10
Qodume Shahri (Lepidium perfoliatum) Seed Gum
Arash Koocheki1 and Mohammad A. Hesarinejad2
1 Department of Food Science and Technology, Ferdowsi University of Mashhad (FUM), PO Box 91775‐1163, Mashhad, Iran
2 Department of Food Processing, Research Institute of Food Science and Technology (RIFST), PO Box 91735‐147, Mashhad, Iran
10.1 Introduction
Lepidium perfoliatum is locally called Qodume Shahri in Iran. The seeds of this plant have been used for hundreds of years in traditional Iranian medicinal prescriptions because of their pharmacological effects [1]. In traditional medicine, mucilage extracts from L. perfoliatum seeds are widely employed as a demulcent for the treatment of dry coughs, whooping cough, and lung infections [2]. Any of the 230 species of herbs constituting the genus Lepidium of the Cruciferae family are distributed throughout the world, and it is native to Egypt, Arabia, Iraq, Iran, and Pakistan. Many, such as L. perfoliatum, are lawn and field weeds, but some are useful salad plants. Most species have long taproots, broad basal leaves differing from the narrow leaves on the flowering stalks, and spike‐like arrangements of small, greenish or whitish, four‐petaled flowers (Figure 10.1a).

Figure 10.1 Pictorial view of (a) Lepidium perfoliatum plant, (b) seeds, and (c) gum powder.
Lepidium perfoliatum seeds are 2 mm long, flat, rounded, ovate‐oblong with reddish brown color and narrowly winged all around (Figure 10.1b). Lepidium perfoliatum seed gum (LPSG) is mainly formed in the outer layer of seeds. When the shell contacts with water, it quickly produces a viscid, turbid, and insipid liquid. The dried LPSG has a brownish‐yellow color (Figure 10.1c).
Considering the cost, availability, and functional characteristics of common commercial stabilizers, studies are increasingly being done on the production of new polysaccharides from local sources. Natural plant‐based gums are widely utilized in food industries as thickening, binding, disintegrating, emulsifying, suspending, stabilizing, and gelling agents [3]. Among them, LPSG has shown promise to be considered as a potential novel food thickening agent and is effective for food emulsions [ 1,4]. Therefore, the objective of this chapter is to highlight the unknown potential of LPSG for use in food industries and provide holistic information on its wide spectrum of uses and prospects.
10.2 Gum Extraction Optimization
LPSG occurs mainly in the outermost layer of the hull. This hull is able to release mucilaginous material easily when soaked in water. Therefore, aqueous extraction is one of the most common techniques applied for the extraction of the seed mucilaginous material [1]. The most common method used for the extraction of seed mucilaginous material is the aqueous extraction technique [5–9]. Conventional hot‐water treatment has been used for the extraction of polysaccharides, which is a time‐temperature‐dependent method. The effects of process variables including temperature, time, pH, and the water‐to‐seed ratio are important for extraction of LPSG [1].
LPSG is extracted from whole seeds using deionized water at a water‐to‐seed ratio of 30:1 and pH of 8. The pH is monitored continuously and adjusted by addition of 0.1 mol l−1 NaOH. The water bath temperature is set at 48 ± 1.0 °C during the extraction process. Water is preheated to the desired temperature before the seeds are added. The seed‐water slurry is stirred with an electric mixing paddle throughout the entire extraction period (1.5 h). The mucilage of seeds is discarded, and ultimately the dispersion is dried in a conventional oven (overnight at 45 °C), milled, and sieved using a mesh 18 sifter [1].
This optimum extraction condition is found for maximizing extraction yield, viscosity, hue, and emulsion stability as well as obtaining minimum protein content. While increasing the extraction temperature increases the extraction yield and the protein content of the mucilage, it decreases the viscosity, hue, and stabilizing effect of the gum. Lower pH at the higher water‐to‐seed ratio seems to reduce the apparent viscosity and emulsion stability of the hydrocolloid, but it has no effect on the extraction yield. However, at the higher water‐to‐seed ratio, an increase in pH decreases the hue value. The extraction yield, hue, and protein content of gum increase with an increase in the water‐to‐seed ratio from 30:1 to 60:1, whereas the viscosity and emulsion stability decrease with increase in this variable [1].
Different drying methods affect the apparent viscosity and gelling properties of LPSG. Drying methods for mucilage affect the rigidity and adhesion of LPSG gel. Vacuum oven drying exhibits the highest viscosity reduction. The viscosity of LPSG is not affected by hot air and freeze drying methods. Since the hot air drying requires lower cost, it is preferable to use this process for the production of LPSG with higher viscosity [10].
10.3 Chemical Compositions
LPSG has a high total carbohydrate content (88.23 w/w%) [4], showing that the extracted gum is relatively pure. This polysaccharide is mainly composed of xylose (44.66 ± 0.37), arabinose (31.99 ± 0.48), galactose (12.77 ± 0.01), glucose (7.15 ± 0.33), and rhamnose (3.40 ± 0.21), which is different from most other seed mucilages and is probably an arabinoxylan‐type polysaccharide [11]. The composition of LPSG is similar to that of flaxseed mucilage, which has a mixture of neutral arabinoxylans and strongly acidic rhamnose‐containing polymers [12]. Plantago ovata seed mucilage (also called psyllium) is also an arabinoxylan polysaccharide [13]. Aspinall [14] also stated that the cress seed mucilage contains a xyloarabinan and an acidic polysaccharide in association with cellulose. Arabinoxylans constitute the main part of plant cell walls [15,16]. Arabinoxylans are prebiotic carbohydrates with promising health‐promoting properties that stimulate the activity of specific colon bacteria, particularly bifidobacteria [17]. In other words, they are very important for human nutrition as readily fermentable substrates for gut microbiota [18].
10.4 Functional Properties
The water absorption capacity (WAC) of LPSG (∼20 g/g) is lower than that of guar and locust bean gum, but it is almost similar to that of xanthan. Different WACs could arise due to the differing levels of the polar hydroxyl groups in gums and hence their extent of hydrodynamic interactions. Therefore, a low WAC of an LPSG might be due to the strong chain‐to‐chain interactions among the polysaccharides, and hence their lower interactions with water [4].
LPSG shows lower solubility compared to xanthan, guar, and locust bean gums at temperatures from 30 to 90 °C. The low solubility might be due to the low degree of substitution, which allows the polysaccharide to be more compact. The solubility of LPSG increases as the temperature increases. The solubility of LPSG does not exceed 25%, which is related to the particle size and the impurity of the sample [4].
10.5 Rheological Properties
10.5.1 Flow Properties
In general, LPSG dispersions exhibit non‐Newtonian shear‐thinning behavior at different concentrations and temperatures. LPSG has a great potential as a thickening and stabilizing agent in food systems [ 4,19,20]. Steady shear flow properties show that LPSG has high viscosity, yield stress, and strong shear‐thinning characteristics [4]. This gum is able to bind and immobilize a large amount of water, thus increasing the viscosity, modifying the texture, and stabilizing the product consistency.
10.5.1.1 Effect of Concentration
Figure 10.2 illustrates the effect of different LPSG concentrations on the apparent viscosity at 25 °C and the on consistency coefficient at different temperatures. It can be seen that the apparent viscosity of LPSG decreases with increasing shear rate (Figure 10.2a). The reduction in viscosity is sharp at the beginning and smoothened at high shear rates. At low shear rates, the gum molecules are disarranged and only partially aligned, resulting in a higher viscosity. As the shear rate is increased, the molecules become completely oriented and aligned, thus decreasing the inner friction and lowering the viscosity. A comparison of the apparent viscosity of LPSG with that of other commercial gums shows that at identical applied shear rates, the viscosity of this gum is lower than that of guar gum, almost similar to that of xanthan gum, and higher than that of locust bean gum [4]. Increasing the concentration of LPSG increases the apparent viscosity of the solution [4]. LPSG can maintain its stabilizing and thickening effect during slow or fast freezing (slow and fast conditions). The apparent viscosity of LPSG increases insignificantly after the fast freezing condition [21]. Since the main mechanism of emulsion stability by hydrocolloids is the increase in solution viscosity, it is expected that emulsion stability will be preserved by the addition of LPSG under fast freezing conditions [21].
