23
Edible/Biodegradable Films and Coatings from Natural Hydrocolloids
Younes Zahedi
Department of Food Science and Technology, Faculty of Agriculture and Natural Resources, University of Mohaghegh Ardabili, P.O. Box 56199‐11367, Ardabil, Iran
23.1 Introduction
The use of synthetic plastics for packaging of foods, pharmaceutical, and other products has increased extensively in the past 70 years, causing environmental pollution by plastic wastes. On the other hand, the ever‐shrinking oil reservoirs, concerns about toxic migration, and the increasing trend for safe and organic foods are the reasons for the growing demand for sustainable/green packaging in the last decades. Renewable agricultural and biomass feedstock, which are safe and of natural origin, are being used to develop non‐polluting biodegradable plastics and biopolymers [1]. Materials that can be used to make edible or biodegradable films include polysaccharides, proteins, lipids, and polyesters or combinations of these materials. Edible, biodegradable films and coatings can act as barriers to control the transfer of moisture, oxygen, carbon dioxide, lipids, and flavor components and thus maintain the quality and increase the shelf life of food products [2]. Starch obtained from different plant sources is the most common polysaccharide used, either alone or in combination with other substances, for film production. Also, other commercial hydrocolloids, for example, xanthan, alginate, agar, carrageenan, locust bean gum, guar, and pectin, have been extensively utilized for the preparation of edible films. In recent years, new plant sources of polysaccharides with different structure and composition have been identified, and their application potential in various fields of the food industry are being investigated. Use of the novel hydrocolloids as biopackaging materials is one of the interesting fields being explored. Some of these hydrocolloids such as basil seed gum (BSG), brea gum, and gum Cordia have shown satisfactory film‐making ability. Furthermore, in some studies, to improve the pure film properties, some modifications have been performed by incorporating lipid substances or nanoparticles into the matrix of the film, resulting in emulsified or nanocomposite films, respectively. This chapter will focus on the film preparation process from the novel natural hydrocolloids, a comparison of the physical and mechanical characteristics of the produced films with those of conventional synthetic films, and finally, an evaluation of their food preservation capability.
23.2 Film Preparation
Biodegradable packaging can be prepared by two customary methods: dry (thermoplastic) and wet (solvent). Generally, the solvent method is used for fabrication of polysaccharide‐based edible/biodegradable films, with the exception of starch, which could be generated by both methods of film preparation. Due to the hydrophilic nature of polysaccharides, water is a common solvent for film making that finally must be separated for the formation of the film layer.
Gum or mucilage extraction is an essential step in the preparation of films or coatings from natural hydrocolloids. First, seeds or fruits are usually soaked in distilled water at a temperature of 25–80 °C to complete hydration. Then, the swollen seeds or fruits are often stirred with a rod paddle blender to scrape the gum or mucilage layer off the surface. After separation of the gum or mucilage, precipitation by alcohol or acid may be required. The product is generally dried by an air circulating oven, milled, and sieved to obtain fine particles. Sometimes, it might be preferred to dry gum or mucilage solution with a freeze‐dryer rather than with an oven to achieve a high‐quality product with a light color. The isolated gum or mucilage is composed of mostly a carbohydrate fraction as well as small fractions of residual protein, lipid, and ash. The impurities are unavoidable even in the commercial hydrocolloids and may influence the physicochemical properties of the final films or coatings.
The dried gums have been commonly used at low concentration (about 1% w/v) to prevent the problems caused by the high viscosity of gum solutions during the various steps of film making (mixing, degassing, and casting). Nevertheless, in some cases, such as brea gum, a high concentration (10% w/v) is needed to obtain appropriate films [3]. For film fabrication, first, gum powder is gradually added to distilled or deionized water (25–80 °C) under stirring to disintegrate aggregates and thereby form a homogeneous dispersion. Sometimes, proteins or other polysaccharides can be combined with gum powder to modify the film's mechanical properties. Polysaccharide‐based films and coatings are often quite brittle and stiff due to extensive intermolecular interactions, and thus a suitable plasticizer must be added to decrease film or coating brittleness; also, the stretchability of the film increases, and its cohesion and mechanical resistance decrease. Glycerol is predominantly used as plasticizers for gum‐based edible films and coatings, which is often added to the filmogenic solution after complete dissolution of the powder. The gum solution is often maintained for 24 h to provide enough time to complete hydration and possibly react with plasticizer molecules for any configurational changes [4]. The hydration can be performed at different steps of film production, immediately after powder dissolving up to the casting time.
To make emulsified films, a lipid material and surfactants such as Tween 80 or lecithin are incorporated into the film solution that is previously heated to above than melting point of the lipid. Next, the mixture is homogenized for several minutes for effective dispersion of lipid molecules. Antimicrobials, essential oils, antioxidants, and so on, are the components that can be included to promote the film or coating effectiveness. The final film solution usually has many bubbles because of high consistency; therefore, degassing is an important step to eliminate the bubbles. The existence of these bubbles can negatively affect the appearance, attractiveness, and physical characteristics of the film. Lastly, the gum‐based film or coating is formed by applying the prepared formulation to the desired casting or product surface and allowing the solvent to evaporate. For film production with an adequate thickness, a certain weight or volume of the formulation must be spread evenly on a surface that will release the film after drying. The mold is exposed to ambient or oven conditions until a uniformed dried film is ensured.
23.3 Film Characteristics
23.3.1 Thickness
Film and coating thickness is a macroscopic parameter which must be determined because of its influence on some physical properties of the film (water vapor permeability (WVP), mechanical characteristics, and opacity) and likewise its food shelf life and quality. Usually, packaging materials with a thickness lower than 250 microns are called films and those greater than 250 microns are called sheets. Control of film and coating thickness is necessary during application on respiratory products in order to prevent anaerobic respiration and synthesis of unfavorable substances. Coating thickness is influenced by solution properties such as surface tension, density, and viscosity, and coating methods (brushing, spraying, panning, dipping, etc.) [5]. Film thickness depends on different factors, mainly the solid content of the film‐forming solution at casting. Usually, the higher concentration of the film solution results in thicker films. In addition, the nature and composition of the films affect the thickness [6]. As shown in Table 23.1, the thickness of the films fabricated from natural hydrocolloids varied over a wide range from 28 microns for BSG films [12] to 163 microns for brea gum [14]. Many investigations showed that upon a constant casting mass, increase in plasticizer or other additive content caused thickening of the films prepared from various natural hydrocolloids [ 4, 6,7,9,11, 12,18,19,22,23]. Glycerol, as a general plasticizer, could absorb a considerable amount of water, thereby producing swelled films with enhanced thickness [15, 22]. However, in some studies, the effect of increasing the concentration of additives on the gum‐based film's diameter has been reported to be insignificant [8,10, 15,26,27]. It has been observed that the plasticizer type could influence the film thickness; sage seed gum (SSG) films containing sorbitol were thicker than the ones with glycerol [23]. Differences in the density of the phases and interactions among different components of the emulsified films could change the thickness [4]. Mohammad Amini et al. [4] found that incorporation of long‐chain fatty acids (25% w/w gum) into BSG edible film enhanced the thickness of the emulsified films from 65 to 95 micron. This can be explained by the presence of lipid molecules between the polysaccharides chains, preventing the formation of an ordered and compact structure and expanding the film matrix [ 4,16]. In other studies, the thickness of the films made from BSG showed a different behavior upon essential oil addition: thyme essential oil (thymol) thickened the films, whereas oregano and Zataria multiflora essential oils (ZMEOs) did not effectively change the thickness [ 10– 12, 26].
Table 23.1 Water vapor permeability (WVP) values of gum‐based films and some synthetic plastic films.
| Gum/polymer | Plasticizer (% w/w) | Additive | Film thickness (mm) | Test conditions (°C, % RH inside/outside) | WVP (×10−11 g/m Pa s) | Reference |
| AHSG | Glycerol (0–45) | — | 0.092–0.134 | 25, 0/53 | 4.93–6.41 | [7] |
| AHSG | Glycerol (50) | PVA (0–100% w/w) | 0.09–0.12 | 23, 0/97 | 51.03–135.53 | [8] |
| BSG | Glycerol (100) | Palmitic acid (0–25% w/w) | 0.065–0.071 | 24, 0/97 | 1.34–5.96 | [4] |
| Glycerol (100) | Stearic acid (0–25% w/w) | 0.065–0.076 | 24, 0/97 | 0.67–5.96 | [4] | |
| Glycerol (100) | Oleic acid (0–25% w/w) | 0.065–0.095 | 24, 0/97 | 0.11–5.96 | [4] | |
| Glycerol (40–100) | — | 0.055–0.065 | 24, 0/97 | 4.23–6.61 | [4] | |
| Sorbitol (40–100) | — | 0.047–0.058 | 24, 0/97 | 3.14–6.72 | [4] | |
| BSG | Glycerol (0–50) | — | 0.058–0.074 | 25,0/75 | 16.9–22.4 | [9] |
| BSG | Glycerol (30) | OVES (0–6% w/w) | 0.06–0.07 | 30,0/100 | 3.69–4.33 | [10] |
| BSG | Glycerol (35) | Thymol (0–10% w/w) | 0.066–0.079 | 25, 0/75 | 21.3–115.6 | [11] |
| BSG | Glycerol (30) | Thymol (0–3% w/w) | 0.028–0.056 | 25, 0/50 | 157.40–378.47 | [12] |
| BG | Glycerol (0–30) | — | — | — | 80–250 | [3] |
| BG | Glycerol (25) | Montmorillonite (0–5% w/w) | — | 25, 100/0 | 37.9–71.7 | [13] |
| BG | Glycerol (0–40) | Beeswax (0–40% w/w) | 0.159–0.163 | 32,0/75 | 2–4.7 | [14] |
| CSM | Glycerol (25–75) | — | 0.054–0.060 | 25,0/75 | 3.638–12.277 | [15] |
| GC | PEG 400 (20) | GMS (0–0.02% w/w) + Beeswax (0–20% w/w) | 0.073–0.074 | 25, 0/58–90 | 0.9–6.3 | [16] |
| GC | Glycerol (10–30) | — | — | 25, 0/100 | 18–54 | [17] |
| Sorbitol (10–30) | — | — | 25, 0/100 | 13–39 | [17] | |
| PEG 200 (10–30) | — | — | 25, 0/100 | 22–25 | [17] | |
| PEG 400 (10–30) | — | — | 25, 0/100 | 9.5–12 | [17] | |
| CSG | Glycerol (35) | ZMEO (0–4% w/w) | 0.067–0.122 | 25, 0/75 | 9.5–32.1 | [18] |
| CSG | Glycerol (25–50) | — | 0.067–0.079 | 25, 0/75 | 21–24.3 | [19] |
| LPSG | Glycerol (40–70) | — | 0.069–0.071 | 25, 0/97 | 20.49–24.14 | [20] |
| LPSG | Glycerol (60) | Palmitic acid (0–30% w/w) | — | 25, 0/97 | 17.1–22.6 | [21] |
| LPSG | Glycerol (60) | Stearic acid (0–30% w/w) | — | 25, 0/97 | 16.8–22.6 | [21] |
| PSG | Glycerol (15–35) | — | 0.050–0.072 | 25, 0/75 | 11.6–18.8 | [22] |
| SSG | Glycerol (40–100) | — | 0.090–0.117 | 22, 0/97 | 4.23–5.71 | [23] |
| SSG | Sorbitol (40–100) | — | 0.100–0.123 | 22, 0/97 | 2.98–4.09 | [23] |
| GT | — | LBG (0–100% w/w) | 0.044–0.047 | 25, 0/100 | 7.49–10.55 | [24] |
| Polyester | — | — | 0.252 | [ 15,25] | ||
| HDPE | — | — | 0.033 | [ 15, 25] | ||
| LDPE | 0.014 | 23, 55/100 | 1.13 | [1] | ||
| LDPE | — | 28, 0/100 | 0.036 | |||
| PVDC (Saran) | 0.012 | 23, 55/100 | 0.083 | |||
| Cellophane | — | 38, 0/90 | 8.41 | |||
| PVDC (Saran) | — | 28, 0/100 | 0.022 | |||
| PVC | — | 28, 0/100 | 0.713 |
AHSG: Alyssum homolocarpum seed gum, BSG: Basil seed gum, BG: Brea gum, CSM: Chia seed mucilage, GC: Gum Cordia, CSG: Cress seed gum, LPSG: Lepidium perfoliatum seed gum, PSG: Psyllium seed gum, SSG: Sage seed gum, GT: Gum tragacanth, LDPE: Low‐density poly ethylene, HDPE: High‐density polyethylene, PVDC: Polyvinylidene chloride, PVC: Polyvinyl chloride, PVA: Polyvinyl alcohol, GMS: Glycerol monostearate, LBG: Locust bean gum, OVES: Origanum vulgare (oregano) essential oil, ZMEO: Zataria multiflora essential oil.
