virial coefficient (A2 = 1.45 × 10−4 mol ml g−2), the solutions of different salts and sugars are poor solvents, as indicated by a monotonous decrease in intrinsic viscosity, swollen specific volume, shape function, hydration parameter, and coil dimensions. Further, it has been reported that the hydrogel content and Huggins constant, as parameters representing the interactions of BSG molecules with different co‐solutes, increase significantly as the ionic strength and sugar concentrations are increased from 0.005 to 0.05 M and from 2.5% to 40% w/v, respectively. Briefly, it can be concluded from the results of determining the molecular parameters that any increase in temperature, ionic strength, and sugar concentration led the BSG molecules to assume a less extended conformation, thus contracting to a compact sphere‐like shape with an increased amount of intermolecular aggregation and, finally, a reduced hydrodynamic volume. The decreasing order of BSG molecular parameters was determined to be Ca2+ > Na+ > lactose > sucrose > temperature [19].
7.4.2 Steady Shear Properties
The steady shear flow behavior of BSG solutions has been investigated in some studies [ 4, 11, 15,20,21]. Generally, it has been concluded on the basis of fitting time‐independent models on shear rate–shear stress data that BSG exhibits a pronounced pseudoplastic or shear‐thinning behavior, characterized by small values of the flow behavior index along with a high consistency coefficient determined by the power‐law and Herschel–Bulkley models (Table Table 7.3). Surprisingly, the values determined for BSG are comparable to those of commercial hydrocolloids under similar conditions. The strong pseudoplasticity of BSG solutions has been attributed to its rather semi‐rigid chain conformation that gives rise to a highly entangled macromolecular solution and the presence of a gel‐like structure, which is related to the tendency of molecular association [4].
Table 7.3 Steady shear flow properties of 1% aqueous solution of BSG at 20 °C and shear rate range of 0.01–1000s−1.
Source: Reproduced from Razavi et al. [4] with permission from Elsevier.
| Model a | k (Pa sn) | n (−) | τ 0 (Pa) | η (Pa s) |
| Power‐law: |
4.2 | 0.29 | — | — |
| Herschel–Bulkley: |
2.8 | 0.35 | 1.64 | — |
| Bingham: |
— | — | 5.66 | 0.044 |
| Casson: |
— | — | 4.6 | 0.019 |
a τ, k, n, τ 0, and η are shear stress, consistency coefficient, flow behavior index, yield stress, and plastic viscosity, respectively.
By fitting viscoplastic models on BSG rheological data, it has been shown that there is a yield stress (Table Table 7.3) indicating a cross‐linked or an interactive structure with intermolecular associations which must be broken down before hydrocolloid solution can flow appropriately. It is believed that the yield stress is a useful characteristic when binding, stabilizing, and fat‐replacing applications are needed [4]. In this regard, different direct and indirect methods of determining the yield stress have been assessed, and it has been concluded that BSG solutions exhibit yield stress. The indirect method, that is, viscometry, seemed inappropriate for yield stress measurements because it has not detected the concentration dependence of the yield stress of BSG over the range 1%–1.4%. On the other hand, the inclined plane and cylindrical penetrometer methods showed significant differences between the yield values of BSG at different concentrations [21].
The viscosity profile of BSG has been described by different shear‐thinning models by determining the zero‐shear and infinite‐shear viscosities (Table Table 7.4). The value of the zero‐shear viscosity for BSG has been observed to be higher than that of commercial galactomannans like locust bean gum and guar. The higher the zero‐shear viscosity, as an indicator of the microstructural nature of BSG during storage, the greater the number of linkages between the macromolecules. On the other hand, the infinite‐shear viscosity indicates the consistency of the hydrocolloid during processing, so that a greater value shows that a greater energy is required for processing [4].
Table 7.4 Parameters of shear‐thinning models for 1% solution of BSG at 20 °C and shear rate range of 0.01–1000 s−1.
Source: Reproduced from Razavi et al. [4] with permission from Elsevier.
| Model a | η 0 (Pa s) | η ∞ (Pa s) | k (s−1) | m (−) |
| Carreau: |
67.21 | 0.64 | 14.47 | 0.61 |
| Cross: |
67.44 | 1.31 | 9.53 | 0.84 |
| Williamson: |
68.09 | — | 6.69 | 0.69 |
a η 0, η ∞, k, and m are zero‐shear viscosity, infinite‐shear viscosity, relaxation time, and power index, respectively.
Estimation of the relaxation time (k), which is an indicator of the rate of breakdown of macromolecules in a shear environment, showed that BSG exhibits a higher gel strength compared to that of the galactomannans (Table Table 7.4). The lower the value of the relaxation time, the higher the resistance of the macromolecule to the applied shear. In addition, on the basis of the parameter m obtained by the Cross and Williamson models, it has been observed that BSG tends to form shear‐thinning rather than Newtonian‐like solutions, as indicated by m tending to unity [4].
The time‐dependent rheological characteristics of BSG solutions (4%), characterized by the forward–backward shear method, revealed that thixotropic behavior appeared at concentrations of 4% or higher. Alternatively, assessing the time‐dependent properties of BSG by the constant shear method revealed thixotropic behavior. From the results of fitting time‐dependent models, it has been observed that the first‐order stress decay model with a nonzero equilibrium stress value is more appropriate compared to models such as the Weltman model, structural kinetic model, and first‐order stress decay model with zero equilibrium stress [20].
