14
Brea Tree (Cercidium praecox) Exudate Gum
María A. Bertuzzi and Aníbal M. Slavutsky
Instituto de Investigaciones para la Industria Química, Facultad de Ingeniería, Universidad Nacional de Salta, CP: A4408FVY Salta, Argentina
14.1 Introduction
Brea gum is an exudate gum from the brea tree, also known as “palo verde” or “chañar brea.” The accepted name of this species is Parkinsonia praecox (Ruiz & Pav.) Hawkins or Cercidium praecox (Ruiz & Pav.) Harm, as a synonym. This species belongs to the Fabaceae family and grows from northern Patagonia to the southern United States of America, mainly in tropical and subtropical arid habitats. The scientific name Cercidium, from the Greek “kerkidion,” refers to the similarity between the fruit and a weaving shuttle, and praecox means “precocious” [1]. The small trees or large shrubs reach up to 8 m tall (Figure 14.1). The leaves develop after the first rains and shed shortly afterward. Thus, the tree depends on the photosynthetic activity of the light green bark of the stem and twigs. Generally, at each knot, there are solitary thorns up to 2 cm in length. The leaves are small, composed, and characterized by sprouting after the beginning of flowering. The flowers appear in spring and summer, and the tree fructifies in late summer and early autumn [2,3].

Figure 14.1 Brea tree and branch with exudate.
This species has a deep and strong root system that allows it to grow on rocky slopes. Brea is a colonizing species of degraded environments, constituting the native vegetation of the arid and semi‐arid zones of America. Intensive grazing and unsustainable forest use in some subtropical arid and semi‐arid regions of South America have led to the degradation of soils, favoring the establishment of this species [4]. The desertification of soils also generates an important social impact affecting the economy of the region.
Brea wood decomposes fast, so it has no use as wood or coal. Nevertheless, the plant exudes a water‐soluble gummy exudate, naturally or through wounds in its trunk or main branches (Figure 14.1). This exudate has similar characteristics to gum arabic, produced by species of the genus Acacia in African countries, which has applications as a thickener in food and pharmaceutical products, and adhesives, among other uses. Therefore, brea gum, as non‐wood forest product, is a sustainable productive alternative for the inhabitants of these regions, allows diversification of production, and a sustainable use of the environment by vulnerable rural communities.
Coirini et al. [5] studied the conditions of gum extraction and concluded that the best yield was obtained from trees with trunks between 13 and 18 cm in diameter. The authors recommend that to favor the extraction of the gum, helical cuts should be made on the stem with a saw. A direct relationship between productivity and wound size was found, and a 25–30 cm2 incision area was suggested. However, the adopted criterion is that the wound size should not excessively weaken the tree. The gum begins to flow within one to three days after making the incision. After 12 or 15 days, the secretion decreases but does not stop for two months, allowing the collections of 15 to 30 days to be separated. An adult tree can produce between 100 and 300 g of brea gum per year [6]. The production and composition of the brea gum are complex and varies to some extent depending on the geographical origin, climatic conditions, and age of the trees.
From an anatomical point of view, the gum comes from the cells of the parenchyma surrounding the vessels of the secondary xylem and causes total or partial occlusion of its lumen, all produced by some traumatism [7].
The recently secreted gum is pale golden yellow, with semi‐liquid consistency, and weakly sweetened. As the gum solidifies in contact with air, it takes on different colorations until it reaches a reddish yellow at the point of higher consistency. After solidification, tears are harvested from the trees. The tree produces gum throughout the year, although with significant differences depending on the season. Productivity variation throughout the year is related to the vegetative activity of the tree. The lowest harvests were found during the winter months and the highest during the hot and dry season. Heavy rains wash away virtually all production [6]. The gum is manually collected and pre‐cleaned to remove the bark, sand, and other impurities. Some simple treatments such us manual cleaning, sieving, and grinding are common before the sale. The gum is easily soluble in water, forms a homogeneous solution, viscous and clear, and is insoluble in organic solvents [8].
Rural people have traditionally used brea gum as a “woodland candy” since pre‐Columbian times without harmful consequences. Von Müller et al. [9] reported a toxicological evaluation of brea gum in mice. Their results suggest that feeding mice at levels up to 5% of brea gum does not produce any toxicological effects, supporting its potential use as a food additive for human consumption. Thus, in August 2013, brea gum was authorized as a food additive in Argentina, with its incorporation into the Argentinian Food Code [10].
Currently, the world market for vegetable gums used as food additives is led by gum arabic, a non‐wood forest product obtained from Acacia Senegal (L.) Willd., also known as “hashab.” Sudan produces around 90 000 tonnes per year, which is mainly exported to Europe and the United States of America [11,12]. Small‐scale farmers are the main producers of gum arabic in the traditional rain‐fed farming areas, both from natural forests, and small plantations. It represents an income diversification strategy to mitigate crop failure. For these populations, which are among the poorest and most vulnerable in Sudan, gum arabic contributes up to 50% of their total cash earnings. Over the past five years, demand for gum arabic has increased by an estimated 2.5% annually and is expected to continue growing at a similar rate. Major drivers of this growth include new product development and the substitution of synthetic thickeners, because consumers are looking for natural ingredients with clean labeling. The countries with the largest food and drink industries are also the largest markets for gum arabic. These markets are strongly dependent on a politically unstable country, such as Sudan. In 2015, Europe, mainly France, imported approximately 50 000 tonnes of gum arabic from Sudan and then re‐exported it. Sudan dictates the global prices for gum arabic, and in the past decade, political unrest in Sudan has caused strong price fluctuations. The prices for cleaned and spray‐dried gum arabic from Acacia senegal varies between 2700 and 8000 dollars per ton. Currently, prices amounted to 3200 dollars per ton [11]. All this is a very favorable scenario for the introduction in the market of brea gum, as a replacement of gum arabic.
Von Müller et al. [13] estimated a price of 1500 dollars per ton for brea gum (without refining) produced in the arid Chaco from Córdoba, Argentina, while Alesso et al. [6] reported a price between 1000 and 2500 dollars per ton for raw brea gum, recollected in arid Chaco from Santiago del Estero, Argentina. These prices demonstrate the competitive advantage of brea gum over gum arabic, showing its potential as a non‐wood forest product of secondary forests.
Brea tree belongs to the Fabaceae or Leguminosae family. Therefore, in addition to the exploitation of the exudate from the trunk, its seeds can be used for oil extraction. Ortega‐Nieblas et al. [14] studied the oxidation process of raw and refined oils extracted from seeds of Cercidium praecox during 122 days storage at room temperature. Corn and soybean oils were used as controls. Crude oil of brea seeds showed similar oxidation indexes to those for the corn raw oil and lower than those of soybean raw oil. Refined oils exhibited a similar performance. The rancid odor was detected after 5 days storage in soybean oil, while brea oil exhibited this odor after 62 days. These results indicate that legume seed of brea tree could be a good alternative as a source of oil.
14.2 Physicochemical Characteristics
14.2.1 Compositions
Gum is harvested from the tree as nodules. The crude exudate of brea gum is purified by dissolution in water. After removing the insoluble material (bark, sand, and other contaminants) by decantation, centrifugation, or filtration, the solution is subjected to drying in order to separate the gum from the solvent. Depending on the drying method, a subsequent grinding and sieving may be required.
The composition of brea gum exhibits variability depending on botanical and agronomical features. The precise chemical and molecular structure of the gum differs according to the botanical origin, soil, and climate characteristics, among others, and these differences are reflected in some of the analytical properties of the gum.
Some authors have studied the composition of brea gum collected from brea trees in different places in America such as Argentina, Venezuela, and México [ 8 15–17]. They found an acidic heteropolysaccharide formed by L‐arabinose, D‐xylose, D‐glucuronic, and 4‐O‐methyl‐D‐glucuronic acid, which also contains between 4% and 10% of proteins and around 4% of ash (Table 14.1). According to Anderson et al. [17] and Bertuzzi et al. [16], brea gum has a comparatively high amount of calcium and magnesium and low sodium, in relation to other similar gums.