It is established that thickness usually exhibits a direct relationship with the WVP of the hydrophilic films [28]. The water vapor transmission rate (WVTR) is independent of thickness, but the WVP increases upon film thickening. The results of Abdul Haq [29] indicate that the WVP of gum Cordia film is elevated as a function of film thickening. When the film or coating carries active components, the thickness could be an effective factor in the release; in the thinner films or coatings, the transfer rate is high because of the lower distance from the surface [30].
23.3.2 Permeability
23.3.2.1 Water Vapor Permeability (WVP)
The WVP is the most important property of biopolymeric films owing to the fact that an insufficient water vapor barrier performance might cause serious problems in food products, such as deteriorative reactions and crispness decrease induced by water gain or water loss. For this reason, the WVP is the most extensively studied property of edible films and coatings. The WVP of edible films depends on many factors including the diffusivity and solubility of water molecules in the film matrix, the hydrophobic or hydrophilic nature of biopolymers, the presence of voids or cracks in their structure, polymeric chain mobility, specific interactions between the functional groups of the polymers, the integrity of the film structure, the hydrophilic/hydrophobic and crystalline/amorphous ratios, the type and level of plasticizer, film thickness, water sensitivity, ambient conditions (temperature and relative humidity (RH)), and so on [ 6, 8, 12, 20, 22]. From the standpoint of these factors, conflicting results reported by different researchers are the norm. Due to the hydrophilic nature of natural hydrocolloids, edible films prepared from them do not act as an efficient water vapor barrier. Furthermore, the brittleness of these films can be improved by different plasticizers, which mainly show a high tendency to moisture. In fact, polyols, especially glycerol and sorbitol, as general plasticizers improve the flexibility of films at the expense of the water vapor barrier. Glycerol was mostly used as a plasticizing agent in the preparation of many gum‐based films, which increased the WVP as a function of glycerol concentration [ 3, 6, 7, 9, 11, 15, 17, 19, 22, 23]. The main reason for the WVP increase was that glycerol molecules could penetrate into the intermolecular space of macromolecules, thereby reducing intermolecular bonds and enhancing segmental motions, which consequently facilitate the diffusion of water molecules [ 3, 4, 6, 7, 15, 19, 22]. Other plasticizers used for film making from natural hydrocolloids are sorbitol and polyethylene glycol (PEG). The results of studies showed that the WVP of gum‐based films was importantly influenced by the plasticizer type [ 17, 23]. According to the findings of Razavi et al. [23], the WVP values of SSG film prepared with glycerol were higher than those of films containing sorbitol. Also, the order of the WVP increase induced in gum Cordia films by incorporation of plasticizer was glycerol > sorbitol > PEG 200 > PEG 400 [17]. Interestingly, at low content of PEGs, the WVP of the plasticized films was higher than that of neat gum Cordia films [17].
Although lipid materials possess a hydrophobic nature, fabrication of stand‐alone films has not been yet attempted because their monomeric structure gives rise to very brittle films. Nevertheless, lipids have been extensively employed in some film formulations aiming at WVP improvement. Besides, some lipids exhibit a good plasticizing effect and have been categorized as a plasticizer. Note that water molecules permeate through the hydrophilic part of the film matrix, and thus the percentage WVP decrease by lipids is proportional to the hydrophilic‐to‐hydrophobic ratio of the film composition [21]. The water vapor barrier of some gum‐based films have been modified by various lipids as follows: the WVP of BSG and Lepidium perfoliatum seed gum (LPSG) films incorporated with long‐chain fatty acids decreased up to 98% and 25%, respectively, and the WVP of brea gum and gum Cordia films containing beeswax reduced up to 57% and 85%, respectively [ 4, 14, 16, 21].
Edible films and coatings have great carrying capacity for functional components due to both natural origin and direct contact with food. Essential oils might be incorporated into edible films as antimicrobial and antioxidant agents in order to extend the storage life of food. Additionally, the existing variety of hydrophobic components in essential oils can sometimes promote the water vapor barrier of films. Hashemi et al. [10] observed that the WVP values of BSG coatings were reduced by oregano essential oil due to the interference of distributed oil globules in the transmission of water molecules through the coating matrix.
One of the most effective strategies to solve the drawbacks of synthetic and natural packaging films is to incorporate nanoscale materials into the matrix of the film. Compared to lipids, nanomaterials can modify the moisture, aroma, and gas barriers by different mechanisms without a noticeable increase in the edible/biodegradable film's opacity. A novel nanocomposite film was developed by loading montmorillonite nanoparticles to brea gum film solution [ 13, 27]. Depending on the montmorillonite content, the WVP of the films decreased from 24% to 47% by two mechanisms: (1) establishment of extensive interactions between brea gum and nanoclay prevents the diffusion of water molecules through the film matrix and (2) generation of a tortuous pathway by nanoclay lengthens the path of water vapor diffusion [ 13, 27].
It is concluded that incorporation of lipids and nanomaterials can be considered as effective methods for achieving the desired WVP for films based on natural hydrocolloids. In spite of this, as with most polysaccharide‐based films, films fabricated from natural hydrocolloids exhibit restricted moisture barrier ability because of their hydrophilic nature, and WVP values are still higher than those measured for synthetic plastic films (Table 23.1). Data collected for the WVP of the best gum‐based film (gum Cordia) show values that are about 40‐fold higher than those of the best synthetic film (polyvinylidene chloride) and about ninefold lower than the worst synthetic film (cellophane), and BSG film is found to be the most susceptible to water vapor transmission versus cellophane.
23.3.2.2 Oxygen Permeability (OP)
The permeability of packaging materials to oxygen is measured to evaluate the ability to preserve different foods against harmful reactions (oxidation, enzymatic browning, etc.) and microbial growth. On the other hand, some foods, for example, meat, fruit, and vegetables, need a controlled level of oxygen in order to inhibit respiration, blooming, and growth of putrefying bacteria. However, the oxygen transmission rate has to be minimized in most foods to extend storage life. Due to the nature of synthetic and biopolymer‐based films, different gases can always pass through the films, and a gas‐impermeable film is almost impossible to create. The OP of packaging films is influenced by many factors, especially the RH and temperature. Moreover, polymer polarity, concentration, and type of modifying agent (plasticizers, lipids, nanostructures, antimicrobials, etc.), crystallization degree, and orientation process affect the permeability of films to gases.
The effect of plasticizer on the OP of gum‐based films has been investigated [ 7, 17]. Mohammadi Nafchi et al. [7] reported that the OP of Alyssum homolocarpum seed gum (AHSG) films increased with enhancing glycerol content. This is because hydrogen and covalent polymeric bonds among adjacent polymer chains are disrupted by plasticizers, thereby increasing polymer mobility [31]. A similar result was found for gum Cordia films plasticized by various plasticizers [17]. The authors also deduced that glycerol and PEG 400 were the worst and the best, respectively, for the oxygen barrier, and sorbitol and PEG 200 were in between.
Different natural or synthetic additives may be loaded into the edible films and coatings to produce active packaging materials. This can be accompanied by changes in the permeability of edible films or coatings. Khazaei et al. [11] found that the OP of BSG film decreased as a result of thymol addition. In contrast, gum Cordia films containing butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and α‐tocopherol antioxidants demonstrated a reduced OP compared with the control film [29].
As mentioned earlier, WVP improvement in biopolymers could have been achieved by lipids. On the other hand, OP increase is the next result of lipid incorporation into films. For instance, the OP of gum Cordia film was greatly affected by beeswax and ranged from 0.39 × 10−15 to 23.1 × 10−15 g (m s Pa)−1 [16]. Diffusion and solution are the two essential conditions for gas and vapor permeation through the film. The polarity of gas determines the solubility value, and film structure affects diffusivity [4]. Hydrocolloid‐based bioplastics possess more polar structure compared with many synthetic plastics, leading to a higher barrier for nonpolar gases such as O2, CO2, and N2. According to the observations of Abdul Haq et al. [16], the addition of a hydrophobic substance to gum Cordia film increased the OP in two ways: (1) decreasing the film polarity and (2) facilitating O2 diffusion within the reconstructed emulsified film.
The OP values of gum‐based films and conventional synthetic plastic films are tabulated in Table 23.2. It is necessary to measure the OP of all films under the same test conditions for proper comparison; if not, the conditions under which the measurements were obtained must be considered. As can be seen, the measurements have been mostly carried out at 23–25 °C, but at different RHs. At higher RHs, hydrophilic polymers easily absorb environmental moisture, thus enhancing gas permeability [1]. Considering the RH, the OPs of all the tabulated gum‐based films are lower than those of low‐density polyethylene (LDPE) and methylcellulose and are close to those of polyamide 6 and polyethylene terephthalate (PET), which are potent oxygen barriers. The OP of the ethylene vinyl alcohol (EVOH) film was lower than that of the films developed from BSG and gum Cordia, probably because of greater polarity. Overall, the oxygen barrier characteristics of gum‐based edible films are in the range of the values reported for other bio‐based and synthetic packaging films.