The flow behavior of BSG mixtures with xanthan, guar gum, and locust bean gum has been evaluated at 1:3, 1:1, and 3:1 blending ratios to describe the interactions through steady shear and relative viscosities. It was shown that there were no significant differences between the consistency coefficient of guar and locust bean solutions and their blends at 1:3 ratio (25% substitution). Also, from a comparison of the apparent viscosity (at 293 s−1), it has been concluded that the investigated commercial gums can be substituted by 25% and 50% BSG to provide the same consistency [22].
The influence of drying methods (air drying, freeze drying, and vacuum air drying) applied to extracted BSG on the apparent viscosity has been investigated, and it has been reported that the drying process generally decreased the viscosity, and that the most drastic decrease was caused by the air drying method at 80 °C (Figure 7.2). The viscosity reduction has been attributed to the substantial impact of the drying process on the chemical composition of BSG, altering the balance of the soluble and insoluble fractions of the macromolecule [15].

Figure 7.2 Apparent viscosity of Balangu seed gum (BSG) at shear rate of 60 s−1 as affected by air drying (AD), freeze drying (FD), and vacuum oven drying (VO) methods in comparison with non‐dried (CS) BSG.
Source: Adapted from Salehi and Kashaninejad [15] with permission from Taylor & Francis.
Table 7.5 Rheological parameters of BSG as a function of freeze–thaw cycles at different temperatures and concentrations.
Source: Reproduced from Khodaei et al. [24] with permission from Elsevier.
| Concentration (%) | Temperature (°C) | Freeze–thaw cycle | τ 0 (Pa) a | k (Pa sn) a | n (−) a | Relative hysteresis (%) |
| 0.25 | Control | — | 0.66 | 0.15 | 0.60 | 7.22 |
| −18 | 1st | 0.71 | 0.24 | 0.56 | 10.32 | |
| 2nd | 0.70 | 0.21 | 0.60 | 10.89 | ||
| 3rd | 0.73 | 0.23 | 0.60 | 11.80 | ||
| −30 | 1st | 0.78 | 0.18 | 0.60 | 10.95 | |
| 2nd | 0.80 | 0.17 | 0.61 | 11.04 | ||
| 3rd | 0.89 | 0.17 | 0.60 | 13.02 | ||
| 0.50 | Control | — | 1.38 | 1.07 | 0.46 | 13.42 |
| −18 | 1st | 1.64 | 0.99 | 0.47 | 11.65 | |
| 2nd | 1.60 | 1.13 | 0.45 | 15.44 | ||
| 3rd | 2.19 | 1.00 | 0.48 | 17.34 | ||
| −30 | 1st | 1.60 | 0.98 | 0.45 | 12.75 | |
| 2nd | 1.68 | 1.26 | 0.43 | 15.19 | ||
| 3rd | 1.90 | 1.16 | 0.47 | 16.56 | ||
| 0.75 | Control | — | 4.06 | 1.87 | 0.44 | 13.97 |
| −18 | 1st | 4.14 | 2.56 | 0.41 | 14.88 | |
| 2nd | 4.91 | 2.70 | 0.42 | 17.81 | ||
| 3rd | 4.44 | 2.79 | 0.41 | 17.95 | ||
| −30 | 1st | 3.65 | 2.41 | 0.40 | 14.15 | |
| 2nd | 5.09 | 2.52 | 0.43 | 17.13 | ||
| 3rd | 5.15 | 2.57 | 0.42 | 18.46 |
a τ 0, k, and n denote yield stress, consistency coefficient, and flow behavior index, respectively.
In another study, the influence of different sugars (sucrose, glucose, fructose, and lactose) and salts (NaCl and CaCl2) at 1%–4% concentrations on the flow behavior and apparent viscosity of BSG solutions (1%) has been investigated. It has been concluded that the apparent viscosity of BSG solutions has been improved in the presence of different sugars, with the lowest viscosity enhancement being observed for fructose. On the other hand, the addition of salts and increase of their concentration decreased the apparent viscosity of BSG solutions, in which the presence of NaCl with concentrations higher than 0.25% had a small additional effect on reducing the viscosity [15]. This is in accordance with the results of Mohammad Amini and Razavi [19], which investigated the influence of sugars and salts on the intrinsic viscosity of BSG. The viscosity reduction of BSG solutions containing salts may be attributed to the reduction of the intermolecular repulsions in presence of counterions, thus inhibiting the macromolecules of BSG from forming an expanded structure.
On the basis of the power‐law model, it has been reported that the consistency coefficient (k) of BSG solutions has been enhanced by the addition of sugars, whereas the presence of salts did not have an obvious impact on k. On the other hand, sucrose, fructose, and lactose did not induce a significant change in the flow behavior index (n), except glucose, which increased it. The presence of salts, in contrast, decreased the value of n, indicating an improvement in the pseudoplasticity of BSG solutions [23].