Table 14.1 Compositions of brea gum reported by different authors (g kg−1).
| Composition | Anderson et al. [17] | Bertuzzi et al. [16] | Castel et al. [18] | De Pinto et al. [8] |
| Moisture | 127 | 135 (±5) | 48.3 (±1.3) | 130.8 (±27.9) |
| Protein | 106 | 62 (±2) | 75.2 (±0.9) | 44 (±12.3) |
| Ash | 48 | 38 (±3) | 42.3 (±0.9) | 42.5(±9.5) |
| Fat | — | — | 1.1 (±0.1) | — |
Cerezo et al. [15], de Pinto et al. [ 8,19], and Anderson et al. [17] analyzed the carbohydrate fraction of brea gum. Their studies showed a main chain that is a β‐(1,4)‐linked D‐xylan backbone with short branches, possibly (1,2)‐linkages, containing residues of D‐xylose, L‐arabinose, and D‐glucuronic acid. Anderson et al. [17] found galactose and galacturonic acid, instead of glucuronic acid and its methyl ether (Table 14.2). The aqueous solution of brea gum is levorotatory.
Table 14.2 Monosaccharide composition of brea gum (% dry weight).
Polysaccharides of brea gum are divided into two fractions. The first fraction (84%) is a polysaccharide of 2.79 kDa molecular mass, and the second fraction (16%) forms a polysaccharide–protein complex with a molecular mass of 1.92 × 102 kDa. In addition, there is a set of proteins with molecular masses ranging from 6.5 to 66 kDa [20]. The amino acid composition of proteins present in brea gum contains alanine, aspartic acid, hydroxyproline, leucine, threonine, serine, glutamic acid, valine, lysine, glycine, histidine, phenylalanine, isoleucine, and methionine, in descending order of quantity [17]. In native conditions, protein forms aggregates through disulfide bridges [20].
Proteins and carbohydrate–protein complexes are responsible for some of the most relevant gum functionalities, such as interfacial stabilization through hydrophobic protein groups acting as cleavage points to form viscoelastic films at interfaces [20]. Brea gum exhibits a particularly high amount of protein compared to other gums, which are almost solely made up of polysaccharides. This hydrocolloid has three times more protein than gum arabic, which contains between 1.5% and 2.6% of proteins [ 20,21]. Some authors suggested that a higher protein content favorably affects the interfacial properties, producing better emulsifying properties [22,23].
Brea gum is amber color, has semi‐liquid consistency, and a faintly sweet flavor. It is highly soluble in water. Solutions are homogeneous and exhibit an acidic character. The natural pH of brea gum is around 4 (3.9–4.3), resulting from the glucuronic acid residues.
Considering that brea gum has been proposed as a replacement for gum arabic, it is relevant to describe its main characteristics of the latter. Gum arabic is a complex slightly acidic polysaccharide, obtained from the stems and branches of Acacia senegal. The highly branched backbone of 1,3‐linked galactopyranosyl residues are substituted by arabinose, galactose, rhamnose, glucuronic acid, and its methyl ether. This hydrocolloid also contains around 2% of proteins, forming an arabinogalactan–protein complex, and cations like calcium, magnesium, and potassium [ 21,24]. The gum is highly soluble in water and insoluble in ethanol. It forms solutions over a wide range of concentrations without becoming highly viscous. The combination of high solubility in water and low viscosity confers on gum arabic its highly valued emulsifying, stabilizing, thickening, and suspending properties. Its main areas of application are food, pharmaceutical, and technical (adhesive) uses [ 21,25].
14.2.2 Color
Color attributes are of prime importance because they directly influence consumer acceptability. The vision sensations sent to the brain create the three dimensions of color judgment response that is known as the three‐dimensional color space. In the CIE Lab system, these dimensions are expressed as L*, related to lightness varying from black (zero) to white (100), and the other two related to chromaticity, a* from green (−a*) to red (+a*), and b* from blue (−b*) to yellow (+b*).
The color of gum solution is often difficult to predict from the color of tears or powder. The size and conditions of the lumps and powder affect judgment considerably. An appropriate color comparison can then be made in solutions of defined concentrations. The color of brea gum solutions at different concentrations between 1% to 28% w/v was studied. Small changes in a* values (+7 to +17) were observed, whereas b* values (+8 to +77) increased significantly with the increase in the brea gum content (1% to 28%). The decrease in L* values (60 to 34) showed a decrease in the solution luminosity of the solution with increasing concentration [16]. These findings suggest a net increase in amber color and a decrease in the brightness of the solution with increasing brea gum concentration. These results are in agreement with data reported by Rao et al. [26], where chitosan films were prepared with different proportions of guar gum. They found a net increase in yellow‐brown color because of incorporation of guar gum. They also reported that the increase in the concentration of guar gum in the film resulted in a significant decrease in L*. Despite this, the influence of the color of gum powder or solution could be insignificant when it is incorporated into different products. López et al. [27] studied the effect of incorporation of brea gum in fresh bread and observed that the addition of 1% of brea gum to a basic bread formulation does not produce significant differences in the color of the final product.
14.2.3 Density
The solution density can be determined by using pycnometers or buoyancy‐type densitometers at a constant temperature. Temperature and brea gum concentration affect the solution density. Increasing temperature decreases the solution density within the temperature range (25–50 °C) and concentration range (0.0–1.5%) studied. Other hydrocolloids show a similar trend. The decrease in solution density with temperature is due to the increase in specific volume caused by the rupture of the hydrogen bonds of the associated polymer molecules in the presence of water molecules [28]. Besides, the solution density increases linearly with gum concentration until saturation values (Figure 14.2). This linear relationship between density and brea gum concentration was maintained even at high concentrations. Brea gum solutions exhibited density values similar to that exhibited by other common hydrocolloids in the temperature range studied. At 1% polymer concentration and 25 °C, the density of guar gum is 1.0014 g mL−1, sodium alginate is 1.0029 g mL−1, and hydroxypropyl methylcellulose (HPMC) is 1.0002 g mL−1 [28].

Figure 14.2 Solution density as a function of brea gum concentration at 25 °C.
14.3 Functional Properties
Gums are used as a thickening, gelling, emulsifying, and stabilizing agents because of their ability to alter the rheological properties of the solvent in which they are dissolved, usually water. Changes in viscosity occur because of the hydrodynamic volume of these high‐molecular‐weight polymers and the interactions between polymer chains when gums are dissolved or dispersed [29,30]. These properties have been exploited for their functionality in food systems, including textural attributes and mouthfeel [21].
14.3.1 Solubility
Solubility is the capacity of a material (solute) to dissolve in a solvent. Gum solubility is an important factor that determines the applications of the gum. Brea gum is highly soluble in water, and its solubility increase with increasing temperature. The rate of gum solubilization depends on particle size and temperature. The high surface tension of very small particles hinders dissolution, as do low temperatures. In aqueous solutions, saturation occurs at 12.8% of brea gum at 5 °C, 28.8% at 25 °C, and 33.0% at 45 °C [16]. Similar behavior was exhibited by other gums like guar gum, alginate, and gum arabic. Generally, other gums cannot exceed 5% concentration in water because of the high viscosity developed (e.g., carboxymethyl cellulose, and κ‐carrageen). This characteristic property of brea gum makes it useful for applications where a high solid content is required. However, brea gum powder is insoluble in alcohols like glycerol and ethanol, vegetal oils, and organic solvents such as hexane, acetone, and petroleum ether [16]. Mantell [31] reported that gum arabic was also insoluble in organic solvents.
14.3.2 Surface Properties
The interfacial properties of polymers solutions can be studied through surface tension analysis. The formation and stabilization of foams and emulsions depend on this property. The surface tension of water was significantly reduced by brea gum addition. The surface tension decreased from 72 mN m−1 (pure water) to 51.75 mN m−1, when hydrocolloid concentration in water solution reaches 5% (Figure 14.3). For higher concentrations, the surface tension remained practically unaltered, showing that the critical micelle concentration was reached at 5% of brea gum [16]. Klein et al. [32] reported that the surface tension of aqueous solution of gum arabic at 5% concentration was 51.7 mN m−1, while 5% whey protein isolate reduced the surface tension to 47.8 mN m−1. They also observed that greater concentration did not show any further reduction in the surface tension of water.