Table 23.2 Oxygen permeability (OP) values of gum‐based films and some synthetic plastic films.
| Gum/polymer | Plasticizer (% w/w) | Additive | Film thickness (mm) | Test conditions (°C, %RH) | OP (×10−15 g/m Pa s) | Reference |
| AHSG | Glycerol (0–45) | — | 0.092–0.134 | 25, 50 | 0.010–0.016 | [7] |
| AHSG | Glycerol (50) | PVA (0–100% w/w) | 0.09–0.12 | 25, 50 | 0.00046–0.7065 | [8] |
| BSG | Glycerol (35) | Thymol (0–10% w/w) | 0.066–0.079 | 23, 50 | 0.756–0.868 | [11] |
| GC | PEG 400 (20) | GMS (0–0.02% w/w) + Beeswax (0–20% w/w) | 0.073–0.074 | 25, 58 | 0.39–23.1 | [16] |
| GC | Glycerol (10–30) | — | — | 25, 58 | 0.26–5.31 | [17] |
| Sorbitol (10–30) | — | — | 25, 58 | 0.27–2.20 | [17] | |
| PEG 200 (10–30) | — | — | 25, 58 | 0.28–2.42 | [17] | |
| PEG 400 (10–30) | — | — | 25, 58 | 0.16–0.84 | [17] | |
| GC | Glycerol (20) | — | — | 25, 58 | 0.38 | [29] |
| Glycerol (20) | BHA | — | 25, 58 | 0.51 | [29] | |
| Glycerol (20) | BHT | — | 25, 58 | 0.45 | [29] | |
| Glycerol (20) | α‐tocopherol | — | 25, 58 | 0.43 | [29] | |
| MC | 30, 0 | 16.704 | [1] | |||
| Chitosan | 25, 0 | 0.0182 | ||||
| Wheat gluten | 25, 0 | 0.0396 | ||||
| MC/B | 25, 0 | 15.968 | ||||
| Cellophane | 23, 0 | 0.0428 | ||||
| LDPE | 23, 0 | 32.096 | ||||
| HDPE | 23, 100 | 7.168 | ||||
| Rigid PVC | 23, 0 | 0.512 | ||||
| EVOH | 23, 0 | 0.0064 | ||||
| EVOH | 23, 95 | 0.192 | ||||
| PA6 | 23, 0 | 0.380 | ||||
| PET | 23, 0 | 0.380 |
AHSG: Alyssum homolocarpum seed gum, BSG: basil seed gum, GC: Gum Cordia, MC: Methylcellulose, MC: Methylcellulose/beeswax, LDPE: Low‐density polyethylene, HDPE: High‐density polyethylene, PVC: Polyvinyl chloride, EVOH: Ethylene vinyl alcohol, PVA: Polyvinyl alcohol, PA6: Polyamide 6, PET: Polyethylene terephthalate, GMS: Glycerol monostearate.
23.3.3 Density
Density is an additional structural feature, sometimes measured in the edible films, giving more information about film compactness. Density is mainly governed by molecular weight, structure, conformation and packing arrangement, and also film composition. Permeability to water vapor, gases, and aromatic compounds are the attributes that may be affected by density; the higher the compactness of a film's structure, the harder the diffusion of permeants through the film. Density measurements in the gum‐based films (Table Table 23.3) show that using hydrophilic plasticizers (glycerol and sorbitol) decreased the density [ 4, 6, 7, 9 19– 21]. The authors hypothesized that moisture absorption by plasticizers may account for the film thickening and compactness reduction [ 6, 23]. In other work, the small molecular size of polyvinyl alcohol (PVA) in comparison with AHSG polysaccharides and water absorption by hydrophilic PVA were primarily responsible for expanding the tight molecular packing of AHSG film [8]. It was expected to reduce the emulsified film's density due to the low density of lipids compared with the film's other components, whereas the opposite effect was seen in LPSG film [21]. It seems that fatty acid molecules fill the gaps and voids of the biopolymer matrix, and produce a more compact morphology compared to the neat LPSG film. Nonetheless, the density of BSG film was not significantly changed by the addition of fatty acids [4].
Table 23.3 Water‐related (moisture content, water solubility, and moisture uptake) and density values of gum‐based films.
| Gum/mucilage | Plasticizer (% w/w) | Additive | Moisture content (%) | Water solubility (%) | Moisture uptake (%) | Density (g/cm3) | Reference |
| AHSG | Glycerol (0–45) | — | 14.2–19.1 | 38.1–53.2 | 6.9–8.43 a | 1.18–1.29 | [7] |
| AHSG | Glycerol (50) | PVA (0–100% w/w) | 23.1–54.1 | 13–75 | — | 1.12–1.47 | [8] |
| BSG | Glycerol (100) | Palmitic acid (0–25% w/w) | 38.3–48.7 | 37.6–55.4 | 107.4–146.0 | 1.33–1.34 | [4] |
| Glycerol (100) | Stearic acid (0–25% w/w) | 35.8–48.7 | 31.4–55.4 | 106.1–146.0 | 1.33–1.34 | [4] | |
| Glycerol (100) | Oleic acid (0–25% w/w) | 42.4–48.7 | 27.2–55.4 | 91.1–146.0 | 1.33–1.34 | [4] | |
| Glycerol (40–100) | — | 26.8–48.7 | 39–50 | 107.6–143.1 | 1.34–1.37 | [4] | |
| Sorbitol (40–100) | — | 10.1–11.5 | 35–46 | 69.5–85.5 | 1.38–1.43 | [4] | |
| BSG | Glycerol | ZMEO (1–3% v/v) | — | 23.8–29.3 | — | — | [26] |
| BSG | Glycerol (0–50) | — | 16.2–18.5 | 47.3–53.7 | — | — | [9] |
| BSG | Glycerol (30) | OVES (0–6% w/w) | 17.5–17.9 | — | — | — | [10] |
| BSG | Glycerol (30) | OVES (0–5% w/w) | 17.8–17.8 | — | — | — | [32] |
| CF | Glycerol (16) | Maize starch (0–200% w/w) | 7.2–12.2 | 23.4–55.5 | — | — | [25] |
| CSM | Glycerol (25–75) | — | 18.1–41.8 | 52.7–84.5 | — | — | [15] |
| CSG | Glycerol (35) | ZMEO (0–4% w/w) | 14.0–16.8 | 35.2–42.5 | — | — | [18] |
| CSG | Glycerol (25–50) | — | 15.4–18.7 | 48.3–54.1 | — | 1.22–1.30 | [19] |
| LPSG | Glycerol (40–70) | — | 23.2–7.66 | 38.9–58.0 | 127.4–155.1 | 0.88–0.93 | [20] |
| LPSG | Glycerol (60) | GPP (20–80% w/w) | 22.1–25.1 | 27.4–58.1 | 56.1–104.9 | 1.33–1.42 | [6] |
| Glycerol (70) | GPP (20–80% w/w) | 24.9–30.4 | 30.5–62.4 | 59.6–108.5 | 1.28–1.40 | [6] | |
| Glycerol (80) | GPP (20–80% w/w) | 29.1–37.1 | 38.8–67.1 | 64.2–115.5 | 1.19–1.27 | [6] | |
| LPSG | Glycerol (60) | Palmitic acid (0–30% w/w) | 26.6–32.7 | 40.0–56.8 | 57.3–152.6 | 0.91–1.05 | [21] |
| Glycerol (60) | Stearic acid (0–30% w/w) | 24.3–32.7 | 35.5–56.8 | 56.9–152.6 | 0.91–1.05 | [21] | |
| PSG | Glycerol (15–35) | — | 13.5–16.7 | 47.7–52.9 | — | — | [22] |
| SSG | Glycerol (40–100) | — | 26.6–48.8 | 79–83 | 110.8–139.1 | 1.32–1.40 | [23] |
| Sorbitol (40–100) | — | 13.4–14.1 | 65–84 | 68.6–91.9 | 1.27–1.41 | [23] | |
| GT | — | LBG (0–100% w/w) | 12.0–16.2 | — | 43.8–95.8 | 0.91–1.04 | [24] |
AHSG: Alyssum homolocarpum seed gum, BSG: Basil seed gum, CF: Chia flour, CSM: Chia seed mucilage, CSG: Cress seed gum, LPSG: Lepidium perfoliatum seed gum, PSG: Psyllium seed gum, SSG: Sage seed gum, GT: Gum tragacanth, PVA: Polyvinyl alcohol, ZMEO: Zataria multiflora essential oils, OVES: Origanum vulgare (oregano) essential oil, GPP: Grass pea protein, LBG: Locust bean gum.
a g/g dried film.
23.3.4 Water‐Related Properties
23.3.4.1 Moisture Content
The presence of moisture in the structure of most edible films and coatings is unavoidable because water is used as a solvent. One of the best functions of water is the plasticizing effect on biopolymer‐based films. On the other hand, the moisture content of the edible films may negatively influence water vapor and gas permeability, and films with high moisture are not appropriate for sensitive foods [8]. Hydrocolloid‐based films and coatings are expected to capture more water owing to their hydrophilic nature. Gum‐based films are not an exception to this rule, and their moisture content ranged from 10% to 54% (Table Table 23.3). In addition to their biopolymer nature, other components of the film (plasticizer, essential oil, lipid, nanoparticle, etc.) may influence the moisture content. It has been proved by the findings of different studies that the moisture content of all gum‐based films substantially increased in response to glycerol increase, but there was no significant change upon sorbitol increase [ 4, 6, 7, 9, 15, 19, 20, 22, 23]. This increase was attributed to the hygroscopic character of glycerol, which gives it an excellent capacity for water binding [ 4, 9, 20]. Because of this, sorbitol may be the preferred plasticizer for gum‐based films when high moisture content is harmful. Lipid derivatives may decrease the film's moisture by promoting matrix hydrophobicity [ 4, 21]. This is observed in the researches of Mohammad Amini et al. [4] and Seyedi et al. [21] for BSG and LPSG films, respectively. Also, incorporation of functional additives might affect the film's water content, as reported in the following studies. Oregano essential oil had no effect on the moisture content of BSG films [10], whereas this parameter was reduced by addition of ZMEO into cress seed gum (CSG) films [18]. Interactions among the hydroxyl groups of CSG film and the essential oil components, as well as a decrease in the film's hydrophilicity, may account for the limited water holding capacity, and the subsequent moisture content reduction [ 18,33].