In another work, the influence of multiple freeze–thaw cycles (up to three times) at −18 °C and − 30 °C on the steady shear rheological properties of BSG has been studied. Similarly, pseudoplastic flow behavior has been observed for BSG solutions: the consistency coefficient and yield stress increased when the concentration was increased, whereas the flow behavior index decreased, showing a stronger shear‐thinning behavior at higher concentrations. On the basis of the Herschel–Bulkley model, it has been reported that the yield stress of BSG solutions increased slightly when the number of repeated freeze–thaw cycles was increased, which was more pronounced at a lower temperature (−30 °C) compared to −18 °C (Table Table 7.5). On the other hand, no specific change in the flow behavior index and consistency coefficient of BSG has been observed in regard to the increased number of freeze–thaw cycles. Overall, the rheological parameters of BSG solutions, regardless of their concentration, remained almost unchanged, and the freeze–thaw process had no pronounced destructive influence. Using the hysteresis loop method, the time‐dependent rheological characteristics of BSG solutions in the concentration range 0.25%–0.75% has been shown; an increase in the concentration and number of cycles of the freeze–thaw process increased the relative hysteresis, which was more pronounced at −30 °C (Table Table 7.5). The constant shearing (50 s−1, 25 °C) method and modeling of the first‐order stress decay with the nonzero equilibrium model showed that 1% BSG solutions exhibited thixotropic behavior in which application of multiple freeze–thaw cycles increased the initial and equilibrium stress values, whereas the decay rate constant was not affected. This has been attributed to the exclusion of BSG polymer chains from the growing ice crystals during the freezing, resulting in polymer‐rich regions within the unfrozen concentrated phase that promote chain association, thus enhancing thixotropic behavior [24].
7.4.3 Dynamic Shear Properties
The limiting or critical strain, which determines the maximum applicable deformation for a system before structural failure occurs, has been obtained through the strain sweep test. It has been observed that the storage modulus (G′) is independent of the strain amplitude up to 5%, which corresponds to the critical strain for BSG solution (Figure 7.3a). As a measure of the yield stress, the stress value at the crossover point of G′ and loss modulus (G″), where the material behavior changes from solid to viscous, has been determined to be 2.15 Pa, which seems close to the value of the yield stress estimated using steady shear data (Table Table 7.3a). Also, it has been demonstrated that BSG exhibits a gel structure at 1% concentration on the basis of the ratio of G′ to G″ (1.38–1.63) in the linear viscoelastic region [4].
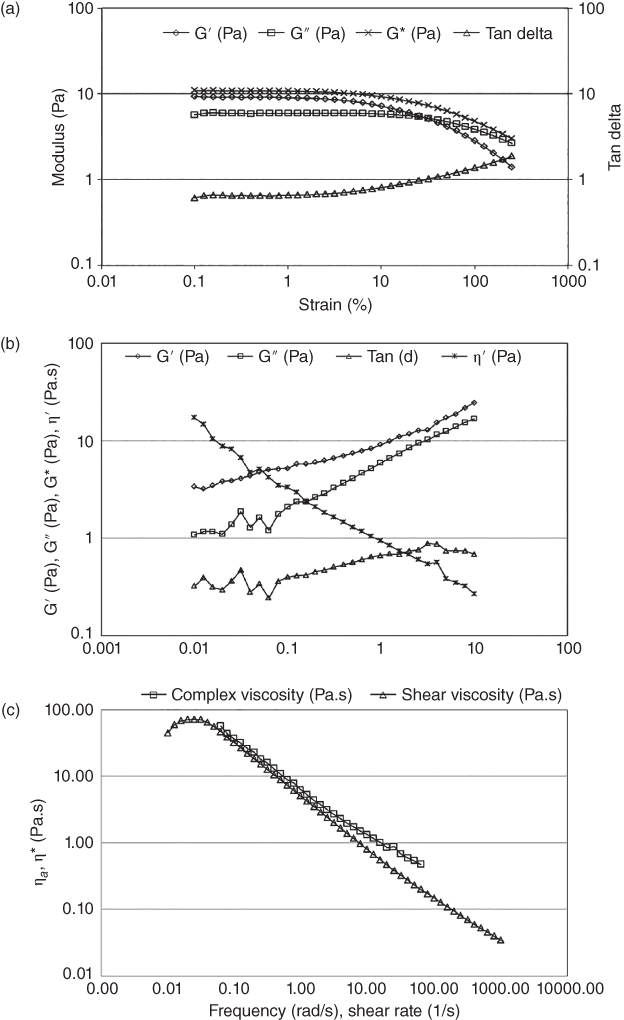
Figure 7.3 Mechanical spectra of 1% Balangu seed gum solution determined at 20 °C: (a) strain sweep test (1 Hz frequency) and (b) frequency sweep at 0.5% strain, and (c) Cox–Merz plot.
Source: Adopted from Razavi et al. [4] with permission from Elsevier.
The results of the frequency sweep test have shown that BSG behaves like a weak gel, which can be inferred from the parallel curves of G′ and G″ over a wide range of frequency with G′ > G″, a linear reduction in the complex viscosity in the logarithmic scale, and a lower‐than‐unity loss tangent (tan δ) (Figure 7.3b). On the basis of the Cox–Merz relationship, which relates the dynamic viscosity and shear viscosity (Figure 7.3c), it has been revealed that BSG exhibits a flexible and ordered conformation between random coil and semi‐rigid‐ chain (refer to Section 7.3), showing strong inter‐chain association and pseudoplastic flow behavior [4].