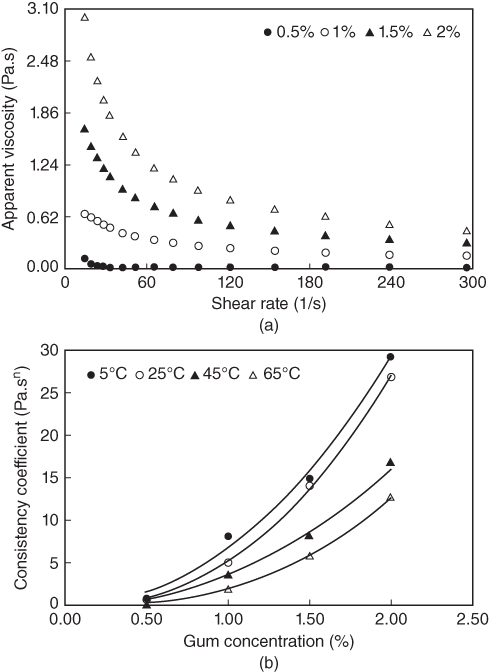
Figure 10.2 Effect of gum concentration on (a) the apparent viscosity at 25 °C and (b) the consistency coefficient at different temperatures of LPSG.
Source: Adapted from Koocheki et al. [4] with permission from Elsevier.
As seen in Figure 10.2b, the consistency coefficient (k) of LPSG, obtained by the power‐law model, increases nonlinearly as the gum concentration increases. The increase in the k value with gum concentration might be due to the increase in the water‐binding capacity of LPSG [4]. A power‐law equation (k = aC b ) is successful in describing the concentration dependency of the LPSG consistency coefficient. When the LPSG concentration increases, both “a” and “b” parameters increase. An increase in the “b” value indicates a higher dependency of k on concentration [ 4,22].
The flow behavior index, n, also decreases progressively as the gum concentration increases, indicating its increasing tendency toward pseudoplastic behavior [4]. While non‐Newtonian behavior is observed for the LPSG solution (n < 1), a solution containing 0.5% LPSG is found to be less pseudoplastic at 65 °C (n = 0.82). The shear‐thinning behavior (pseudoplasticity) of the LPSG solution represents an irreversible structural breakdown and the molecular alignment which takes place within such substance. Solutions containing 1%–2% LPSG at selected temperatures (5, 25, 45, and 65 °C) and 0.5% gum solution at temperatures below 25 °C demonstrate a flow behavior index less than 0.6, which is an important factor for sensory properties and food formulation [4]. The pseudoplastic nature of the gum and the onset of entanglement result in the shear‐thinning becoming progressively more pronounced as the concentration is increased. Magnitudes of the yield stress from the Mizrahi–Berk model for LPSG solutions are also dependent on the concentration of the gum. At higher LPSG concentrations, a higher yield stress is obtained [4].
10.5.1.2 Effect of Temperature
As temperature increases from 5 to 65 °C, the apparent viscosity of LPSG decreases. This decrease may be related to the decrease in the intermolecular interactions, which in turn decreases the energy needed for the flow and thus decreases the interference of the hydrodynamic domains [ 4,23,24]. The LPSG solutions are less pseudoplastic (higher n values) when the temperature is higher. As seen in Figure 10.2b, a decrease in the consistency coefficient is observed when the temperature is increased at all levels of solid concentration, indicating the temperature dependency of the apparent viscosity of LPSG [4]. The yield stress value obtained from the Mizrahi–Berk model for LPSG decreases with temperature, since the stress level in the fluid increases and the structure responsible for the yield stress is destroyed [4].
The activation energy, estimated by an Arrhenius‐type model for LPSG, decreases from 31.61 kJ mol−1 at 0.5% concentration to 11.43 kJ mol−1 for 2% concentration. The decrease in activation energy, as a result of the increase in concentration, shows the lower sensitivity of LPSG to temperature at higher concentrations and can be interpreted by the Eyring theory. The Eyring theory postulates that the activation energy of a flow process is due to the formation of some extra space becoming available for molecular flow. As the concentration increases, the space available for molecular flow decreases, thus decreasing the activation energy [ 4,25].
10.5.1.3 Effect of Salt
The effect of ion concentration on viscosity is important for the determination of the polyelectrolytic behavior of mucilage and estimation of the functional and rheological properties [4]. The LPSG solutions show a rapid reduction in viscosity values when 0.2% of NaCl, KCl, CaCl2, and MgCl2 are added. The decreasing effect is more pronounced for solutions containing higher salt concentrations (Figure 10.3). However, NaCl concentrations higher than 0.6% have little additional effect on the reduction of mucilage viscosity. Divalent salts have more ability to decrease the apparent viscosity of LPSG solutions than other tested salts [4]. LPSG has a negative charge at pH 7, and the measured zeta potential in aqueous suspension is −43.7 ± 2.68 mV [20]. When the hydrocolloid is consists of negatively charged polyelectrolyte molecules, the addition of positive ions reduces the repulsion forces and molecular expansion, which significantly reduces the viscosity [26]. Data suggest that the affinity of LPSG for metal ions depends on the charge/ion‐radius ratios, and small ions with high charge have a stronger affinity to chain binding sites [ 4,27,28].

Figure 10.3 Effect of different NaCl, KCl, MgCl2, and CaCl2 concentrations on the apparent viscosity of 1% (w/w) LPSG solution at the shear rate of 46.16 s−1 and 25 °C.
Source: Adapted from Koocheki et al. [4] with permission from Elsevier.
10.5.1.4 Effect of pH
At low pH levels (3–5), charge suppression results in a smaller conformation of the polymer chains because the acidic components exist in the free acid form [4]. At shear rates lower than 60 s−1, the maximum viscosity of LPSG is maintained over the pH range 7–9 (Figure 10.4). This occurrence is, presumably, due to the ionization of carboxyl groups in the LPSG structure which increases its viscosity [4]. The viscosity will be at its maximum when the LPSG molecular chains are in a state close to the rod conformation in the solution [27]. In other words, as the pH rises, the functional groups in LPSG chains induce electrostatic repulsion that tends to keep the molecules in an extended form and therefore produce a highly viscous solution [29]. Conversely, in the more alkaline regions (pH 11), the solution viscosity drops at 0.5%–2% concentrations. At a high pH (11), the decrease in viscosity may be related to the alkaline depolymerization reactions [30]. At higher shear rates (over 60 s−1), changes in pH values do not have a considerable influence on the viscosity.
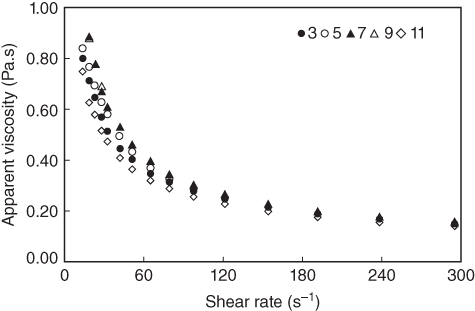
Figure 10.4 Effect of different pHs on viscosity of 1% LPSG at 25 °C.
Source: Adapted from Koocheki et al. [4] with permission from Elsevier.