23.3.4.2 Water Solubility
Unlike synthetic polymers, which are mostly characterized as hydrophobic materials, biopolymers are hydrophilic. Hence, the water solubility of edible/biodegradable films and coatings is of interest for pharmaceutical and food applications. Generally, higher water solubility would imply lower water resistance and greater hydrophilicity of the film components. Films with high water solubility cannot serve as appropriate packaging for foods with high moisture content or for foods stored at high RH. However, greater water solubility may be beneficial in some cases. For example, film solubility is advantageous in situations when the films will be consumed with a product that is heated prior to consumption such as dry soup mixes, and or controlled release of drugs. Furthermore, it was proved that the water solubility of films facilitates their disintegration and biodegradability [34,35].
Similar to other members of the polysaccharide family, gum‐based films usually possess intermediate or high water solubility (Table Table 23.3). Combining gums with various polymers may result in films whose behavior varies in the water solubility test. Some protein‐based films such as native proteins of milk also exhibit high solubility. Heating is a common treatment during film fabrication which mainly leads to protein unfolding, exposing the hydrophobic parts of the protein, and the formation of new covalent SS bonding during drying, thereby reducing film water solubility. The result of a study showed that addition of grass pea protein isolate (GPPI) to LPSG films containing 60% glycerol decreased water solubility from 58% to 27% due to the influence of heat‐denatured proteins [6]. In another study, blending PVA, a synthetic polymer rich in hydroxyl groups, with AHSG in the ratio 0%–80% increased the water solubility of the composite films by more than four orders of magnitude [8].
Edible films and coatings are more susceptible to disintegration in water in the presence of hydrophilic plasticizers. There are many reports of an increase in the water solubility of gum‐based films with increasing plasticizer concentration [ 6, 7, 9, 15, 17, 19, 20, 22, 23]. The authors hypothesized that the plasticizers can easily penetrate into the interface of biopolymer chains due to their small molecular size, restricting intermolecular linkages between them and consequently facilitating solubility in water [36]. A research into the effect of plasticizer type (glycerol, sorbitol, PEG 200, and PEG 400) on the water solubility of films made from gum Cordia revealed no relationship between plasticizer type and solubility changes [17]. This result was confirmed by the findings of other researchers for SSG and BSG films plasticized by both glycerol and sorbitol [ 4, 23].
A negative effect of different additives on the water solubility of gum‐based films was reported by different workers. Lipid derivatives such as beeswax and long‐chain fatty acids could improve the resistance of gum‐based films to disintegration in water due to the hydrophobicity enhancement of BSG, LPSG, brea gum, and gum Cordia [ 4, 14, 21, 29,37]. However, the solubility of gum Cordia films was not influenced by oil‐soluble antioxidants, probably owing to their small amounts [29]. Moreover, essential oils and nanoclay can contribute to reducing the solubility in water of gum‐based films [ 12, 13, 18, 26]. Establishment of hydrogen bonds between the exfoliated structure of nanoclay particles and the polysaccharide chains was described by Slavutsky et al. [13] as a reason for the decreased solubility of brea gum nanocomposites.
In conclusion, film‐making treatment, the quantity of free ‐OH in the film matrix, plasticizer content, additives, solubilizing conditions (water temperature, agitation, and time), and crystallinity, polarity, and cross‐linking of the biopolymer are the main factors influencing the solubility of hydrocolloid‐based films and coatings [ 8, 15, 22]. And, depending on the application, the water solubility of the films can be modified by considering these factors.
23.3.4.3 Water Contact Angle
Contact angle measurement provides information regarding surface energy and surface tension [38]. The water droplet contact angle characteristic can be used as the hydrophilicity index of edible/biodegradable films and coating surfaces, too [34]. A contact angle smaller than 90° implies that water will spread over a large area on the surface (high wettability); contact angles greater than 90° indicate that water will minimize its contact with the surface (low wettability), and the corresponding material is defined as hydrophobic (Figure 23.1) [39,40]. Synthetic packaging films commonly exhibit fairly high contact angles, and therefore their surfaces have to be modified prior to their printing [38]. However, a high value for water contact angle is of interest in packaging films used for food preservation.
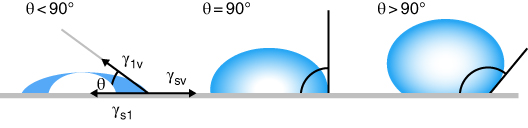
Figure 23.1 Illustration of contact angles formed by sessile liquid drops on a smooth homogeneous solid surface.
Source: Adapted from Yuan and Lee [39] with permission from Springer.
The water contact angles of some polymeric and biopolymeric films are shown in Table 23.4. It can be seen that the values are in the range 51°–105.4° for synthetic films and 27°–130.4° for hydrocolloid‐based edible films. Among the synthetic films, the ones with more polar groups such as PVA, polyvinyl acetate, and nylon 6 show a lower contact angle, and upon decreasing the polarity of these films, the contact angle tends to reduce. The water contact angle may decrease when the surface is highly hydrophilic, that is, in hydrocolloid‐based films. In fact, the neat films, or the films made from pristine or native hydrocolloids, usually exhibit a high affinity for moisture, which justifies the need for surface hydrophobization by means of chemical modification [40]. Nevertheless, some hydrocolloids produce films with adequate contact angles (gliadin films = 85–105° and agar film = 92.6°), which obviate the surface modification requirements [42,43]. A number of studies have been conducted to modify starch, hemicellulose, chitosan, and chitin polysaccharides, with the aim of imparting higher hydrophobicity to the surface of the film [40]. As a result, for example, the water contact angle was shifted from 43° for the native starch to 123° with the modified starch (Table 23.4). Various studies have shown that the type and concentration of plasticizer and hydrophobic substances can deeply influence this characteristic, besides the nature of the biopolymer as the most effective factor. The contact angle of many gum‐based films was in the range 30°–80°. It is expected that with increasing hydrophilic plasticizer content, the surface of the film would be easily wetted, as observed in different studies [ 4, 7, 9, 19, 20, 22]. However, findings for brea gum, LPSG, and SSG films, plasticized with sorbitol and glycerol, were inconsistent. No logical reason was presented for this phenomenon [ 3, 6, 23]. Generally, organic components increase the surface hydrophobicity, obviating the need for chemical modification (hydrophobization).
Table 23.4 Water contact angle values of gum‐based films and some synthetic plastic films.
| Gum/mucilage | Plasticizer (% w/w) | Additive | Water contact angle (°) | Reference | Plastic | Water contact angle (°) | Reference |
| AHSG | Glycerol (0–45) | — | 48.8–75.6 | [7] | PVA | 51 | [41] |
| AHSG | Glycerol (50) | PVA (0–100% w/w) | 30.9–74.6 | [8] | Nylon 6 | 62.6 | |
| BSG | Glycerol (100) | Palmitic acid (0–25% w/w) | 52.7–81.5 | [4] | PMMA | 70.9 | |
| Glycerol (100) | Stearic acid (0–25% w/w) | 52.7–97.33 | [4] | Nylon 12 | 72.4 | ||
| Glycerol (100) | Oleic acid (0–25% w/w) | 52.7–93.6 | [4] | PET | 72.5 | ||
| Glycerol (40–100) | — | 51.3–74.3 | [4] | PVDC (Saran) | 80 | ||
| Sorbitol (40–100) | — | 41.8–63.0 | [4] | PC | 82 | ||
| BSG | Glycerol (0–50) | — | 39.1–83.4 | [9] | PVC | 85.6 | |
| BSG | Glycerol (30) | OVES (0–5% w/w) | 48.5–82.0 | [32] | PS | 87.4 | |
| BG | Glycerol (0–30) | — | 67.3–71.2 | [3] | Nylon 10,10 | 94 | |
| CSG | Glycerol (25–50) | — | 43.7–79.8 | [19] | PE | 96 | |
| LPSG | Glycerol (40–70) | — | 68.3–72.9 | [20] | PP | 102.1 | |
| LPSG | Glycerol (60) | GPP (20–80% w/w) | 60.5–75.9 | [6] | LDPE | 105.4 | [42] |
| LPSG | Glycerol (60) | Palmitic acid (0–30% w/w) | 70.2–122.9 | [21] | LDPE | 100.7 | [43] |
| LPSG | Glycerol (60) | Stearic acid (0–30% w/w) | 70.2–130.4 | [21] | Chitin | 50–84 | [40] |
| PSG | Glycerol (15–35) | — | 41.0–84.4 | [22] | Chitosan | 27–82 | [40] |
| SSG | Glycerol (40–100) | — | 32.2–55.9 | [23] | Starch | 43–123 | [40] |
| SSG | Sorbitol (40–100) | — | 41.5–103.1 | [23] | Hemicellulose | 70–123 | [40] |
| Agar | Glycerol (15) | 15 | 92.6 | [42] | Zein | 62.3 | [44] |
| CS | Glycerol (15) | 15 | 50.4 | [42] | Zein plasticized with glucose | 52.3 | [44] |
| AX | Glycerol (15) | 15 | 65.5 | [42] | Zein plasticized with galactose | 56.2 | [44] |
| Glycerol (15) | 68.6 | [43] | Zein plasticized with fructose | 50.1 | [44] | ||
| Glycerol (15) | Palmitic acid (30% w/w) | 64 | [43] | ||||
| Glycerol (15) | Stearic acid (30% w/w) | 68.6 | [43] | ||||
| Glycerol (15) | Triolein (30% w/w) | 39 | [43] |
AHSG: Alyssum homolocarpum seed gum, BSG: basil seed gum, BG: Brea gum, CSG: Cress seed gum, LPSG: Lepidium perfoliatum seed gum, GPP: Grass pea protein, PSG: Psyllium seed gum, SSG: Sage seed gum, CS: Cassava starch, AX: Arabinoxylan, OVES: Origanum vulgare (oregano) essential oil, PVA: Polyvinyl alcohol, LDPE: Low‐density poly ethylene, EVOH: Ethylene vinyl alcohol, PET: Polyethylene terephthalate, PMMA: Polymethyl methacrylate, PVDC: Polyvinylidene chloride, PC: Polycarbonate, PS: Polystyrene, PE: Polyethylene, PP: Polypropylene.
Incorporation of long‐chain fatty acids (C16–C18) into gum‐based films changed the contact angle from 70.2° to 130.4° in LPSG films [21]. This value is close to that of the superhydrophobic surfaces, with the water contact angle being greater than 150°, indicating hardly any contact between the deposited liquid droplet and the surface [39]. On the other hand, the hydrophilicity of some films such as cellophane is so large that water droplets are immediately absorbed, and the contact angle cannot be measured [43]. Increasing film hydrophobicity is not always the reason for contact angle increase. For example, Hashemi et al. [32] suggested that covering hydrophilic groups of BSG active film with oregano essential oil increased the contact angle by more than 30°. The values displayed in Table 23.4 are mostly the initial water contact angles, and it is expected to decrease the angles with lapse of time for hydrophilic surfaces due to rapid changes in the surface properties [34].