7.4.4 Textural Properties
Large‐deformation texture analysis has established that BSG solutions with 4% concentration had the lowest hardness value in comparison with xanthan, guar gum, and locust bean gum according to penetration, back extrusion, and texture profile analysis (TPA) tests. The penetration test showed that substitution of guar gum with 25% BSG decreased the hardness, while replacing locust bean gum and xanthan with 25%–75% BSG improved the hardness. In the back extrusion test, the hardness of the xanthan and locust bean gum mixed gels was increased with an increase in BSG concentration, whereas the highest values were observed in guar and locust bean gum mixtures with 50% substitution level of BSG. On the basis of the TPA test, the hardness of mixed gels decreased significantly at all substitution levels of BSG. On the other hand, the value of the apparent modulus of elasticity, cohesiveness, and gumminess did not change with BSG replacement. The springiness of xanthan and guar gum exhibited an increase in the presence of BSG, whereas it did not change for locust bean gum mixed gels. Although the chewiness of xanthan was not affected by BSG substitution, the presence of BSG in guar gum and locust bean gum mixed gels increased and decreased the chewiness, respectively [25].
In another study, textural parameters have been investigated with the penetration test for BSG gels with 3% concentration as a function of gum drying method (Table Table 7.6). It has been reported that the lowest hardness, stickiness, consistency, and adhesiveness values were exhibited by air‐dried BSG at elevated temperatures, while freeze‐ and vacuum‐dried gums exhibited the highest values [15].
Table 7.6 Influence of drying methods on textural characteristics of 3% BSG solutions.
Source: Adopted from Salehi and Kashaninejad [15] with permission from Taylor & Francis.
| Drying method a | Hardness (g) | Stickiness (g) | Consistency (g s) | Adhesiveness (g s) |
| AD‐40 °C | 39.6 | 11.2 | 386.3 | 91.7 |
| AD‐50 °C | 40.4 | 11.4 | 364.8 | 91.6 |
| AD‐60 °C | 34.4 | 10.1 | 324.2 | 69.9 |
| AD‐70 °C | 33.1 | 10.7 | 284.7 | 69.1 |
| AD‐80 °C | 33.6 | 9.9 | 245.3 | 64.1 |
| FD | 46.9 | 14.6 | 487.8 | 130.8 |
| VO | 46.6 | 12.3 | 426.8 | 95.4 |
a AD, FD, and VO denote air drying, freeze drying, and vacuum oven drying, respectively.
By investigating the influence of multiple freeze–thaw cycles on the textural parameters of 1% BSG gels at 25 °C using the TPA test, Khodaei et al. [24] reported that hardness, stiffness, cohesiveness, springiness, and gumminess have been increased slightly, more profoundly at lower temperatures (−30 °C). The authors attributed the high resistance of BSG gels to the freeze–thaw process to the weak gel characteristics, and therefore they concluded that BSG has excellent potential for controlling texture and reducing the destructive effects of ice crystals during temperature fluctuations of food systems.
7.5 Functional Properties
7.5.1 Stabilizing
The potential of replacing commercial hydrocolloids (palmate‐tuber Salep (PTS) and carboxymethylcellulose (CMC)) with BSG as a stabilizer in hardened ice cream formulation has been studied. It has been reported that the presence of BSG in formulation increased the viscosity of ice cream mix so that the highest values were observed in the case of 75% replacement of CMC and 50% substitution of PTS with BSG. The higher viscosity of ice cream mix is believed to be an important factor for its melting resistance and smoothness. In contrast, the presence of BSG caused a significant reduction in ice cream overrun, due to the increased viscosity of the mixture, while the sensory attributes of ice cream containing BSG, whether alone or in combination with PTS and CMC, were improved or at least not changed compared to the control [26,27].
In another study, the influence of different concentrations of BSG as a stabilizer for soft ice cream compared to PTS and CMC gums has been investigated. It has been reported that the viscosity of ice cream increased as the level of stabilizer increased from 0.3% to 0.5%, and viscosity enhancement followed this order: CMC > BSG > PTS (Figure 7.4).
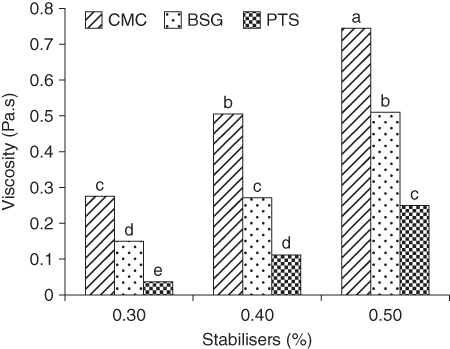
Figure 7.4 Viscosity of soft ice cream mix as a function of stabilizer type and level. CMC, carboxymethylcellulose; BSG, Balangu seed gum; PTS, palmate‐tuber Salep.
Source: Adapted from Bahramparvar et al. [28] with permission from Wiley.
Similarly, BSG reduced the overrun value of ice cream as its concentration increased, and it also caused greater reduction in overrun compared to other gums. The results of sensory attributes have established that ice creams containing BSG were acceptable at all addition levels [ 28,29].
In a similar study, it has been found that soft ice cream mixes containing BSG behaved as a pseudoplastic fluid, and on the basis of the power‐law model, the apparent viscosity and the consistency coefficient increased, while the flow behavior index decreased in response to increasing addition levels. Comparing the flow properties, BSG enhanced the rheological properties of ice cream mix to a greater extent than CMC and Salep. This, in turn, will guarantee a better mouthfeel, which is desirable in ice creams. The sensory analysis showed that BSG acts as a very suitable ice cream stabilizer, which is comparable with CMC, a well‐known commercial stabilizer [ 29,30].