10.5.2 Dynamic Rheological Properties
Dynamic rheology is one of the most extensively used methods to assess the viscoelastic behavior of polysaccharide solutions, dispersions, and gels [31].
10.5.2.1 Strain Sweep Measurements
At low gum concentrations (1.5%–2.5%), the elastic modulus (G′) remains constant at strains of up to about 1%. As the gum concentration increases, the strain at which the elastic modulus decreases, increases to more than 1%. The values of the elastic and loss moduli (G′ and G″) at the linear viscoelastic (LVE) region also increase with increasing LPSG concentration. This indicates that increasing LPSG concentration increases the strength of the system and makes it more rigid. The increase in temperature has the same effect as a concentration increase. As the temperature is raised from 5 to 85 °C, the limiting values of the strain are also increased. This value is high for 3% LPSG at 85 °C, implying higher stability of the viscoelastic material under the strain amplitude [31]. Accordingly, raising the temperature from 5 to 85 °C increases the structural strength (G′ at LVE) of the gum solution at constant concentrations [31]. Increasing gum concentration increases the yield stress values at the flow point, meaning that the gel network formed by LPSG becomes stronger. The yield stress values for LPSG decrease with temperature increase, which might be due to the increase in the stress level in the fluid and the destruction of the structure responsible for the yield stress [31].
10.5.2.2 Frequency Sweep Measurements
The LPSG solution has a typical weak gel‐like behavior where the magnitudes of G′ and G″ slightly increase with a small frequency dependency when the frequency is increased from 0.0628 to 62.8 rad s−1. The storage modulus for LPSG is always higher than its loss modulus within the experimental range of frequencies (0.01–10 Hz) with no crossover point. Therefore, LPSG behaves more like a solid; that is, the deformations will be essentially elastic and recoverable [31].
At 85 °C, the G′ and G″ values for 2.5% LPSG concentration are lower than that of 2% LPSG, showing that the elastic properties can be decreased at the special concentration. The reason for this phenomenon is unknown. Increasing the temperature from 5 to 85 °C increases the elastic modulus and decreases the viscous modulus for LPSG solutions. However, at both temperatures, G′ is always greater than G″ throughout the frequency range covered (Figure 10.5). This means that LPSG solutions can show a weak gel behavior even at high temperatures, and their structure is not highly sensitive to temperature change [31].
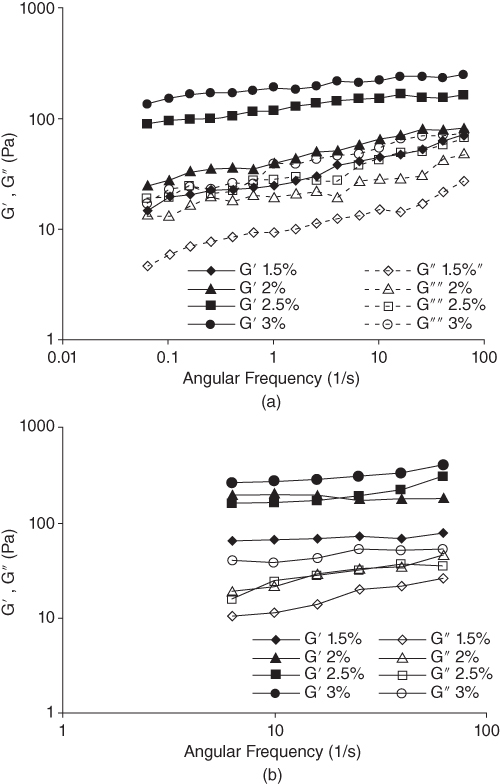
Figure 10.5 Effect of LPSG concentration on storage (G′) and loss (G″) moduli as functions of frequency at (a) 5 °C and (b) 85 °C.
Source: Adapted from Hesarinejad et al. [31] with permission from Elsevier.
The loss factor (tan δ) values for LPSG solutions lie between 0.1 and 0.5 (tan δ < 1) within the experimental frequency range (0.0628–62.8 rad s−1), with a weak dependence on frequency for all the samples, indicating that the mixtures are more elastic than viscous [31].
The complex viscosity (G*) increases as the LPSG concentration increases from 1.5% to 3%. These results confirm the high potential of this hydrocolloid as a thickener or stabilizer in increasing the consistency of food systems. The complex viscosity of LPSG is also affected by temperature, so that an increase in the complex viscosity is observed when the temperature is increased from 5 to 85 °C [31].
10.5.2.3 Temperature Sweep Measurements
The LPSG shows a higher storage modulus than the loss modulus, in the entire range of temperatures from 5 to 85 °C. There is no crossover point for G′ and G″, which means that the LPSG solution behavior is predominantly elastic and remains in the solid‐like state at all temperatures. During the initial heating from 40 to 50 °C, G′ decreases slowly as temperature increases, reaching to its minimum at around 50 °C [31]. The initial decrease in G′ can be related to the increase in fluidity with increasing temperature. This decrease may also be attributed to the energy dissipation movement of the molecules and decrease in the intermolecular interactions, which in turn decrease the energy needed for flow [ 23, 24].
The increasing trend for G′ depends on the concentration of LPSG. As the temperature increases from 50 to 85 °C, the loss modulus for higher gum concentrations (2.5%–3%) starts to increase with a uniform trend [31]. However, for low LPSG concentrations, an increase in temperature has no significant effect on the storage modulus. The increase in G′ can be related to the formation of a three‐dimensional network structure and to the conversion of the sol fraction into a gel. In LPSG solutions, the increase in G′ during heating may also be caused by the thickening effect of the gums, which restricts the mobility of fluids. Keeping the samples at 85 °C has no further effect on G′ [31] (Figure 10.6).
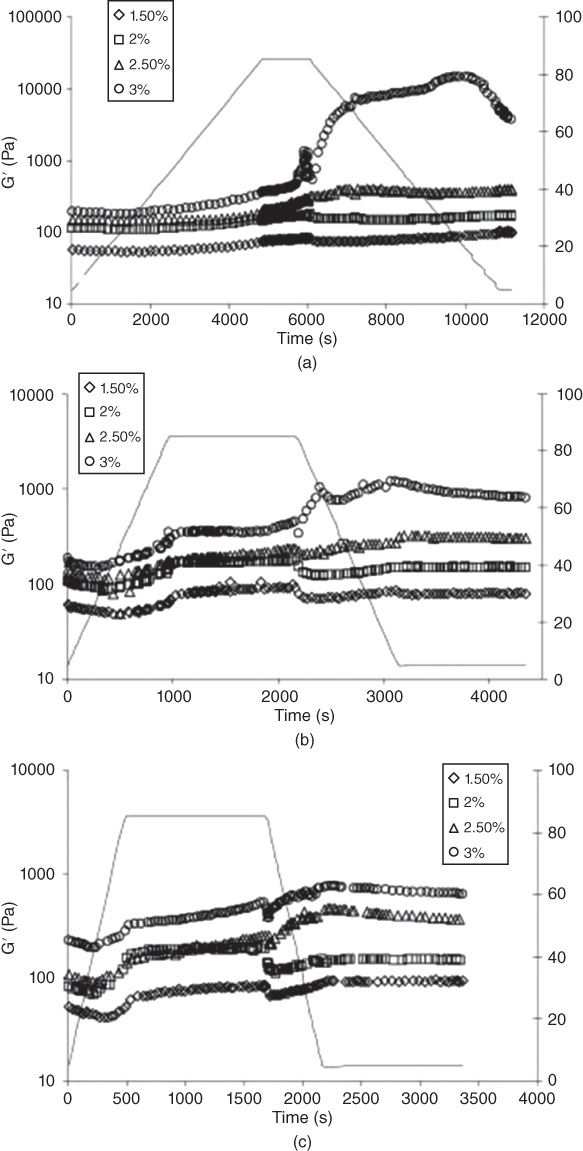
Figure 10.6 Variation of storage modulus at different LPSG concentrations and heating‐cooling rate of (a) 1 °C min−1, (b) 5 °C min−1, and (c) 10 °C min−1.