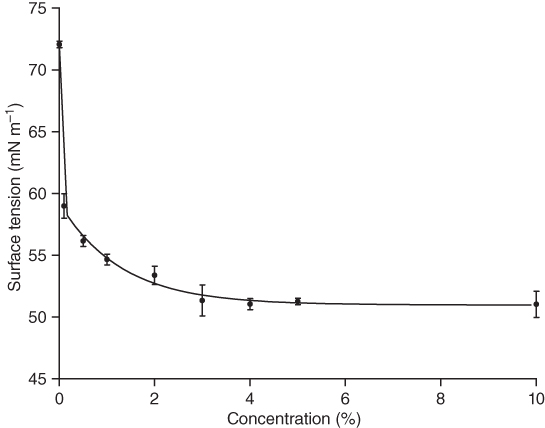
Figure 14.3 Surface tension as a function of the concentration of brea gum solutions at 25 °C.
14.3.3 Rheological Properties
Brea gum, as well as gum arabic, exhibits exceptional behavior between vegetal gums because of the high solubility and low viscosity of their solutions. This ability to form highly concentrated solutions contributes to the emulsifying and stabilizing properties of these gums. These unusual characteristics, when compared to those exhibited by other similar‐molecular‐weight polysaccharides, are related to the branched and more compact molecular structure of brea gum.
The rheological properties of brea gum solution are affected by system state variables such as temperature and polymer concentration. The Ostwald viscometer was used to study the effect of temperature (25–50 °C) and brea gum concentration (0%–1.5%) on kinematic viscosity. It was found that the kinematic viscosity of brea gum solution increases with concentration and decreases with temperature. Besides, a more marked effect of temperature on solution viscosity as the concentration increases was detected. Brea gum solution displays a kinematic viscosity between 0.72 and 1.63 cm2 s−1 for gum concentrations between 0.2% and 1.5% at 25 °C. At a brea gum content of 1%, the kinematic viscosity falls from 1.44 to 0.94 cm2 s−1 when the temperature rises from 25 to 50 °C [16]. The Mark–Houwink parameters show that the hydrocolloid acquires a spherical form in water solution. The intrinsic viscosity of brea gum solutions at 25 °C is 52.7 mL g−1 and, the average molecular weight is 6.28 kDa [33].
The viscosity of brea gum solutions of higher concentrations was measured using a rotational rheometer. It was observed that brea gum concentrations lower than 10% and shear rates up to 100 s−1 produce Newtonian behavior, and viscosity values do not depend on the shear rate [16]. William et al. [34] reported low viscosity even at high concentrations of gum arabic solutions, but at a concentration above 30%, the viscosity of gum arabic solutions increases exponentially.
Figure 14.4 represents the viscosity of different hydrocolloids, measured at 100 s−1, as a function of polymer concentration. Our data are compared with those reported by Glicksman [35] for starch and gum arabic, at the same temperature. Brea gum exhibits similar viscosity values than gum arabic in the concentration range studied. The viscosities of gum arabic and brea gum are notoriously lower than those of gelatinized starch solutions and much lower than those of other vegetal gums like gum tragacanth or gum ghatti. Owing to the compact and branched structure, and therefore, small hydrodynamic volume, brea gum solutions are characterized by a low viscosity, allowing the use of high gum concentration. It could be of interest in some applications.
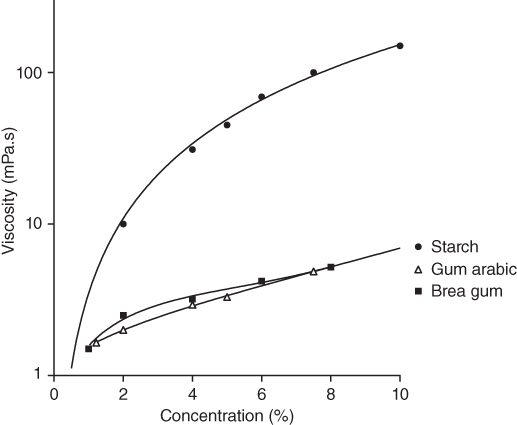
Figure 14.4 Apparent viscosity as a function of hydrocolloid concentration measured at 100 s−1 and 25 °C.
The effect of pH on the viscosity of brea gum was studied at 20% concentration and 25 °C. The natural pH of brea gum was between 3.90 and 4.30, resulting from the glucuronic acid residues. The pH of polysaccharide solution affects its viscosity behavior. Acid polysaccharides exist in aqueous solutions in the form of macro‐ions, and their charge depends on the pH. The coil dimension of these polyelectrolytes may be expanded by electrostatic repulsions or contracted by electrostatic attraction between chain segments. The addition of acid or alkali modifies the solution viscosity because of the changes in the electrostatic charges on the macromolecule. At the natural pH (around 4), the viscosity of brea gum solution reaches a maximum. At lower pH values, the reduced ionization of acid residues (mainly glucuronic acid) results in a more compact polymer volume, a decrease in the hydrodynamic volume, and thus a decrease in solution viscosity. Above pH 4, additional alkali raises the ionic strength of the solution, which in turn masks the repulsive electrostatic charges and regenerates a compact conformation similar to an acidic condition, lowering the viscosity. William et al. [34] reported the same behavior for 20% gum arabic solution, which exhibited a maximum viscosity at around pH 5.0–5.5.
14.3.4 Foaming Properties
A foam is a coarse dispersion of gas in a liquid, most of the phase volume being gas, with the liquid in thin sheets called lamellae between the gas bubbles [36]. The foaming properties can be described in terms of foam expansion (FE) and foam capacity (FC). FE is the percentage in the volume of foam formed, related to the initial liquid, and FC is the percentage in the volume of liquid incorporated into the foam.
Foam formation involves three stages. First, the soluble hydrocolloid (proteins or carbohydrate–proteins complexes of brea gum) diffuses to the air–water interface, becomes concentrated, and reduces the surface tension. Second, macromolecules located at the interface orientate polar moieties toward the water and nonpolar groups toward the air. Third, hydrocolloid molecules interact, forming and stabilizing the film around the bubbles. Polypeptides can interact to form a film with possible partial denaturation and coagulation [37]. Thus, foaming ability strongly depends on brea gum concentration. It was observed that FC and FE increase up to 5% of brea gum concentration and then drop sharply. FE increases from 49% to 99% when the brea gum concentration rises from 1% to 5%. After that, FE falls to 51%, when the hydrocolloid concentration reaches 10%. Similarly, FC increases from 650% to 1075% when brea gum content rises up to 5%, and then drops to 475% when the gum concentration reaches 10%. These results evinced that a 5% brea gum content is the critical micelle concentration [16].
Initially, when foams are formed, the air cells are spherical and the lamella is thick with the large amount of solution contained in them. As time passes, the liquid drains from the foam, the lamella thins, and the air cells pack closer and assume polyhedral shapes. Drainage of fluid from the lamella is the main destabilizing force as it allows disproportionation, and large cells grow at the expense of small ones. As the brea gum concentration increases up to 5%, the foam density also rises, and the size of the air cells decreases. The liquid content of the freshly prepared foam increases with brea gum concentration, resulting in thicker lamellas as higher brea gum concentrations are used [16].
Ostwald ripening contributes to foam instability during aging. This involves smaller bubbles shrinking and disappearing at the expense of the growth of bigger bubbles. The thermodynamic driving force for this process is the difference in chemical potentials of molecules in the smaller and larger bubbles [38]. After 24 h, the solution volume retained in the foam is approximately 35% of the volume retained initially when 5% of brea gum is used. For higher concentrations, the drainage of the fluid increases progressively. In foams formed with 10% brea gum solution, after aging, the foam contains 26% of the liquid volume retained into the freshly prepared foam. Foams prepared with 20% brea gum solution contain only 10% of the solution incorporated into the fresh foam, after 24 h of drainage. The Ostwald ripening phenomenon is observed more clearly in high‐concentration solutions. These results are consistent with the reduction in FE and FC for concentrations above 5% [16].
The surface properties of brea gum solutions are affected by nanoclay addition because nanoparticles also absorb at the interphase and interact with the hydrocolloid. The effect of the incorporation of montmorillonite (MMT) as nanoclay on the surface tension and FC of 10% brea gum solutions was studied by adding MMT in concentrations ranging between 0% and 5% w/w of the polymer [39]. The results indicate a reduction in FC of brea gum/MMT solutions as the MMT concentration increases. This could be explained by the increase in surface tension of gum solutions (from 51.75 to 62.15 mN m−1) caused by the increasing MMT content. Brea gum has around 7% proteins in its composition that are considered surface‐active colloids in foam stabilization. Thus, there may be interactions between brea gum and MMT or competitive adsorption on the foam surface, leading to an antagonistic effect on foam stability. Moreover, when brea gum–MMT solution was shaken and aged, the formation of a stable gel was observed. This gel consists of a three‐dimensional structure capable of capturing water in its interior. These results provide evidence about the capacity of MMT to interact with the polymeric matrix, and they prove that a structure with greater resistance and stability was formed [39]. Zhang et al. [40] found a synergistic effect with a nonionic surfactant and Laponite, and an antagonistic effect using silica in concentrations lower than 8%.