In another study, the influence of BSG as yogurt stabilizer at 0.15%–0.25% concentration has been assessed in comparison with gelatin. It has been reported that incorporating BSG in yogurt formulation has led to acceptable physicochemical characteristics (pH, titratable acidity, syneresis, water‐holding capacity, hardness, and viscosity) and sensory perception with lower concentrations compared to gelatin (0.5%). Microbiological examinations showed that the microbial count was affected by incorporation of BSG, with minimum count and negative test results for coliforms during storage being observed. The best results for physicochemical parameters have been achieved at 0.25% concentration, while the highest sensory scores were obtained at 0.20% concentration [31].
The stabilizing effect of 0.15%–0.30% BSG was recently investigated in an oil‐in‐water emulsion containing whey protein. It was established that increasing the BSG concentration resulted in higher viscosity, zeta potential, and polydispersity index (PDI) while decreasing the mean droplet diameter and creaming rate, resulting in an overall enhancement of emulsion stability during two days of storage. The optimum BSG concentration is suggested to be 0.15% or less because of the depletion flocculation phenomenon [32,33].
7.5.2 Fat Replacement
The incorporation of BSG as a fat replacer (at least 25% fat replacement) along with different sweeteners in low‐calorie pistachio butter (isomalt and sucrose) has been compared to basil seed gum and xanthan on the basis of steady shear rheological properties. Pseudoplastic behavior has been reported for pistachio butter containing BSG so that increasing the gum level from 0.01% to 0.04% increased the consistency coefficient and yield stress while decreasing the flow behavior index [34]. Alternatively, time‐dependent rheological parameters have been investigated through the forward–backward shearing method for the same system and similar BSG levels in pistachio butter formulation, which revealed thixotropic behavior, decreasing the magnitude of the hysteresis loop with increasing gum level and emulsion stability for three‐month storage [35].
In another study, dynamic shear and large‐deformation rheological measurements at 5–65 °C on reduced‐fat pistachio butter containing 0.136% BSG have been performed by comparing formulations containing 0.4% xanthan and 0.092% basil seed gum. From dynamic spectra, a viscoelastic solid structure has been reported for pistachio butter containing gums. It has been revealed by the temperature sweep test that raising the temperature changed the elastic structure of pistachio butter to viscous behavior, characterized by an increase in the loss tangent. From the time sweep test, it has been observed that the complex modulus diminishes as the temperature rises, where the value of the complex modulus remains almost constant over time at 45 and 65 °C but shows an increasing trend at room temperature (Figure 7.5). For TPA test, the highest hardness and adhesiveness values have been reported for pistachio butter samples containing BSG [36].
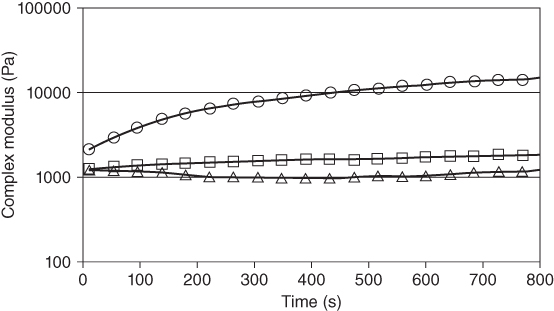
Figure 7.5 Complex modulus as a function of time for low‐calorie pistachio butter containing Balangu seed gum: (○) 25 °C, (□) 45 °C, and (∆) 65 °C.
Source: Adopted from Emadzadeh et al. [36] with permission from Taylor and Francis.
The results of steady shear rheological measurements at 5–65 °C revealed that the apparent viscosity and consistency of reduced‐fat pistachio butter containing BSG declined when the test temperature was increased, but remained higher than the values determined for formulations containing xanthan. Also, the structural recovery evaluations proved weak thixotropic behavior [37].
In another work, the potentials of BSG at 0.3%–0.5% concentration as a fat replacer in model O/W emulsions with 25%–35% fat content has been studied. The mean particle size of emulsions containing BSG ranged from 2.19 to 2.75 μm so that when the gum concentration was increased, the mean particle size decreased at all fat content levels. When the BSG concentration was increased, the specific surface area of the emulsions increased at all fat content levels, indicating an increase in emulsion stability. The steady shear rheological assessment of emulsions indicated a pseudoplastic behavior in which the pseudoplasticity decreased with increasing gum concentration, while the apparent viscosity, consistency coefficient, and yield stress increased. On the basis of dynamic rheological characterizations, it has been shown that emulsions exhibit weak gel structure with loss tangent values higher than 0.1, while the loss tangent decreases with increasing gum concentration and fat content. The sensory evaluations revealed that by when the BSG concentration was increased, the sensory consistency and oiliness increased, while the smoothness of the emulsions remained unchanged. The visual assessment of emulsions revealed that they were stable for up to three weeks when stored at 4 and 25 °C without any phase separation, except at the lower BSG concentrations, while the emulsions containing the highest concentration of BSG were stable for at least 40 days. The authors related the observations to viscosity enhancement, the existence of yield stress, and strong steric layers in boundaries caused by the presence of BSG and increasing BSG concentration [38].