23.3.4.4 Moisture Uptake and Moisture Sorption Isotherm
Moisture uptake characteristics could be a good indicator of the edible/biodegradable film's water sensitivity. At high RH, water uptake can soften the film structure through plasticization, making it easier for diffusing molecules to pass through the film. Accordingly, it is essential to minimize the tendency of the film to absorb moisture in order to increase the shelf life of foods. The uptake of moisture by films depends on both the chemical structure (interactions, number of accessible sites for absorption, film components such as plasticizers, different additives and modifying agents) and film morphology [ 8, 24, 34]. Also, due to the hydrophilic nature of hydrocolloid‐based edible films, all their properties are commonly governed by the RH of the surrounding environment. For this reason, determination of water uptake has been performed under two RH conditions: constant and variable. At constant RH, a saturated salt solution with a relatively high RH such as potassium sulfate or sodium chloride is used for testing the moisture uptake of films pieces. In the second method, moisture sorption isotherms can be generated over a wide range of water activities (aw) by using different saturated salt solutions. The determination of the moisture sorption isotherms can be useful for predicting the durability of edible films and also of wrapped foods during preservation at stores with various RH values [6]. For instance, the result of one study indicated that brea gum films disintegrated at RH values higher than 75% owing to great hygroscopicity [3]. In general, moisture absorption of hydrophilic films increases as a function of water activity, but the moisture absorption rate may be different within a wide range of aw [6].
Similar to foodstuffs, the sorption behavior of edible films and coatings has been extensively elucidated by using two well‐known isotherm equations of Guggenheim, Anderson, and de Boer (GAB) and Brunauer, Emmett, and Teller (BET) [45]. Despite the fact that a much broader range of aw values (0.05–0.8) is covered by GAB compared with BET, both of them have been employed for fitting experimental sorption isotherm data of edible films. Monjazeb et al. [8] investigated the sorption isotherms of composite films made from AHSG and PVA in the aw range 0.11–0.90. The obtained data for absorptions were fitted with the GAB equation, and a sigmoidal shape was produced for all films (Figure 23.2). They observed a slow rate of moisture absorption at low and intermediate aw values, and then a steep rise in the films' moisture content at aw > 0.53. In another study, sorption isotherms of films made from brea gum, fitted well with the BET model, showed a small tendency to water absorption at aw < 0.50, and exponentially increased with RH increase [3]. But, in the case of gum Cordia films, there was a linear rise in moisture content until RH 70% was reached, implying lower hydrophilicity of these films compared with brea gum and AHSG films [17]. As with most foods, the hydrophilic character of gum‐based films often produces a sigmoidal shape for their sorption isotherms [ 8, 17].
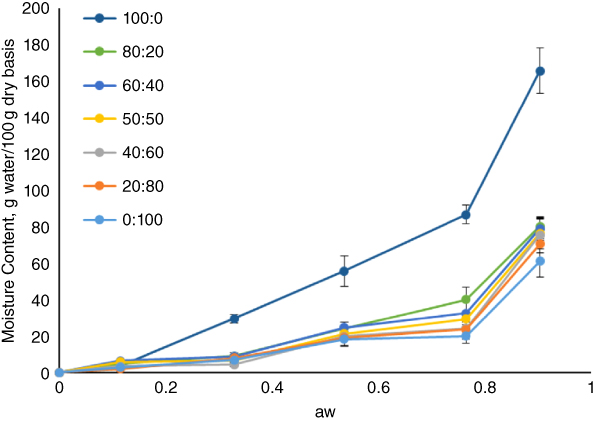
Figure 23.2 Moisture absorption isotherms of PVA–AHSG blend films at 25 °C.
Source: Adapted from Monjazeb Marvdashti et al. [8] with permission from Elsevier.
The use of hygroscopic plasticizers can easily enhance susceptibility to moisture absorption and swelling of hydrocolloid‐based films, which was confirmed by many studies. In these studies, moisture uptake of films fabricated from gum or mucilage of AHSG, BSG, LPSG, SSG, brea gum, and gum Cordia gum was increased by different concentrations of glycerol, sorbitol, PEG 200, and PEG 400 plasticizers [ 3, 4, 6, 7, 16, 20, 21, 23]. This was because of the hydrophilic character of the abovementioned plasticizers originating from their hydroxyl groups, which show high affinity for binding with the water molecules of the surrounding atmosphere. Although the type and content of the plasticizer were unimportant factors to influence the sorption isotherms, they dramatically influenced the moisture uptake, and with an increase in plasticizer concentration, water absorption of gum‐based films increased [ 3, 4, 7, 20, 23]. According to the findings, glycerol acted as a potent hygroscopic material compared with sorbitol in BSG and SSG films, and the water absorption value of BSG film was 1.5–1.7 orders of magnitude larger than that of SSG film [ 4, 23]. This claim was confirmed by the research of Abdul Haq et al. [46]. They observed that the moisture susceptibility of gum Cordia films including various plasticizers at different RH values was in the order of glycerol > sorbitol > PEG 200 > PEG 400 [46]. This can be explained by the molecular size of the plasticizers, which determines how a given plasticizer may contribute in the film structure [ 46,47]. Furthermore, at the same concentration of plasticizers, the number of hydroxyl groups per unit mass is an influential factor in moisture uptake, and this ratio of glycerol is higher than that of others [46].
Film properties are usually influenced by the addition of modifying substances. These materials can modify the sorption behavior of edible films. Lipids and nanomaterials are the common additives which reduce the water absorption of hydrophilic films by two different mechanisms. The sorption behavior of some gum‐based emulsified or nanocomposite films was examined. In one study, incorporation of 10% w/w palmitic or stearic fatty acids into LPSG film led to a water uptake drop by about 60% [20]. In another study, the same lipids at the level of 25% decreased the absorption value of BSG up to 27% [4]. The authors hypothesized that fatty acids partially cover the hydrophilic sites of the biopolymer, and as a result, the accessibility of water molecules was restricted [ 4, 20]. However, montmorillonite, a common nanomaterial and rich in hydroxyl groups, directly binds to hydrophilic groups of the biopolymer and reduces the chances of water molecule absorption. Plots of the sorption isotherms of brea gum nanocomposite films by Slavutsky and Bertuzzi [ 13, 27] supported the highlighted role of nanoclay in diminishing water uptake.
In conclusion, taking into consideration the high water uptake of gum‐based films, especially at RH > 50%, it is suggested that these films be used for wrapping foods in regions with a dry and semi‐dry climate. In contrast, synthetic films are resistant or less susceptible to water absorption and are thus preferred to package foods subject to intermediate and high RHs.
23.3.5 Mechanical Properties
Food packaging is responsible for protecting food against a variety of external stresses until it is consumed. Hence, proper selection of packaging material with adequate mechanical characteristics is of great importance in order to maintain food quality [34]. An edible film or coating may provide some mechanical protection for a food, reducing bruising and breakage and thus improving food integrity [1]. The mechanical properties of edible/biodegradable films are evaluated by measuring the three usual parameters: tensile strength (TS), elongation at break (EB), and elastic or Young's modulus (EM or YM) as an index of stiffness. Some other tests like the puncture test may be sometimes performed in certain films, too. The mechanical properties of films are mainly associated with the distribution and density of the intermolecular and intramolecular interactions allowed by the primary and spatial structures [1]. These interactions appear to be very important in protein films compared with polysaccharide ones due to the individual characteristics of proteins, such as four levels of structure, heat sensitivity, enzyme‐induced bonds, and so on. The presence of protein and protein derivatives as impurities in the commercial and natural gums might interfere with intermolecular interactions during film preparation, and thereby stabilize the films' network.
Films usually become dry, weak, and brittle without the contribution of plasticizers in the film fabrication process. For this reason, different types and concentrations of plasticizers have been tested to modify brittleness and flexibility, and to achieve films with adequate mechanical properties. Besides mechanical attributes, other attributes of the film will often be changed upon plasticizer interference. Plasticization with polyols usually decreases the stiffness and increases the extensibility of films. The results of mechanical measurements showed that with increasing levels of glycerol, sorbitol, PEG 200, and PEG 400 in the natural hydrocolloids films, EB always increased at the expense of the TS and EM [ 3, 4, 7, 9, 15, 17, 19, 20, 22, 23]. Plasticizer type may be an important factor that influences film structure, as reported for gum‐based films. The films made from SSG, BSG, and gum Cordia containing glycerol showed higher EB and lower TS compared with those plasticized with sorbitol owing to the greater number of hydroxyl groups at the same concentration [ 4, 17, 23]. Indeed, the greater amount of water absorbed by hydrophilic glycerol enhances the spatial distance between biopolymer chains, thereby reducing the TS [17]. Moreover, gum Cordia films became stiffer upon increasing the plasticizers' molecular weight, and the magnitude of mechanical variations, as a function of plasticizer content, was lower in comparison to low‐molecular‐weight plasticizers [17]. In contrast to polyol plasticizers, the mechanical behavior of edible films does not commonly follow a predictable trend in response to materials with inherited plasticizing capability, for example, fatty acids, essential oils, and other lipid derivatives. This unexpected behavior was observed in gum‐based films, too. Incorporation of Zataria multiflora and thyme essential oils into CSG and BSG films, respectively, decreased the TS [ 12, 18], while ZMEO, in other work, increased the TS of BSG film [26]. In these studies, the flexibility of all films was improved by the aforementioned essential oils [ 12, 18, 26]. Long‐chain fatty acids and beeswax can often degrade the mechanical characteristics of edible emulsified films. This phenomenon was also reported for the films prepared from LPSG, brea gum, and gum Cordia [ 14, 16, 21]. Lipids generally produce very brittle and weak films because of their monomeric structure; the protein or polysaccharide‐based films casted from emulsified solutions possess a non‐continuous matrix due to weak interactions between the biopolymer – a polar molecule – and lipid – a nonpolar molecule – resulting in decreased TS and EB [2]. However, Mohammad Amini et al. [4] found that the incorporation of palmitic, stearic, or oleic acids in emulsified BSG films is accompanied by enhanced TS and reduced EB.
One of the common methods of film fabrication is blending two different polymers, with the aim of producing a new film (copolymer) with modified properties. Novel biocomposite films were made by blending some natural hydrocolloids with other hydrocolloids such as whey protein concentrate (WPC), locust bean gum (LBG), maize starch, and grass pea protein as well as PVA [ 6, 8, 24, 25,48]. Mechanical investigation of the novel films showed that increasing mucilage or gum ratio in Salvia hispanica/WPC [48] and LPSG–grass pea protein [6] blended films achieves the desired mechanical properties, while it decreased cohesion and mechanical resistance in gum tragacanth–LBG [24] and chia flour–maize starch [25] blend films. In the films from AHSG–PVA, increasing the gum concentration reinforced the film network and reduced extensibility [8].