14.3.5 Emulsifying Properties
The emulsions are widely used in the formulation of food, pharmaceutical, and cosmetic products. Moreover, in microencapsulation technology, emulsification is one of the important steps. The emulsion stability and droplet size are highly relevant in the retention of flavors and volatile compounds in microencapsulation by a spray dryer.
Most hydrocolloids can act as stabilizers of oil‐in‐water emulsions, but only a few can act as emulsifiers. The latter functionality requires substantial surface activity at the oil–water interface, and hence the ability to facilitate the formation and stabilization of fine droplets during and after emulsification [38]. gum arabic and brea gum form a polysaccharide–protein complex that has been shown to be responsible for the interface activity and the emulsifying properties of these gums. The proteinaceous components of these complexes would embed in the oil phase, while the carbohydrates would extend out of the surface into the aqueous phase [41,42]. When the proteinaceous part is removed, the gum tends to lose its surface activity and emulsification capability [43]. In the case of gum arabic, only a small part of the gum mixture is responsible for its activity, and therefore large amounts of gum are required to obtain stability. Brea gum has three times more protein than gum arabic, and so should exhibit a similar increase in emulsifying capacity. The protein diffuses, adsorbs on the surface, reduces the interfacial tension, and provides an interfacial barrier that offers protection against coalescence. The adsorption process is rapid under stirred conditions or at high protein concentration. Protein must unfold to a certain degree and reorient at the interphase, with polar groups directed toward water and the nonpolar groups directed toward oil. A continuous film is formed because of polypeptides' protein–protein interactions, and associations through electrostatic, hydrophobic interactions, and hydrogen bonds between protein and polysaccharide components. It appears that the spheroidal shape of these gums together with the proteinaceous portion attached to the polysaccharides facilitate alignment and close packing of the gum at the oil–water interface. Spatial separation of the relatively hydrophobic portions of the gum molecule (i.e., methyl groups) from the hydrophilic hydroxyl and ionic regions appears to have a significant effect on its surface activity [44]. In the same sense, Castel et al. [20] indicated that the polysaccharide–protein complex present in the brea gum molecular structure is responsible for their high interfacial activity and good emulsifying properties. Besides, the low pH of brea gum solution and high ionic strength is beneficial for protein emulsification properties, because it favors its unfolding in the interface.
Oil‐in‐water emulsions in the ratio 1:4 were formulated with brea gum solutions of different concentrations in the range 1%–20% w/w. The emulsion was prepared using a homogenizer at 5000 rpm for 5 min [16]. Emulsions show a decrease in droplet diameter and an increase in droplet number with increasing brea gum concentration up to a limit of 5%. However, at higher gum concentrations, the number of droplets decreases, and the droplet size increased sharply. 5% of brea gum was the emulsifier concentration required to produce the minimum mean droplet size (maximum surface area per oil volume unit).
Castel et al. [22] reported that an oil‐in‐water emulsion prepared with brea gum in the ratio 1:9 showed a droplet size distribution similar to that of the emulsion stabilized with gum arabic at the same concentration. But, according to these authors, the brea‐gum‐based emulsion was more stable than the gum arabic emulsion, possibly due to the higher viscosity of the brea gum emulsion. Nevertheless, considering that there are no major differences between the viscosities reached by both gums at that concentration, but there are important differences in the protein content in both, it is therefore more likely that the greater emulsion stability of brea gum is caused by its higher protein content. For his part, Dickinson [38] indicated that a 10% of gum arabic is necessary to reach the minimum droplet diameter in an emulsion of oil‐in‐water of 1:6 and pH = 3. This concentration of gum arabic is twice that required to reach the minimum droplet size for a 1:4 O/W emulsion with brea gum. Wareing [29] reported that at least 12% of gum arabic is necessary to obtain 1:4 O/W emulsion, confirming that the greater protein content of brea gum is responsible for its emulsifying properties.
Moreover, the rheological characterization of brea gum solution indicates that the solution viscosity increases with increasing brea gum concentration. Thus, a high concentration of brea gum can improve emulsion stability by retarding the destabilization, due to the increase in the viscosity of the aqueous phase [ 16, 22]. This is favored by the high solubility of brea gum in water.
During aging, emulsion stability increase with brea gum concentration (up to 5% brea gum content). In this range, no observable changes are detected after 24 h storage at 25 °C. Creaming is only observed after five days, and the volume of the top phase increases with brea gum concentration. At concentrations above 5% brea gum, despite the protective effect of the higher viscosity of the water phase, the higher size of oil droplets causes enhanced emulsion separation. A clear aqueous phase separation from the rest of the emulsion is observed after five days of storage.
Castel et al. [22] studied the rheological behavior of an O/W emulsion of brea gum and gum arabic. They found that all emulsion flow curves exhibited shear‐thinning behavior at low shear rates and a Newtonian plateau at high shear rates. They also reported that mechanical spectra show droplets tending to arrange themselves as a network in the emulsions, which was related to the high stability. Brea gum emulsion exhibited higher viscosity and stability than gum arabic emulsion at the same concentration.
14.4 Applications
14.4.1 Edible Film
An edible film or coating is defined as a thin and continuous layer of edible material formed on the food (coating) or separately and then applied to the food (film). Edible films and coatings generate a modified atmosphere by creating a semi‐permeable barrier against O2, CO2, moisture, volatile compounds, and solute movement, thus reducing respiration, water and aroma loss, and oxidation reaction rates [45]. Many polysaccharides were used as film‐forming materials, including exudate gums, such as gum arabic [46], and mesquite gum [47]. Some authors studied the formulation of edible films based on brea gum and their formation with the casting method [ 39 48–50]. Generally, brea gum films exhibit good visual aspect, transparency, and amber color. Microscopic observation shows a dense and homogeneous structure without pores (Figure 14.5). In all cases, glycerol was used as a plasticizer of the film structure, in concentrations that ranged from 0% to 50% w/w based on polymer content. Brea gum films exhibit high solubility in water (80% at 25 °C), and this parameter increases with temperature until full solubilization. Film wettability of brea gum studied through measurements of contact angle indicates a variation from 71° to 67° as the glycerol content increases. This reduction in the contact angle manifests as an increase in wettability or the hydrophilic character of the film produced by the plasticizer, which has a hydrophilic nature [48].
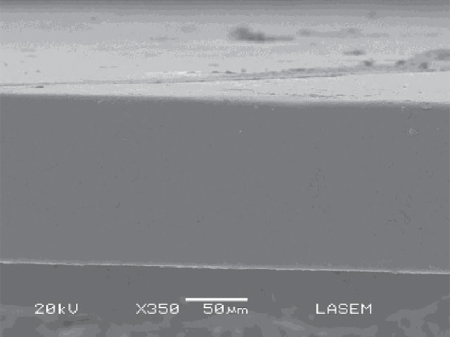
Figure 14.5 Scanning electron microscopy (SEM) microphotography of brea‐gum‐based film.
Water sorption isotherms of films, measured at 25 °C, show that at high‐water‐activity values (above 0.50), the film matrix swells, altering its structure and properties. This is shown by isotherm curves that exhibit a slight slope at low values of water activity, but take an exponential course at water activities higher than 0.5 (Type III of the Brunauer, Emmett, and Teller (BET) classification). Moreover, due to the high solubility of brea gum, film moisture content data above 0.75 of water activity cannot be obtained. At constant water activity, the moisture content of films increases with glycerol content. This increment is more pronounced above 15% of glycerol content [48].
Water vapor permeability remains practically constant up to 20% of glycerol content at a value of 8 × 10−10 gm−1s−1Pa−1 and then increases linearly, with a value of 25 × 10−10 gm−1s−1Pa−1 at 30% plasticizer content. Glycerol produces the plasticization on brea gum films by increasing chain mobility within the network, producing a decrease of tensile strength and an increase in elongation. Brea gum films are brittle due to the extensive intermolecular forces between the polymeric chains and require plasticization to improve their mechanical properties. Films without plasticization cannot be tested. Tensile strength decreases linearly with glycerol content varying from 12.4 MPa at 10% of glycerol to 2.3 MPa at 55% of a plasticizer. Film elongation is maintained almost constant at around 11% at glycerol concentrations ranging between 20% and 40%. Above 40%, the elongation increases up to 22% when the glycerol content reaches 55% [48].