Recently, the fat‐replacing potential of BSG at 0.1%–0.3% concentration has been assessed in Iranian white cheese and compared to full‐fat (3.8%) cheese. It has been concluded that BSG improved the texture of cheese so that adding 0.2% BSG showed the most likeness to full‐fat cheese, which gained the highest acceptance based on sensory evaluation [39].
7.5.3 Emulsifying
The emulsifying properties of BSG solution at 0.1%–0.5% concentration has been studied [24]. It has been established that the emulsification capacity and emulsion stability were increased with increasing concentration; also, they were improved to some extent by applying repeated freeze–thaw cycles. The results of the emulsification potential of BSG are in accordance with the measurements of surface tension performed by Razavi et al. [4]. It is believed that an increase in emulsion stability could be attributed to the inter‐chain association and concentration of polymer chains in the non‐frozen phase of the solution, which prevented oil droplets from aggregating. Also, the stabilization of the emulsion has been related to the presence of protein in BSG along with the large molecular size of its polymer chains, which can form a thick layer to protect oil droplets against aggregation [24].
7.5.4 Foaming
The foaming potential of BSG has been evaluated in comparison with xanthan, Qodume Shahri (Lepidium perfoliatum) seed gum, basil seed gum, and cress (Lepidium sativum) seed gum in the white button mushroom puree. It has been revealed that foam density and foam stability increased when the gum level was raised from 0.1% to 0.9% in foam formulations. While low concentrations of BSG have been reported to be less effective on foam stability, greater effects have been observed at higher concentrations [40].
In another work, the foaming capacity and foam stability of BSG at 0.5%–1% concentration was studied. It was reported that BSG cannot form stable foams at concentrations below 0.5%, but at higher concentrations, foaming capacity enhanced by increasing the BSG concentration. On the other hand, it was observed that the presence of BSG increases the stability of foam after applying multiple freeze–thaw cycles, more significantly at a lower temperature (−30 °C) [24].

Figure 7.6 Micrographs of the cross‐section of films containing 35% glycerol fabricated with (a) 0.4% BSG, (b) 1.2% BSG, and (c) 1.6% BSG.
Source: Adopted from Sadeghi‐Varkani et al. [41] with permissions from Elsevier.
7.5.5 Edible Films
The properties of a new edible film prepared from 0.4% to 1.6% BSG solutions containing 10%–60% glycerol as plasticizer have been recently studied. It has been reported that increasing the plasticizer concentration increases the water solubility of films, while increasing the concentration of BSG decreased it. On the other hand, the lowest water vapor permeability (WVP) has been observed for BSG films with 1.2% concentration, and increasing glycerol caused an increase in WVP. The higher solubility and WVP of films has been attributed to the integrity disruption caused by the inclusion of low‐molecular‐weight plasticizer, resulting in a porous structure in the films. Also, a higher concentration of BSG made fabricated films more permeable to water vapor (Figure 7.6). The transparency and whiteness index of films decreased as the BSG concentration increased, while tensile strength and elongation at break enhanced with increasing the concentration of BSG up to 1.2%, but higher concentration decreased them. This behavior has been explained in terms of increased molecular chain interactions resulting in greater inhomogeneity in the film structure. The glass transition temperature of BSG films decreased with increasing glycerol concentration, and its value has been reported to be higher than for commercial films like high‐density polyethylene. It was concluded that the optimal BSG and glycerol concentrations for fabricating BSG edible film are 1.2% and 35%, respectively. The oxygen permeability (50% relative humidity for 24 h) of BSG film has been reported to be 6.25 cm3 μm m−2 kPa. Overall, the conclusion was that BSG films exhibit mechanical, oxygen permeability, and WVP properties that are comparable to those of some polysaccharide‐based edible films [41].
7.5.6 Other Applications
The influence of the BSG at 0.25% and 0.5% concentrations on the rheological properties of wheat flour dough and quality attributes of loaf bread have been investigated. It has been shown from farinograph and extensograph examinations that water absorption, stability, resistance to mixing, strength, and extensibility of dough are enhanced by adding BSG. Further, the water activity, firmness, porosity (Figure 7.7), anti‐staling, and organoleptic properties of bread are improved [11].

Figure 7.7 Images of bread samples containing Balangu seed gum [12].
The stability of 1% BSG gel over multiple freeze–thaw cycles has been investigated by measuring syneresis. It has been observed that BSG gel can strongly withstand multiple freeze–thaw cycles without any syneresis, indicating strong water‐holding capacity. Therefore, BSG has been suggested for use as a gelling agent in frozen dairy and bakery products [24].
Recently, the influence of BSG concentration (0.3% and 0.6%) in sponge cake formulations was determined. It was concluded that the moisture content and lightness of cake increased as a result of BSG. The formulation containing 0.3% BSG had the highest amount of specific volume, porosity, and sensory scores; also, BSG had anti‐staling and texture‐softening properties [42].
7.6 Conclusions and Future Trends
The extracted gum from Balangu (L. royleana) seeds, that is, BSG, has been the subject of important studies in the past few years. As a novel hydrocolloid, BSG is mainly composed of arabinose, galactose, and rhamnose with semi‐rigid conformation, high intrinsic viscosity, strong pseudoplastic flow behavior and high shear viscosity, and a weak gel structure, which are comparable to most commercial hydrocolloids. On the basis of the knowledge that is available to date, BSG can be viewed as an interesting option for food and pharmaceutical applications. Although different chemical, rheological, and technological features of BSG have been assessed, further investigations on the molecular structure of its polysaccharides and some other potential functionalities like emulsifying and foaming properties for broader applications are required.