Source: Adapted from Hesarinejad et al. [31] with permission from Elsevier.
A hysteresis in the rheological measurements between the heating and cooling curves is found for LPSG solutions. The storage modulus of LPSG increases during cooling, which indicates that hydrogen bonds are formed, and the network becomes stronger. The increase in G′ during cooling is due to the strengthened hydrophobic interactions. The effect of the cooling process on the storage modulus is more pronounced for 3% LPSG, and G′ is much larger than those obtained during the heating process. Such a greater increase in G′ can be attributed to the formation of a three‐dimensional network structure due to the strong interactions in LPSG solutions. This fact seems to show that structural change occurs during cooling of 3% LPSG solution.
G′ also decreases by increasing the heating–cooling rates, which indicates that the molecules of LPSG do not have enough time to develop a firm network. The effect of the heating–cooling rate on the gelling characteristics of LPSG solutions is observed especially during cooling; an increase in G′ during cooling of 3% LPSG after heating is much higher for samples with a 1 °C min−1 cooling rate [31]. It increases rapidly and shows a peak of 1 °C min−1 cooling rate due to the formation of a thermo‐irreversible network structure, while G′ curves for all LPSG solutions (1.5%–3%) show an almost plateau region at heating–cooling rates of 5 and 10 °C min−1. Results suggest that the heating–cooling rate dependence of G′ is negligible for 1.5%–2.5% LPSG. Therefore, we can propose a critical concentration of 3% LPSG for gel formation, because at this concentration, a thermo‐irreversible network structure will be formed, and the structural strength will be increased at low heating–cooling rates.
10.6 Applications
10.6.1 Emulsions
The incorporation of LPSG into oil‐water emulsions greatly enhances the stability against phase separation [4]. When the LPSG concentration increases from 0.5% to 0.75%, the stability of these emulsions slightly increases, but remains constant at concentrations higher than 0.75% [4]. Stoke's law states that the velocity of a moving droplet is inversely related to the viscosity of the solution. Thus, by increasing the viscosity of the emulsion through the addition of LPSG, the stability of the emulsion to gravitational separation can be increased [32].
At low concentrations (0.5%–0.75%), LPSG also has a higher emulsion stabilizing effect than locust bean gum; nevertheless, these values are lower than those for guar and xanthan gums. Comparatively, the emulsion stability of LPSG is almost similar to that of guar gum at 1%; however, the stabilizing property of LPSG is lower than that of xanthan and locust bean gums at this concentration. For the LPSG‐stabilized emulsion, when the concentration increases from 0.5% to 0.75%, the stability slightly increases due to the increase in the emulsion viscosity, but remains constant at 1% LPSG [4].
Oil dispersions are well stabilized in the aqueous phase by whey protein concentrate (WPC) and LPSG [19]. It is found that the LPSG concentration should be considered as a primary factor for the stabilization of corn oil‐in‐water emulsion. The LPSG concentration significantly reduces the creaming in corn oil‐in‐water emulsions [19]. The effective size of the droplets also decreases because of the high viscosity of the aqueous phase. However, the addition of 0.4% LPSG to WPC emulsions promotes droplet flocculation through a depletion mechanism. Furthermore, this process leads to faster coalescence of droplets within the flocculated emulsion [19].
At neutral pH, the addition of a very low level of LPSG (0.2%) to emulsions made with WPC causes the droplet's flocculation, which leads to rapid creaming and phase separation. The flocculation could be assumed to occur by a depletion mechanism. However, at higher gum concentrations beyond this value, the creaming rate and the droplet size are lowered because of the high viscosity of the aqueous phase. This effect is more pronounced for emulsions stored at 4 °C. The emulsions containing LPSG are relatively stable against droplet aggregation or growth during storage at 4 °C compared to the ambient temperature. As far as the rheological properties of the emulsions are concerned, the consistency coefficient and yield stress are found to be very low in the absence of gum. Following gum addition, the consistency coefficient and yield stress of emulsions increase considerably. Addition of LPSG has no significant effect on the zeta potential values of such emulsions, and the content remains at −33.15 ± 6.15 mV. This shows that LPSG has high acidic fractions. The surface and interfacial tension increases with the addition of LPSG to WPC‐coated oil droplets. Therefore, for emulsions stabilized with WPC, there is no or very little adsorption of LPSG at the interface [20].
Increasing the oil volume fraction in O/W emulsions stabilized by LPSG increases the droplet size of the emulsion. In addition, at high oil volume fractions, the high viscosity caused by the increase in the packing fraction can limit the motion of the oil droplets and hence decrease the creaming rate [19]. It can be concluded that LPSG and WPC can be used to produce low‐fat emulsions with good stability during storage [19].
Corn oil‐in‐water emulsions stabilized with WPC in the presence of LPSG have a small droplet size under ultrasound treatment, meaning that ultrasonic waves have the ability to reduce the emulsion's droplet size [33]. Adding LPSG to the emulsion during homogenization can increase the adsorption and coverage of oil droplet surfaces by a protein, which effectively inhibits droplet aggregation or coalescence and enhances the formation of small particles in the emulsion. This is due to the increase in the viscosity of the continuous phase, giving enough time to the protein to be absorbed on the surface of the oil. The viscosity of the emulsions increases as the LPSG content increases. The presence of high amounts of high‐molecular‐weight molecules increases the resistance to flow and prevents the creaming of oil droplets. As the concentration of LPSG is increased, the yield stress of the emulsion also increases [33]. The high yield stress is a beneficial property for the gum when it is applied as a binder because it prevents the material from undergoing phase separation, sedimentation, or aggregation [34].
Different processing treatments such as freezing, pasteurization, and sterilization affect the oil droplet size, creaming, flow properties, and stability of O/W emulsions prepared by WPC and stabilized by LPSG. The shear‐thinning behavior for emulsions at all thermal treatments is also observed. Increasing the LPSG concentration decreases the flow behavior index at all thermal processing. This indicates that the shear‐thinning behavior of the emulsions increases when the LPSG concentration is increased [35]. Also, with increasing LPSG concentration, the consistency coefficient of the emulsions increases in all treatments [35]. The addition of LPSG to WPC emulsions in pasteurization and sterilization treatments causes flocculation of oil droplets, which results in a marked increase in particle size and the creaming rate. However, at higher LPSG concentrations, the particle size remains constant, apparently because of the high viscosity of the aqueous phase [35]. It should also be noted that freezing conditions (slow and fast conditions) have no significant effect on the LPSG emulsion particle size [ 21, 35]. The results show that adding LPSG to the emulsion can produce a product which tolerates the thermal processes and create a relatively stable system [35].
10.6.2 Edible Film
LPSG can be a new potential source for the manufacture of films and coatings with modified properties. LPSG‐based films can be produced by adjusting the glycerol content as a plasticizer [36]. The density of films ranged between 0.88 and 0.93 g cm−3. However, no significant decrease is observed by increasing the glycerol concentration. Increasing glycerol concentration has also no significant effect on the thickness of the film. Therefore, any changes in film density and thickness are not due to the presence of glycerol [36]. Images taken from electron scanning microscopy indicate that LPSG film has a uniform surface comprising compact sheets with no holes or fracture in its structure [36].