Formulation of the antimicrobial edible film could be a convenient alternative to the addition of antimicrobials to foods, such as ham, hard, and semi‐hard cheeses, with the advantage of concentrating the additive in the zone where the majority of the contamination takes place. In this direction, anti‐Listeria active films were prepared using brea gum, as well as other biopolymers like gelatin and wheat gluten, as film matrix, and the enterocin produced by E. faecium CRL1385 at a concentration of ca. 500 UA cm−2. Results showed that the gelatin and wheat gluten films with enterocin addition can control Listeria contamination. However, brea gum and the enterocin were found to be incompatible since antimicrobial activity was lost in this films [51].
Various strategies of film formulation have been used to improve the properties of brea gum films, such as nanocomposite films and emulsion‐based films. Spotti et al. [50] studied the formulation of emulsion‐based brea gum films with the addition of beeswax (20% and 40% w/w of brea gum) and plasticized with glycerol at 0%, 20%, and 40%. This beeswax addition was devoted to improving water barrier properties, considering that the water vapor permeability of edible films plays an important role in food deterioration reactions. Emulsion‐based films are homogeneous, without phase separation. Their transparency decreases with increasing beeswax concentration. Moreover, beeswax decreases the water solubility of films considerably because of its hydrophobic nature. Film solubility is reduced from 100% to 87% by the addition of 40% beeswax. Water vapor permeability decreases by almost half when 20% of beeswax is added and by even a little more when 40% of wax is used. Beeswax incorporation results in improvement of water vapor permeability, but at the expense of the mechanical properties of brea gum films. Tensile strength and elongation decrease from 7.6 to 1.5 MPa and from 8% to 3.5%, respectively, when beeswax is added [50]. These values make the film very difficult to manipulate.
The functional properties of brea‐gum‐based films can also be improved by nanocomposite film formulation. MMT is a layered smectite clay mineral with a platelet structure. MMT consists of 1‐nm‐thick aluminosilicate layers surface‐substituted with metal cations and stacked in 10‐mm‐sized multilayer stacks. Naturally occurring MMT is hydrophilic [52]. MMT can strongly interact with brea gum and reinforce the film structure. The clay content and dispersion feature of the MMT layers influence the properties of the nanocomposite films. Depending on the technique used for MMT incorporation, an exfoliated structure can be obtained in the film matrix. However, microscopic observation cannot detect any change in the dense film matrix when the exfoliated structure is formed.
Electrostatic interaction between MMT and the hydrocolloid allows the formation of a network that strengthens the film matrix and reduces film solubility and water sorption capacity. Sorption isotherms of nanocomposite films are adequately fitted by the BET model. MMT decreases film water adsorption in all the water activity ranges. Then, the water content of the monolayer, calculated with the BET equation, reduces with MMT content at all temperatures, indicating a lower affinity to water. The entropy change and net isosteric heat of adsorption show a well‐defined peak at monolayer water content, and in both cases indicate a more stable and ordered structure when MMT was added. Negative values of Gibb's energy changes show the process spontaneity and indicate that the final structure of brea gum–MMT film has less affinity for water than brea gum films. The permeability depends on the diffusivity (the tortuosity of the pathway formed by the MMT nanoparticles) and solubility of the water molecules. The decrease in water vapor permeability with MMT content confirms the adequate dispersion of the nanoclay particles, causing the water molecules to move through a tortuous path as they diffuse through the polymer matrix. The incorporation of 5% MMT decreases the water vapor permeability from 8−10 to 3.7−10 gm−1s−1Pa−1. Thus, MMT incorporation reduces the hydrophilic character of brea‐gum‐based films, and as a consequence their water vapor permeability [49].
MMT incorporation increases film opacity and affects the mechanical properties of brea gum–MMT films. The increase in Young's modulus indicates that the film becomes more rigid, resistant to lengthening or stretching with increasing MMT content. The tensile strength also increases from 7.45 to 20 MPa, accompanied by a loss in elongation from 19% to 9%. Gas permeability measurements indicate that MMT addition reduces not only film permeability to O2, N2, and CO2, but also the permselectivity (CO2/O2) of brea gum film from 0.93 to 0.58 [39]. Nanotechnology provides an alternative technique for the improvement of both the barrier and mechanical properties of brea‐gum‐based films. The brea gum–MMT nanocomposite films, with improved water vapor and gas barrier and mechanical properties, could be used as environmentally friendly food packaging materials for extending food shelf life.
14.4.2 Encapsulation
Encapsulation may be defined as the process of entrapping one substance (active agent) within another substance (wall material). In the food industry, an encapsulation process can be applied as a useful tool to improve delivery of bioactive molecules (e.g., antioxidants, minerals, vitamins, phytosterols, fatty acids), and living cells (e.g., probiotics) into foods. Capsules usually have diameters of a few nanometers to a few millimeters. Since encapsulating compounds are often in liquid form, different technologies based on drying like spray drying, fluid‐bed coating, spray‐chilling, spray‐cooling, or melt injection are used [53]. Other techniques such as complex coacervation are also applied. Biomolecules are mostly used as the material for encapsulation in the food sector. Materials have to provide maximum protection of the active compound, to hold actives within the capsule's structure during processing and storage, not react with the fill, and have good rheological characteristics at high concentration to facilitate the drying process [53].
The widest biopolymers used for encapsulation in food applications are polysaccharides, such as starch and its derivatives and plant exudates. The capability of brea gum and gum arabic to achieve high concentration and low viscosity make them highly suitable as an external phase for encapsulation.
Gum Arabic showed better efficiency as the encapsulating material of fish oil than hemicellulose by spray drying [54]. Butstraen and Salaün [55] prepared microcapsules by complex coacervation of gum arabic and chitosan containing a commercially available blend of triglycerides as the core. They found an optimum formation of the complex at pH 3.6 and a weight ratio of chitosan to gum arabic mixtures of 1:4.
Brea gum and gelatin were used for microencapsulation of peppermint essence by complex coacervation. Brea gum and gelatin were cross‐linked with glutaraldehyde during formation of the capsules. The capsules were applied onto paper and cloth using polyvinyl alcohol (PVA) adhesive. The capsules based on brea gum and gelatin were compared with those obtained with gum arabic and gelatin. The results indicated that microcapsules obtained with both gums are similar and present high encapsulation efficiency of peppermint essence. However, when capsules were prepared with brea gum, it was necessary only the 60% of the amount of gum arabic required for the same quantity of gelatin [56].
Castel et al. [18] analyzed the capability of brea gum to encapsulate corn oil by spray drying. They studied the effect of inulin incorporation into the emulsion on the resulting capsules. The powders were almost spherical particles, with contractions, but without cracks or apparent porosity, providing good protection and retention of the encapsulated material. Inulin addition increased the encapsulation efficiency by more than 100%. Their results showed that the combination of brea gum and inulin is a good alternative for the external phase that microencapsulates hydrophobic compounds.
14.4.3 Hydrogels
Polyelectrolytes are macromolecules that possess a relatively large number of functional groups that either are charged or under suitable conditions can become charged. Polyelectrolyte complexes are formed by mixing solutions of oppositely charged polyelectrolytes without any chemical covalent cross‐linker. These complexes not only include electrostatic and dipole–dipole association, but also hydrogen and hydrophobic bonds [57]. Polyelectrolyte complexes are studied for drug delivery, pH indicator, temperature indicator prototype, and hydrogels [58–62].
Hydrogels are a group of polymeric materials of hydrophilic structure, which make them capable of holding large amounts of water in their three‐dimensional networks. These products are extensively employed in a wide number of industrial and environmental applications. A gel is commonly defined as a cross‐linked network of polymers, which can absorb solvent, but which is insoluble therein. The interactions responsible for this solvent absorption correspond to capillarity forces, osmosis, and polymer–solvent molecular interactions, among others [63]. Some gels have the property of undergoing phase transitions when immersed in certain solvents and under certain conditions. These phase transitions change the gel matrix volume by reversible extension or contraction. Volume changes can reach very large values, such as 1000 times the volume of the dry gel, because of changes in external variables, such as temperature, ionic strength, pH or selected solvent.