References
- 1 Naghibi, F., Mosaddegh, M., Mohammadi Motamed, S. et al. (2005). Labiatae family in folk medicine in Iran: from ethnobotany to pharmacology. Iranian Journal of Pharmaceutical Research 2 (2): 63–79.
- 2 Mahmood, S., Hayat, M.Q., Sadiq, A. et al. (2013). Antibacterial activity of Lallemantia royleana (Benth.) indigenous to Pakistan. African Journal of Microbiology Research 7 (31): 4006–4009.
- 3 Khare, C.P. (2007). Indian Medicinal Plants: An Illustrated Dictionary. New York: Springer‐Verlag, Ltd.
- 4 Razavi, S.M.A., Cui, S.W., and Ding, H. (2016). Structural and physicochemical characteristics of a novel water‐soluble gum from Lallemantia royleana seed. International Journal of Biological Macromolecules 83: 142–151.
- 5 Bozorgi, M. and Vazirian, M. (2016). Antioxidant activity of Lallemantia royleana (Benth.) seed extract. Traditional and integrative Medicine 1 (4): 147–150.
- 6 Jasmine, F. (2016). Antioxidant properties and phytochemical analysis of some medicinal plants. Ph.D. thesis. Integral University.
- 7 Jasmine, F., Shazia, M., Ali, S.M. et al. (2016). Phyto‐chemical analysis, in‐vitro antioxidant potential and GC‐MS of Lallemantia royleana seeds. International Journal of Scientific and Research Publications 6 (2): 407–411.
- 8 Ghannadi, A., Movahedian, A., and Jannesary, Z. (2015). Hypocholesterolemic effects of Balangu (Lallemantia royleana) seeds in the rabbits fed on a cholesterol‐containing diet. Avicenna Journal of Phytomedicine 5 (3): 167–173.
- 9 Atabaki, R. and Hassanpour‐ezatti, M. (2014). Improvement of lidocaine local anesthetic action using Lallemantia royleana seed mucilage as an excipient. Iranian Journal of Pharmaceutical Research 13 (4): 1431–1436.
- 10 Razavi, S.M.A., Mohammadi Moghaddam, T., and Mohammad Amini, A. (2008). Physico‐mechanic properties and chemical composition of Balangu (Lellemantia royleana (Benth. In Walla.)) seed. International Journal of Food Engineering 4 (5): 1–10.
- 11 Mohammad Amini, A. (2007). Optimization of the Lallemantia royleana gum extraction and investigation into its effects on rheological and organoleptic properties of bread in comparison with xanthan gum. Master thesis. Ferdowsi University of Mashhad.
- 12 Mohammad Amini, A., (2007). Modeling and optimization of mucilage extraction from Lallemantia royleana: a response surface‐genetic algorithm approach. Poster presented at the EFFOST/EHEDG Joint Conference, Lisbon, November 14–16, 2007.
- 13 Emadzadeh, B., Razavi, S.M.A., Mohammad Amini, A. (2008) Evaluation of the hydrocolloid extraction of Lallemantia royleana seed by image analysis method. Poster presented at the 9th International Hydrocolloids Conference (9th IHC), Singapore, June 15–19, 2008.
- 14 Salehi, F. and Kashaninejad, M. (2013). Modelling gum extraction from Balangu (Lallemantia royleana) seed. Innovative Food Technologies (JIFT) 1 (1): 13–20. (in Persian).
- 15 Salehi, F. and Kashaninejad, M. (2014). Effect of different drying methods on rheological and textural properties of Balangu seed gum. Drying Technology 32: 720–727.
- 16 Ali, S., Parvez, N., and Sharma, P.K. (2016). Extraction and evaluation of Lallemantia royleana seed mucilage. World Journal of Pharmacy and Pharmaceutical Sciences 5 (6): 1056–1066.
- 17 Piazza, L., Bertini, S., and Milany, J. (2010). Extraction and structural characterization of the polysaccharide fraction of Launaea acanthodes gum. Carbohydrate Polymers 79: 449–454.
- 18 Farhadi, N. (2017). Structural elucidation of a water‐soluble polysaccharide isolated from Balangu Shirazi (Lallemantia royleana) seeds. Food Hydrocolloids 72: 263–270.
- 19 Mohammad Amini, A. and Razavi, S.M.A. (2012). Dilute solution properties of Balangu (Lallemantia royleana) seed gum: effect of temperature, salt, and sugar. International Journal of Biological Macromolecules 51: 235–243.
- 20 Razavi, S.M.A. and Karazhiyan, H. (2009). Flow properties and thixotropy of selected hydrocolloids: experimental and modeling studies. Food Hydrocolloids 23 (3): 908–912.
- 21 Razavi, S.M.A., Emadzadeh, B., and Zahedi, Y. (2011). Direct and indirect methods to evaluate the yield stress of selected food hydrocolloids. EJEAFChe 10 (11): 3132–3142.