The mechanical properties of films developed from natural hydrocolloids as well as some commercial synthetic films are presented in Table 23.5. Two main advantages of synthetic films compared with the biopolymeric ones are the WVP and mechanical characteristics. In spite of the many attempts that have been made to improve the structure of the bio‐based film and make it comparable to that of the synthetic films, the synthetic ones are still under the better conditions. Similar to most biopolymers, gum‐based films are relatively weak from the mechanical standpoint. Brea gum and gum Cordia films displayed the lowest (1.3 MPa) and highest (55 MPa) resistance in the tensile test, respectively. Therefore, the value of the strongest gum‐based film was very far from that of the strongest synthetic films (polystyrene = 175 MPa). On the other hand, the TS values of LDPE (9–17 MPa), the weakest synthetic film, were between those of the gum‐based films. The lowest extensibility belonged to gum tragacanth–LBG blend films (0.7%–1.1%), which was close to the EB of polystyrene film (1%). From the other point of view, the EB value of LDPE film (500%) was more than seven orders of magnitude greater than the most flexible gum‐based film, that is, CSG (EB ≈ 70%).
Table 23.5 Mechanical properties values of gum‐based films and some synthetic plastic films. a
| Gum/polymer | Plasticizer (% w/w) | Additive | Tensile strength (MPa) | Young's modulus (MPa) | Elongation at break (%) | Reference |
| AHSG | Glycerol (0–45) | — | 11.8–19.4 | 279–438 | 25–43 | [7] |
| AHSG | Glycerol (50) | PVA (0–100% w/w) | 26.0–37.2 | 50–3526 | 1.4–370 | [8] |
| BSG | Glycerol (100) | Palmitic acid (0–25% w/w) | 5.7–8.4 | — | 29.9–37.1 | [4] |
| Glycerol (100) | Stearic acid (0–25% w/w) | 5.7–9.9 | — | 25.8–37.1 | [4] | |
| Glycerol (100) | Oleic acid (0–25% w/w) | 5.7–5.8 | — | 33–37.1 | [4] | |
| Glycerol (40–100) | — | 5.5–15.4 | — | 10.3–22.5 | [4] | |
| Sorbitol (40–100) | — | 5.5–35.4 | — | 3.5–11 | [4] | |
| BSG | Glycerol | ZMEO (1–3% v/v) | 19.7–34.6 | — | 21.5–39.5 | [26] |
| BSG | Glycerol (0–50) | — | 18.1–31.7 | — | 18.5–39.4 | [9] |
| BSG | Glycerol (30) | Thymol (0–3% w/w) | 2.3–9.1 | — | 10.1–12.2 | [12] |
| BG | Glycerol (0–30) | — | 3–13 | — | 24–2 | [3] |
| BG | Glycerol (25) | Montmorillonite (0–5% w/w) | 7.4–20.9 | 392–738 | 9.3–19.9 | [13] |
| BG | Glycerol (0–40) | Beeswax (0–40% w/w) | 1.3–7.5 | — | 3.5–8 | [14] |
| CSM (Salvia hispanica) | Glycerol (50) | WPC (75% w/w) | 3.8–4.7 | — | 16.3–17.3 | [48] |
| CSM (Salvia hispanica) | Glycerol (50) | WPC (80% w/w) | 2.7–3.9 | — | 15.1–15.2 | [48] |
| CSM | Glycerol (25–75) | — | 9.4–17.7 | 105–778 | 1.9–15.9 | [15] |
| GC | PEG 400 (20) | GMS (0–0.02% w/w) + Beeswax (0–20% w/w) | 3–15 | 190–900 | 5–28 | [16] |
| GC | Glycerol (10–30) | — | 3–55 | 5–1900 | 0–62 | [17] |
| Sorbitol (10–30) | — | 12–55 | 5–1900 | 0–50 | [17] | |
| PEG 200 (10–30) | — | 17–55 | 600–1900 | 0–40 | [17] | |
| PEG 400 (10–30) | — | 24–55 | 950–1900 | 0–23 | [17] | |
| CSG | Glycerol (35) | ZMEO (0–4% w/w) | 6.7–9.8 | 20–33.1 | 28.8–69.6 | [18] |
| CSG | Glycerol (25–50) | — | 7.8–12.8 | 17–65 | 19–40 | [19] |
| LPSG | Glycerol (40–70) | — | 3.4–3.9 | 49–110 | 1.2–4.3 | [20] |
| LPSG | Glycerol (60) | Palmitic acid (0–30% w/w) | 11.5–11.9 | — | 3.2–3.7 | [21] |
| LPSG | Glycerol (60) | Stearic acid (0–30% w/w) | 11.3–11.9 | — | 3.2–3.7 | [21] |
| PSG | Glycerol (15–35) | — | 7.8–14.3 | 14–29 | 24.5–34.7 | [22] |
| SSG | Glycerol (40–100) | — | 4.4–16.5 | — | 9–38 | [23] |
| SSG | Sorbitol (40–100) | — | 18.6–26.2 | — | 4–14 | [23] |
| GT | — | LBG (0–100% w/w) | 11.6–24.3 | — | 0.7–1.7 | [24] |
| LDPE | 9–17 | — | 500 | [1] | ||
| HDPE | 17–35 | — | 300 | |||
| PP | 40 | — | 300 | |||
| OPP | 165 | — | 50–75 | |||
| Polyester | 175 | — | 70–100 | |||
| HPMC | 69 | — | 10 | |||
| PS | 35–55 | — | 1 | |||
| MC | 62 | 10 | ||||
| Amylose | 70 | — | 23 | |||
| Cellophane | 55–124 | — | 16–60 |
AHSG: Alyssum homolocarpum seed gum, BSG: Basil seed gum, BG: Brea gum, CSM: Chia seed mucilage, GC: Gum Cordia, CSG: Cress seed gum, LPSG: Lepidium perfoliatum seed gum, PSG: Psyllium seed gum, SSG: Sage seed gum, GT: Gum tragacanth, LDPE: Low‐density poly ethylene, HDPE: High‐density poly ethylene, PP: Polypropylene, OPP: Oriented polypropylene, HPMC: Hydroxypropyl methylcellulose, PS: Polystyrene, MC: Methylcellulose, PVA: Polyvinyl alcohol, GMS: Glycerol monostearate, WPC: Whey protein concentrate, LBG: Locust bean gum, ZMEO: Zataria multiflora essential oils.
a Test conditions: 25 °C, RH = 50%.
23.3.6 Visual Characteristics
The visual characteristics (glossiness, color, transparency, etc.) of edible/biodegradable films and coatings are important factors in consumer acceptability and even food quality due to interactions with light. The color of films or coatings is important from the psychological point of view, too. Color carries meaning and can influence consumers' thoughts, feelings, and behaviors [49]. Synthetic packaging films might sometimes be colored by colorants for different purposes. Coloration may be also done for edible films and coatings because they have the carrier potential for various nutraceutical and functional additives such as color. The marketability and light protection of wrapped foods could be positively influenced by adding the permitted and food‐grade coloring agents to films.
The filmogenic solutions from natural hydrocolloids leave colorful or colorless, see‐through films after drying, which increases consumer confidence (Figure 23.3). The color of the final edible films and coatings is strongly dependent on the nature and purity of the biopolymer. For instance, the films developed from CMC are colorless and transparent, while the gum‐based films exhibit a variety of colors depending on the gum or mucilage impurities. The color developed by the extracted mucilage probably originates from the tegument pigments or tannic substances [50]. The extraction process is occasionally followed by partial purification of gum or mucilage, often by alcoholic precipitation, thereby producing a fairly pure product. But the gum powder still retains some impurities like protein and pigments. There are such impurities even within commercial gums, which, for example, induce a pale yellow color for gum arabic and guar gum. Also, according to the literature, the protein content of commercial guar, locust bean, and Tara gums is 10%, 8%, and 3.13%, respectively [50]. The remaining pigments (directly) and proteins (indirectly) – Millard reactions during heating of film‐forming solution – may affect the film's color. Besides the impurities, hydrocolloid extraction conditions, the plasticizer content, the ratio of major components (in the composite or blended films), and the film‐making technique have been shown to influence color [51].

Figure 23.3 Psyllium seed gum film plasticized with glycerol.
Source: Adapted from Ahmadi et al. [22] with permission from Elsevier.
The L*, a*, and b* CIE Lab color values, total color difference (ΔE), and whiteness and yellowness indexes are used to describe film color and its changes. L* refers to the lightness component, which ranges from 0 to 100, and parameters a* (green to red or redness) and b* (blue to yellow or yellowness) are two chromatic components which range from −120 to +120 [52]. The result of color measurements showed that the L*, a*, and b* values of gum‐based films ranged from 57 to 83, −18.26 to 14.9, and −6.4 to 50, respectively [ 3, 4, 7, 9 12– 15 19– 23, 46]. The relatively high L* value of these films suggests that their lightness is acceptable. The film lightness may vary slightly or significantly depending on the plasticizer concentration; unusually, for chia seed mucilage (CSM) [15] and LPG [20] films, the L* value exhibited an upward trend as the level of plasticizer increased [ 3, 4, 7, 9, 14, 19, 22, 23, 46]. On the other hand, nanoclay, essential oils, and lipid derivatives, as film additives, negatively influenced the lightness of some gum‐based films [ 12– 14, 21].
The gum‐based films' color became yellowish (greater value for b*) in the presence of higher plasticizer levels, except for the films from brea gum and gum Cordia [ 3, 46]. Variations of a* in the films did not follow the specific pattern. The deepest variations for color occurred for brea gum films containing 25% glycerol, in which the a* and b* values changed by three and four orders of magnitude, respectively [3], and for BSG films containing 50% glycerol, in which L* increased from 64 to 79 [9]. It seems that glycerol is probably and interestingly the most effective agent for color changes in gum‐based films.
Besides color, light transmittance from films has been received more attention and is described by the terms transparency and opacity (cloudiness and turbidity). Although the product content could be viewed behind the transparent films, destructive UV light also pass through them and adversely affect food by generating free radicals in products by a wide variety of organic photochemical reactions [35]. On the other hand, opaque films can protect food well against UV light at the expense of film attractiveness. Some treatments for improving the permeability and mechanical properties of films might be associated with unwanted changes in opacity. Nanosized materials (nanoclay, metal oxides, etc.) and lipids with a high melting point such as waxes and saturated long‐chain fatty acids can usually cause cloudy films (Figure 23.4). For instance, incorporation of stearic and palmitic acids into LPSG and BSG films increased turbidity, while the contribution of oleic acid in BSG film turbidity was small [ 4, 23]. Researchers have deduced that the function of lipid molecules in the light transmission and, consequently, film opacity, originates from their dispersion in the film matrix and light scattering. This phenomenon was observed for brea gum films in the presence of montmorillonite [13]. The light transmittance behavior of films when loaded with transparent liquid plasticizers and essential oils is unpredictable, and transparency may increase or decrease. This was evidenced by measurement of transparency in gum‐based films by different workers [ 3, 12, 15, 20, 32, 46].