Brea gum presents positive charge below pH 3.7, related to its protein fraction. The isoelectric point is observed in a range of pH between 3.6 and 4.0, and above these values, the polymer is negatively charged due to the presence of uronic acids in the polysaccharide [64]. The positive zeta potential of brea gum is explained by the ionization of the protein fraction of brea gum below its isoelectric point. Jayme et al. [65] studied gum arabic as a stabilizer of O/W emulsion and reported an isoelectric point around pH 2 that was independent of the salt concentration of the gum arabic solution.
Hydrogels can be formulated by a combination of pectin and brea gum to form a polyelectrolyte complex. Considering that brea gum presents positive charge below pH 3.7, the formation of a polyelectrolyte complex depends on the pH of the solution mixture. The natural pH of brea gum and pectin solutions is around pH 4. Thus, the pH of brea gum solution must be reduced to 2 in order to ensure the electrostatic interaction and the hydrogel formation. Above a final pH of 3.5, complex formation is not observed, since above this value the zeta potential of the brea gum is negative. Under these conditions, a colloidal solution stabilized by electrostatic repulsions is obtained, considering that pectin is a negative charged colloid. Thus, only when the final solution exhibits a pH below 3.7, the hydrogel is separated from the solution [64].
The addition of nanoparticles of MMT to the hydrogel of brea gum and pectin modifies its properties. The hardness of the hydrogel based on brea gum and gelatin varies from 5.97 to 13 kPa. However, when MMT is incorporated in hydrogel formulation, the hardness values are between 8 and 20 kPa, increasing the effort required to compress the gel structure. Thus, MMT acts as a nano‐reinforcer by interacting with both hydrocolloids in the gel structure, the result being a more stable matrix. This is also reflected by an increase in cohesiveness and a decrease in adhesiveness when MMT is incorporated into the hydrogels [66].
Klein et al. [32] studied the interaction between whey protein isolate and gum arabic. They found that electrostatic interactions occur when the protein charge is partially positive. In this pH range, the protein consists of some positive domains that interact with the negatively charged gum arabic to form weak complexes that do not form precipitating coacervates but rather soluble or colloidal adducts that behave like a large molecular entity with amphiphilic and interfacial characteristics. The charge interactions are sufficient to cause a molecular adduct migration of the two biopolymers together to the air–water or oil–water interface, to improve its adsorption into the interface, enhancing the foam or emulsion stability.
14.4.4 Bread Additive
Bread is a widely consumed product, particularly wheat bread. A decrease in bread quality occurs due to aging, which begins just when loaves are removed from the oven [67]. Hydrocolloids are widely used as additives in the food industry because they are used to modify the rheology and texture of aqueous systems [68]. In addition, the hydrophilicity of the hydrocolloids gives them a high water retention capacity [69].
López and Jiménez [70] studied the effect of different proportions of brea gum on the functional characteristics of wheat flour and its impact on the physical quality of bread. The higher levels of brea gum (1% and 2%) decreased the peak viscosity and increased the stability and setback of the flour. The hydrocolloid competes with starch for water molecules, lowering gelatinization of the starch granules. These changes in the functional characteristics of wheat flour affect bread quality. The loaves added with 1% and 2% brea gum exhibited smaller alveoli, developing more compact, harder, and less aerated crumbs. Nevertheless, the humidity of the samples with 1% and 2% brea gum was higher than the moisture of control bread. On the other hand, the incorporation of brea gum at 0.5% did not affect the pasting parameters and bread quality but increased the moisture of the crumb, so this concentration would be most recommended for baking, since higher humidity could favor the shelf life of the product. Moreover, the effect of the addition of brea gum on the aging of wheat bread was also analyzed. Brea gum reduced the hardening of the crumb in white bread, particularly at 48 h storage. This improvement may be due to the higher moisture content of the crumbs containing brea gum. The higher moisture content would also explain the differences found in the microstructure at 72 h storage. The same effect is observed regardless of the amount of brea gum added. However, the presence of the hydrocolloid seems to favor the retrogradation of amylopectin. The bread firming during storage cannot be explained only by the starch retrogradation or by the moisture content. The organization of polymers within the amorphous region or the repartition of water between the amorphous and crystalline regions may play an important role [27].
López and Goldner [71] analyzed the influence of storage time on the acceptability of bread formulated with lupine protein isolate and brea gum. They formulated different bread samples based on wheat flour with the addition of 10% lupine protein and 0.5% brea gum. The crumbs made with flour mixture (wheat and lupine) exhibited higher moisture content at all storage times, and the addition of brea gum further increased these values. After 24, 48, and 72 h storage, the bread crumbs with lupine protein isolate (with and without brea gum) were less hard. In general, the addition of brea gum made bread more cohesive, gummy, springy, and chewy. At 48 h storage, brea gum improved in acceptability, and this was accentuated at 72 h storage, where 80% of consumers reacted positively because of the “good crumb flavor.” The addition of this hydrocolloid increased the sensory shelf life of the product.
14.4.5 Medium for Fungal Culture
Mesa et al. [72] studied the behavior of the Aspergillus niger strain ATCC 11414, in a culture media based on the exudate of Cercidium praecox. They evaluated the growth of the colonies and the biomass. The macroscopic and microscopic characteristics of the fungus in this medium were similar to those exhibited in Sabouraud Dextrose Agar. This behavior suggests that A. niger produces the enzymes necessary to hydrolyze the acid hetero‐polysaccharides of the gum. The preparation process of the culture medium induced some autohydrolysis in the polymer, releasing arabinose and xylose, substrates used as a carbon source and energy by this microorganism.
14.5 Conclusions
Brea tree (Cercidium praecox) is a constitutive species of the native vegetation of arid and semi‐arid zones of America that cannot be used as wood. The ecological importance of this species lies in its ability to rapidly colonize degraded lands by overgrazing or deforestation. The brea tree grows from northern Patagonia Argentina to the south of the United States of America in arid and hot regions, and it can be utilized as an economic resource by poor and vulnerable populations. However, this requires the greater organization of producers and intensive tree planting on a large scale.
This species produces a water‐soluble exudate through wounds on its trunk or main branches, the brea gum, which can be used instead of gum arabic for a large number of applications in food, pharmacy, and other industrial applications such as glues, pigments, and so on. This would allow the replacement of gum importations in the countries where it grows. In addition, the harvest of brea gum would generate an alternative of genuine income in vast areas of territories with significant signs of biological, physical, and social degradation.
The chemical, physical, and functional characteristics of brea gum make it an excellent substitute for gum arabic. Its protein content, three times greater than that of gum arabic, results in greater surface activity, which produces greater foaming and stabilizing power. Its high solubility and rheological properties allow the formation of solutions with high soluble solids content and low viscosity, which are very important characteristics for the stabilization of aqueous suspensions. The structure of the polymer enables it to form films with high solubility in water. In addition, as the hydrocolloid has an anionic character, it can interact with cationic polymers to form more complex structures.
As the brea tree is a legume, oil can be obtained from its seeds, which in turn can be used be used in the production of biofuels, thus making it an alternative to edible legumes such as soybean.
14.6 Future Trends
The brea tree is a natural and sustainable resource that plays an important role in the recovery of degraded arid or semi‐arid zones, where climatic and ecological conditions are not favorable for agriculture. In Argentina, around 80 000 km2 has potential for the production of the brea tree. In addition to the environmental benefits of the brea tree, brea gum can be obtained from the brea tree in sufficient quantities in these territories to not only replace the national consumption of gum arabic but also to export the surplus.
Considering that these regions are inhabited by very poor people and that gum collection requires intensive labor, the production of brea gum could generate a significant source of income for its inhabitants. Nevertheless, new state policies have to be formulated so as to promote investments to support and finance the intensive plantations, the gum collection, the post‐harvest processing, and the market structure.
Overall, the demand for gum arabic is steadily increasing (2.5% annually). Major drivers of this growth include new product development and substitution of synthetic thickeners. Main suppliers of gum arabic are politically unstable countries like Sudan or Kenya. Thus, the main buyers of gum arabic are attracted by those providers who can guarantee stable supplies of high‐quality gums. This large potential market constitutes an important opportunity to introduce brea gum as an alternative to gum arabic. Finally, in order to introduce brea gum as an alternative to gum arabic, it is necessary to widely disseminate information regarding its characteristics and functional properties among potential users.