- 22 Moahammadi Moghadam, T., Razavi, S.M.A., and Emadzadeh, B. (2011). Rheological interactions of Lallemantia royleana seed extract with selected food hydrocolloids. Journal of the Science of Food and Agriculture 91: 1083–1088.
- 23 Salehi, F., Kashaninejad, M., and Behshad, V. (2014). Effect of sugars and salts on rheological properties of Balangu seed (Lallemantia royleana) gum. International Journal of Biological Macromolecules 67: 16–21.
- 24 Khodaei, D., Razavi, S.M.A., and Haddad Khodaparast, M.H. (2014). Functional properties of Balangu seed gum over multiple freeze‐thaw cycles. Food Research International 66: 58–68.
- 25 Razavi, S.M.A. and Mohammadi Moghadam, T. (2011). Influence of different substitution levels of Balangu seed gum on textural characteristics of selected hydrocolloids. EJEAFChe 10 (9): 2826–2837.
- 26 Bahramparvar, M., Haddad Khodaparast, M.H., and Mohammad Amini, A. (2008). Effect of substitution of carboxymethylcellulose and salep gums with Lallemantia royleana hydrocolloid on ice cream properties. Iranian Food Science and Technology Research Journal 4 (1): 37–47. (in Persian).
- 27 Bahramparvar, M., et al. (2010) Substitution of carboxymethylcellulose and salep gums with Lallemantia royleana hydrocolloid in ice cream formulation. Poster presented at the 10th International Hydrocolloids Conference (10th IHC), Shanghai, June 20–24, 2010.
- 28 Bahram Parvar, M., Haddad Khodaparast, M.H., and Razavi, S.M.A. (2009). The effect of Lallemantia royleana (Balangu) seed, palmate‐tuber Salep and carboxymethylcellulose gums on the physicochemical and sensory properties of typical soft ice cream. International Journal of Dairy Technology 62 (4): 571–576.
- 29 Bahramparvar, M. and Mazaheri Tehrani, M. (2011). Application and functions of stabilizers in ice cream. Food Reviews International 27: 389–407.
- 30 Bahram Parvar, M., Razavi, S.M.A., and Haddad Khodaparast, M.H. (2010). Rheological characterization and sensory evaluation of typical soft ice cream made with selected food hydrocolloids. Food Science and Technology International 16 (1): 79–88.
- 31 Sohail, B., Huma, N., Mehmood, A. et al. (2014). Use of tukhm‐e‐balangu (Lallemantia royleana) as a stabilizer in set type yogurt. Journal of Agroalimentary Processes and Technologies 20 (3): 247–256.
- 32 Hosseini, V.S., Najaf Najafi, M., Mohammadi Sani, A. et al. (2013). Effect of Lallemantia royleana seed gum and whey protein concentrate on stability of oil‐in‐water emulsion. Journal of Research and Innovation in Food Science and Technology 2 (2): 109–120.
- 33 Najaf Najafi, M., Hosaini, V., Mohammadi‐Sani, A. et al. (2016). Physical stability, flow properties and droplets characteristics of Balangu (Lallemantia royleana) seed gum/whey protein stabilized submicron emulsions. Food Hydrocolloids 59: 2–8.
- 34 Emadzadeh, B., Razavi, S.M.A., and Hashemi, M. (2011). Viscous flow behavior of low‐calorie pistachio butter: a response surface methodology. International Journal of Nuts and Related Sciences 2 (1): 37–47.
- 35 Emadzadeh, B., Razavi, S.M.A., and Nassiri Mahallati, M. (2012). Effects of fat replacers and sweeteners on the time‐dependent rheological characteristics and emulsion stability of low‐calorie pistachio butter: a response surface methodology. Food and Bioprocess Technology 5: 1581–1591.
- 36 Emadzadeh, B., Razavi, S.M.A., and Schleining, G. (2013). Dynamic rheological and textural characteristics of low‐calorie pistachio butter. International Journal of Food Properties 16: 512–526.
- 37 Emadzadeh, B., Razavi, S.M.A., Rezvani, E. et al. (2015). Steady shear rheological behavior and thixotropy of low‐calorie pistachio butter. International Journal of Food Properties 18 (1): 137–148.
- 38 Razavi, S.M.A., Emadzadeh, B., Mohammad Amini, A. (2012) Investigation on potentials of some Iranian endemic seed gums as fat replacers. Research project report, Ferdowsi University of Mashhad.
- 39 Rahmani, B.H. and Najaf Najafi, M. (2017). Effect of Lallemantia royleana (Balangu) seed gum on chemical, physical and sensory attributes of low fat cheese. Iranian Journal of Food Science and Technology 14: 173–183. (in Persian).
- 40 Pasban, A., Mohebbi, M., Pourazarang, H. et al. (2014). Effects of endemic hydrocolloids and xanthan gum on foaming properties of white button mushroom puree studied by cluster analysis: a comparative study. Journal of Taibah University for Science 8: 31–38.
- 41 Sadeghi‐Varkani, A., Emam‐Djomeh, Z., and Askari, G. (2018). Physicochemical and microstructural properties of a novel edible film synthesized from Balangu seed mucilage. International Journal of Biological Macromolecules 108: 1110–1119.
- 42 Sheikholeslami, Z., Karimi, M., Davoodi, G. et al. (2017). Evaluation of qualitative, visual and sensory properties of cake containing native gum and natural emulsifier. Journal of Food Science and Technology 14: 237–249.