The moisture content of LPSG film increases as the glycerol concentration increases. Since glycerol acts as a water‐holding agent [37], increasing the glycerol concentration makes the film more hydrophilic. Therefore, the moisture content of LPSG films can be changed due to the formation of glycerol–gum and glycerol–water interactions [36]. Increasing the glycerol content from 40% to 50% does not change LPSG film moisture adsorption, while further increase substantially increases this property. This is due to the high water absorption tendency of glycerol [36]. Glycerol, being a small molecule, can be placed between polymer chains more easily as compared to larger plasticizer molecules, to interrupt the formation of polymer‐polymer hydrogen bonds. At high plasticizer content, glycerol‐rich domains can form within the polymer matrix [38,39]. Therefore, the glycerol might facilitate moisture permeation into LPSG‐based film in high‐moisture atmospheres [36].
Generally, when the water solubility of a film is high, it cannot protect food from moisture or from water loss. Increasing glycerol concentration significantly alters the water solubility of LPSG‐based film. The highest solubility is achieved when a higher concentration of glycerol (70%, w/w) is incorporated [36]. Plasticizers can reduce the crosslinks between biopolymer molecules and thus increase the solubility of the film [40]. Because glycerol is highly hygroscopic, this may explain why glycerol‐plasticized blend films have high water solubility [41].
The water vapor barrier properties of LPSG films are significantly affected by glycerol concentration. Increasing the glycerol content increases the water vapor permeability (WVP) and water vapor transmission rate (WVTR) of the LPSG films. High WVP values in LPSG films can be attributed to the developing hydrophilic nature of the films as the glycerol level increases. This is supported by the differences between the moisture absorptions of LPSG films with different glycerol concentrations [36]. Addition of a plasticizer modifies the properties of the edible film by reducing the intermolecular bonds between the polymer chains, thus increasing the WVP and WVTR of the film. In addition, glycerol as a low‐molecular‐weight substance can probably penetrate into the LPSG network, thereby effectively disrupting the intermolecular interactions among polysaccharide chains [36]. Consequently, there would be greater space for water and other molecules to migrate through the film structure [42].
Increasing the glycerol concentration slightly reduces the water contact angle of LPSG films, which is due to the hydrophilic nature of this plasticizer. The values of the contact angles of LPSG films (>30°) indicate that they would wet, at least partially, the surface of the product [36]. Wettability is one of the most important properties when evaluating the capacity of a solution to coat a surface where higher values of wettability (lower contact angles) are considered the most suitable to coat the surface. An increase in contact angle is known to lower the wetting (relatively higher hydrophobicity) property of the film surface [36]. Measuring the contact angle is a way to specify the surface hydrophilicity of a film. It is well known that a small contact angle below 30° indicates a hydrophilic surface. In other words, films with higher moisture contents have lower contact angles, indicating a greater ability to absorb water and thus higher hydrophilicity [36].
Optical properties are needed to define the ability of films to be applied over the surface of the food since these affect the appearance of the covered product, which is an important quality factor [43]. Among these properties, color attributes are of prime importance because they directly influence consumer acceptability [44]. The color parameters (a* and b*) of LPSG films, with the exception of the L* value, significantly change when the glycerol concentration increases. Incorporation of glycerol into the LPSG film matrix is able to increase the b* value and decrease the a* value. In other words, greenness and yellowness increase with increasing glycerol content [36]. Opacity and transparency are inversely correlated. A higher value of opacity means lower transparency. The transmission of light through the resulting LPSG films does not change with plasticizer concentration [36]. These findings are important since film transparency or opacity are critical properties in various film applications, particularly if the film will be used as a surface food coating or for improving product appearance [45]. In many applications, an increased opacity is desirable; for example, some applications need to provide protection against reactions of deterioration produced by the effect of light, offering some advantage to this type of film.
An increase in plasticizer concentration in LPSG‐based film exerts a great influence over elongation at break values. When the glycerol content increases, the elongation at the break value increases. As the glycerol concentration increases to up to 50%, the tensile strength decreases, and at higher plasticizer concentrations, it will be constant. Moreover, the modulus of elasticity as an index of stiffness also shows a general decrease as the glycerol content increases [36]. Films with low tensile strength and high elongation at break tend to be less brittle. As a result, LPSG films plasticized with glycerol exhibit great flexibility and hence undergo fracture in a slow and sustained pace. Furthermore, the higher the glycerol content, the lower the brittleness and the higher the flexibility of LPSG films. This most likely arises from the penetration of glycerol in the polymer matrix and reduction of inter‐chain interactions, which finally alters the mechanical properties of LPSG‐based film [36]. Generally, plasticizers interfere with polymer chains where, by decreasing intermolecular forces, they soften the rigidity of the film's structure and increase the polymer mobility; this decreases the tensile strength and increases the elongation at break [46].
The variation in the glass transition temperature (Tg) is another effective indicator of the compatibility of polymers. Glycerol is compatible with LPSG, and the effectiveness of plasticization is confirmed since only one Tg is observed. Tg decreases as the glycerol content increases [36]. The increase in glycerol concentration leads to an increase in the free volume and mobility of the polymer network, changing the physical structure of the LPSG film, which is in agreement with the decrease in the Tg values [47]. Tg is the temperature at which the material undergoes a structural transition from the glassy state to a rubbery state [48]. Below Tg, films are rigid and brittle, whereas above it films become flexible and pliable. Tg values for LPSG films are below −61.58 °C and depend on the glycerol concentration of the film. The low Tg values of LPSG films can be attributed to their inherent structural characteristics (high chain mobility) and to their relatively high hydrophilicity, which lets them absorb more water molecules than less hydrophilic films. Tg values are inversely associated with the moisture content of LPSG‐based films; in fact, water acts as a plasticizer and increases the molecular mobility (<Tg values) of the films [36]. Moreover, the melting point (Tm) and enthalpy of melting (ΔHm) also decrease when the glycerol content increases. Such a decrease in thermal stability is affected by the presence of glycerol, which reduces the interactions between polysaccharide chains, and thus stabilizes the network structure; in other words, a higher glycerol concentration requires a lower enthalpy to disrupt inter‐chain interactions [36].
Fatty acids, lipids, and waxes are commonly used in the edible film to reduce its WVP since these materials are hydrophobic and thus are good barriers against moisture migration. LPSG can produce films with good appearance but weak water barrier properties. Since the lipid component can serve as a good barrier to water vapor, stearic (C18) and palmitic (C16) fatty acids can be used to combine the advantages of both lipid and hydrocolloid components. The moisture content of LPSG‐emulsified films decreases as the chain length of fatty acid increases; this behavior can be explained by the higher hydrophobicity of long‐chain fatty acids of stearic acid compared to the short chain of palmitic acid. The water solubility of edible films indicates their water resistance when applied. The type of fatty acid has an effect on the water solubility of the emulsified LPSG films; as the chain length of fatty acids increases, water solubility decreases, which is attributed to the higher hydrophobicity of C18 compared to C16. The increase in fatty acid concentration decreases water solubility. The hydrophobic nature of fatty acids is responsible for the decrease in water solubility. The highest solubility is achieved for non‐emulsified LPSG films. Addition of fatty acids to LPSG film also reduces the moisture absorption for LPSG‐emulsified film. Fatty acids decrease the access of water molecules to hydrophilic sites, which consequently reduce the water absorbency of film. This phenomenon might be due to the immobilization of LPSG chains with fatty acids, which in turn imparts a rigid film structure. Adding the fatty acids to LPSG‐emulsified film slightly increases its density. However, the fatty acid type does not significantly alter the density of the film. The compactness of the film structure associated with the filling of small molecules into LPSG may account for this increase in density [49].