The physical and chemical properties of this polysaccharide allow its application in different fields. In the food industry, brea gum could be used as a stabilizer in the formulation of beverages and to retard the growth of sugar or ice crystals because of its high solubility and low viscosity. Besides, the brea gum solutions are excellent forming and stabilizing agents for emulsions and foams, given their surface properties. In the pharmaceutical industry, brea gum could be utilized as a binder or stabilizer of suspensions.
Brea gum is also a useful material to encapsulate active principles due to its gas and solute barrier properties, or as a sacrificial material to delay dehydration, due to its high water retention capacity. In addition, its ability to form complexes with polyanions such as pectin or alginate allows its use in encapsulation or laminates.
The particular properties of this gum open a wide field for other potential applications that must be studied and developed.
Acknowledgments
The authors thank Ing. Agr. Tania Bertuzzi for technical advice and photographs of the brea tree and the financial support of Consejo de Investigaciones de la Universidad Nacional de Salta (CIUNSa), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
References
- 1 Gómez, M. M., Bogino, S. M. (2005) La brea. Informativo Rural, E.E.A INTA San Luis, 2 (7)55, 1–2.
- 2 Burkart, A. and Carter, A. (1976). Notas sobre el género Cercidium (Caesal/pinoidea) en Sud América. Darwiniana 20 (3–4): 305–311.
- 3 Roth, I. and Linddorf, H. (2002). South American Medicinal Plants: Botany, Remedial Properties and General Use. Springer.
- 4 Páez, S.A. and Marco, D.E. (2000). Seedling habitat structure in dry Chaco forest (Argentina). Journal of Arid Environments 46: 57–68.
- 5 Coirini, R., Zapata, R., Contreras, A., et al. (2015) Goma de Brea (Cercidium praecox) como alternativa productiva en zonas áridas de Latinoamérica. Poster presented in XIV Congreso Forestal Mundial, Durban‐Sudáfrica, September 7, 2015.
- 6 Alesso, S.P., Araujo, P., and Tapias, R. (2003). Aprovechamiento de la goma de brea (Cercidium praecox) en bosques secundarios del Parque Chaqueño Seco. Influencia del tamaño de las heridas sobre la producción. Quebracho‐Revista de Ciencias Forestales 10: 60–70.
- 7 Losano, M.A., Dottori, N., and Cosa, M.T. (2000). Secreciones intravasculares de sustancias gomosas en Cercidium praecox (Fabaceae). Anales del Instituto de Biología, Serie Botánica 71 (1): 1–9.12.
- 8 De Pinto, G., Rodriguez, O., Martinez, M. et al. (1993). Composition of Cercidium praecox gum exudates. Biochemical Systematics and Ecology 21: 297–300.
- 9 Von Müller, A.R., López, C.B., Eynard, A.R. et al. (2009). Subchronical toxicological evaluation of brea gum (Parkinsonia praecox) as food additive in BALB/c mice. Drug and Chemical Toxicology 32 (4): 307–311.
- 10 CCA. Código Alimentario Argentino. (2014) Capítulo XVIII. Artículo 1398. Apartado 72.1, www.anmat.gov.ar/alimentos/normativas_alimentos_caa.asp
- 11 Tarig, E.M., Abdelateif, H.I., Mohamed, E.T. et al. (2016). Recent changes in local marketing patterns of gum arabic in Kordofan, Sudan. Journal of Agricultural Science and Engineering 2 (6): 46–56.
- 12 FAO‐Food and Agriculture Organization Of The United Nations. (1995). Gums, resins, and latexes of plant origin. In on‐wood forest products, http://www.fao.org/docrep/v9236e/V9236e05.htm
- 13 Von Müller, A.R., Coirini, R., Eynard, A.R. et al. (2007). Evaluación socio‐ecónomica de la producción de goma de brea en el Chaco Árido. Multequina 16: 83–98.
- 14 Ortega‐Nieblas, M., Robles‐Burgueño, M.R., and Vázquez‐Moreno, L. (2001). Oxidation indexes in oils from some leguminous seeds from Sonoran desert. Grasas y Aceites 52 (1): 5–9.
- 15 Cerezo, A.S., Stacey, M., and Webber, M.J. (1969). Some structural studies of Brea gum (an exudate from Cercidium australe jonhst.). Carbohydrate Research 9: 505–517.
- 16 Bertuzzi, M.A., Slavutsky, A.M., and Armada, M. (2012). Physicochemical characterisation of the hydrocolloid from Brea tree (Cercidium praecox). International Journal of Food Science and Technology 47 (4): 776–782.
- 17 Anderson, D.M.W. and Morrison, N.A. (1990). The identification of Combretum gums which are not permitted food additives II. Food Additive Contaminant 7: 181–188.
- 18 Castel, V., Buseghín, E., and Carrara, C.R. (2016). Goma brea como material de pared para encapsulación de compuestos hidrofóbicos. Efecto del agregado de inulina. In: VI Congreso Internacional de Ciencia y Tecnología de los Alimentos (ed. León A. E., V. Rosati and C.W. Robledo), 378. Ministerio de Ciencia y Tecnología de la provincia de Córdoba.
- 19 De Pinto, G., Martinez, M., and Rivas, C. (1994). Chemical and spectroscopic studies of Cercidium praecox gum exudate. Carbohydrate Research 260: 17–25.
- 20 Castel, V., Zivanovic, S., Jurat‐Fuentes, J.L. et al. (2016). Chromatographic fractionation and molecular mass characterization of Cercidium praecox (Brea) gum. Journal of the Science of Food and Agriculture 96 (13): 4345–4350.
- 21 Verbeken, D., Dierckx, S., and Dewettinck, K. (2003). Exudate gums: occurrence, production, and applications. Applied Microbiology and Biotechnology 63 (1): 10–21.
- 22 Castel, V., Rubiolo, A.C., and Carrara, C.R. (2017). Droplet size distribution, rheological behavior and stability of corn oil emulsions stabilized by a novel hydrocolloid (Brea gum) compared with gum Arabic. Food Hydrocolloids 63: 170–177.
- 23 Vasile, F.E., Martinez, M.J., Pizones, V.M. et al. (2016). Physicochemical, interfacial and emulsifying properties of a non‐conventional exudate gum (Prosopis alba) in comparison with gum Arabic. Food Hydrocolloids 56: 245–253.
- 24 Sanchez, C., Schmitt, C., Kolodziejczyk, E. et al. (2008). The acacia gum Arabinogalactan fraction is a thin oblate ellipsoid: a new model based on small‐angle neutron scattering and Ab initio calculation. Biophysical Journal 94 (2): 629–639.
- 25 Nie, S.P., Wang, C., Cui, S.W. et al. (2013). A further amendment to the classical core structure of gum Arabic (Acacia Senegal). Food Hydrocolloids 31 (1): 42–48.
- 26 Rao, M.S., Kanatt, S.R., Chawla, S.P. et al. (2010). Chitosan and guar gum composite films: preparation, physical, mechanical and antimicrobial properties. Carbohydrate Polymers 82: 1243–1247.
- 27 López, E.P., Pérez, G.T., De Erramouspe, P.L.J. et al. (2013). Effect of Brea gum on the characteristics of wheat bread at different storage times. Ciencia Y Tecnología de Alimentos 33 (4): 745–752.
- 28 Mutalik, V., Manjeshwar, L.S., Wali, A. et al. (2006). Thermodynamics/hydrodynamics of aqueous polymer solutions and dynamic mechanical characterization of solid films of chitosan, sodium alginate, guar gum, hydroxy ethyl cellulose and hydroxypropyl methylcellulose at different temperatures. Carbohydrate Polymers 65: 9–21.
- 29 Wareing, M.V. (1999). Exudates gums. In: Thickening and Gelling Agents for Food (ed. A. Imeson), 86–118. Gaithersburg, Maryland, USA: Aspen Publishers, Inc.
- 30 Yaseen, E.I., Herald, T.J., Aramouni, F.M. et al. (2005). Rheological properties of selected gum solutions. Food Research International 38: 111–119.
- 31 Mantell, C.L. (1947). The Water‐Soluble Gums. New York: Reinhold Pub.
- 32 Klein, M., Aserin, A., Ishai, P.B. et al. (2010). Interactions between whey proteins isolate and gum Arabic. Colloids and Surfaces. B, Biointerfaces 79: 377–383.