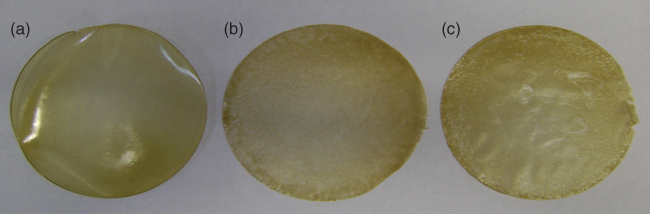
Figure 23.4 Brea gum films (plasticized by glycerol) incorporated with (a) 0, (b) 20, and (c) 40 g/100 g Beeswax.
Source: Adapted from Spotti et al. [14] with permission from Elsevier.
23.3.7 Scanning Electron Microscopy (SEM)
SEM has been used to visualize the surface morphology and microstructure of edible films and coatings. The captured micrographs from the surface or cross section are useful for elucidating film characteristics such as the WVP, mechanical properties, opacity, and density. Generally, there are some bubbles in the structure of natural hydrocolloids films, especially at a higher concentration of materials, or because of ineffective degassing during film preparation, which would negatively influence the characteristics of the film. SEM micrographs of gum‐based films indicated a smooth surface and homogenous structure for many unplasticized films, with a small number of cracks [ 3, 7, 8, 14, 15, 18, 20, 21, 46]. In some cases, the incorporation of plasticizers or lipid derivatives induced a coarse surface, and no crack was revealed due to their plasticizing effect [ 3, 7, 9, 14, 20, 21]. Cross‐sectional studies of emulsified films can provide important information about biopolymer and lipid interactions. Abdul Haq et al. [16] observed a laminar structure in the cross‐sectional image of the gum Cordia–beeswax emulsified films because of the coalescence of lipid globules during film drying. They declared that these lipid layers profoundly affected the WVP of the emulsified films [16]. A structural discontinuity induced by stearic and palmitic fatty acids was also found in the cross‐sectional images of LPSG films, which explains the poor mechanical properties of these emulsified films [21]. Additionally, coalescence of fatty acids in the emulsified LPSG films exposed to drying (or even keeping) occurred and led to surface roughness and structural reorganization [21]. The volatility of most essential oils is problematic for the film matrix, and SEM micrographs verified that the BSG and CSG films loaded with thyme and ZMEOs, respectively, have a loose texture with a sponge‐like structure due to evaporation of essential oils during film drying (Figure 23.5); hence, the authors concluded that one of the reasons for the reduction in the water vapor barrier of these films as a function of the increase in essential oils was the formation of pores and holes in the microstructure of the films [ 12, 18].

Figure 23.5 Scanning electron micrograph of cross section of cress seed gum films: (Left) 0% ZMEO, (right) 4% ZMEO.
Source: Adapted from Kazerani et al. [18] with permission from the publisher.
23.4 Applications
Unlike biodegradable films, edible films and coatings are not meant to fully replace with conventional non‐edible films and coatings. They are used as primary packaging which is in contact with the product but not as secondary or tertiary levels of packaging. Edible films and coatings are an excellent carrier for various functional and nutraceutical components such as antioxidants, antimicrobial, and vitamins, and thus using them in direct contact with fruits, vegetables, and ready‐to‐eat foods will provide high value‐added foods with extended shelf life. Moisture and oil migration, volatility of aromatic compounds, and oxygen permeation are customary mass transfer problems, and nearly all foods are exposed to at least one of them [1]. Coatings not only act as integrity maintainers and appearance enhancers but also decrease mass transfer, especially leaching of liquefied food components during the heating process.
A number of studies have been conducted on the development and use of edible coatings from natural hydrocolloids in order to introduce novel coatings for food preservation. BSG edible coating containing oregano essential oil was used for fresh‐cut apricots. The release of essential oil during storage reduced the microbial population of the fruit due to antimicrobial performance, and effectively retarded the spoilage. The coated apricots exhibited higher soluble phenolics, antioxidant activity, aroma, and overall acceptability compared with uncoated ones [10]. BSG coating loaded with thymol was applied to decrease oil uptake and oxidation in shrimp during deep‐fat frying. The coating considerably lowered both oil uptake and moisture loss; the oxidation indexes (peroxide value and thiobarbituric acid), toughness, and stiffness of the coated shrimps were lower than those of the uncoated ones [53]. In another work, the microbial count of shrimp during cold storage was lessened by thymol‐incorporated BSG coating, without harmful effects on organoleptic properties [11]. Abdul Haq et al. [54] prepared an edible coating by addition of methanolic extract of the fruit of Cordia myxa into gum Cordia coating solution; using this coating increased the shelf life of pine nuts (a rich source of unsaturated fatty acids) by postponing oil oxidation. Applying LPSG edible coating on chiffon cake improved cohesiveness and softness, and retained most of its moisture content [55]. Plantago major seed mucilage coating incorporated with Anethum graveolens (dill) essential oil was employed for increasing the shelf life of beef in refrigerated storage; the coating prevented microbial growth and lipid oxidation owing to the antioxidant and antimicrobial potentials of the essential oil, thereby enhancing the quality and storage life of beef. Also, the color and overall acceptability of coated beef were much better than those of the uncoated samples after 18 days of refrigerated storage [56]. An emulsified coating was fabricated from a combination of Psyllium seed gum and sunflower oil; application of the coating on the fresh‐cut papaya could slow water loss and prolonged shelf life up to two weeks [57]. In order to limit the oil uptake and enhance the quality of deep‐fat fried French‐fries, potato sticks were treated by two hydrocolloid‐based (methylcellulose and tragacanth) coating solutions before frying; these coatings successfully reduced water loss and oil uptake and enhanced product appearance and crispiness [58].
23.5 Conclusions and Future Trends
This chapter focused on the preparation and measurement of films and coatings from novel natural hydrocolloids. Gum‐based films with appropriate quality and adequate thickness were successfully developed in accordance with customary methods. Due to the hydrophilic nature of gum and mucilage powders, the films and coatings are mostly water sensitive, and the WVP, moisture uptake, water solubility, and water contact angle are strongly influenced by water molecules. The easiest way to reduce the water susceptibility of the films is to incorporate lipid derivatives and nanoclay, one of the most well‐known nanomaterials, which improves the abovementioned characteristics at the expense of transparency, OP, and, occasionally, mechanical properties. Also, plasticizer type and especially concentration usually show significant effects on the films' quality. The WVP is comparable with that of other polysaccharide‐based films, but it is more often several orders of magnitude greater than that of synthetic films. The OP of gum‐based films is relatively low due to the inherited polarity of polysaccharides, and the values are in the range reported for most polymers and biopolymers. As expected, and similar to other polysaccharide‐based films, the water solubility of gum‐based films is intermediate, and in some cases it is high, which may be beneficial in some food and pharmaceutical applications, and also after the films are discarded. Measurement of surface hydrophilicity indicates that the water contact angle of natural hydrocolloid films is lower than that of plastic films and is mainly localized in a narrow range. However, hydrophobization by hydrophobic materials made the contact angle of some films equal to that of the most hydrophobic plastics. Investigation of the behavior of films at different RH values revealed relatively high moisture absorption, especially at RH > 50%, which restricts their application. Although gum‐based films are mechanically inferior to synthetic films, the mechanical properties of some of these films are promising. Also, resistance against tensile force is further improved through nanoparticle inclusion. The impurities in gum and mucilage powders impart a light color to films and coatings, thereby increasing attractiveness without detrimental effects on the product. In conclusion, the characteristics of the films from natural hydrocolloids are very close to those of most commercial polysaccharide‐based films and can be used as primary packaging for protecting and extending food shelf life.
More recently, many studies have been published on the development of edible/biodegradable films from new resources, especially natural hydrocolloids. They show considerable potential for film and coating making. Blended, nanocomposite, and emulsified films are derivatives of films made from gums or mucilages that aim for performance improvement. However, more studies are needed to overcome gum‐based films' deficiencies and make them more and more similar to synthetic plastic films. Growing concerns about environmental problems, and also the limitations of oil resources are good motivators for further research on the use of renewable resources for providing packaging materials and will help ensure a promising future for gum‐based films.
References
- 1 Gennadios, A. (2002). Protein‐Based Films and Coatings. CRC Press.
- 2 Zahedi, Y., Ghanbarzadeh, B., and Sedaghat, N. (2010). Physical properties of edible emulsified films based on pistachio globulin protein and fatty acids. Journal of Food Engineering 100 (1): 102–108.
- 3 Bertuzzi, M.A. and Slavutsky, A.M. (2013). Formulation and characterization of film based on gum exudates from brea tree (Cercidium praecox). Journal of Food Science and Engineering 3 (3): 113–122.
- 4 Mohammad Amini, A., Razavi, S.M.A., and Zahedi, Y. (2015). The influence of different plasticisers and fatty acids on functional properties of basil seed gum edible film. International Journal of Food Science & Technology 50 (5): 1137–1143.
- 5 Skurtys, O., Acevedo, C., Pedreschi, F. et al. (2014). Food Hydrocolloid Edible Films and Coatings. Nova Science Publishers.
- 6 Ebrahimi, S.E., Koocheki, A., Milani, E., and Mohebbi, M. (2016). Interactions between Lepidium perfoliatum seed gum‐grass pea (Lathyrus sativus) protein isolate in composite biodegradable film. Food Hydrocolloids 54: 302–314.
- 7 Mohammadi Nafchi, A., Olfat, A., Bagheri, M. et al. (2017). Preparation and characterization of a novel edible film based on Alyssum homolocarpum seed gum. Journal of Food Science and Technology 54 (6): 1703–1710.
- 8 Monjazeb Marvdashti, L., Koocheki, A., and Yavarmanesh, M. (2017). Alyssum homolocarpum seed gum‐polyvinyl alcohol biodegradable composite film: physicochemical, mechanical, thermal and barrier properties. Carbohydrate Polymers 155: 280–293.
- 9 Khazaei, N., Esmaiili, M., Emam Djomeh, Z. et al. (2014). Characterization of new biodegradable edible film made from basil seed (Ocimum basilicum L.) gum. Carbohydrate Polymers 102: 199–206.
- 10 Hashemi, S.M.B., Mousavi Khaneghah, A., Ghaderi Ghahfarrokhi, M., and Es, I. (2017). Basil‐seed gum containing origanum vulgare subsp. Viride essential oil as edible coating for fresh cut apricots. Postharvest Biology and Technology 125: 26–34.
- 11 Khazaei, N., Esmaiili, M., and Emam‐Djomeh, Z. (2017). Application of active edible coatings made from basil seed gum and thymol for quality maintenance of shrimp during cold storage. Journal of the Science of Food and Agriculture 97 (6): 1837–1845.