Water vapor barrier properties improve in the presence of hydrophobic‐type physical obstacles, which are believed to hinder the transfer of water molecules inside the film. Due to the hydrophobic nature of stearic and palmitic fatty acids, their incorporation decreases the WVP and WVTR of the LPSG film. WVP occurs through the hydrophilic portion of the films; therefore, depending on the hydrophilic‐to‐hydrophobic ratio of the film, the presence of fatty acids in films can decrease their WVP. Increasing the concentration of stearic acid slightly decreases the WVP and WVTR of LPSG films, but this reduction is not significant [49].
Adding fatty acids to LPSG increases the hydrophobicity of the composite film. As a result, increasing the fatty acid concentration increases the contact angle of LPSG films. The contact angles of LPSG‐C16 composite films are lower than those of LPSG‐C18 films. This is due to the higher hydrophobicity of stearic acid compared to palmitic acid [49].
However, the opacity increases as the fatty acid level increase to 20%; the fatty acid type does not significantly alter the opacity. Also, no significant difference is observed in film color (L*, a*, b*) when the fatty acid concentration and type are changed. In addition, increasing the fatty acid content significantly increases the yellowness index (YI), while no significant difference in the total color difference (ΔE) and whiteness index (WI) values are detected [49].
Increasing the concentration of fatty acids decreases the tensile strength of LPSG–fatty acid composite films. Also, the tensile strength and elongation at break of the LPSG‐C18 film are lower than those of LPSG‐C16 films. The modulus of elasticity as a factor for stiffness also decreases with increasing fatty acid content. Meanwhile, the modulus of elasticity of LPSG‐C18 film is significantly lower than that of LPSG‐C16 film. This is probably due to the lower polarity of LPSG‐C18 film. The increase in elongation percentage at break with increasing fatty acid content up to 10% can be attributed to its plasticizing effect, while the decrease in elongation at break with increasing fatty acid content up to 30% can be explained by the fact that lipids are unable to form a continuous matrix [49].
In order to improve the film properties simultaneously, two or more biopolymers can be combined. The functional properties of composite films depend on their composition and film‐forming procedure [50,51]. These blends can strongly change the physical and chemical properties of each polymer due to the compatibility/incompatibility between the two macromolecules. Films prepared from binary polymeric blends have different structures, which influence their final properties [52]. LPSG film has an acceptable appearance, but it does not have a firm and flexible structure. Lathyrus sativus protein isolate (LSPI), as a new source of protein, can improve the quality of the LPSG film [53]. LPSG and LSPI form a one‐phase blend because the LPSG–LSPI blend has a single glass transition temperature (Tg). The moisture content, moisture absorption, water solubility, and water vapor barrier of the composite films significantly decrease with the addition of LSPI to LPSG. Increasing the LSPI to LPSG ratio decreases the tensile strength, Young's modulus, and melting point. Addition of LSPI to the composite films increases their transparency, whereas elongation at break significantly increases when the LPSG ratio increases. The lightness of the composite film decreases, and the film becomes greenish and bluish in color when the LSPI proportion increases. With the incorporation of LSPI, the microstructure of films changes to a continuous and uniform network without any pores or cracks. As a result, the mechanical properties of LPSG film are improved by the addition of LSPI to the formulation, and film made from 80:20 LPSG‐to‐LSPI ratio and 60% glycerol can be used as a new biodegradable film with acceptable physical properties [53].
10.6.3 Dairy Products
The two important factors affecting ice cream texture and acceptability are stabilizers and emulsifiers. Stabilizers are ingredients which are used in small amounts in ice cream formulations and impart specific and important functions to the finished product [54]. Different gums such as guar gum, locust bean gum, κ‐carrageenan, and many natural hydrocolloids, due to their useful synergistic effects, are used as stabilizer, fat replacer, and crystal growth inhibitor agents in the ice cream industry [ 54,55]. LPSG added to the ice cream formulation alters its quality attributes including pH, acidity, melting characteristics, overrun, and textural, rheological, and sensory properties. LPSG improves overrun, melting rate, first dripping time, viscosity, hardness, and adhesiveness. The LPSG concentrations beyond 0.2% level lead to a negative effect on the gumminess and chewiness of ice cream. Therefore, LPSG can enhance the quality attributes and texture of ice cream [56]. On the basis of the results of overall acceptance scores, the best ice cream is the one having 0.2% LPSG. This suggests that LPSG is able to impart the favored features in ice cream [56].
10.6.4 Bakery Products
LPSG can improve the quality and shelf life of chiffon cake. There is a positive correlation between the viscosity of batter and cake volume. By increasing the viscosity of batter, LPSG improves the volume of chiffon cake. LPSG increases the cake volume, cohesiveness, and sensory scores, and decreases the firmness and moisture loss during storage of cake [57].
LPSG can be used as an excellent hydrocolloid for the production of pan bread. Adding LPSG has no significant effect on the dough development time. However, the dough mixing tolerance index and the gelatinization temperature decrease with increasing LPSG concentration. With increasing LPSG content, water absorption, dough stability, and viscosity increase. The gas retention value remains constant with the addition of LPSG [58]. Increasing the LPSG concentration also increases the porosity, moisture, lightness (L*), homogeneity, total air cell number, and total air cell area in bread crumbs, but decreases its specific volume, fractal dimensions, entropy, contrast, and size of air cells [ 58,59].
Addition of LPSG also improves the quality of gluten‐free sorghum bread. The analysis of bread crumbs shows that the addition of LPSG improves the structure of this bread. Gluten‐free sorghum bread containing LPSG also has good quality and acceptable sensory properties. As a result, it is possible to produce gluten‐free sorghum bread by adding LPSG [60]. Since functional characteristics of LPSG are similar to those of xanthan gum; using LPSG improves the quality of pan bread, gluten‐free sorghum bread, and chiffon cake [ 57– 59].
10.6.5 Coating of Osmotic Dehydrated Apple
Coating of apple slice using LPSG improves its texture during osmotic dehydration. The solid gain of osmotic dehydrated apples coated with 0.5% LPSG decreases, but at high gum concentration, no significant difference is observed. Scanning electron microscopy of osmotic dehydrated apples shows that the shrinkage decreases in osmotic dehydrated apples containing LPSG. Apples coated with LPSG have porous structures and are puffed. The coating has no significant effect on the textural properties except for its strength. LPSG at 0.5% reduces the color change and increases the puff structure and hardness of apples during osmotic dehydration [61].
10.6.6 Batter in Deep Frying
Addition of LPSG to batter formulations has a favorable effect on the rheological properties of batter and the quality attributes of deep‐fat‐fried chicken nuggets. Batters containing LPSG can be used to improve the quality of chicken nugget. Non‐Newtonian shear‐thinning behavior is observed in batters at all temperatures and LPSG concentrations. Increasing the LPSG concentration decreases the flow behavior index and increases the consistency coefficient of batters. Batter containing 1% LPSG has higher viscosity, pick‐up values, and water content, but imparts low oil absorption and total color changes to the final product. It should be noted that the high viscosity of batter has also some disadvantages which affect the final product's quality and texture. The texture of the chicken nuggets in the presence of LPSG is harder than that of the control. Therefore, LPSG can be recommended for use in batter formulations of chicken nuggets at concentrations not higher than 0.5%. The overall results reflect the suitability of LPSG in producing chicken nuggets with lower oil content. The hydrocolloid provides the highest apparent viscosity and is found to be an effective ingredient in improving the quality parameters of the chicken nuggets [62].