- 33 Potemsky, R., Masuelli, M., Slavutsky, A.M. et al. (2016). Determinación del peso molecular de Chañar brea. In: VI Congreso Internacional de Ciencia y Tecnología de los Alimentos (ed. A.E. León, V. Rosati and C.W. Robledo), 505. Ministerio de Ciencia y Tecnología de la provincia de Córdoba.
- 34 Williams, P.A., Phillips, G.O., and Stephen, A.M. (1990). Spectroscopic and molecular comparisons of three fractions from Acacia Senegal gum. Food Hydrocolloids 4: 305–311.
- 35 Glicksman, M. (1969). Gum Technology in Food Industry. New York: Academic Press.
- 36 Davies, J.T. and Rideal, E.K. (2012). Interfacial Phenomena. Elsevier.
- 37 Zayas, J.F. (1997). Functionality of Proteins in Food. Berlin, Heidelberg: Springer.
- 38 Dickinson, E. (2009). Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocolloids 23: 1473–1482.
- 39 Slavutsky, A.M., Bertuzzi, M.A., Armada, M. et al. (2014). Preparation and characterization of montmorillonite/brea gum nanocomposites films. Food Hydrocolloids 35: 270–278.
- 40 Zhang, S., Sun, D., Dong, X. et al. (2008). Aqueous foams stabilized with particles and nonionic surfactants. Colloids and Surfaces A: Physicochemical and Engineering Aspects 324 (1–3): 1–8.
- 41 Picton, L., Bataille, I., and Muller, G. (2000). Analysis of a complex polysaccharide (gum Arabic) by multi‐angle laser light scattering coupled on‐line to size exclusion chromatography and flow field flow fractionation. Carbohydrate Polymers 42 (1): 23–31.
- 42 Bouyer, E., Mekhloufi, G., Huang, N. et al. (2013). B‐lactoglobulin, gum Arabic, and xanthan gum for emulsifying sweet almond oil: formulation and stabilization mechanisms of pharmaceutical emulsions. Colloids and Surfaces A: Physicochemical and Engineering Aspects 433: 77–87.
- 43 Garti, N. and Reichman, D. (1993). Hydrocolloids as food emulsifiers and stabilizers. Food Structure 12 (4): 411–426.
- 44 Anderson, D.M.W. (1978). Chemotaxonomic aspects of the chemistry of acacia gum exudates. Kew Bulletin 32: 529–536.
- 45 Martínez‐Romero, D., Alburquerque, N., Valverde, J.M. et al. (2006). Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: a new edible coating. Postharvest Biology and Technology 39: 92–100.
- 46 Ali, A., Maqbool, M., Ramachandran, S. et al. (2010). Gum Arabic as a novel edible coating for enhancing shelf‐life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biology and Technology 58: 42–47.
- 47 Bosquez‐Molina, E., Guerrero‐Legarreta, I., and Vernon‐Carter, E.J. (2003). Moisture barrier properties and morphology of mesquite gum ecandelilla wax based edible emulsion coatings. Food Research International 36: 885–893.
- 48 Bertuzzi, M.A. and Slavutsky, A.M. (2013). Formulation and characterization of film based on gum. Journal of Food Science and Engineering 3: 113–122.
- 49 Slavutsky, A.M. and Bertuzzi, M.A. (2015). Thermodynamic study of water sorption and water barrier properties of nanocomposite films based on brea gum. Applied Clay Science 108: 144–148.
- 50 Spotti, M.L., Cecchini, J.P., Spotti, M.J. et al. (2016). Brea gum (from Cercidium praecox) as a structural support for emulsion‐based edible films. LWT‐Food Science and Technology 68: 127–134.
- 51 Ibarguren, C., Vivas, L., Bertuzzi, A. et al. (2010). Edible films with anti‐listeria monocytogenes activity. International Journal of Food Science and Technology 45 (7): 1443–1449.
- 52 Alexandre, M. and Dubois, P. (2000). Polymer‐layered silicate nanocomposites: preparation, properties, and uses of a new class of materials. Materials Science and Engineering: R: Reports 28 (1–2): 1–63.
- 53 Nedovic, V., Kalusevic, A., Manojlovic, V. et al. (2011). An overview of encapsulation technologies for food applications. Procedia Food Science 1: 1806–1815.
- 54 Tatar, F., Tunç, M.T., Dervisoglu, M. et al. (2014). Evaluation of hemicellulose as a coating material with gum Arabic for food microencapsulation. Food Research International 57: 168–175.
- 55 Butstraen, C. and Salaün, F. (2014). Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydrate Polymers 9: 608–616.
- 56 Defain Tesoriero, M. V., Murano, M. & Hermida, L. (2014) Utilización de goma brea para la microencapsulación de fragancias por coacervación compleja. 5° Jornadas de Desarrollo e Inonvación.
- 57 Luo, Y. and Wang, Q. (2014). Recent development of chitosan‐based polyelectrolyte complexes with natural polysaccharides for drug delivery. International Journal of Biological Macromolecules 64: 353–367.
- 58 Bigucci, F., Luppi, B., Cerchiara, T. et al. (2008). Chitosan/pectin polyelectrolyte complexes: selection of suitable preparative conditions for colon‐specific delivery of vancomycin. European Journal of Pharmaceutical Sciences 35 (5): 435–441.
- 59 Coimbra, P., Ferreira, P., de Sousa, H.C. et al. (2011). Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. International Journal of Biological Macromolecules 48 (1): 112–118.
- 60 Maciel, V.B.V., Yoshida, C.M.P., and Franco, T.T. (2014). Development of temperature indicator prototype: card paper coated with chitosan intelligent films. Journal of Agricultural Chemistry and Environment 3 (01): 5–10.
- 61 Maciel, V.B.V., Yoshida, C.M.P., and Franco, T.T. (2015). Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydrate Polymers 132: 537–545.
- 62 Costa, M.P.M., Da Ferreira, I.L.d.M., and Cruz, M.T.d.M. (2016). New polyelectrolyte complex from pectin/chitosan and montmorillonite clay. Carbohydrate Polymers 146: 123–130.
- 63 Ahmed, E.M. (2015). Hydrogel: preparation, characterization, and applications: a review. Journal of Advanced Research 6 (2): 105–121.
- 64 Slavutsky, A.M., Bravo, J.M., and Bertuzzi, M.A. (2016). Argentina. Obtención de complejos de polielectrólitos a base de pectina y goma brea. In: VI Congreso Internacional de Ciencia y Tecnología de los Alimentos (ed. A.E. León, V. Rosati and C.W. Robledo), 441. Ministerio de Ciencia y Tecnología de la provincia de Córdoba.
- 65 Jayme, M., Dunstan, D., and Gee, M. (1999). Zeta potentials of gum Arabic stabilised oil in water emulsions. Food Hydrocolloids 13 (6): 459–465.
- 66 Slavutsky, A.M. and Bertuzzi, M.A. (2016). Obtención De Hidrogeles A Partir De Pectina, Goma Brea Y Montmorillonita. The Journal of the Argentine Chemical Society 103 (1–2), Buenos Aires‐Argentina.
- 67 Bárcenas, M.E. and Rosell, C.M. (2005). Different approach for improving the quality and extending the shelf‐life of the partially baked bread: low temperatures and hydrocolloid addition. Food Chemistry 100: 1594–1601.
- 68 Dziezak, J.D.A. (1991). Focus on gums. Food Technology 45 (3): 115–132.
- 69 Lee, M.H., Baek, M.H., Cha, D.S. et al. (2002). Freeze‐thaw stabilization of sweet potato starch gel by polysaccharide gums. Food Hydrocolloids 16 (4): 345–352.
- 70 López, E.P. and Jiménez, P.L. (2016). Effect of different proportions of brea gum in the functional characteristics of wheat flour starch: impact on the physical quality of bread. Food Science and Technology 36 (1): 1–7.
- 71 López, E.P. and Goldner, M.C. (2015). Influence of storage time for the acceptability of bread formulated with lupine protein isolate and added brea gum. LWT‐Food Science and Technology 64 (2): 1171–1178.
- 72 Mesa, C.L.M., Rodríguez, V.S., Beltrán, F.O. et al. (1997). Behavior of Aspergillus Niger in the gum exudates of cercium praecox and cedrela odorata. Boletín micológico 12 (1/2): 35–39.