- 12 Ramadhani, R.R. (2017). Development of Antioxidant Film from Basil Seed (Ocimum Citriodourum) Mucilage Incorporated with Thyme Essential Oil. Bogor: Ogor Agricultural University.
- 13 Slavutsky, A.M., Bertuzzi, M.A., Armada, M. et al. (2014). Preparation and characterization of montmorillonite/brea gum nanocomposites films. Food Hydrocolloids 35: 270–278.
- 14 Spotti, M.L., Cecchini, J.P., Spotti, M.J., and Carrara, C.R. (2016). Brea gum (from Cercidium praecox) as a structural support for emulsion‐based edible films. LWT‐Food Science and Technology 68: 127–134.
- 15 Dick, M., Costa, T.M.H., Gomaa, A. et al. (2015). Edible film production from chia seed mucilage: effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydrate Polymers 130: 198–205.
- 16 Abdul Haq, M., Hasnain, A., Jafri, F.A. et al. (2016). Characterization of edible gum cordia film: effects of beeswax. LWT‐Food Science and Technology 68: 674–680.
- 17 Abdul Haq, M., Hasnain, A., and Azam, M. (2014). Characterization of edible gum cordia film: effects of plasticizers. LWT‐Food Science and Technology 55 (1): 163–169.
- 18 Kazerani, H., Fahimdanesh, M., Jouki, M. et al. (2016). Physical, mechanical, thermal and antimicrobial properties of active edible film made from cress seed gum incorporated with zataria essential oil. Carpathian Journal of Food Science & Technology 8 (4): 83–90.
- 19 Jouki, M., Khazaei, N., Ghasemlou, M., and Hadinezhad, M. (2013). Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydrate Polymers 96 (1): 39–46.
- 20 Seyedi, S., Koocheki, A., Mohebbi, M., and Zahedi, Y. (2014). Lepidium perfoliatum seed gum: a new source of carbohydrate to make a biodegradable film. Carbohydrate Polymers 101: 349–358.
- 21 Seyedi, S., Koocheki, A., Mohebbi, M., and Zahedi, Y. (2015). Improving the physical and moisture barrier properties of Lepidium perfoliatum seed gum biodegradable film with stearic and palmitic acids. International Journal of Biological Macromolecules 77: 151–158.
- 22 Ahmadi, R., Kalbasi Ashtari, A., Oromiehie, A. et al. (2012). Development and characterization of a novel biodegradable edible film obtained from psyllium seed (Plantago ovata Forsk). Journal of Food Engineering 109 (4): 745–751.
- 23 Razavi, S.M.A., Mohammad Amini, A., and Zahedi, Y. (2015). Characterisation of a new biodegradable edible film based on sage seed gum: influence of plasticiser type and concentration. Food Hydrocolloids 43: 290–298.
- 24 Mostafavi, F.S., Kadkhodaee, R., Emadzadeh, B., and Koocheki, A. (2016). Preparation and characterization of tragacanth‐locust bean gum edible blend films. Carbohydrate Polymers 139: 20–27.
- 25 Dick, M., Henrique Pagno, C., Haas Costa, T.M. et al. (2016). Edible films based on chia flour: development and characterization. Journal of Applied Polymer Science 133 (2): 42455–42464.
- 26 Hashemi Gahruie, H., Ziaee, E., Eskandari, M.H., and Hosseini, S.M.H. (2017). Characterization of basil seed gum‐based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohydrate Polymers 166: 93–103.
- 27 Slavutsky, A.M. and Bertuzzi, M.A. (2015). Thermodynamic study of water sorption and water barrier properties of nanocomposite films based on brea gum. Applied Clay Science 108: 144–148.
- 28 McHugh, T.H., Avena‐Bustillos, R., and Krochta, J. (1993). Hydrophilic edible films: modified procedure for water vapor permeability and explanation of thickness effects. Journal of Food Science 58 (4): 899–903.
- 29 Abdul Haq, M. (2013). Development and Application of Edible Films and Coatings from Gum Cordia (Cordia Myxa)k. University of Karachi.
- 30 Rossi Marquez, G., Han, J.H., Garcia‐Almendarez, B. et al. (2009). Effect of temperature, pH and film thickness on nisin release from antimicrobial whey protein isolate edible films. Journal of the Science of Food and Agriculture 89 (14): 2492–2497.
- 31 Kester, J.J. and Fennema, O.R. (1986). Edible films and coatings: a review. Food Technology 40: 47–59.
- 32 Hashemi, S.M.B. and Mousavi Khaneghah, A. (2017). Characterization of novel basil‐seed gum active edible films and coatings containing oregano essential oil. Progress in Organic Coatings 110: 35–41.
- 33 Jouki, M., Tabatabaei Yazdi, F., Mortazavi, S.A., and Koocheki, A. (2014). Quince seed mucilage films incorporated with oregano essential oil: physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocolloids 36: 9–19.
- 34 Oleyaei, S.A., Zahedi, Y., Ghanbarzadeh, B., and Moayedi, A.A. (2016). Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. International Journal of Biological Macromolecules 89: 256–264.
- 35 Zahedi, Y., Fathi Achachlouei, B., and Yousefi, A.R. (2018). Physical and mechanical properties of hybrid montmorillonite/zinc oxide reinforced carboxymethyl cellulose nanocomposites. International Journal of Biological Macromolecules 108: 863–873.
- 36 Ghasemlou, M., Khodaiyan, F., and Oromiehie, A. (2011). Physical, mechanical, barrier, and thermal properties of polyol‐plasticized biodegradable edible film made from kefiran. Carbohydrate Polymers 84 (1): 477–483.
- 37 Cecchini, J.P., Spotti, M.J., Piagentini, A.M. et al. (2017). Development of edible films obtained from submicron emulsions based on whey protein concentrate, oil/beeswax and brea gum. Food Science and Technology International 23 (4): 371–381.
- 38 Silvestre, C. and Cimmino, S. (2013). Ecosustainable Polymer Nanomaterials for Food Packaging: Innovative Solutions, Characterization Needs, Safety and Environmental Issues. CRC Press.
- 39 Yuan, Y. and Lee, T.R. (2013). Contact angle and wetting properties. In: Surface Science Techniques, 3–34. Springer.
- 40 Cunha, A.G. and Gandini, A. (2010). Turning polysaccharides into hydrophobic materials: a critical review. Part 2. Hemicelluloses, chitin/chitosan, starch, pectin and alginates. Cellulose 17 (6): 1045–1065.
- 41 Critical Surface Tension and Contact Angle with Water for Various Polymers, https://www.accudynetest.com/polytable_03.html?sortby=contact_angle (Accessed 29 November 2017).
- 42 The, D.P., Debeaufort, F., Voilley, A., and Luu, D. (2009). Biopolymer interactions affect the functional properties of edible films based on agar, cassava starch and arabinoxylan blends. Journal of Food Engineering 90 (4): 548–558.
- 43 Peroval, C., Debeaufort, F., Despre, D., and Voilley, A. (2002). Edible arabinoxylan‐based films. 1. Effects of lipid type on water vapor permeability, film structure, and other physical characteristics. Journal of Agricultural and Food Chemistry 50 (14): 3977–3983.
- 44 Ghanbarzadeh, B., Musavi, M., Oromiehie, A. et al. (2007). Effect of plasticizing sugars on water vapor permeability, surface energy and microstructure properties of zein films. LWT‐Food Science and Technology 40 (7): 1191–1197.
- 45 Timmermann, E., Chirife, J., and Iglesias, H. (2001). Water sorption isotherms of foods and foodstuffs: BET or GAB parameters? Journal of Food Engineering 48 (1): 19–31.
- 46 Abdul Haq, M., Jafri, F.A., and Hasnain, A. (2016). Effects of plasticizers on sorption and optical properties of gum cordia based edible film. Journal of Food Science and Technology 53 (6): 2606–2613.
- 47 Donhowe, I.G. and Fennema, O. (1993). The effects of plasticizers on crystallinity, permeability, and mechanical properties of methylcellulose films. Journal of Food Processing and Preservation 17 (4): 247–257.
- 48 Munoz, L., Aguilera, J., Rodriguez‐Turienzo, L. et al. (2012). Characterization and microstructure of films made from mucilage of Salvia hispanica and whey protein concentrate. Journal of Food Engineering 111 (3): 511–518.
- 49 Labrecque, L.I., Patrick, V.M., and Milne, G.R. (2013). The marketers prismatic palette: a review of color research and future directions. Psychology & Marketing 30 (2): 187–202.
- 50 Koocheki, A., Taherian, A.R., Razavi, S.M., and Bostan, A. (2009). Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocolloids 23 (8): 2369–2379.
- 51 Su, J.‐F., Yuan, X.‐Y., Huang, Z. et al. (2012). Physicochemical properties of soy protein isolate/carboxymethyl cellulose blend films crosslinked by Maillard reactions: color, transparency and heat‐sealing ability. Materials Science and Engineering: C 32 (1): 40–46.
- 52 Sedaghat, N. and Zahedi, Y. (2012). Application of edible coating and acidic washing for extending the storage life of mushrooms (Agaricus bisporus). Food Science and Technology International 18 (6): 523–530.
- 53 Khazaei, N., Esmaiili, M., and Emam‐Djomeh, Z. (2016). Effect of active edible coatings made by basil seed gum and thymol on oil uptake and oxidation in shrimp during deep‐fat frying. Carbohydrate Polymers 137: 249–254.
- 54 Abdul Haq, M., Alam, M.J., and Hasnain, A. (2013). Gum Cordia: a novel edible coating to increase the shelf life of Chilgoza (Pinus gerardiana). LWT‐Food Science and Technology 50 (1): 306–311.
- 55 Amirabadi, S., Koocheki, A., and Mohebbi, M. (2015). Effect of xanthan and Lepidium perfoliatum seed gums on quality and shelf‐life of chiffon cake. Iranian Food Science and Technology Research Journal 10 (4): 375–386.
- 56 Alizadeh Behbahani, B., Shahidi, F., Tabatabaei Yazdi, F. et al. (2017). Use of Plantago major seed mucilage as a novel edible coating incorporated with Anethum graveolens essential oil on shelf life extension of beef in refrigerated storage. International Journal of Biological Macromolecules 94: 515–526.
- 57 Yousuf, B. and Srivastava, A.K. (2015). Psyllium (Plantago) gum as an effective edible coating to improve quality and shelf life of fresh‐cut papaya (Carica papaya). International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering 9 (7): 765–770.
- 58 Hoseinabadi, V., Badii, F., Gharachorloo, M., and Heshmati, A. (2012). Effects of blanching and hydrocolloid coating of pot atoeswith methyl cellulose and tragacanth on French‐fries oil uptake and qualitative properties. Iranian Journal of Nutrition Sciences & Food Technology 6 (4): 71–81.