10.7 Conclusions and Future Trends
The functional and rheological properties of LPSG have been reviewed in this chapter. The benefits of LPSG solutions are influenced by their extraction procedure (time, temperature, pH, and seed‐to‐water ratio) and the measurement conditions (shear, concentration, temperature, pH, and the presence of salts such as NaCl, KCl, CaCl2, and MgCl2). LPSG can be considered as an appropriate thickening and stabilizing agent in food and pharmaceutical systems due to its high consistency, yield stress, and shear‐thinning behavior. The reduction in LPSG viscosity provides processing advantage during high‐shear processing operations such as pumping and filling, whereas the high apparent viscosity during mastication provides a desirable mouthfeel upon consumption. LPSG is able to bind with a large amount of water and increase the product consistency. This gum can be used effectively in the formation of low‐fat emulsions having a relatively high stability during storage. LPSG improves the texture and stability of bakery products and can reduce the absorption of oil in batter's deep frying. This emerging hydrocolloid has weak gel behavior at low (0.3%–0.7%) and high (1.5%–3%) concentrations and can also play an important role in texture modification. LPSG can also promisingly be applied in producing edible films with improved quality properties. The functional and rheological characteristics of LPSG prove its potential for application as a new food hydrocolloid source.
Industrial applications of LPSG will be possible due to its ability to bind with water. Thus, it will mainly be used as a thickener and stabilizer. But the future trends are related to industrial development and the ability to produce competitive products from the standpoint of performance and price. A detailed survey of all production‐cost aspects is essential to appraise the economic value of this gum in comparison to its counterparts. This ambitious challenge is crucial for a more sustainable approach to the production of novel food hydrocolloid source for food industries.
References
- 1 Koocheki, A., Taherian, A.R., Razavi, S.M. et al. (2009). Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocolloids 23 (8): 2369–2379.
- 2 Amin, G.H. (2005). Medicinal Plants of Iran. Tehran, Iran: Tehran University Publication.
- 3 Malviya, R. (2011). Extraction characterization and evaluation of selected mucilage as pharmaceutical excipient. Polimery w medycynie 41 (3): 39–44.
- 4 Koocheki, A., Taherian, A.R., and Bostan, A. (2013). Studies on the steady shear flow behavior and functional properties of Lepidium perfoliatum seed gum. Food Research International 50 (1): 446–456.
- 5 Amin, A.M., Ahmad, A.S., Yin, Y.Y. et al. (2007). Extraction, purification and characterization of durian (Durio zibethinus) seed gum. Food Hydrocolloids 21 (2): 273–279.
- 6 Brummer, Y., Cui, W., and Wang, Q. (2003). Extraction, purification and physicochemical characterization of fenugreek gum. Food Hydrocolloids 17 (3): 229–236.
- 7 Ibañez, M.C. and Ferrero, C. (2003). Extraction and characterization of the hydrocolloid from Prosopis flexuosa DC seeds. Food Research International 36 (5): 455–460.
- 8 Koocheki, A., Mortazavi, S.A., Shahidi, F. et al. (2010). Optimization of mucilage extraction from Qodume Shirazi seed (Alyssum homolocarpum) using response surface methodology. Journal of Food Process Engineering 33 (5): 861–882.
- 9 Sepúlveda, E., Sáenz, C., Aliaga, E. et al. (2007). Extraction and characterization of mucilage in Opuntia spp. Journal of Arid Environments 68 (4): 534–545.
- 10 Momeni, L., Alaedini, B., and Koocheki, A. (2016). Effect of different drying methods on viscosity and gelation of Lipidium perfoliatum seed gum. Iranian Journal of Biosystems Engineering 47 (1): 159–166. (In the Persian Language).
- 11 Hesarinejad, M. A. (2012). Viscoelastic characterization of Qodume Shirazi ( Alyssum homolocarpum ) and Qodume Shahri ( Lepidium perfoliatum ) seeds gum. Master's thesis (M.Sc.). Ferdowsi University of Mashhad, Iran.
- 12 Warrand, J., Michaud, P., Picton, L. et al. (2005). Structural investigations of the neutral polysaccharide of Linum usitatissimum L. seeds mucilage. International Journal of Biological Macromolecules 35 (3–4): 121–125.
- 13 Yu, L., Yakubov, G.E., Zeng, W. et al. (2017). Multi‐layer mucilage of Plantago ovata seeds: rheological differences arise from variations in arabinoxylan side chains. Carbohydrate Polymers 165: 132–141.
- 14 Aspinall, G.O. (1970). Arabinans and xylans. In: Polysaccharides, 103–115. New York, EU: Pergamon Elmsford.
- 15 Butardo, V.M. and Sreenivasulu, N. (2016). Tailoring grain storage reserves for a healthier rice diet and its comparative status with other cereals. International Review of Cell and Molecular Biology 323: 31–70.
- 16 Izydorczyk, M.S. and Biliaderis, C.G. (1995). Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydrate Polymers 28 (1): 33–48.
- 17 Rivière, A., Moens, F., Selak, M. et al. (2014). The ability of bifidobacteria to degrade arabinoxylan oligosaccharide constituents and derived oligosaccharides is strain dependent. Applied and Environmental Microbiology 80 (1): 204–217.
- 18 Broekaert, W.F., Courtin, C.M., Verbeke, K. et al. (2011). Prebiotic and other health‐related effects of cereal‐derived arabinoxylans, arabinoxylan‐oligosaccharides, and xylooligosaccharides. Critical Reviews in Food Science and Nutrition 51 (2): 178–194.
- 19 Soleimanpour, M., Koocheki, A., and Kadkhodaee, R. (2013). Influence of main emulsion components on the physical properties of corn oil in water emulsion: effect of oil volume fraction, whey protein concentrate and Lepidium perfoliatum seed gum. Food Research International 50 (1): 457–466.
- 20 Soleimanpour, M., Koocheki, A., and Kadkhodaee, R. (2013). Effect of Lepidium perfoliatum seed gum addition on whey protein concentrate stabilized emulsions stored at cold and ambient temperature. Food Hydrocolloids 30 (1): 292–301.
- 21 Mahfoozy, M., Koocheki, A., and Razavi, S.M.A. (2017). Effect of freezing on functional properties of Lepidium perfoliatum seed gum. Iranian Food Science and Technology Research Journal 13 (2): 240–250. (In Persian language).
- 22 Marcotte, M., Hoshahili, A.R.T., and Ramaswamy, H.S. (2001). Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Research International 34 (8): 695–703.
- 23 Bohdanecky, M. and Kovar, J. (1982). Viscosity of Polymer Solutions. Amsterdam: Polymer Science Library 2, Elsevier Scientific Publishing Company.
- 24 Lapasin, R. and Pricl, S. (1995). Rheology. In: Rheology of Industrial Polysaccharides: Theory and Applications, 162–249. Boston, MA: Springer.
- 25 Haminiuk, C.W.I., Sierakowski, M.R., Vidal, J.R.M.B. et al. (2006). Influence of temperature on the rheological behavior of whole araçá pulp (Psidium cattleianum sabine). LWT‐Food Science and Technology 39 (4): 427–431.
- 26 Medina‐Torres, L., Brito‐De La Fuente, E., Torrestiana‐Sanchez, B. et al. (2000). Rheological properties of the mucilage gum (Opuntia ficus indica). Food Hydrocolloids 14 (5): 417–424.
- 27 Feng, T., Gu, Z.B., and Jin, Z.Y. (2007). Chemical composition and some rheological properties of Mesona Blumes gum. Food Science and Technology International 13 (1): 55–61.
- 28 Oliveira, J.D., Silva, D.A., De Paula, R.C.M. et al. (2001). Composition and effect of salt on rheological and gelation properties of Enterolobium contortisilliquum gum exudate. International Journal of Biological Macromolecules 29 (1): 35–44.
- 29 Launay, B., Doublier, J.L., and Cuvelier, G. (1985). Flow properties of aqueous solutions and dispersions of polysaccharides. In: Functional Properties of Food Macromolecules (ed. S.E. Hill, J.R. Mitchell and D.A. Ledward), 1–78. London: Elsevier Applied Science Publishers.
- 30 Fedeniuk, R.W. and Biliaderis, C.G. (1994). Composition and physicochemical properties of linseed (Linum usitatissimum L.) mucilage. Journal of Agricultural and Food Chemistry 42 (2): 240–247.
- 31 Hesarinejad, M.A., Koocheki, A., and Razavi, S.M.A. (2014). Dynamic rheological properties of Lepidium perfoliatum seed gum: effect of concentration, temperature and heating/cooling rate. Food Hydrocolloids 35: 583–589.
- 32 McClements, D.J. (2015). Food Emulsions: Principles, Practices, and Techniques. Boca Raton: CRC Press.
- 33 Soleimanpour, M., Kadkhodaee, R., Koocheki, A. et al. (2013). Effect of Qodumeh Shahri seed gum on physical properties of corn‐oil in water emulsion prepared by high intensity ultrasound. Iranian Food Science and Technology Research Journal 9 (1): 21–30.
- 34 Balmaceda, E., Rha, C., and Huang, F. (1973). Rheological properties of hydrocolloids. Journal of Food Science 38 (7): 1169–1173.
- 35 Koocheki, A. and Hesarinejad, M.A. (2016). Effect of freezing, pasteurization and sterilization on physical properties of oil‐in‐water stabilized with Lepidium perfoliatum seed gum and whey protein concentrate. Iranian Journal of Food Science and Technology 64: 21–31.
- 36 Seyedi, S., Koocheki, A., Mohebbi, M. et al. (2014). Lepidium perfoliatum seed gum: a new source of carbohydrate to make a biodegradable film. Carbohydrate Polymers 101: 349–358.
- 37 Arvanitoyannis, I. and Biliaderis, C.G. (1999). Physical properties of polyol‐plasticized edible blends made of methyl cellulose and soluble starch. Carbohydrate Polymers 38 (1): 47–58.
- 38 Chen, P. and Zhang, L. (2005). New evidences of glass transitions and microstructures of soy protein plasticized with glycerol. Macromolecular Bioscience 5 (3): 237–245.
- 39 Sothornvit, R. and Krochta, J.M. (2005). Plasticizers in edible films and coatings. In: Innovations in Food Packaging, 403–433. UK: Academic Press.
- 40 Ghasemlou, M., Khodaiyan, F., and Oromiehie, A. (2011). Physical, mechanical, barrier, and thermal properties of polyol‐plasticized biodegradable edible film made from kefiran. Carbohydrate Polymers 84 (1): 477–483.
- 41 Tong, Q., Xiao, Q., and Lim, L.T. (2013). Effects of glycerol, sorbitol, xylitol and fructose plasticisers on mechanical and moisture barrier properties of pullulan–alginate–carboxymethylcellulose blend films. International Journal of Food Science and Technology 48 (4): 870–878.
- 42 Sothornvit, R. and Krochta, J.M. (2000). Water vapor permeability and solubility of films from hydrolyzed whey protein. Journal of Food Science 65 (4): 700–703.
- 43 Mali, S., Grossmann, M.V.E., Garcı&c.acute;a, M.A. et al. (2004). Barrier, mechanical and optical properties of plasticized yam starch films. Carbohydrate Polymers 56 (2): 129–135.
- 44 Rao, M.S., Kanatt, S.R., Chawla, S.P. et al. (2010). Chitosan and guar gum composite films: preparation, physical, mechanical and antimicrobial properties. Carbohydrate Polymers 82 (4): 1243–1247.
- 45 Gontard, N., Guilbert, S., and Cuq, J.L. (1992). Edible wheat gluten films: influence of the main process variables on film properties using response surface methodology. Journal of Food Science 57 (1): 190–195.
- 46 Leceta, I., Guerrero, P., and De la Caba, K. (2013). Functional properties of chitosan‐based films. Carbohydrate Polymers 93 (1): 339–346.
- 47 Roos, Y. and Karel, M. (1991). Plasticizing effect of water on thermal behavior and crystallization of amorphous food models. Journal of Food Science 56 (1): 38–43.
- 48 Yang, L. and Paulson, A.T. (2000). Mechanical and water vapour barrier properties of edible gellan films. Food Research International 33 (7): 563–570.
- 49 Seyedi, S., Koocheki, A., Mohebbi, M. et al. (2015). Improving the physical and moisture barrier properties of Lepidium perfoliatum seed gum biodegradable film with stearic and palmitic acids. International Journal of Biological Macromolecules 77: 151–158.
- 50 Osés, J., Fabregat‐Vázquez, M., Pedroza‐Islas, R. et al. (2009). Development and characterization of composite edible films based on whey protein isolate and mesquite gum. Journal of Food Engineering 92 (1): 56–62.
- 51 Osés, J., Fernández‐Pan, I., Mendoza, M. et al. (2009). Stability of the mechanical properties of edible films based on whey protein isolate during storage at different relative humidity. Food Hydrocolloids 23 (1): 125–131.
- 52 Guerrero, P., Garrido, T., Leceta, I. et al. (2013). Films based on proteins and polysaccharides: preparation and physical–chemical characterization. European Polymer Journal 49 (11): 3713–3721.
- 53 Ebrahimi, S.E., Koocheki, A., Milani, E. et al. (2016). Interactions between Lepidium perfoliatum seed gum–grass pea (Lathyrus sativus) protein isolate in composite biodegradable film. Food Hydrocolloids 54: 302–314.
- 54 Bahramparvar, M. and Mazaheri Tehrani, M. (2011). Application and functions of stabilizers in ice cream. Food Reviews International 27 (4): 389–407.
- 55 BahramParvar, M., Tehrani, M.M., and Razavi, S.M.A. (2013). Effects of a novel stabilizer blend and presence of κ‐carrageenan on some properties of vanilla ice cream during storage. Food Bioscience 3: 10–18.
- 56 Azari‐Anpar, M., Tehrani, N.S., Aghajani, N. et al. (2017). Optimization of the new formulation of ice cream with native Iranian seed gums (Lepidium perfoliatum and Lepidium sativum) using response surface methodology (RSM). Journal of Food Science and Technology 54 (1): 196–208.
- 57 Amirabadi, S., Koocheki, A., and Mohebbi, M. (2015). Effect of xanthan and Lepidium perfoliatum seed gums on quality and shelf‐life of chiffon cake. Iranian Food Science and Technology Research Journal 10 (4): 375–386. (In Persian language).
- 58 Bagheri, H., Koocheki, A., and Mohebbi, M. (2016). Effects of Leidium perfoliatum seed and xanthan gums on physical properties of pan bread. Iranian Journal of Food Science and Technology 58: 117–129. (In Persian language).
- 59 Bagheri, H., Koocheki, A., and Mohebbi, M. (2016). Use of image texture and fractal dimensions analysis for evaluation of the effects of Qudome shahri and xanthan on crumb structure of pan bread. Journal of Food Research 26 (3): 397–409. (In Persian language).
- 60 Bagheri, H., Koocheki, A., and Mohebbi, M. (2016). Evaluation of gluten free bread by using sorghum flour and Qodume shahri and xanthan gums. Journal of Food Technology and Nutrition 13 (2): 75–86. (In Persian language).
- 61 Mohammadkhani, M., Koocheki, A., and Mohebbi, M. (2018). Effect of Lepidium perfoliatum seed gum coating on mass transfer and quality of osmotic dehydrated apple. Iranian Journal of Food Science and Technology 14: 97–105. (In Persian language).
- 62 Mehr, H.M., Koocheki, A., and Mohebbi, M. (2016). Performance of Lepidium perfoliatum seed gum in deep‐fried battered chicken nugget: effect of gum concentration and batter temperature. Journal of Food Measurement and Characterization 10 (1): 166–176.
